mTOR Signaling and Potential Therapeutic Targeting in Meningioma
Abstract
1. Introduction
2. The mTOR Complex 1 (TORC1)
2.1. The NF2 Pathway
2.2. The PI3K/Akt Pathway
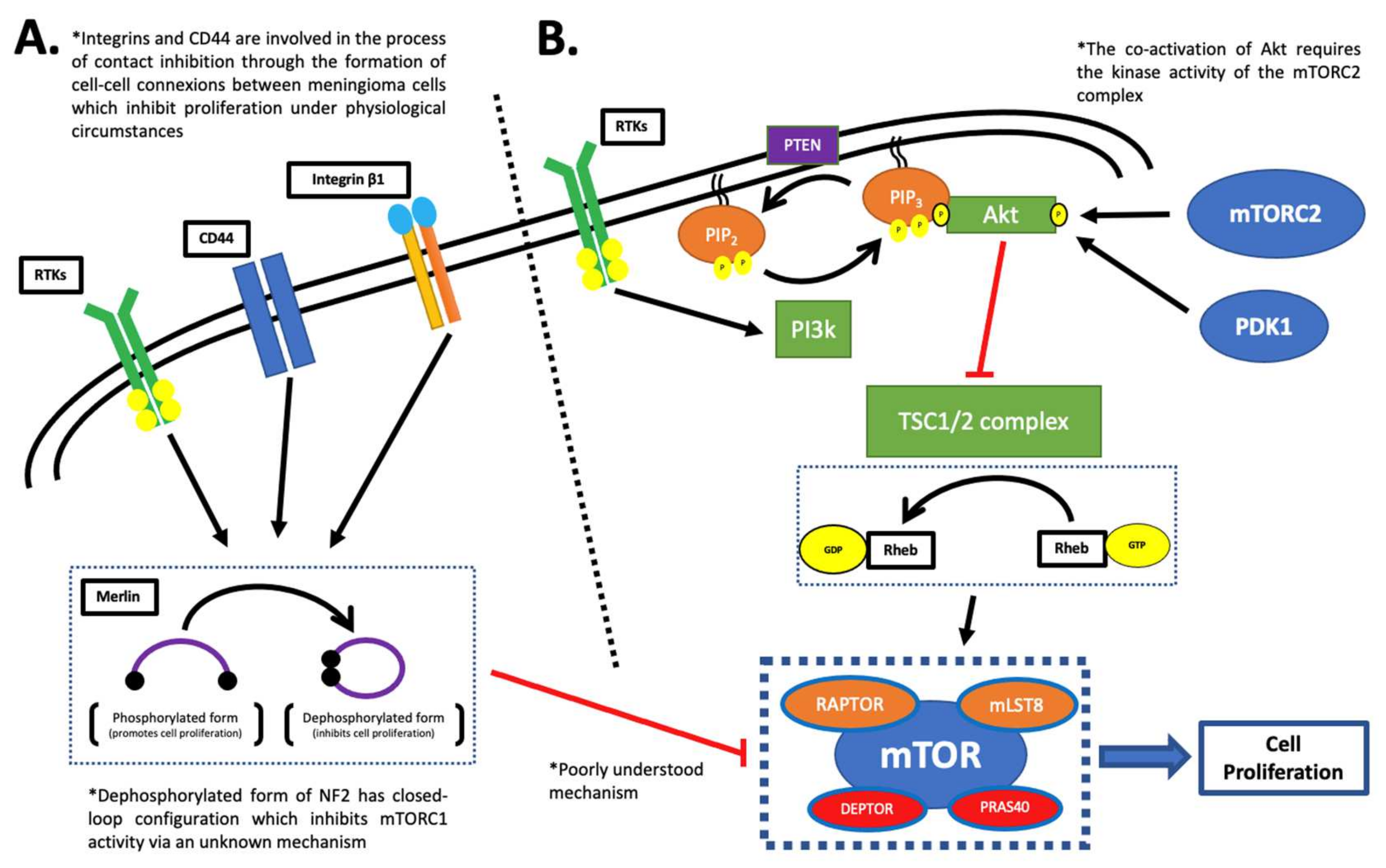
3. The mTOR Complex 2 (mTORC2)
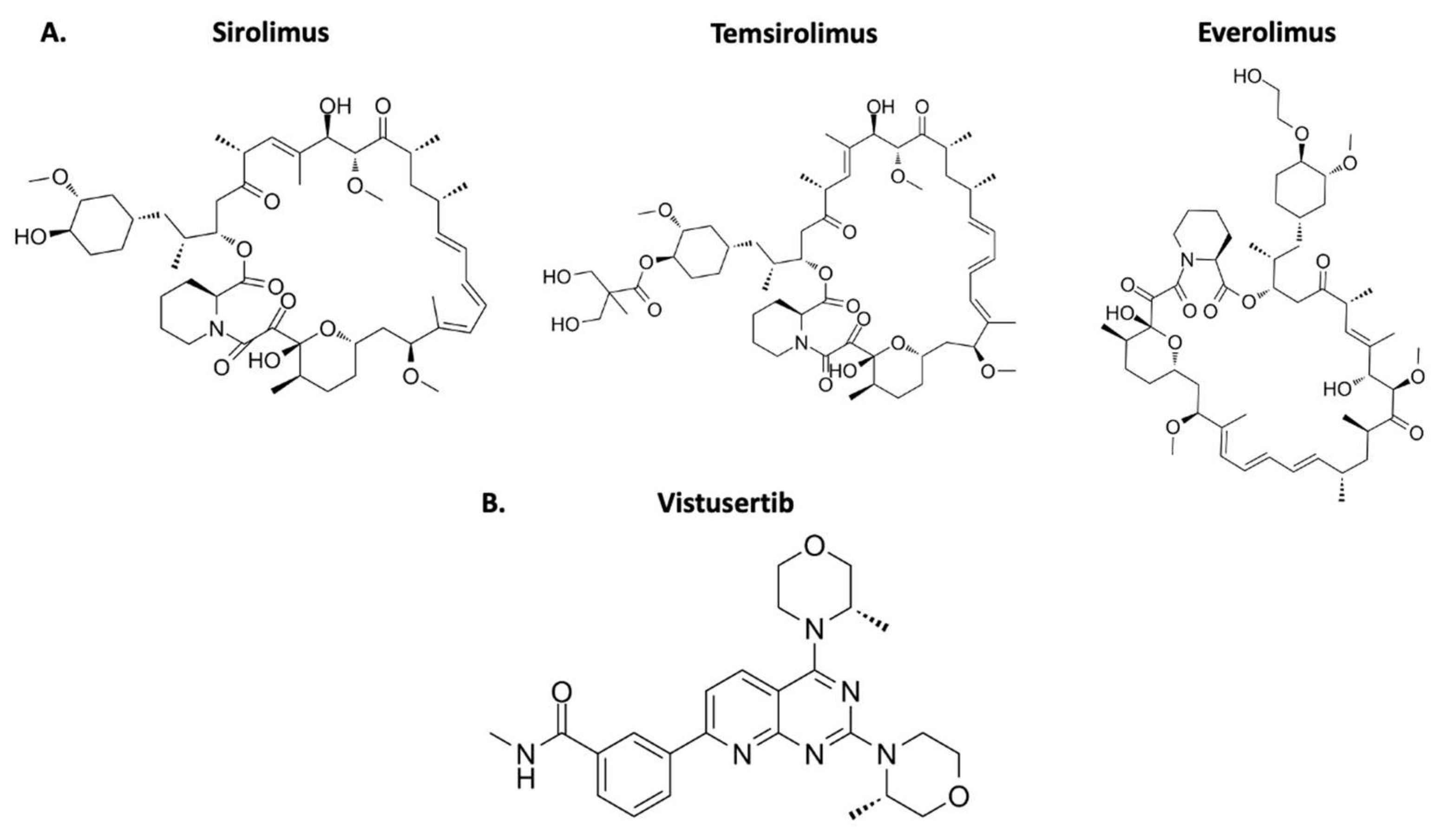
4. mTOR Inhibitors
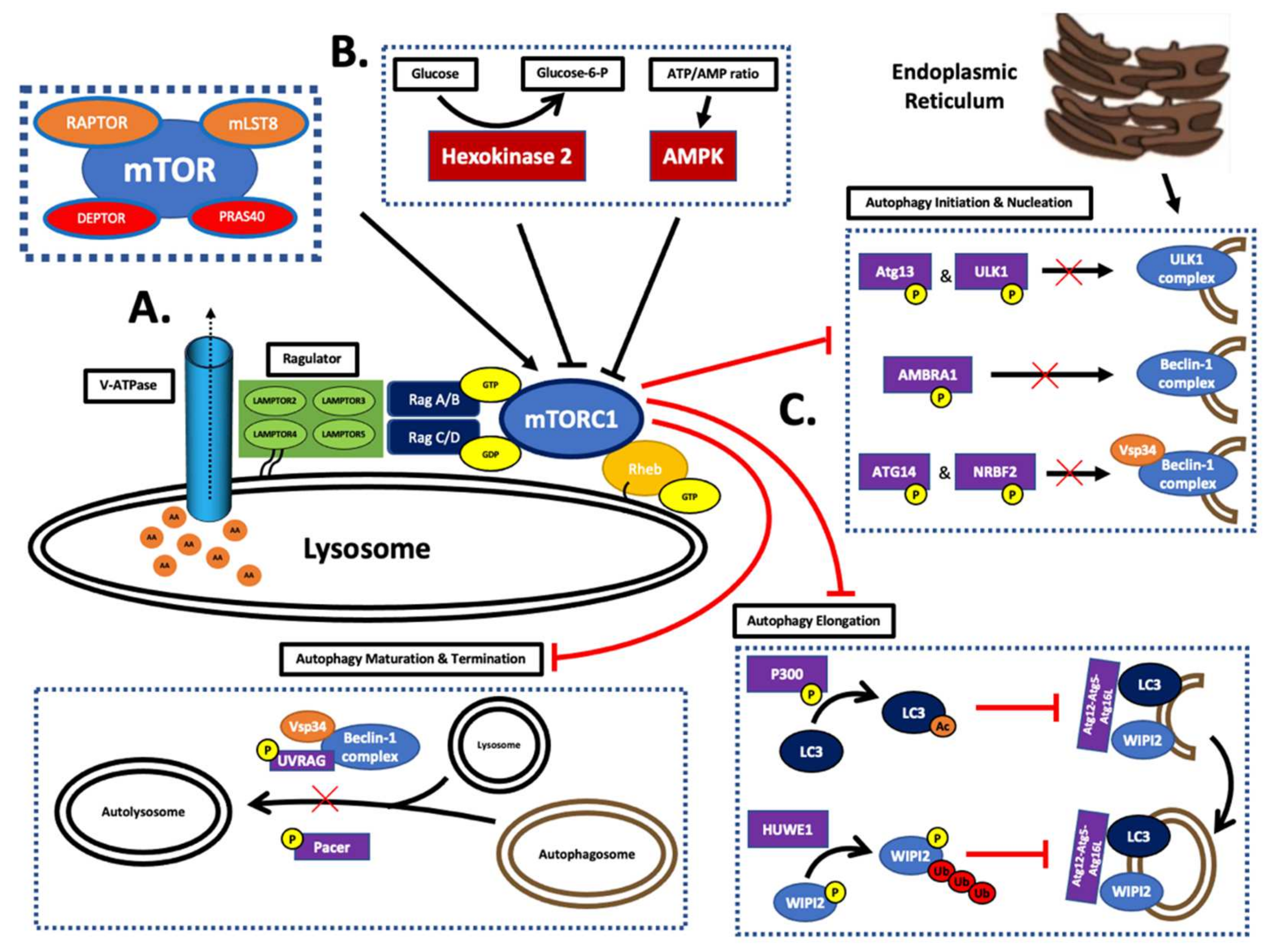
5. Redox Homeostasis and mTOR
5.1. mTOR Control
5.2. Macroautophagy
5.3. The Thioredoxin–Thioredoxin Reductase System
6. Therapeutical Application
7. Application as a Biomarker
8. Prospective
8.1. Lycopene Treatment
8.2. Methylation Targeting
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ostrom, Q.T.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro-Oncology 2021, 23, iii1–iii105. [Google Scholar] [CrossRef] [PubMed]
- Brodbelt, A.R.; Barclay, M.E.; Greenberg, D.; Williams, M.; Jenkinson, M.D.; Karabatsou, K. The outcome of patients with surgically treated meningioma in England: 1999–2013. A cancer registry data analysis. Br. J. Neurosurg. 2019, 33, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Van Alkemade, H.; De Leau, M.; Dieleman, E.M.T.; Kardaun, J.W.P.F.; Van Os, R.; Vandertop, W.P.; Van Furth, W.R.; Stalpers, L.J.A. Impaired survival and long-term neurological problems in benign meningioma. Neuro-Oncology 2012, 14, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Adeberg, S.; Hartmann, C.; Welzel, T.; Rieken, S.; Habermehl, D.; Von Deimling, A.; Debus, J.; Combs, S.E. Long-term outcome after radiotherapy in patients with atypical and malignant meningiomas–clinical results in 85 patients treated in a single institution leading to optimized guidelines for early radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Magil, T.S.; Lee, D.S.; Yen, A.J.; Lucas, C.-H.G.; Raleigh, D.R.; Aghi, M.K.; Theodosopoulos, P.V.; McDermott, M.W. Surgical outcomes after reoperation for recurrent skull base meningiomas. J. Neurosurg. 2018, 130, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Vera, E.A.; Iorgulescu, J.B.; Raper, D.M.S.; Madhavan, K.; Lally, B.E.; Morcos, J.; Elhammady, S.; Sherman, J.; Komotar, R. Review of Stereotactic Radiosurgery Practice in the Management of Skull Base Meningiomas. J. Neurol. Surg. 2014, 75, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, A.; Khan, R.; Ghosh, M.K. Blood Brain Barrier: A Challenge for Effectual Therapy of Brain Tumors. BioMed Res. Int. 2015, 2015, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kim, L. A narrative review of targeted therapies in meningioma. Chin. Clin. Oncol. 2020, 9, 76. [Google Scholar] [CrossRef]
- Graillon, T.; Sanson, M.; Campello, C.; Idbaih, A.; Peyre, M.; Peyrière, H.; Noémie, B.; Basset, N.; Autran, D.; Roche, C.; et al. Everolimus and Octreotide for Patients with Recurrent Meningioma: Results from the Phase II CEVOREM Trial. Clin. Cancer Res. 2020, 26, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Falzone, L.; Salomone, S.; Libra, M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front. Pharmacol. 2018, 9, 1300. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Li, X.; Zhang, J. mTOR Signaling in Cancer and mTOR Inhibitors in Solid Tumor Targeting Therapy. Int. J. Mol. Sci. 2019, 20, 755. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. mTOR signaling pathway and mTOR inhibitors in cancer: Progress and challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Kong, Q.; Zhang, H.; Wang, H.; Wang, J.; Luo, T.; Jiang, Y. Targeting mTOR for cancer therapy. J. Hematol. Oncol. 2019, 12, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Popova, N.; Jücker, M. The Role of mTOR Signaling as a Therapeutic Target in Cancer. Int. J. Mol. Sci. 2021, 22, 1743. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administrtation. Search Orphan Drug Designations and Approvals. Available online: https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=379312 (accessed on 31 December 2021).
