Role of Silica Nanoparticles in Abiotic and Biotic Stress Tolerance in Plants: A Review
Abstract
1. Introduction
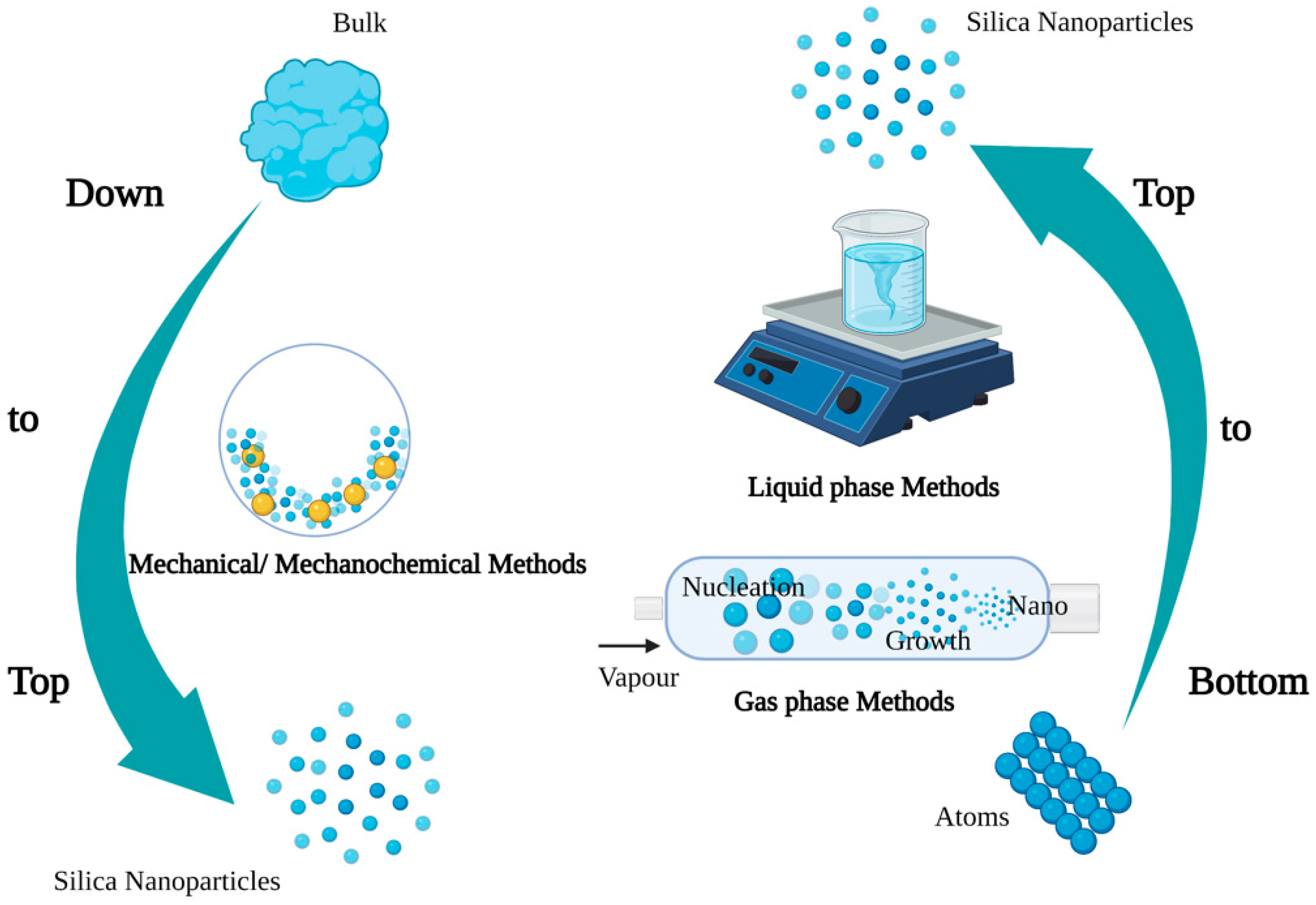
2. Synthesis and Characteristics of SNPs
3. Absorption and Transmission of SNPs in Plants
4. SNPs Improve Plant Stress Tolerance and Its Mechanism
4.1. Disease Stress
4.2. Pest Stress
4.3. Heavy Metal Stress
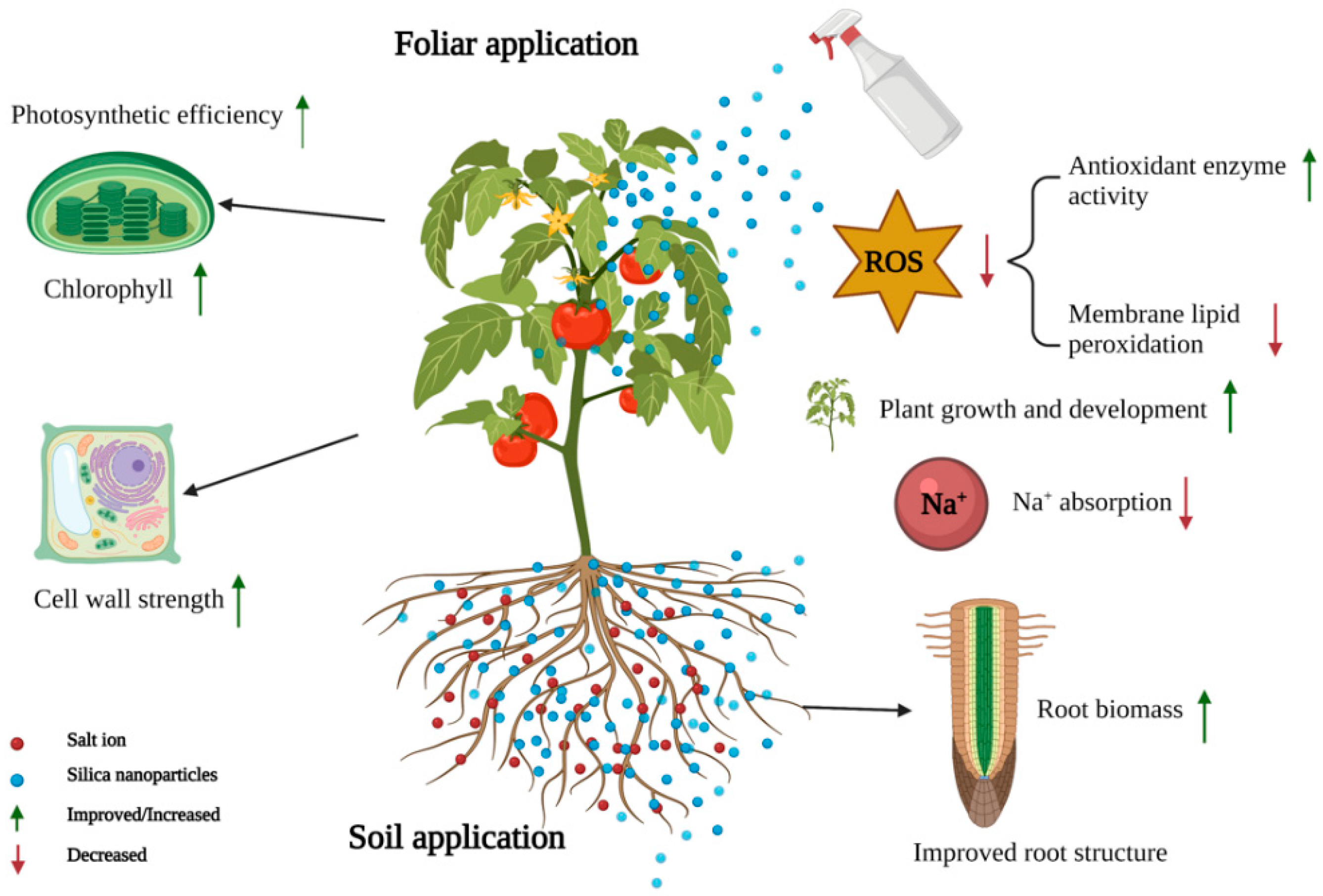
4.4. Salt Stress
4.5. Drought Stress
5. SNPs Toxic to the Plant
6. Conclusions
- The study of the behavior of SNPs in plants and their interaction mechanism, influence, and agricultural application is at its early stage [21,93]. Previous studies have mainly focused on enhancing physical barriers, promoting plant growth, inducing plant resistance, and activating resistance enzyme activity, but rarely involved the effect of SNPs on plant metabolites and soil microbial community under stress. The “SNPs–plant–soil–microorganism” system should be considered as a whole system, and in-depth research should be conducted on the molecular mechanisms of SNPs that increase the plant’s resistance to adversity from the physiological, biochemical, molecular, proteomic, and metabonomic levels through omics methods.
- The combination of SNPs and other methods such as surface modification of SNPs, SNPs loaded with fungicides, and the combination of SNPs with beneficial microorganisms could increase the effect of SNPs in improving plant stress.
- It is necessary to establish a synthesis–structure–toxicity relationship. The synthesis of SNPs affects their structure and surface chemical characteristics, thereby controlling the degradation of SNPs in plants. After being absorbed by plants, SNPs exist internally within plants’ and are slowly released, thus influencing the life activities of plants. The release of SNPs and their biological toxicity need further attention. The interaction between SNPs and environmental microorganisms also requires special attention.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tyczewska, A.; Woźniak, E.; Gracz, J.; Kuczyński, J.; Twardowski, T. Towards food security: Current state and future prospects of agrobiotechnology. Trends Biotechnol. 2018, 36, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Mwobobia, E.G.; Sichangi, A.W.; Thiong’o, K.B. Characterization of wheat production using earth-based observations: A case study of Meru County, Kenya. Model. Earth Syst. Environ. 2020, 6, 13–25. [Google Scholar] [CrossRef]
- Oshunsanya, S.O.; Nwosu, N.J.; Li, Y. Abiotic Stress in Agricultural Crops under Climatic Conditions//Sustainable Agriculture, Forest and Environmental Management; Springer: Singapore, 2019; pp. 71–100. [Google Scholar]
- Servin, A.; Elmer, W.; Mukherjee, A.; Arnab, M.; Roberto, D.T.; Helmi, H.; White, J.C.; Prem, B.; Christian, D. A review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield. J. Nanopart. Res. 2015, 17, 92. [Google Scholar] [CrossRef]
- Chhipa, H. Nanofertilizers and nanopesticides for agriculture. Environ. Chem. Lett. 2017, 15, 15–22. [Google Scholar] [CrossRef]
- Jeelani, P.G.; Mulay, P.; Venkat, R.; Ramalingam, C. Multifaceted application of silica nanoparticles. A review. Silicon 2020, 12, 1337–1354. [Google Scholar] [CrossRef]
- Rastogi, A.; Tripathi, D.K.; Yadav, S.; Chauhan, D.K.; Živčák, M.; Ghorbanpour, M.; El-Sheery, N.I.; Brestic, M. Application of silicon nanoparticles in agriculture. 3Biotech 2019, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Salajegheh, M.; Yavarzadeh, M.; Payandeh, A.; Payandeh, A.; Akbarian, M.M. Effects of titanium and silicon nanoparticles on antioxidant enzymes activity and some biochemical properties of Cuminum cyminum L. under drought stress. Banat’s J. Biotechnol. 2020, 11, 19–25. [Google Scholar] [CrossRef]
- Joseph, S.; Anawar, H.M.; Storer, P.; Blackwell, P.; Chia, C.; Lin, Y.; Munroe, P.; Donne, S.; Horvat, J.; Wang, J.; et al. Effects of enriched biochars containing magnetic iron nanoparticles on mycorrhizal colonisation, plant growth, nutrient uptake and soil quality improvement. Pedosphere 2015, 25, 749–760. [Google Scholar] [CrossRef]
- Cai, L.; Chen, J.; Liu, Z.; Wang, H.; Yang, H.; Ding, W. Magnesium oxide nanoparticles: Effective agricultural antibacterial agent against Ralstonia solanacearum. Front. Microbiol. 2018, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Elmer, W.; Shen, Y.; Zuverza-Mena, N.; Ma, C.; Botella, P.; White, J.C.; Haynes, C.L. Silica nanoparticle dissolution rate controls the suppression of fusarium wilt of watermelon (Citrullus lanatus). Environ. Sci. Technol 2021, 55, 13513–13522. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Sun, Q.; Chen, J.; Wang, G.; Wang, D.; Zeng, X.; Wang, J. Citric acid-assisted ultrasmall CeO2 nanoparticles for efficient photocatalytic degradation of glyphosate. Chem. Eng. J. 2021, 425, 130640. [Google Scholar] [CrossRef]
