Improved Production of 5-Hydroxymethylfurfural in Acidic Deep Eutectic Solvents Using Microwave-Assisted Reactions
Abstract
:1. Introduction
2. Results and Discussion
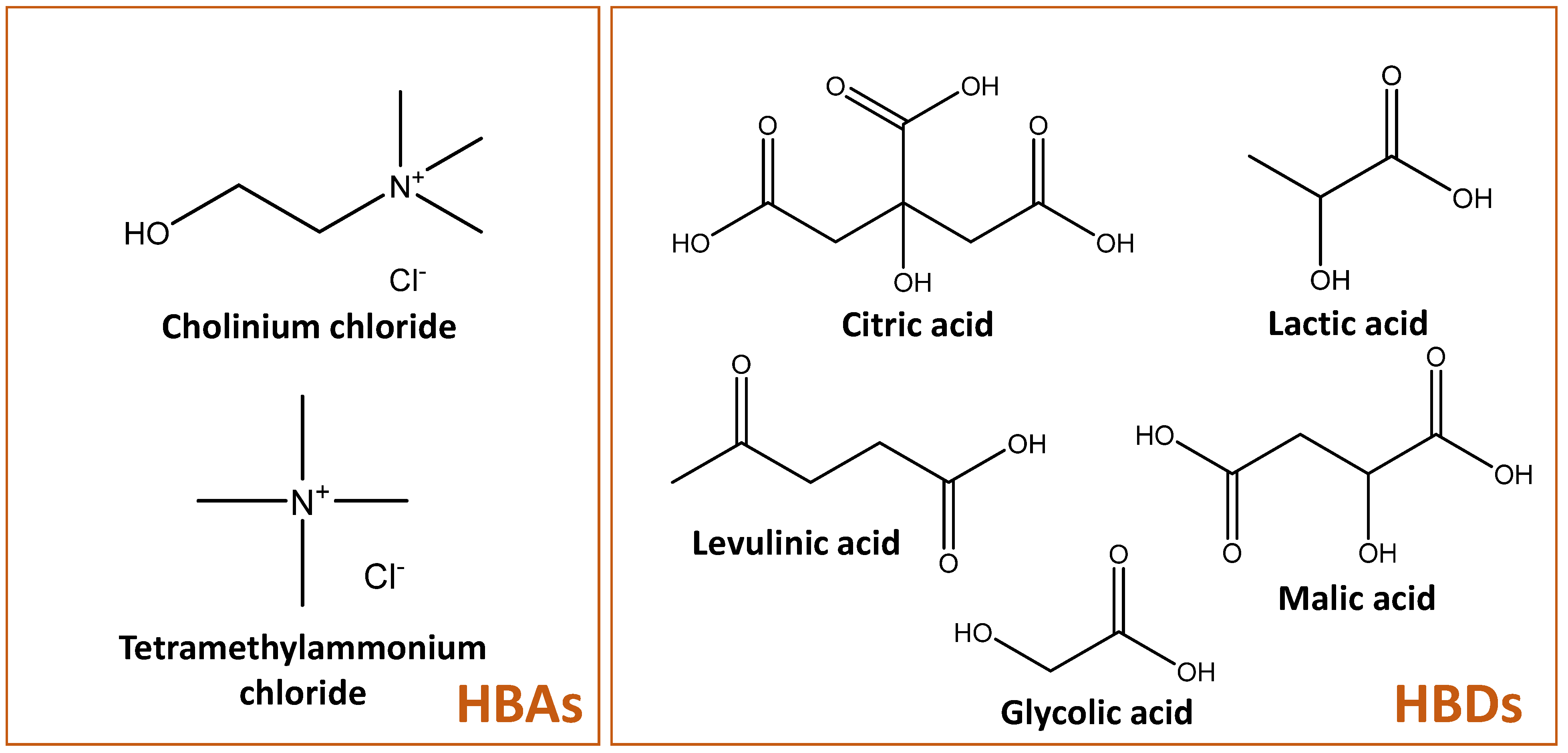
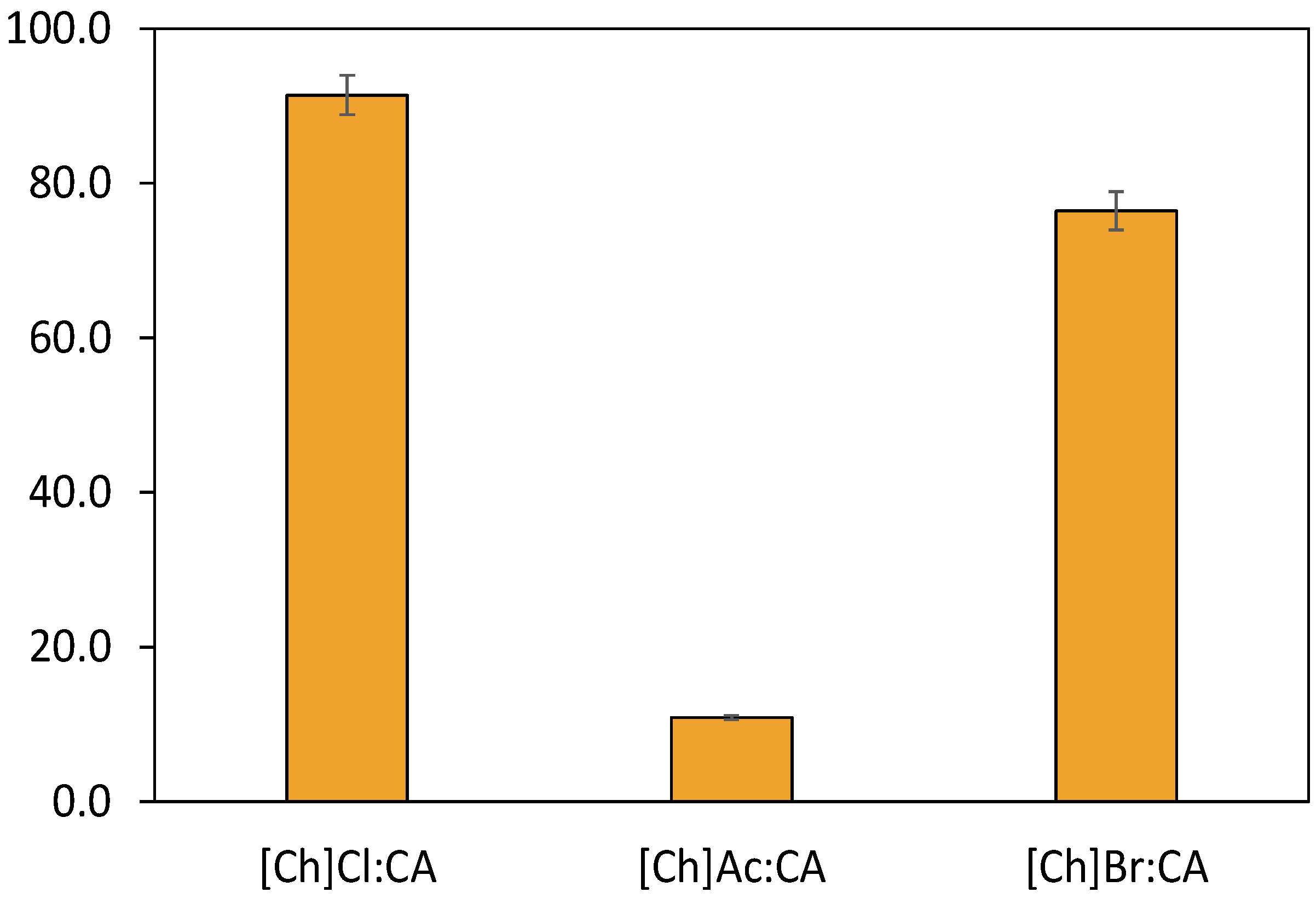
2.1. Initial DES Screening
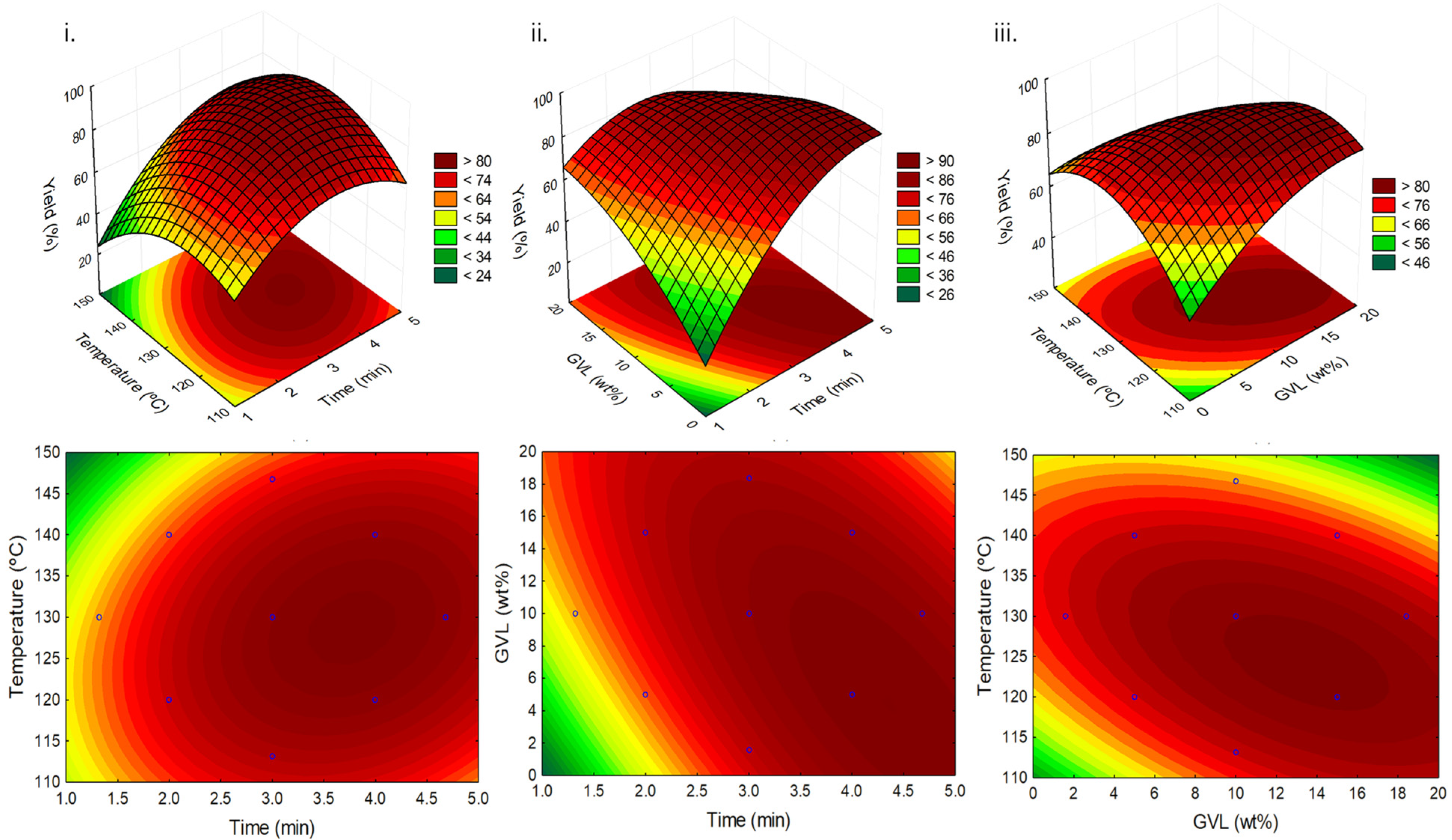
2.2. Reaction Optimization through Response Surface Methodology
3. Materials and Methods
3.1. Chemicals
3.2. DES Preparation
3.3. Production of 5-HMF
3.4. 5-HMF Quantification
3.5. Reaction Conditions Optimization by Response Surface Methodology (RSM)
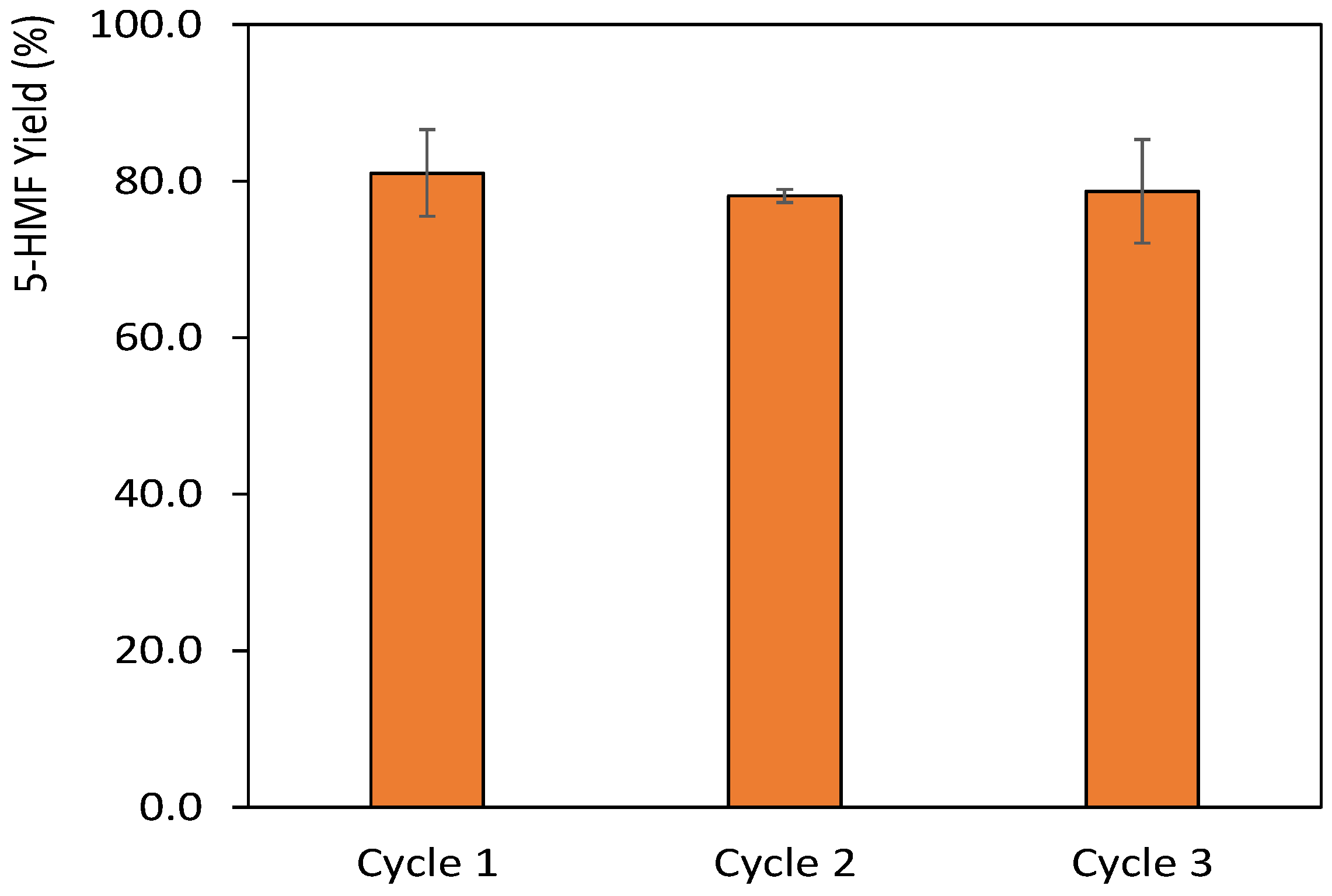
3.6. Recovery of the 5-HMF and DES Reuse
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Román-Leshkov, Y.; Barrett, C.J.; Liu, Z.Y.; Dumesic, J.A. Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates. Nature 2007, 447, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, G.; Ripa, M.; Parthenope, N. Chemicals from biomass: Technological versus environmental. Biofuels Bioprod. Biorefining 2016, 11, 195–214. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E factor 25 years on: The rise of green chemistry and sustainability. Green Chem. 2016, 19, 18–43. [Google Scholar] [CrossRef]
- Kamm, B.; Kamm, M. Principles of biorefineries. Appl. Microbiol. Biotechnol. 2004, 64, 137–145. [Google Scholar] [CrossRef]
- Zhao, H.; Holladay, J.E.; Brown, H.; Zhang, Z.C. Metal Chlorides in Ionic Liquid Solvents Convert Sugars to 5-Hydroxymethylfurfural. Science 2007, 316, 1597–1600. [Google Scholar] [CrossRef]