- U.S. Food & Drug Administrtation. Search Orphan Drug Designations and Approvals. Available online: https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=196404 (accessed on 31 December 2021).
- Drugs.com. Afinitor FDA Approval History. Available online: https://www.drugs.com/history/afinitor.html (accessed on 31 December 2021).
- James, M.F.; Han, S.; Polizzano, C.; Plotkin, S.R.; Manning, B.D.; Stemmer-Rachamimov, A.O.; Gusella, J.F.; Ramesh, V. NF2/merlin is a novel negative regulator of mTOR complex 1, and activation of mTORC1 is associated with meningioma and schwannoma growth. Mol. Cell. Biol. 2009, 29, 4250–4261. [Google Scholar] [CrossRef] [PubMed]
- Brastianos, P.K.; Horowitz, P.M.; Santagata, S.; Jones, R.T.; McKenna, A.; Getz, G.; Ligon, K.L.; Palescandolo, E.; Van Hummelen, P.; Ducar, M.D.; et al. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat. Genet. 2012, 45, 285–289. [Google Scholar] [CrossRef]
- Pachow, D.; Andrae, N.; Kliese, N.; Angenstein, F.; Stork, O.; Wilisch-Neumann, A.; Kirches, E.; Mawrin, C. mTORC1 inhibitors suppress meningioma growth in mouse models. Clin. Cancer Res. 2013, 19, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; You, L.; Giancotti, F.G. Shedding light on Merlin’s wizardry. Trends Cell Biol. 2007, 17, 222–229. [Google Scholar] [CrossRef]
- Okada, T.; Lopez-Lago, M.; Giancotti, F.G. Merlin/NF-2 mediates contact inhibition of growth by suppressing recruitment of Rac to the plasma membrane. J. Cell Biol. 2005, 171, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Morrison, H.; Sherman, L.S.; Banine, F.; Isacke, C.; Haipek, C.A.; Gutmann, D.H.; Ponta, H.; Herrlich, P. The NF2 tumor suppressor gene product, merlin, mediates contact inhibition of growth through interactions with CD44. Genes Dev. 2001, 15, 968–980. [Google Scholar] [CrossRef]
- Ariyannur, S.A.; Vikkath, N.; Pillai, A.B. Cerebrospinal Fluid Hyaluronan and Neurofibromatosis Type 2. Cancer Microenviron. 2018, 11, 125–133. [Google Scholar] [CrossRef] [PubMed]
- López-Lago, A.M.; Okada, T.; Murillo, M.M.; Socci, N.; Giancotti, F.G. Loss of the Tumor Suppressor Gene NF2, Encoding Merlin, Constitutively Activates Integrin-Dependent mTORC1 Signalling. Mol. Cell. Biol. 2009, 29, 4235–4249. [Google Scholar] [CrossRef] [PubMed]
- Nigim, F.; Kiyokawa, J.; Gurtner, A.; Kawamura, Y.; Hua, L.; Kasper, E.M.; Brastianos, P.K.; Cahill, D.P.; Rabkin, S.D.; Martuza, R.L.; et al. A Monoclonal Antibody Against β1 Integrin Inhibits Proliferation and Increases Survival in an Orthotopic Model of High-Grade Meningioma. Target Oncol. 2019, 14, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, M.I.; Kolev, V.N.; Vidal, C.M.; Kadariya, Y.; Ring, J.E.; Wright, Q.; Weaver, D.T.; Menges, C.; Padval, M.; McClatchey, A.; et al. Merlin deficiency predicts for FAK inhibitor sensitivity: A synthetic lethal relationship. Sci. Transl. Med. 2014, 6, 237ra68. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Yan, F.; Ren, X. The role and mechanism of CRL4 E3 ubiquitin ligase in cancer and its potential therapy implications. Oncotarget 2015, 6, 42590–42602. [Google Scholar] [CrossRef] [PubMed]
- Van Rensburg, H.J.J.; Yang, X. Essential signaling in NF2 loss-related tumours: The therapeutic potential of CRL4DCAF1 and mTOR combined inhibition. J. Thorac. Dis. 2017, 9, 3533–3536. [Google Scholar] [CrossRef] [PubMed]
- Kma, L.; Baruah, T.J. The interplay of ROS and the PI3K/Akt pathway in autophagy regulation. Biotechnol. Appl. Biochem. 2021. [Google Scholar] [CrossRef] [PubMed]