- Choppin, G.R.; Pathak, P.; Thakur, P. Polymerization and complexation behavior of silicic acid: A review. Main Group Met. Chem. 2008, 31, 53–72. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. Functions and transport of silicon in plants. Cell. Mol. Life Sci. 2008, 65, 3049–3057. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Gong, H. Beneficial effects of silicon on salt and drought tolerance in plants. Agron. Sustain. Dev. 2014, 34, 455–472. [Google Scholar] [CrossRef]
- Debona, D.; Rodrigues, F.A.; Datnoff, L.E. Silicon’s role in abiotic and biotic plant stresses. Annu. Rev. Phytopathol. 2017, 55, 85–107. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.; Saleh, A.M.; Habeeb, T.H.; Hassan, Y.M.; Zrieq, R.; Wadaan, M.A.M.; Hozzein, W.N.; Selim, S.; Matos, M.; AbdElgawad, H. Silicon dioxide nanoparticles ameliorate the phytotoxic hazards of aluminum in maize grown on acidic soil. Sci. Total Environ. 2019, 693, 133636. [Google Scholar] [CrossRef] [PubMed]
- El-Shetehy, M.; Moradi, A.; Maceroni, M.; Maceroni, M.; Reinhardt, D.; Petri-Fink, A.; Rothen-Rutishauser, B.; Mauch, F.; Schwab, F. Silica nanoparticles enhance disease resistance in Arabidopsis plants. Nat. Nanotechnol. 2020, 16, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.I.; Yi, Z.; Rookes, J.E.; Kong, L.X.; Cahill, D.M. Mesoporous silica nanoparticles as a biomolecule delivery vehicle in plants. J. Nanopart. Res. 2013, 15, 1676. [Google Scholar] [CrossRef]
- Dubchak, S.; Ogar, A.; Mietelski, J.W.; Turnau, K. Influence of silver and titanium nanoparticles on arbuscular mycorrhiza colonization and accumulation of radiocaesium in Helianthus annuus. Span. J. Agric. Res. 2010, 1, 103–108. [Google Scholar] [CrossRef]
- Suriyaprabha, R.; Karunakaran, G.; Yuvakkumar, R.; Prabu, P.; Rajendran, V.; Kannan, N. Growth and physiological responses of maize (Zea mays L.) to porous silica nanoparticles in soil. J. Nanopart. Res. 2012, 14, 1294. [Google Scholar] [CrossRef]
- Suriyaprabha, R.; Karunakaran, G.; Kavitha, K.; Yuvakkumar, R.; Rajendran, V.; Kannan, N. Application of silica nanoparticle in maize to enhance fungal resistance. IET Nanobiotechnol. 2014, 8, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.K.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Dubey, N.K. Silicon nanoparticles (SiNp) alleviate chromium (VI) phytotoxicity in Pisumsativum (L.) seedlings. Plant Physiol. Biochem. 2015, 96, 189–198. [Google Scholar] [CrossRef]
- Cui, J.; Liu, T.; Li, F.; Yi, J.; Liu, C.; Yu, H. Silica nanoparticles alleviate cadmium toxicity in rice cells: Mechanisms and size effects. Environ. Pollut. 2017, 228, 363–369. [Google Scholar] [CrossRef]
- Abdel-Haliem, M.E.F.; Hegazy, H.S.; Hassan, N.S.; Naguib, D.M. Effect of silica ions and nano silica on rice plants under salinity stress. Ecol. Eng. 2017, 99, 282–289. [Google Scholar] [CrossRef]
- Rajput, N. Methods of preparation of nanoparticles—A review. Int. J. Adv. Eng. Technol. 2015, 7, 1806. [Google Scholar]
- Ayuk, E.L.; Ugwu, M.O.; Aronimo, S.B. A review on synthetic methods of nanostructured materials. Chem. Res. J. 2017, 2, 97–123. [Google Scholar]
- Lam, C.; Zhang, Y.F.; Tang, Y.H.; Lee, C.S.; Bello, I.; Lee, S.T. Large-scale synthesis of ultrafine Si nanoparticles by ball milling. J. Cryst. Growth 2000, 220, 466–470. [Google Scholar] [CrossRef]
- Yadav, T.P.; Yadav, R.M.; Singh, D.P. Mechanical milling: A top down approach for the synthesis of nanomaterials and nanocomposites. Nanosci. Nanotechnol. 2012, 2, 22–48. [Google Scholar] [CrossRef]
- Habibullah, G.; Viktorova, J.; Ruml, T. Current Strategies for Noble Metal Nanoparticle Synthesis. Nanoscale Res. Lett. 2021, 16, 47. [Google Scholar] [CrossRef] [PubMed]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Hu, A.; Yao, Z.; Yu, X. Phase behavior of a sodium dodec anolallyl sulfosuccinicdiester/n-pentanol/methyl acrylate/butyl acrylate/water microemulsion system and preparation of acrylate latexes by microemulsion polymerization. J. Appl. Polym. Sci. 2009, 113, 2202–2208. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y.L. Highly pure amorphous silica nano-disks from rice straw. Powder Technol. 2012, 225, 149–155. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous silica nanoparticles: A comprehensive review on synthesis and recent advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Zamani, H.; Jafari, A.; Mousavi, S.M.; Darezereshki, E. Biosynthesis of silica nanoparticle using Saccharomyces cervisiae and its application on enhanced oil recovery. J. Pet. Sci. Eng. 2020, 190, 107002. [Google Scholar] [CrossRef]