- Putten, R.; Waal, J.C.; Jong, E.; Rasrendra, C.B.; Heeres, H.J.; Vries, J.G. De Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass. Us Nrel 2004, 1, 76. [Google Scholar] [CrossRef] [Green Version]
- Chun, J.; Lee, J.; Yi, Y.; Hong, S.; Chung, C. Direct conversion of starch to hydroxymethylfurfural in the presence of an ionic liquid with metal chloride. Starch Stärke 2010, 62, 326–330. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, Z.; Zhou, Y.; Han, B.; Fan, H.; Li, W.; Song, J.; Xie, Y. Conversion of fructose to 5-hydroxymethylfurfural using ionic liquids prepared from renewable materials. Green Chem. 2008, 10, 1280–1283. [Google Scholar] [CrossRef]
- Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Liquid-Phase Catalytic Processing of Biomass-Derived Oxygenated Hydrocarbons to Fuels and Chemicals. Angew. Chem. Int. Ed. 2007, 46, 7164–7183. [Google Scholar] [CrossRef]
- Esteban, J.; Vorholt, A.J.; Leitner, W. An overview of the biphasic dehydration of sugars to 5-hydroxymethylfurfural and furfural: A rational selection of solvents using COSMO-RS and selection guides. Green Chem. 2020, 22, 2097–2128. [Google Scholar] [CrossRef]
- Mika, L.; Cséfalvay, E.; Németh, Á. Catalytic Conversion of Carbohydrates to Initial Platform Chemicals: Chemistry and Sustainability. Chem. Rev. 2018, 118, 505–613. [Google Scholar] [CrossRef] [PubMed]
- Overton, J.C.; Engelberth, A.S.; Mosier, N.S. Single-Vessel Synthesis of 5-Hydroxymethylfurfural (HMF) from Milled Corn. ACS Sustain. Chem. Eng. 2020, 8, 18–21. [Google Scholar] [CrossRef]