- Pópulo, H.; Lopes, J.M.; Sooares, P. The mTOR signalling pathway in human cancer. Int. J. Mol. Sci. 2012, 13, 1886–1918. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, R.L.; James, M.F.; DeSouza, P.A.; Wagh, V.; Zhao, W.-N.; Jordan, J.T.; Stemmer-Rachamimov, A.O.; Plotkin, S.R.; Gusella, J.F.; Haggarty, S.J.; et al. A high-throughput kinome screen reveals serum/glucocorticoid- regulated kinase 1 as a therapeutic target for NF2-deficient meningiomas. Oncotarget 2015, 6, 16981–16997. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, Z.; Jiang, Y.; Meng, Z.; Lu, M. mTOR Signaling: The Interface Linking Cellular Metabolism and Hepatitis B Virus Replication. Virol. Sin. 2021, 36, 1303–1314. [Google Scholar] [CrossRef] [PubMed]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K pathway in human disease. Cell Press 2018, 170, 605–635. [Google Scholar] [CrossRef]
- Tumaneng, K.; Russel, R.C.; Guan, K.-M. Organ Size Control by Hippo and TOR Pathways. Curr. Biol. 2012, 22, R368–R379. [Google Scholar] [CrossRef]
- Koundouros, N.; Poulogiannis, G. Phosphoinositide 3-Kinase/Akt Signaling and Redox Metabolism in Cancer. Front. Oncol. 2018, 8, 160. [Google Scholar] [CrossRef]
- Dai, H.; Thompson, A.W. The “other” mTOR complex: New insights into mTORC2 immunobiology and their implications. Am. J. Transplant. 2019, 19, 1614–1621. [Google Scholar] [CrossRef]
- An, P.; Xu, W.; Luo, J.; Luo, Y. Expanding TOR Complex 2 Signaling: Emerging Regulators and New Connections. Front. Cell Dev. Biol. 2021, 9, 713806. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Hall, M.N. Regulation of mTORC2 Signaling. Genes 2020, 11, 1045. [Google Scholar] [CrossRef]
- Ebner, M.; Sinkovics, B.; Szczygieł, M.; Ribeiro, D.W.; Yudushkin, I. Localization of mTORC2 activity inside cells. J. Cell Biol. 2017, 216, 343–353. [Google Scholar] [CrossRef] [PubMed]
- James, M.F.; Stivison, E.; Bauchamp, R.; Han, S.; Li, H.; Wallace, M.R.; Gusella, F.F.; Stemmer-Rachamimov, A.O.; Ramesh, V. Regulation of mTOR Complex 2 Signaling in Neurofibromatosis 2-Deficient Target Cell Types. Mol. Cancer Res. 2012, 10, 649–659. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Ali, S.M.; Sengupta, S.; Sheen, J.-H.; Phsu, P.P.; Bagley, A.F.; Markhard, A.L.; Sabatini, D.M. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell 2006, 22, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Lousberg, L.; Jerusalem, G. Safety, Efficacy, and Patient Acceptability of Everolimus in the Treatment of Breast Cancer. Breast Cancer Clin. Res. 2016, 10, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.C.; Garcia, C.A.; Wu, S. Discontinuation of Everolimus Due to Related and Unrelated Adverse Events in Cancer Patients: A Meta-Analysis. Cancer Investig. 2017, 35, 552–561. [Google Scholar] [CrossRef]
- Arena, C.; Bizzoca, M.E.; Caponio, V.C.A.; Troiano, G.; Zhurakivska, K.; Leuci, S.; Lo Muzio, L. Everolimus therapy and side-effects: A systematic review and meta-analysis. Int. J. Oncol. 2021, 59, 54. [Google Scholar] [CrossRef]
- MedChemExpress. Rapamycin. Available online: https://www.medchemexpress.com/Rapamycin.html (accessed on 31 December 2021).
- MedChemExpress. Temsirolimus. Available online: https://www.medchemexpress.com/Temsirolimus.html (accessed on 31 December 2021).
- MedChemExpress. Everolimus. Available online: https://www.medchemexpress.com/Everolimus.html (accessed on 31 December 2021).
- MedChemExpress. Vistusertib. Available online: https://www.medchemexpress.com/AZD2014.html (accessed on 31 December 2021).