- Croissant, J.G.; Butler, K.S.; Zink, J.I.; Brinker, C.J. Synthetic amorphous silica nanoparticles: Toxicity, biomedical and environmental implications. Nat. Rev. Mater. 2020, 5, 886–909. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.; Li, P.Z.; Nguyen, K.T.; Wang, X.; Luo, Z.; Zhang, H.; Tan, N.S.; Zhao, Y. Biocompatible, uniform, and redispersible mesoporous silica nanoparticles for cancer-targeted drug delivery in vivo. Adv. Func. Mater. 2014, 42, 2450–2461. [Google Scholar] [CrossRef]
- Li, L.; Liu, T.; Fu, C.; Tan, L.; Meng, X.; Liu, H. Biodistribution, excretion, and toxicity of mesoporous silica nanoparticles after oral administration depend on their shape. Nanomed. NBM 2015, 11, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Van, B.A.; Kentgens, A.P.M. Particle morphology and chemical microstructure of colloidal silica spheres made from alkoxysilanes. J. Non-Cryst. Solids 1992, 149, 161–178. [Google Scholar]
- Ganguly, A.; Ahmad, T.; Ganguli, A.K. Silica mesostructures: Control of pore size and surface area using a surfactant-templated hydrothermal process. Langmuir 2010, 26, 14901–14908. [Google Scholar] [CrossRef] [PubMed]
- Croissant, J.G.; Fatieiev, Y.; Khashab, N.M. Degradability and clearance of silicon, organosilica, silsesquioxane, silica mixed oxide, and mesoporous silica nanoparticles. Adv. Mater. 2017, 29, 1604634. [Google Scholar] [CrossRef] [PubMed]
- Godin, B.; Gu, J.; Serda, R.E.; Bhavane, R.; Tasciotti, E.; Chiappini, C.; Liu, X.; Tanaka, T.; Decuzzi, P.; Ferrari, M. Tailoring the degradation kinetics of mesoporous silicon structures through PEGylation. J. Biomed. Mater. Res. Part A 2010, 94, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Showkat, A.; Zhang, Y.P.; Kim, M.S.; Gopalan, A.I.; Reddy, K.R.; Lee, K. Analysis of heavy metal toxic ions by adsorption onto amino-functionalized ordered mesoporous silica. Bull. Korean Chem. Soc. 2007, 28, 1985–1992. [Google Scholar]
- Nguyen, N.T.; Nguyen, D.H.; Pham, D.D.; Dang, V.P.; Nguyen, Q.H.; Hoang, D.Q. New oligochitosan-nanosilica hybrid materials: Preparation and application on chili plants for resistance to anthracnose disease and growth enhancement. Polym. J. 2017, 49, 861–869. [Google Scholar] [CrossRef]
- Popp, C.; Burghardt, M.; Friedmann, A.; Riederer, M. Characterization of hydrophilic and lipophilic pathways of Hedera helix L. cuticular membranes: Permeation of water and uncharged organic compounds. J. Exp. Bot. 2005, 56, 2797–2806. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, L. Polar paths of diffusion across plant cuticles: New evidence for an old hypothesis. Ann. Bot. 2005, 95, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
- Eichert, T.; Kurtz, A.; Steiner, U.; Goldbach, H.E. Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water-suspended nanoparticles. Physiol. Plant. 2008, 134, 151–160. [Google Scholar] [CrossRef]
- Hu, P.; An, J.; Faulkner, M.M.; Wu, H.; Li, Z.; Tian, X.; Giraldo, J.P. Nanoparticle charge and size control foliar delivery efficiency to plant cells and organelles. ACS Nano 2020, 14, 7970–7986. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, X.; Zhao, J.; Liu, X.; Feng, W.; White, J.C.; Xing, B. Xylem-and phloem-based transport of CuO nanoparticles in maize (Zea mays L.). Environ. Sci. Technol. 2012, 46, 4434–4441. [Google Scholar] [CrossRef]
- Lv, J.; Christie, P.; Zhang, S. Uptake, translocation, and transformation of metal-based nanoparticles in plants: Recent advances and methodological challenges. Environ. Sci. Nano 2019, 6, 41–59. [Google Scholar] [CrossRef]
- Rico, C.M.; Majumdar, S.; Duarte-Gardea, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 2011, 59, 3485–3498. [Google Scholar] [CrossRef]
- Kurepa, J.; Paunesku, T.; Vogt, S.; Arora, H.; Rabatic, B.M.; Lu, J.; Wanzer, M.B.; Woloschak, G.E.; Smalle, J.A. Uptake and distribution of ultrasmall anatase TiO2 Alizarin red S nanoconjugates in Arabidopsis thaliana. Nano Lett. 2010, 10, 2296–2302. [Google Scholar] [CrossRef]
- Chang, F.P.; Kuang, L.Y.; Huang, C.A.; Jane, W.; Hung, Y.; Yu-ie, C.; Mou, C. A simple plant gene delivery system using mesoporous silica nanoparticles as carriers. J. Mat. Chem. B 2013, 1, 5279–5287. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Hussain, H.I.; Yi, Z.; Siegele, R.; Cresswell, T.; Kong, L.; Cahill, D.M. Uptake and cellular distribution, in four plant species, of fluorescently labeled mesoporous silica nanoparticles. Plant Cell Rep. 2014, 33, 1389–1402. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.K.; Singh, S.; Singh, S.; Pandey, R.; Singh, V.P.; Sharma, N.C.; Prasad, S.M.; Dubey, N.K.; Chauhan, D.K. An overview on manufactured nanoparticles in plants: Uptake, translocation, accumulation and phytotoxicity. Plant Physiol. Biochem. 2017, 110, 2–12. [Google Scholar] [CrossRef]
- Sun, D.; Hussain, H.I.; Yi, Z.; Rookes, J.E.; Kong, L.; Cahill, D.M. Mesoporous silica nanoparticles enhance seedling growth and photosynthesis in wheat and lupin. Chemosphere 2016, 152, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef]
