METTL21A, a Non-Histone Methyltransferase, Is Dispensable for Spermatogenesis and Male Fertility in Mice
Abstract
:1. Introduction
2. Results
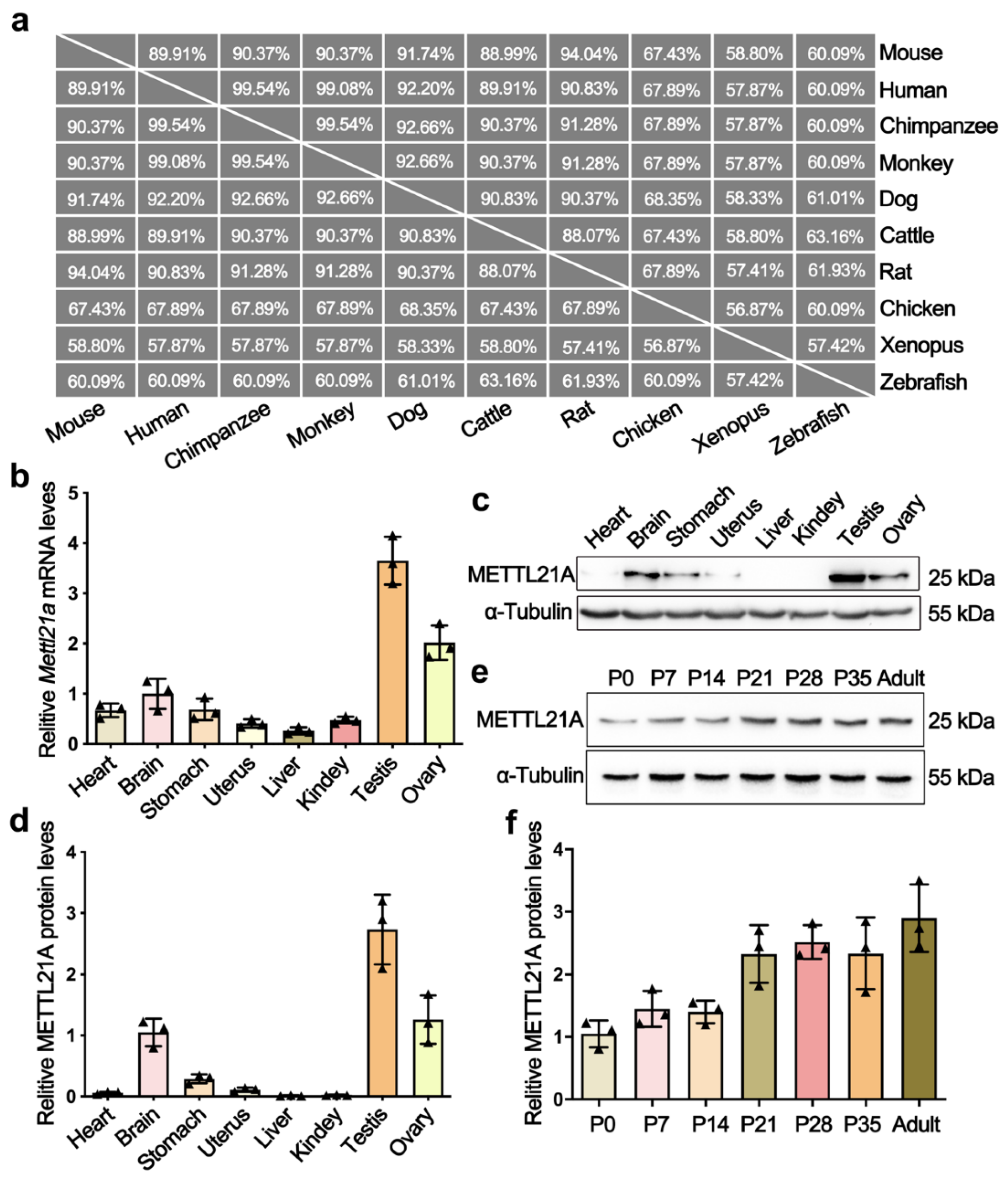
2.1. Mettl21a Is Preferentially Expressed in Mouse Testes
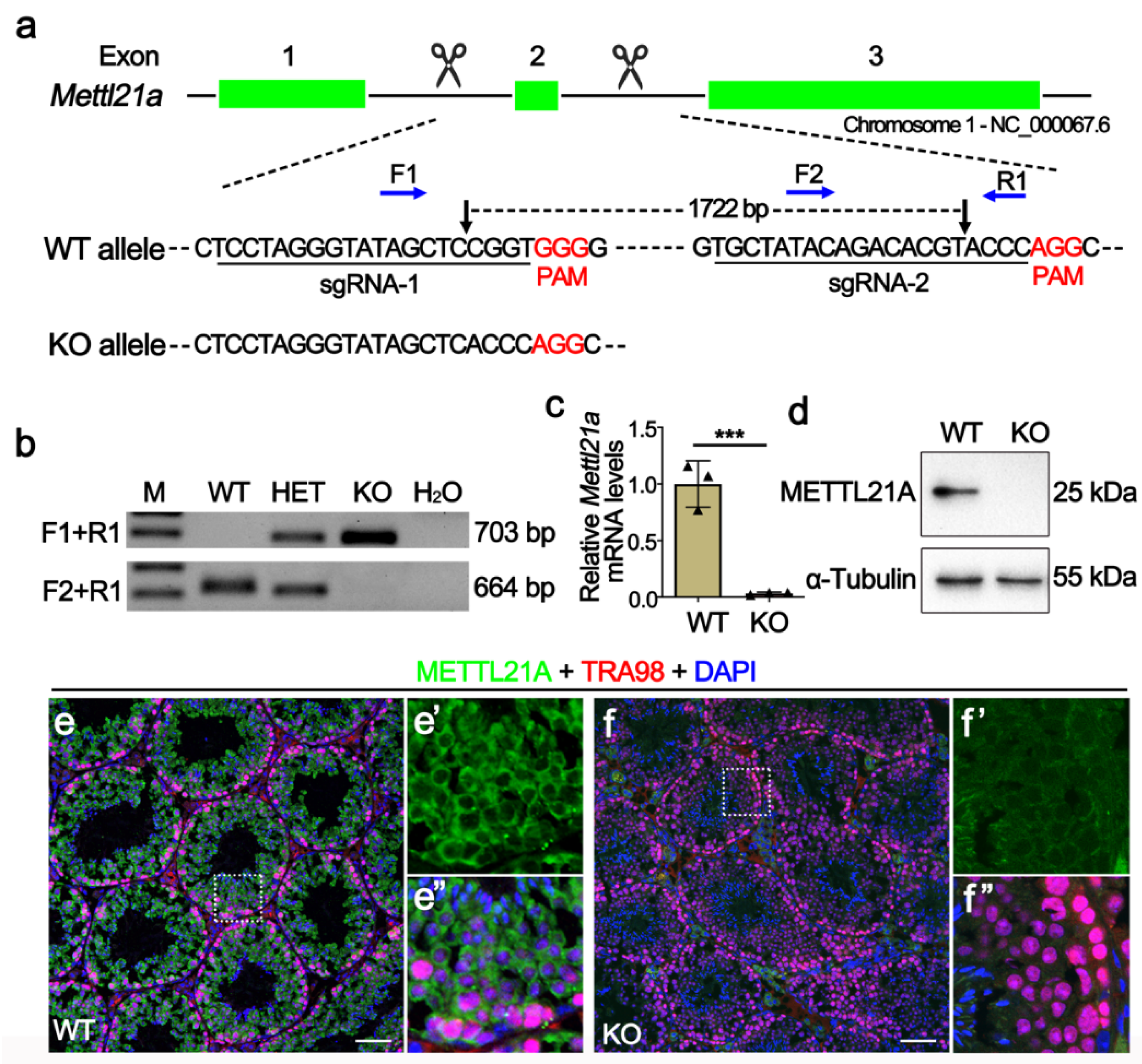
2.2. Generation of Mettl21a Knockout Mice
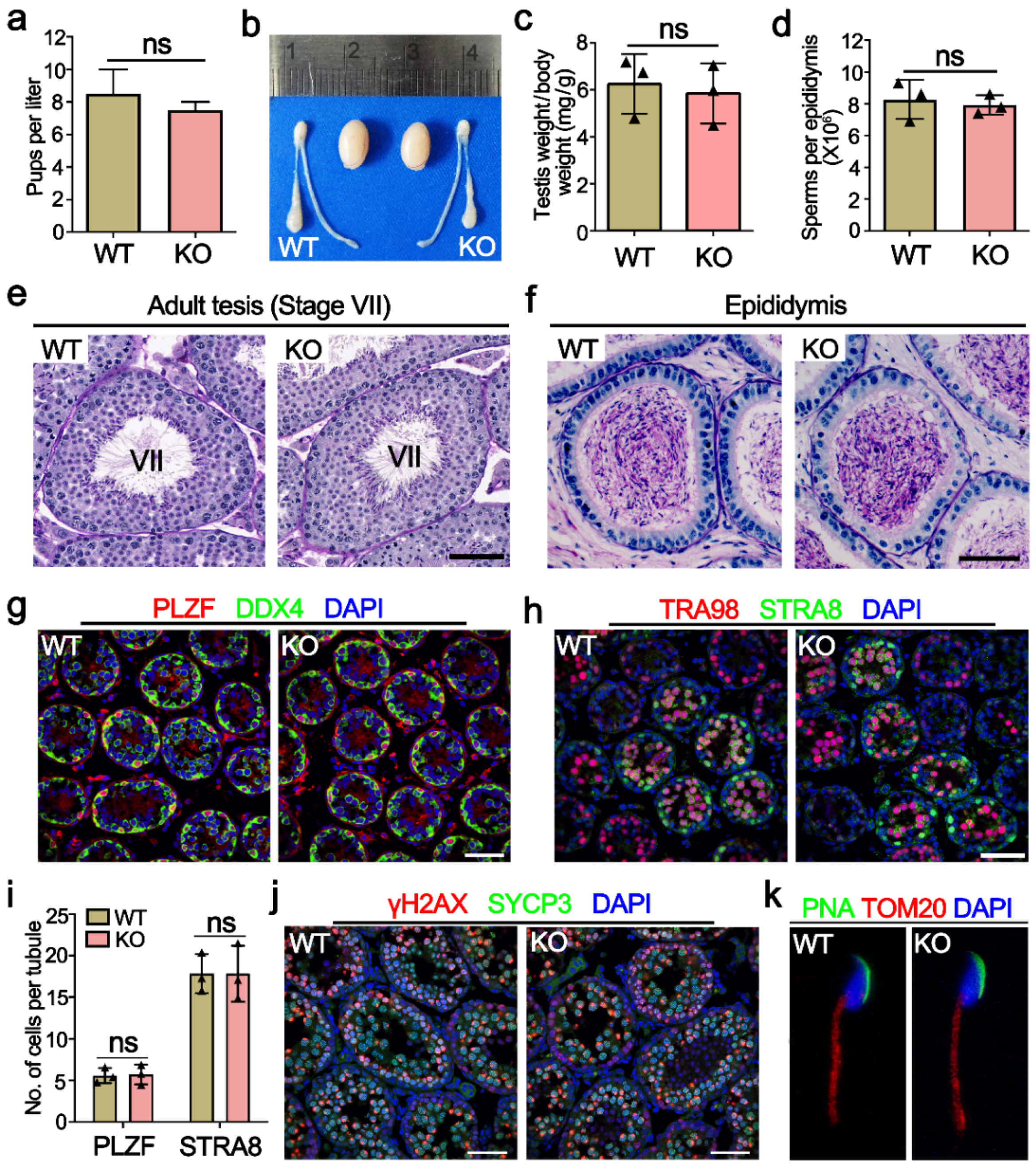
2.3. Mettl21a Is Dispensable for Spermatogenesis
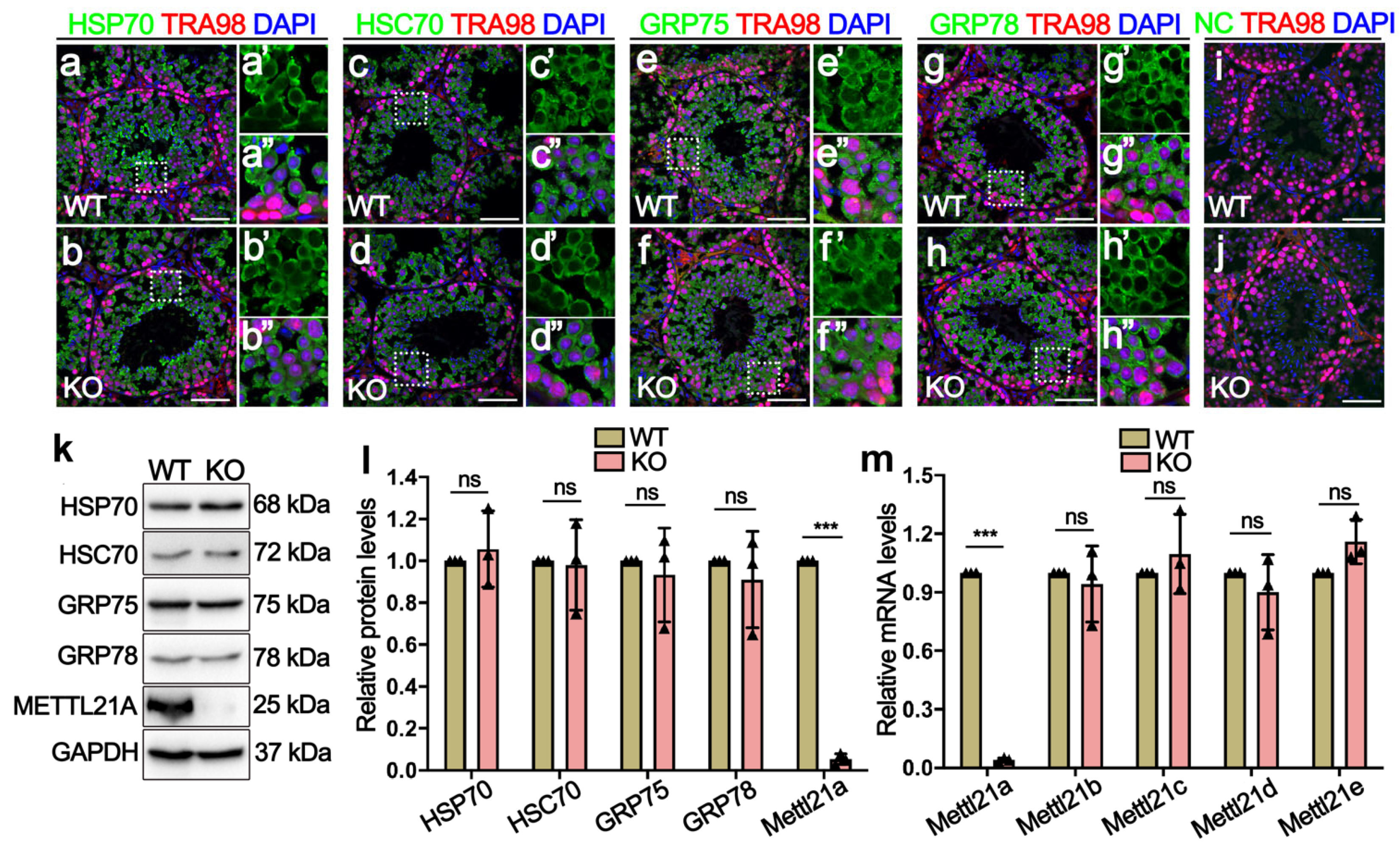