- Jin, Y.-P.; Valenzuela, N.M.; Ziegler, M.E.; Rozengurt, E.; Reed, E.F. Everolimus Inhibits Anti-HLA I Antibody-Mediated Endothelial Cell Signaling, Migration and Proliferation More Potently than Sirolimus. Am. J. Transplant. 2014, 14, 806–819. [Google Scholar] [CrossRef]
- Lee, J.; Giordano, S.; Zhang, J. Autophagy, mitochondria and oxidative stress: Cross-talk and redox signalling. Biochem. J. 2012, 441, 523–540. [Google Scholar] [CrossRef]
- Dossou, S.A.; Basu, A. The Emerging Roles of mTORC1 in Macromanaging Autophagy. Cancers 2019, 11, 1422. [Google Scholar] [CrossRef]
- Esen, H.; Feyzioglu, B.; Erdi, F.; Keskin, F.; Kaya, B.; Demir, L.S. High thioredoxin reductase 1 expression in meningiomas undergoing malignant progression. Brain Tumor Pathol. 2015, 32, 195–201. [Google Scholar] [CrossRef]
- Wen, C.; Wang, H.; Wu, X.; He, L.; Zhou, Q.; Wang, F.; Chen, S.; Huang, L.; Chen, J.; Wang, H.; et al. ROS-mediated inactivation of the PI3K/AKT pathway is involved in the antigastric cancer effects of thioredoxin reductase-1 inhibitor chaetocin. Cell Death Dis. 2019, 10, 809. [Google Scholar] [CrossRef] [PubMed]
- Goldbrunner, R.; Minniti, G.; Preusser, M.; Jemkinson, M.D.; Sallabanda, K.; Houdart, E.; Von Deimling, A.; Starvrinou, P.; Lefranc, F.; Lund-Johansen, M.; et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016, 17, e383–e391. [Google Scholar] [CrossRef]
- Nassiri, F.; Liu, J.; Patil, V.; Mamatjan, Y.; Wang, J.Z.; Hugh-White, R.; Macklin, A.M.; Khan, S.; Singh, O.; Karimi, S.; et al. A clinically applicable integrative molecular classification of meningiomas. Nature 2021, 597, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D.; Okedli, E.; Woodard, A.; Steven, A.T.; Allen, G.S. Evidence for phosphatidylinositol 3-kinase-Akt-p7S6K pathway activation and transduction of mitogenic signals by platelet-derived growth factor in meningioma cells. J. Neurosurg. 2002, 97, 668–675. [Google Scholar] [CrossRef]
- Pachow, D.; Wick, W.; Gutmann, D.H.; Mawrin, C. The mTOR signaling pathway as a treatment target for intracranial neoplasms. Neuro-Oncology 2015, 17, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Du, Z.; Hu, C.; Greenwald, N.F.; Abedalthagafi, M.; Agar, N.Y.R.; Dunn, G.P.; Bi, W.L.; Santagata, S.; Dunn, I.F. Osteoglycin promotes meningioma development through downregulation of NF2 and activation of mTOR signaling. Cell Commun. Signal. 2017, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Birle, D.C.; Tannock, I.F. Effects of the mammalian target of rapamycin inhibitor CCI-779 used alone or with chemotherapy on human prostate cancer cells and xenografts. Cancer Res. 2005, 65, 2825–2831. [Google Scholar] [CrossRef]
- Frost, P.; Moatamed, F.; Hoang, B.; Shi, Y.; Gera, J.; Yan, H.; Frost, P.; Gibbons, J.; Lichtenstein, A. In vivo antitumor effects of the mTOR inhibitor CCI-779 against human multiple myeloma cells in a xenograft model. Blood 2004, 104, 4181–4187. [Google Scholar] [CrossRef]
- Bertolini, F.; Pecchi, A.; Stefani, A.; Fontana, A.; Rosssi, G. Everolimus effectively blocks pulmonary metastases from meningioma. Neuro-Oncology 2015, 17, 1301–1302. [Google Scholar] [CrossRef][Green Version]
- Shih, C.K.; Chowdhary, S.; Rosenblatt, P.; Weir, A.B.; Shepard, G.C.; Williams, J.T.; Shastry, M.; Burris, H.A.; Hainsworth, J.D. A phase II trial of bevacizumab and everolimus as treatment for patients with refractory, progressive intracranial meningioma. J. Neuro-Oncol. 2016, 129, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Graillon, T.; Romano, D.; Defilles, C.; Saveanu, A.; Mohamed, A.; Figarella-Branger, D.; Roche, P.-H.; Fuentes, S.; Chinot, O.; Dufour, H.; et al. Octreotide therapy in meningiomas: In vitro study, clinical correlation, and literature review. J. Neurosurg. 2017, 127, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Yesilöz, Ü.; Kirches, E.; Hartmann, C.; Scholz, J.; Kropf, S.; Sahm, F.; Nakamura, M.; Mawrin, C. Frequent AKT1E17K mutations in skull base meningiomas are associated with mTOR and ERK1/2 activation and reduced time to tumor recurrence. Neuro-Oncology 2017, 19, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Barresi, V.; Lionti, S.; La Rocca, L.; Caliri, S.; Caffo, M. High p-mTOR expression is associated with recurrence and shorter disease-free survival in atypical meningiomas. Neuropathology 2018, 39, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D.; O’Connell, M.; Vito, F.; Bakos, R.S. Increased STAT-3 and synchronous activation of Raf-1-MEK-1-MAPK, and phosphatidylinositol 3-Kinase-Akt-mTOR pathways in atypical and anaplastic meningiomas. J. Neuro-Oncol. 2009, 92, 129–136. [Google Scholar] [CrossRef] [PubMed]