- Nazaralian, S.; Majd, A.; Irian, S.; Najafi, F.; Ghahremaninejad, F.; Landberg, T.; Greger, M. Comparison of silicon nanoparticles and silicate treatments in fenugreek. Plant Physiol. Biochem. 2017, 115, 25–33. [Google Scholar] [CrossRef]
- Asgari, F.; Majd, A.; Jonoubi, P.; Najafi, F. Effects of silicon nanoparticles on molecular, chemical, structural and ultrastructural characteristics of oat (Avena sativa L.). Plant Physiol. Biochem. 2018, 127, 152–160. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Vishwakarma, K.; Singh, V.P.; Prakash, V.; Sharma, S.; Muneer, S.; Nikolic, M.; Deshmukh, R.; Vaculik, M.; Corpa, F.J. Silicon crosstalk with reactive oxygen species, phytohormones and other signaling molecules. J. Hazard. Mater. 2021, 408, 124820. [Google Scholar] [CrossRef]
- Aslani, F.; Bagheri, S.; MuhdJulkapli, N.; Juraimi, A.S.; Hashemi, F.S.G.; Baghdadi, A. Effects of engineered nanomaterials on plants growth: An overview. Sci. World J. 2014, 2014, 641759. [Google Scholar] [CrossRef]
- Slomberg, D.L.; Schoenfisch, M.H. Silica nanoparticle phytotoxicity to Arabidopsis thaliana. Environ. Sci. Technol. 2012, 46, 10247–10254. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.W.; Slomberg, D.L.; Rao, K.S.; Schoenfisch, M.H. Influence of scaffold size on bactericidal activity of nitric oxide-releasing silica nanoparticles. ACS Nano 2011, 5, 7235. [Google Scholar] [CrossRef]
- Gilroy, S.; Suzuki, N.; Miller, G.; Choi, W.; Toyota, M.; Devireddy, A.R.; Mittler, R. A tidal wave of signals: Calcium and ROS at the forefront of rapid systemic signaling. Trends Plant Sci. 2014, 19, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Blumwald, E. The roles of ROS and ABA in systemic acquired acclimation. Plant Cell 2015, 27, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, F.K.; Devireddy, A.R.; Azad, R.K.; Shulaev, V.; Mittler, R. Local and systemic metabolic responses during light-induced rapid systemic signaling. Plant Physiol. 2018, 178, 1461–1472. [Google Scholar] [CrossRef]
- Kollist, H.; Zandalinas, S.I.; Sengupta, S.; Nuhkat, M.; Kangasjärvi, J.; Mittler, R. Rapid responses to abiotic stress: Priming the landscape for the signal transduction network. Trends Plant Sci. 2018, 24, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Fichman, Y.; Mittler, R. Rapid systemic signaling during abiotic and biotic stresses: Is the ROS wave master of all trades? Plant J. 2020, 102, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Rangaraj, S.; Gopalu, K.; Muthusamy, P.; Rathinam, Y.; Venkatachalam, R.; Narayanasamy, K. Augmented biocontrol action of silica nanoparticles and Pseudomonas fluorescens bioformulant in maize (Zea mays L.). RSC Adv. 2014, 4, 8461–8465. [Google Scholar] [CrossRef]
- Namjoyan, S.; Sorooshzadeh, A.; Rajabi, A.; Aghaalikhani, M. Nano-silicon protects sugar beet plants against water deficit stress by improving the antioxidant systems and compatible solutes. Acta Physiol. Plant. 2020, 42, 157. [Google Scholar] [CrossRef]
- Buchman, J.T.; Elmer, W.H.; Ma, C.; Landy, K.M.; White, J.C.; Haynes, C.L. Chitosan-Coated mesoporous silica nanoparticle treatment of Citrullus lanatus (watermelon): Enhanced fungal disease suppression and modulated expression of stress-related genes. ACS Sustain. Chem. Eng. 2019, 7, 19649–19659. [Google Scholar] [CrossRef]
- Derbalah, A.; Shenashen, M.; Hamza, A.; Mohamed, A.; El-Safty, S. Antifungal activity of fabricated mesoporous silica nanoparticles against early blight of tomato. Egypt. J. Basic Appl. Sci. 2018, 5, 145–150. [Google Scholar] [CrossRef]
- El-Shewy, E.S. The efficacy of copper oxide, tri-calcium phosphate and silicon dioxide nanoparticles in controlling black scurf disease of potato. Ann. Agric. Sci. Moshtohor 2019, 57, 129–138. [Google Scholar] [CrossRef]
- Hasan, K.A.; Soliman, H.; Baka, Z.; Shabana, Y.M. Efficacy of nano-silicon in the control of chocolate spot disease of Vicia faba L. caused by Botrytis fabae. Egypt. J. Basic Appl. Sci. 2020, 7, 53–66. [Google Scholar] [CrossRef]
- Khan, M.R.; Siddiqui, Z.A. Use of silicon dioxide nanoparticles for the management of Meloidogyne incognita, Pectobacterium betavasculorum and Rhizoctonia solanidisease complex of beetroot (Beta vulgaris L.). Sci. Hortic. 2020, 265, 109–211. [Google Scholar] [CrossRef]
- Elamawi, R.M.; Tahoon, A.M.; Elsharnoby, D.E.; El-Shafey, R.A. Bio-production of silica nanoparticles from rice husk and their impact on rice bakanae disease and grain yield. Arch. Phytopathol. Pflanzenschutz 2020, 53, 459–478. [Google Scholar] [CrossRef]
- El-Shabrawy, E.M. Use Silica nanoparticles in controlling late wilt disease in maize caused by Harpophora Maydis. Egypt. J. Appl. Sci. 2021, 36, 1–19. [Google Scholar]