- Hansen, T.S.; Woodley, J.M.; Riisager, A. Efficient microwave-assisted synthesis of 5-hydroxymethylfurfural from concentrated aqueous fructose. Carbohydr. Res. 2009, 344, 2568–2572. [Google Scholar] [CrossRef]
- Bicker, M.; Hirth, J.; Vogel, H. Dehydration of fructose to 5-hydroxymethylfurfural in sub-and supercritical acetone. Green Chem. 2003, 5, 280–284. [Google Scholar] [CrossRef]
- Tarabanko, V.E.; Chernyak, M.Y.U.; Nepomnyashchiy, I.V.; Smirnova, M.A. High temperature 5-hydroxymethylfurfural synthesis in a flow reactor. Chem. Sustain. Dev. 2006, 14, 49–53. [Google Scholar]
- Li, Y.; Lu, X.; Yuan, L.; Liu, X. Fructose decomposition kinetics in organic acids-enriched high temperature liquid water. Biomass Bioenergy 2009, 33, 1182–1187. [Google Scholar] [CrossRef]
- Ohara, M.; Takagaki, A.; Nishimura, S.; Ebitani, K. Syntheses of 5-hydroxymethylfurfural and levoglucosan by selective dehydration of glucose using solid acid and base catalysts. Appl. Catal. A Gen. 2010, 383, 149–155. [Google Scholar] [CrossRef]
- Yan, H.; Yang, Y.; Tong, D.; Xiang, X.; Hu, C. Catalytic conversion of glucose to 5-hydroxymethylfurfural over SO42-/ZrO2 and SO42-/ZrO2-Al2O3 solid acid catalysts. Catal. Commun. 2009, 10, 1558–1563. [Google Scholar] [CrossRef]
- Binder, J.B.; Raines, R.T. Simple chemical transformation of lignocellulosic biomass into furans for fuels and chemicals. J. Am. Chem. Soc. 2009, 131, 1979–1985. [Google Scholar] [CrossRef]
- Delbecq, F.; Wang, Y.; Muralidhara, A.; El Ouardi, K.; Marlair, G.; Len, C. Hydrolysis of Hemicellulose and Derivatives—A Review of Recent Advances in the Production of Furfural. Front. Chem. 2018, 6, 1–29. [Google Scholar] [CrossRef] [PubMed]