- El-Habr, E.A.; Levidou, G.; Trigka, E.-A.; Sakalidou, J.; Piperi, C.; Chatziandreou, I.; Spyropoulou, A.; Soldatos, R.; Tomara, G.; Petraki, K.; et al. Complex interactions between the components of the PI3K/AKT/mTOR pathway, and with components of MAPK, JAK/STAT and Notch-1 pathways, indicate their involvement in meningioma development. Eur. J. Pathol. 2014, 465, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, M.; Wawrzyniak, D.; Rolle, K.; Chomczyński, P.; Oziewicz, S.; Jurga, S.; Barciszewski, J. Let food be your medicine: Nutraceutical properties of lycopene. Food Funct. 2019, 10, 3090–3102. [Google Scholar] [CrossRef] [PubMed]
- Marzocco, S.; Singla, R.K.; Capasso, A. Multifaceted Effects of Lycopene: A Boulevard to the Multitarget-Based Treatment for Cancer. Molecules 2021, 26, 5333. [Google Scholar] [CrossRef]
- Puah, B.P.; Jalil, J.; Attiq, A.; Kamisah, Y. New Insights into Molecular Mechanism behind Anti-Cancer Activities of Lycopene. Molecules 2021, 26, 3888. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lu, X.; Yu, R. Lycopene Inhibits Epithelial–Mesenchymal Transition and Promotes Apoptosis in Oral Cancer via PI3K/AKT/m-TOR Signal Pathway. Drug Des. Dev. Ther. 2020, 14, 2461–2471. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, B.; Yu, L.; Li, Q.; Li, S.; Wang, P.; Jing, T.; Men, T. Lycopene Attenuates Hypoxia-Induced Testicular Injury by Inhibiting PROK2 Expression and Activating PI3K/AKT/mTOR Pathway in a Varicocele Adult Rat. Evid.-Based Complement. Altern. Med. 2021, 3471356. [Google Scholar] [CrossRef] [PubMed]
- Ip, B.C.; Liu, C.; Ausman, L.M.; Von Lintig, J.; Wang, Z.-D. Lycopene attenuated hepatic tumorigenesis via differential mechanisms depending on carotenoid cleavage enzyme in mice. Cancer Prev. Res. 2014, 7, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Sahin, K.; Orhan, C.; Tuzcu, M.; Sahin, N.; Ali, S.; Bahcecioglu, I.H.; Guler, O.; Ozercan, I.; Ilhan, N.; Kucuk, O. Orally administered lycopene attenuates diethylnitrosamine-induced hepatocarcinogenesis in rats by modulating Nrf-2/HO-1 and Akt/mTOR pathways. Nutr. Cancer 2014, 66, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Pungsrinont, T.; Kallenbach, J.; Baniahmad, A. Role of PI3K-AKT-mTOR Pathway as a Pro-Survival Signaling and Resistance-Mediating Mechanism to Therapy of Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 11088. [Google Scholar] [CrossRef] [PubMed]
- Toren, P.; Zoubeidi, A. Targeting the PI3K/Akt pathway in prostate cancer: Challenges and opportunities (review). Int. J. Oncol. 2014, 45, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Kremer, C.L.; Klein, R.R.; Mendelson, J.; Browne, W.; Samadzedeh, L.K.; Vanpatten, K.; Highstrom, L.; Pestano, G.A.; Nagle, R.B. Expression of mTOR signaling pathway markers in prostate cancer progression. Prostate 2006, 66, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Mettlin, C.; Selenskas, S.; Natarajan, N.; Huben, R. Intake of carotenoids and retinol in relation to risk of prostate cancer. Cancer 1989, 64, 605–612. [Google Scholar] [CrossRef]
- Mirahmadi, M.; Azimi-Hashemi, S.; Saburi, E.; Kamali, H.; Pishbin, M.; Hadizadeh, F. Potential inhibitory effect of lycopene on prostate cancer. Biomed. Pharmacother. 2020, 129, 110459. [Google Scholar] [CrossRef] [PubMed]
- Kucuk, O.; Sarkar, F.H.; Sakr, W.; Djuric, Z.; Pollak, M.N.; Khachik, F.; Li, Y.W.; Banerjee, M.; Grignon, D.; Bertram, J.S.; et al. Phase II randomized clinical trial of lycopene supplementation before radical prostatectomy. Cancer Epidemiol. Biomark. Prev. 2001, 10, 861–868. [Google Scholar]
- Barber, N.J.; Zhang, X.; Zhu, G.; Pramanik, R.; Barber, J.A.; Martin, F.L.; Morris, J.D.H.; Muir, G.H. Lycopene inhibits DNA synthesis in primary prostate epithelial cells in vitro and its administration is associated with a reduced prostate-specific antigen velocity in a phase II clinical study. Prostate Cancer Prostatic Dis. 2006, 9, 407–413. [Google Scholar] [CrossRef]