- Nisaq, G.J.; Sudarsono, S.; Sukma, D. Nano silica spray increase Phalaenopsis pulcherrima growth and resistance against Dickeya dadantii infection. IOP Conf. Ser. Earth Environ. Sci. 2021, 694, 012040. [Google Scholar] [CrossRef]
- Nawrath, C. Unraveling the complex network of cuticular structure and function. Curr. Opin. Plant Biol. 2006, 9, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Łaźniewska, J.; Macioszek, V.K.; Kononowicz, A.K. Plant-fungus interface: The role of surface structures in plant resistance and susceptibility to pathogenic fungi. Physiol. Mol. Plant Pathol. 2012, 78, 24–30. [Google Scholar] [CrossRef]
- Hartley, S.E.; Fitt, R.N.; McLarnon, E.L.; Wade, R.N. Defending the leaf surface: Intra- and inter-specific differences in silicon deposition in grasses in response to damage and silicon supply. Front. Plant Sci. 2015, 6, 35. [Google Scholar] [CrossRef]
- Hayasaka, T.; Fujii, H.; Ishiguro, K. The role of silicon in preventing appressorial penetration by the rice blast fungus. Phytopathology 2008, 98, 1038–1044. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Madany, M.M.Y.; Saleh, A.M.; Habeeb, T.H.; Hozzein, W.N.; AbdElgawad, H. Silicon dioxide nanoparticles alleviate the threats of broomrape infection in tomato by inducing cell wall fortification and modulating ROS homeostasis. Environ. Sci. Nano 2020, 7, 1415–1430. [Google Scholar] [CrossRef]
- Kandhol, N.; Singh, V.P.; Peralta-Videa, J.; Corpas, F.J.; Tripathi, D.K. Silica nanoparticles: The rising star in plant disease protection. Trends Plant Sci. 2021, 27, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Z.; Xu, S.A.; Wen, L.X.; Liu, F.; Liu, A.Q.; Wang, Q.; Sun, H.Y.; Yu, W.; Chen, J.F. Controlled release of avermectin from porous hollow silica nanoparticles: Influence of shell thickness on loading efficiency, UV-shielding property and release. J. Control. Release 2006, 111, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Barik, T.K.; Sahu, B.; Swain, V. Silica nanoparticle—From medicine to pest control. Parasitol. Res. 2008, 103, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Athanassiou, C.G.; Kavallieratos, N.G.; Benelli, G.; Losic, D.; Rani, P.U.; Desneux, N. Nanoparticles for pest control: Current status and future perspectives. J. Pest Sci. 2018, 91, 1–15. [Google Scholar] [CrossRef]
- Dayan, F.E.; Cantrell, C.L.; Duke, S.O. Natural products in crop protection. Bioorg. Med. Chem. 2009, 17, 4022–4034. [Google Scholar] [CrossRef] [PubMed]
- Madhusudhanamurthya, J.; Usha, R.P.I.; Raob, K.R.S. Organic-inorganic hybrids of nanosilica and certain botanical compounds for their improved bioactivity against agricultural pests. Curr. Trends Biotechnol. Pharm. 2013, 7, 615–624. [Google Scholar]
- Usha, S.; Nair, D.G.; Vishnudas, S. Geopolymer binder from industrial wastes: A review. Int. J. Civ. Eng. Technol. 2014, 5, 219–225. [Google Scholar]
- Bapat, G.; Zinjarde, S.; Tamhane, V. Evaluation of silica nanoparticle mediated delivery of protease inhibitor in tomato plants and its effect on insect pest Helicoverpa armigera. Colloids Surf. B 2020, 193, 111079. [Google Scholar] [CrossRef]
- El-Helaly, A.A.; El-Bendary, H.M.; Abdel-Wahab, A.S.; El-Sheikh, M.A.K.; Elnagar, S. The silica-nano particles treatment of squash foliage and survival and development of Spodoptera littoralis (Bosid.) larvae. Pest Control 2016, 5, 6. [Google Scholar]
- Debnath, N.; Das, S.; Seth, D.; Chandra, R.; Bhattacharya, S.C.; Goswami, A. Entomotoxic effect of silica nanoparticles against Sitophilus oryzae (L.). J. Pest Sci. 2011, 84, 99–105. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Abdelsalam, N.R.; Fouda, M.M.G.; Mackled, M.I.; Al-Jaddadi, M.A.M.; Ali, H.M.; Siddiqui, M.H.; Kandil, E.E. Soil application of nano silica on maize yield and its insecticidal activity against some stored insects after the post-harvest. Nanomaterials 2020, 10, 739. [Google Scholar] [CrossRef] [PubMed]
- Vani, C.; Brindhaa, U. Silica nanoparticles as nanocides against Corcyra cephalonica (S.), the stored grain pest. Int. J. Pharma Bio Sci. 2013, 4, 1108–1118. [Google Scholar]
- Balabanidou, V.; Grigoraki, L.; Vontas, J. Insect cuticle: A critical determinant of insecticide resistance. Curr. Opin. Insect Sci. 2018, 27, 68–74. [Google Scholar] [CrossRef]
- Debnath, N.; Mitra, S.; Das, S.; Goswami, A. Synthesis of surface functionalized silica nanoparticles and their use as entomo toxic nanocides. Powder Technol. 2012, 221, 252–256. [Google Scholar] [CrossRef]
- Ayoub, H.A.; Khairy, M.; Rashwan, F.A.; Abdel-Hafez, H.F. Synthesis and characterization of silica nanostructures for cotton leaf worm control. J. Nanostructure Chem. 2017, 7, 91–100. [Google Scholar] [CrossRef]
- Benelli, G. Mode of action of nanoparticles against insects. Environ. Sci. Pollut. Res. 2018, 25, 12329–12341. [Google Scholar] [CrossRef]