- François, J.; Vigier, K.D.O. Catalytic Conversion of Carbohydrates to Furanic Derivatives in the Presence of Choline Chloride. Catalysts 2017, 7, 218. [Google Scholar] [CrossRef] [Green Version]
- Hansen, T.S.; Mielby, J.; Riisager, A. Synergy of boric acid and added salts in the catalytic dehydration of hexoses to 5-hydroxymethylfurfural in water. Green Chem. 2011, 13, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Chheda, J.N.; Román-Leshkov, Y.; Dumesic, J.A. Production of 5-hydroxymethylfurfural and furfural by dehydration of biomass-derived mono- and poly-saccharides. Green Chem. 2007, 9, 342–350. [Google Scholar] [CrossRef]
- McNeff, C.V.; Nowlan, D.T.; McNeff, L.C.; Yan, B.; Fedie, R.L. Continuous production of 5-hydroxymethylfurfural from simple and complex carbohydrates. Appl. Catal. A Gen. 2010, 384, 65–69. [Google Scholar] [CrossRef]
- Qi, X.; Watanabe, M.; Aida, T.M.; Smith, R.L. Catalytic dehydration of fructose into 5-hydroxymethylfurfural by ion-exchange resin in mixed-aqueous system by microwave heating. Green Chem. 2008, 10, 799–805. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; Silva, F.A.; Quental, M.V.; Mondal, D.; Freire, M.G.; Coutinho, J.A.P. Ionic-Liquid-Mediated Extraction and Separation Processes for Bioactive Compounds: Past, Present, and Future Trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef]
- Zakrzewska, M.E.; Bogel-Łukasik, E.; Bogel-Łukasik, R. Ionic liquid-mediated formation of 5-hydroxymethylfurfural-A promising biomass-derived building block. Chem. Rev. 2011, 111, 397–417. [Google Scholar] [CrossRef]