- Bowen, P.; Chen, L.; Stacewicz-Sapuntzakis, M.; Duncan, C.; Sharifi, R.; Ghosh, L.; Kim, H.-S.; Christov-Tzelkov, K.; Van Breemen, R. Tomato sauce supplementation and prostate cancer: Lycopene accumulation and modulation of biomarkers of carcinogenesis. Exp. Biol. Med. 2002, 227, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Kucuk, O.; Sarkar, F.H.; Djuric, Z.; Sakr, W.; Pollak, M.N.; Khachik, F.; Benerjee, M.; Bertram, J.S.; Wood, D.P., Jr. Effects of lycopene supplementation in patients with localized prostate cancer. Exp. Biol. Med. 2002, 227, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Vaishampayan, U.; Hussain, M.; Banerjee, M.; Seren, S.; Sarkar, F.H.; Fontana, J.; Forman, J.D.; Cher, M.L.; Powell, I.; Pontes, J.E.; et al. Lycopene and soy isoflavones in the treatment of prostate cancer. Nutr. Cancer 2007, 59, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.S.; Gupta, N.P. Lycopene: A novel drug therapy in hormone refractory metastatic prostate cancer. Urol. Oncol. 2004, 22, 415–420. [Google Scholar] [CrossRef]
- Locasale, J.W. Serine, glycine and one-carbon units: Cancer metabolism in full circle. Nat. Rev. Cancer 2013, 13, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.-D.; Wu, W.K.K.; Wang, H.-Y.; Li, X.-X. Serine and one-carbon metabolism, a bridge that links mTOR signaling and DNA methylation in cancer. Pharmacol. Res. 2019, 149, 104352. [Google Scholar] [CrossRef]
- Harachi, M.; Masui, K.; Honda, H.; Muragaki, Y.; Kawamata, T.; Cavenee, W.K.; Mischel, P.S.; Shibata, N. Dual Regulation of Histone Methylation by mTOR Complexes Controls Glioblastoma Tumor Cell Growth via EZH2 and SAM. Mol. Cancer Res. 2020, 18, 1142–1152. [Google Scholar] [CrossRef] [PubMed]
- Stögbauer, L.; Thomas, C.; Wagner, A.; Warneke, N.; Bunk, E.C.; Grauer, O.; Canisius, J.; Paulus, W.; Stummer, W.; Senner, V.; et al. Efficacy of decitabine in malignant meningioma cells: Relation to promoter demethylation of distinct tumor suppressor and oncogenes and independence from TERT. J. Neurosurg. 2020, 135, 845–854. [Google Scholar] [CrossRef] [PubMed]
| Drug | Disease | FDA Approval |
|---|---|---|
| Sirolimus | Lymphangioleiomyomatosis (LAM) | May 2015 |
| Temsirolimus | Renal cell carcinoma (advanced disease) | May 2007 |
| Everolimus | Renal cell carcinoma (advanced disease) | March 2009 |
| Breast cancer (advanced HR+ tumor) | July 2012 | |
Neuroendocrine carcinoma
| February 2016 | |
Tuberous sclerosis-associated cancers
| April 2012 |
| Study Title | Drug | Publication Date | NTC Number |
|---|---|---|---|
| mTORC1 Inhibitors Suppress Meningioma Growth in Mouse Models (A.) | Temsirolimus | March 2013 | N/A |
| Everolimus Effectively Blocks Pulmonary Metastases from Meningioma (B.) | Everolimus | September 2015 | N/A |
| A Phase II Trial of Bevacizumab and Everolimus as Treatment for Patients with Refractory, Progressive Intracranial Meningioma (C.) | Everolimus Bevacizumab | June 2016 | NCT00972335 |
| Everolimus and Octreotide for Patients with Recurrent Meningioma: Results from the Phase II CEVOREM Trial (D.) | Everolimus Octreotide | February 2020 | NCT02333565 |
| Study Title | Drug | Phase | Start Date | Completion Date | Trial Identifier |
|---|---|---|---|---|---|
| AZD2014 In NF2 Patients with Progressive or Symptomatic Meningiomas (A.) | Vistusertib | Phase 2 | 13 July 2016 | 22 December 2020 | NCT02831257 |
| Vistusertib (AZD2014) For Recurrent Grade II-III Meningiomas (B.) | Vistusertib | Phase 2 | 17 October 2017 | 25 July 2024 | NCT03071874 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinker, B.; Barciszewska, A.-M. mTOR Signaling and Potential Therapeutic Targeting in Meningioma. Int. J. Mol. Sci. 2022, 23, 1978. https://doi.org/10.3390/ijms23041978
Pinker B, Barciszewska A-M. mTOR Signaling and Potential Therapeutic Targeting in Meningioma. International Journal of Molecular Sciences. 2022; 23(4):1978. https://doi.org/10.3390/ijms23041978
Chicago/Turabian StylePinker, Benjamin, and Anna-Maria Barciszewska. 2022. "mTOR Signaling and Potential Therapeutic Targeting in Meningioma" International Journal of Molecular Sciences 23, no. 4: 1978. https://doi.org/10.3390/ijms23041978
APA StylePinker, B., & Barciszewska, A.-M. (2022). mTOR Signaling and Potential Therapeutic Targeting in Meningioma. International Journal of Molecular Sciences, 23(4), 1978. https://doi.org/10.3390/ijms23041978