- Sushila, N.; Pavitra, S.A.G.; Ashoka, J.; Sharanagouda, H. Biosynthesis and effect of green silica nanoparticles on tobacco caterpillar, Spodoptera litura on cotton. J. Entomol. Zool. Stud. 2020, 8, 1564–1570. [Google Scholar]
- Shoaib, A.; Elabasy, A.; Waqas, M.; Lin, L.; Cheng, X.; Zhang, Q.; Shi, Z. Entomotoxic effect of silicon dioxide nanoparticles on Plutella xylostella (L.) (Lepidoptera: Plutellidae) under laboratory conditions. Toxicol. Environ. Chem. 2018, 100, 80–91. [Google Scholar] [CrossRef]
- Thabet, A.F.; Galal, O.A.; Tuda, M.; EI-Samahy, M.F.M.; Fujita, R.; Hion, M. Silica nanoparticle effect on population parameters and gene expression of an internal feeder, American serpentine leafminer. Preprints 2020, 2020100369, 1–15. [Google Scholar]
- Liu, C.; Li, F.; Luo, C.; Liu, X.; Wang, S.; Liu, T.; Li, X. Foliar application of two silica sols reduced cadmium accumulation in rice grains. J. Hazard. Mater. 2009, 161, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, F.; Gao, S. Foliar application with nano-silicon alleviates Cd toxicity in rice seedlings. Environ. Sci. Pollut. Res. 2015, 22, 2837–2845. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Rizwan, M.; Hussain, A.; Rehman, M.Z.; Ali, B.; Yousaf, B.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Silicon nanoparticles enhanced the growth and reduced the cadmium accumulation in grains of wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2019, 140, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, N.; Liang, X.; Bai, X.; Zheng, L.; Zhao, J.; Li, Y.; Zhang, Z.; Gao, Y. Silica nanoparticles alleviate mercury toxicity via immobilization and inactivation of Hg (ii) in soybean (Glycine max). Environ. Sci. Nano 2020, 7, 1807–1817. [Google Scholar] [CrossRef]
- Fatemi, H.; Pour, B.E.; Rizwan, M. Foliar application of silicon nanoparticles affected the growth, vitamin C, flavonoid, and antioxidant enzyme activities of coriander (Coriandrum sativum L.) plants grown in lead (Pb)-spiked soil. Environ. Sci. Pollut. Res. 2021, 28, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhang, C.; Zhao, Y.; Huang, Y.; Liu, Z. Foliar application with nano-silicon reduced cadmium accumulation in grains by inhibiting cadmium translocation in rice plants. Environ. Sci. Pollut. Res. 2018, 25, 2361–2368. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Chauhan, R.; Korram, J.; Satnami, M.L.; Keshavkant, S. Silica nanoparticle minimizes aluminium imposed injuries by impeding cytotoxic agents and over expressing protective genes in Cicer arietinum. Sci. Hortic. 2020, 260, 108885. [Google Scholar] [CrossRef]
- Mithöfer, A.; Schulze, B.; Boland, W. Biotic and heavy metal stress response in plants: Evidence for common signals. FEBS Lett. 2004, 566, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Khan, A.L.; Waqas, M.; Lee, I. Silicon regulates antioxidant activities of crop plants under abiotic-induced oxidative stress: A review. Front. Plant Sci. 2017, 8, 510. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.K.; Singh, S.; Singh, V.P.; Prasad, M.; Chauhan, D.K.; Dubey, N.K. Silicon nanoparticles more efficiently alleviate arsenate toxicity than silicon in maize cultiver and hybrid differing in arsenate tolerance. Front. Environ. Sci. 2016, 4, 46. [Google Scholar] [CrossRef]
- Yadav, S.K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Singh, S.; Prasad, S.M.; Sharma, S.; Dubey, N.K.; Ramawat, N.; Prasad, R.; Singh, V.P.; Tripathi, D.K.; Chauhan, D.K. Silicon and nitric oxide-mediated mechanisms of cadmium toxicity alleviation in wheat seedlings. Physiol. Plant. 2020, 13065. [Google Scholar] [CrossRef] [PubMed]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Ghori, N.H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; ur Rehman, M.Z.; Malik, S.; Adrees, M.; Qayyum, M.F.; Alamri, S.A.; Alyemeni, M.N.; Ahmad, P. Effect of foliar applications of silicon and titanium dioxide nanoparticles on growth, oxidative stress, and cadmium accumulation by rice (Oryza sativa). Acta Physiol. Plant. 2019, 41, 35. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Haghighi, M.; Pessarakli, M. Influence of silicon and nano-silicon on salinity tolerance of cherry tomatoes (Solanum lycopersicum L.) at early growth stage. Sci. Hortic. 2013, 161, 111–117. [Google Scholar] [CrossRef]
- Sabaghnia, N.; Janmohammadi, M. Graphic analysis of nano-silicon by salinity stress interaction on germination properties of lentil using the biplot method. Agric. For. 2014, 60, 29–40. [Google Scholar]
- Tantawy, A.S.; Salama, Y.A.M.; El-Nemr, M.A. Nano silicon application improves salinity tolerance of sweet pepper plants. Int. J. Chemtech Res. 2015, 8, 11–17. [Google Scholar]
- Kalteh, M.; Alipour, Z.T.; Ashraf, S.; Aliabadi, M.M.; Nosratabadi, A.F. Effect of silica nanoparticles on basil (Ocimum basilicum) under salinity stress. J. Chem. Health Risks 2018, 4, 49–55. [Google Scholar]