- Okano, T.; Qiao, K.; Bao, Q.; Tomida, D.; Hagiwara, H.; Yokoyama, C. Dehydration of fructose to 5-hydroxymethylfurfural (HMF) in an aqueous acetonitrile biphasic system in the presence of acidic ionic liquids. Appl. Catal. A Gen. 2013, 451, 1–5. [Google Scholar] [CrossRef]
- Khandelwal, S.; Tailor, Y.K.; Kumar, M. Deep eutectic solvents (DESs) as eco-friendly and sustainable solvent/catalyst systems in organic transformations. J. Mol. Liq. 2016, 215, 345–386. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun 2003, 1, 70–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Zhang, Z.; Zhou, Y.; Song, J.; Fan, H.; Han, B. Direct conversion of inulin to 5-hydroxymethylfurfural in biorenewable ionic liquids. Green Chem. 2009, 11, 873–877. [Google Scholar] [CrossRef]
- Zuo, M.; Wang, X.; Wang, Q.; Zeng, X.; Lin, L. Aqueous-Natural Deep Eutectic Solvent (A-NADES) Enhanced 5-Hydroxymethylfurfural (HMF) Production from Glucose, Starch, and Food Wastes. ChemSusChem 2021, 1–13. [Google Scholar] [CrossRef]
- Poerwono, H.; Higashiyama, K.; Kubo, H.; Brittain, A.; Suharjono, T.P.; Sudiana, K.; Indrayanto, G. Citric acid. Anal. Profiles Drug Subst. Excip. 2001, 28, 1–76. [Google Scholar]
- Mellmer, M.A.; Sanpitakseree, C.; Demir, B.; Ma, K.; Elliott, W.A.; Bai, P.; Johnson, R.L.; Walker, T.W.; Shanks, B.H.; Rioux, R.M.; et al. Effects of chloride ions in acid-catalyzed biomass dehydration reactions in polar aprotic solvents. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- He, O.; Zhang, Y.; Wang, P.; Liu, L.; Wang, Q.; Yang, N.; Li, W.; Champagne, P.; Yu, H. Experimental and kinetic study on the production of furfural and HMF from glucose. Catalysts 2021, 11, 11. [Google Scholar] [CrossRef]
- Zhang, Z. Synthesis of γ-Valerolactone from Carbohydrates and its Applications. ChemSusChem 2016, 9, 156–171. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morais, E.S.; Freire, M.G.; Freire, C.S.R.; Silvestre, A.J.D. Improved Production of 5-Hydroxymethylfurfural in Acidic Deep Eutectic Solvents Using Microwave-Assisted Reactions. Int. J. Mol. Sci. 2022, 23, 1959. https://doi.org/10.3390/ijms23041959
Morais ES, Freire MG, Freire CSR, Silvestre AJD. Improved Production of 5-Hydroxymethylfurfural in Acidic Deep Eutectic Solvents Using Microwave-Assisted Reactions. International Journal of Molecular Sciences. 2022; 23(4):1959. https://doi.org/10.3390/ijms23041959
Chicago/Turabian StyleMorais, Eduarda S., Mara G. Freire, Carmen S. R. Freire, and Armando J. D. Silvestre. 2022. "Improved Production of 5-Hydroxymethylfurfural in Acidic Deep Eutectic Solvents Using Microwave-Assisted Reactions" International Journal of Molecular Sciences 23, no. 4: 1959. https://doi.org/10.3390/ijms23041959
APA StyleMorais, E. S., Freire, M. G., Freire, C. S. R., & Silvestre, A. J. D. (2022). Improved Production of 5-Hydroxymethylfurfural in Acidic Deep Eutectic Solvents Using Microwave-Assisted Reactions. International Journal of Molecular Sciences, 23(4), 1959. https://doi.org/10.3390/ijms23041959