- Alsaeedi, A.; El-Ramady, H.; Alshaal, T.; El-Garawani, M.; Elhawat, N.; Al-Otaibie, A. Exogenous Silica nanoparticles improves germination and growth of cucumber by maintaining K+/Na+ ratio under elevated Na+ stress. Plant Physiol. Biochem. 2018, 125, 164–171. [Google Scholar] [CrossRef]
- Naguib, D.M.; Abdalla, H. Metabolic status during germination of nano silica primed Zea mays seeds under salinity stress. J. Crop Sci. Biotechnol. 2019, 22, 415–423. [Google Scholar] [CrossRef]
- González, A.; Ayerbe, L. Effect of terminal water stress on leaf epicuticular wax load, residual transpiration and grain yield in barley. Euphytica 2010, 172, 341–349. [Google Scholar] [CrossRef]
- Samuels, A.L.; Glass, A.D.M.; Ehret, D.L.; Menzies, J.G. The effects of silicon supplementation on cucumber fruit: Changes in surface characteristics. Ann. Bot. 1993, 72, 433–440. [Google Scholar] [CrossRef]
- Avestan, S.; Ghasemnezhad, M.; Esfahani, M.; Byrt, C.S. Application of nano-silicon dioxide improves salt stress tolerance in strawberry plants. Agronomy 2019, 9, 246. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Nayyar, H.; Walia, D.P. Water stress induced proline accumulation in contrasting wheat genotypes as affected by calcium and abscisic acid. Biol. Plant. 2003, 46, 275–279. [Google Scholar] [CrossRef]
- Almutairi, Z.M. Effect of nano-silicon application on the expression of salt tolerance genes in germinating tomato (Solanum lycopersicum L.) seedlings under salt stress. Plant Omics 2016, 9, 106–114. [Google Scholar]
- Behboudi, F.; Sarvestani, T.Z.; Kassaee, M.Z.; Sanavi, S.A.M.M.; Sorooshzadeh, A. Improving growth and yield of wheat under drought stress via application of SiO2 nanoparticles. J. Agric. Sci. Technol. 2018, 20, 1479–1492. [Google Scholar]
- Ashkavand, P.; Zarafshar, M.; Tabari, M.; Mirzaie, J.; Nikpour, A.; Bordbar, S.K.; Struve, D.; Striker, G.G. Application of SiO2 nanoparticles as pretreatment alleviates the impact of drought on the physiological performance of Prunus mahaleb (Rosaceae). Bol. Soc. Argent. Bot. 2018, 53, 207–219. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Moharrami, F.; Sarikhani, S.; Padervand, M. Selenium and silica nanostructure-based recovery of strawberry plants subjected to drought stress. Sci. Rep. 2020, 10, 17672. [Google Scholar] [CrossRef] [PubMed]
- Hellala, F.; Amerb, A.K.; El-Sayeda, S.; El-Azab, K. Mitigation The negative effect of water stress on barley by nano silica application. Plant Arch. 2020, 20, 3224–3231. [Google Scholar]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, M.; Hausman, J.F.; Lutts, S.; Guerriero, G. Silicon and plants: Current knowledge and technological perspectives. Front. Plant Sci. 2017, 8, 411. [Google Scholar] [CrossRef]
- Boaretto, L.F.; Carvalho, G.; Borgo, L.; Creste, S.; Landell, M.G.A.; Mazzafera, P.; Azevedo, R.A. Water stress reveals differential antioxidant responses of tolerant and non-tolerant sugarcane genotypes. Plant Physiol. Biochem. 2014, 74, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Parveen, A.; Liu, W.; Hussain, S.; Asghar, J.; Perveen, S.; Xiong, Y. Silicon priming regulates morpho-physiological growth and oxidative metabolism in maize under drought stress. Plants 2019, 8, 431. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Sustainable Agric. 2009, 29, 153–188. [Google Scholar]
- Ashkavand, P.; Tabari, M.; Zarafshar, M.; Tomášková, I.; Struve, D. Effect of SiO2 nanoparticles on drought resistance in hawthorn seedlings. Leś. Pr. Bad. 2015, 76, 350–359. [Google Scholar] [CrossRef]
- Ghormade, V.; Deshpande, M.V.; Paknikar, K.M. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol. Adv. 2011, 29, 792–803. [Google Scholar] [CrossRef]
- Peters, R.; Kramer, E.; Oomen, A.G.; Herrera Rivera, Z.E.; Oegema, G.; Tromp, P.C.; Fokkink, R.; Rietveld, A.; Marvin, H.J.P.; Weigel, S.; et al. Presence of nano-sized silica during in vitro digestion of foods containing silica as a food additive. ACS Nano 2012, 6, 2441–2451. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, S.; Krystek, P.; Peters, R.J.; Lankveld, D.P.; Bokkers, B.G.; van Hoeven-Arentzen, P.H.; Bouwmeester, H.; Oomen, A.G. Presence and risks of nanosilica in food products. Nanotoxicology 2011, 5, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Liljenström, C.; Lazarevic, D.; Finnveden, G. Silicon-Based Nanomaterials in a Life-Cycle Perspective, including a Case Study on Self-Cleaning Coatings. 2013. Available online: https://www.researchgate.net/profile/David-Lazarevic/publication/280264076_Silicon-based_nanomaterials_in_a_life-cycle_perspective_including_a_case_study_on_self-cleaning_coatings/links/55afbf5408aeb9239916f5f3/Silicon-based-nanomaterials-in-a-life-cycle-perspective-including-a-case-study-on-self-cleaning-coatings.pdf (accessed on 6 February 2022).
- Van, H.K.; De Schamphelaere, K.A.C.; Van, M.P.; Lcucas, S.; Janssen, C.R. Ecotoxicity of silica nanoparticles to the green alga Pseudokirchneriella subcapitata: Importance of surface area. Environ. Toxicol. Chem. 2008, 27, 1948–1957. [Google Scholar]
- Manzo, S.; Buono, S.; Rametta, G.; Miglietta, M.; Schiavo, S.; Di Francia, G. The diverse toxic effect of SiO2 and TiO2 nanoparticles toward the marine microalgae Dunaliella tertiolecta. Environ. Sci. Pollut. Res. 2015, 22, 15941–15951. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Mahendra, S.; Zodrow, K.; Li, D.; Tsai, Y.; Braam, J.; Alvarez, P.J.J. Developmental phytotoxicity of metal oxide nanoparticles to Arabidopsis thaliana. Environ. Toxicol. Chem. 2010, 29, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, H.; White, J.C.; Chen, X.; Li, H.; Qu, X.; Ji, R. Metabolomics reveals that engineered nanomaterial exposure in soil alters both soil rhizosphere metabolite profiles and maize metabolic pathways. Environ. Sci. Nano 2019, 6, 1716–1727. [Google Scholar] [CrossRef]

| Size and Concentration | Plants | Applied Method | Disease | Mechanism | References |
|---|---|---|---|---|---|
| 20–40 nm, 5, 10, and 15 kg·ha−1 | Corn | Soil application | Fusarium oxysporum, Aspergillusniger | Increase phenolic content, activated defense-related enzymes | [22] |
| 30–50 nm 60 mg·L−1 | Chili | Foliar spray | Colletotrichum sp. | _ | [44] |
| 100, 200, 300, and 400 mg·L−1 | Tomato | Foliar spray | Alternaria solani | Killed germs | [72] |
| <50 nm 50, 100, 150, and 200 µL·L−1 | Potato | Soaked potato tubers | Rhizoctonia solani | activated defense-related enzymes | [73] |
| 36–39 nm 500 mg·L−1 | Watermelon | Root dip | Fusarium oxysporum f. sp. niveum | Reduced the expression of stress-related genes | [71] |
| 30–60 nm, 1.5 and 3 mM | Broad bean | Foliar spray | Botrytis fabae | Increased defense compounds and activated defense-related enzymes | [74] |
| 54 ± 7 nm, 25, 100, 400, and 1600 mg SiO2·L−1 | Arabidopsis | Foliar spray | P. syringae | Induce plant resistance | [18] |
| 5–15 nm, 100 and 200 mg·L−1 | Beet | Foliar spray and Seed soaking | Meloidogyne incognita, Pectobacterium betavasculorum, and Rhizoctoniasolani | Promoted growth, improved photosynthesis, activated defense-related enzymes | [75] |
| 15 nm 50 mg·L−1 | Rice | Foliar spray | Fusarium fujikuroi | Improved peroxidase activity | [76] |
| 1500 mg·L−1 | Watermelon | leaf immersion method | Fusarium oxysporum | _ | [11] |
| 20, 40.2, 70.2, and 95.5 nm 0.5, 2.5, 5, and 10 ppm | Maize | In vitro experiment | Harpophora maydis | inhibited the mycelia growth | [77] |
| 0, 7.5, 15, 22.5, and 30 ppm | Phalaenopsis | Foliar spray | Dickeya dadantii | Promoted growth | [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Ning, C.; Pan, T.; Cai, K. Role of Silica Nanoparticles in Abiotic and Biotic Stress Tolerance in Plants: A Review. Int. J. Mol. Sci. 2022, 23, 1947. https://doi.org/10.3390/ijms23041947
Wang L, Ning C, Pan T, Cai K. Role of Silica Nanoparticles in Abiotic and Biotic Stress Tolerance in Plants: A Review. International Journal of Molecular Sciences. 2022; 23(4):1947. https://doi.org/10.3390/ijms23041947
Chicago/Turabian StyleWang, Lei, Chuanchuan Ning, Taowen Pan, and Kunzheng Cai. 2022. "Role of Silica Nanoparticles in Abiotic and Biotic Stress Tolerance in Plants: A Review" International Journal of Molecular Sciences 23, no. 4: 1947. https://doi.org/10.3390/ijms23041947
APA StyleWang, L., Ning, C., Pan, T., & Cai, K. (2022). Role of Silica Nanoparticles in Abiotic and Biotic Stress Tolerance in Plants: A Review. International Journal of Molecular Sciences, 23(4), 1947. https://doi.org/10.3390/ijms23041947