2.4. Deletion of Mettl21a Did Not Alter the Expression of Its Target Proteins and Other Mettl21 Members
3. Discussions
4. Materials and Methods
4.1. Animals and Ethics Statement
4.2. CRISPR-Cas9 Mediated Gene Targeting and Genotyping
4.3. Fertility Test
4.4. Tissue Collection and Histological Analyses
4.5. Sperm Counting
4.6. Total RNA Extraction and RT-qPCR
4.7. Immunostaining
4.8. Protein Extract and Western Blot
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neto, F.T.L.; Bach, P.V.; Najari, B.B.; Li, P.S.; Goldstein, M. Spermatogenesis in humans and its affecting factors. Semin. Cell Dev. Biol. 2016, 59, 10–26. [Google Scholar] [CrossRef] [PubMed]
- McSwiggin, H.; O’Doherty, A. Epigenetic reprogramming during spermatogenesis and male factor infertility. Reproduction 2018, 156, R9–R21. [Google Scholar] [CrossRef] [PubMed]
- Biggar, K.K.; Li, S.S.-C. Non-histone protein methylation as a regulator of cellular signalling and function. Nat. Rev. Mol. Cell Biol. 2015, 16, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.W.; Cho, Y.; Bae, G.-U.; Kim, S.-N.; Kim, Y.K. Protein arginine methyltransferases: Promising targets for cancer therapy. Exp. Mol. Med. 2021, 53, 788–808. [Google Scholar] [CrossRef]
- Mahgoub, M.; Paiano, J.; Bruno, M.; Wu, W.; Pathuri, S.; Zhang, X.; Ralls, S.; Cheng, X.; Nussenzweig, A.; Macfarlan, T. Dual histone methyl reader ZCWPW1 facilitates repair of meiotic double strand breaks in male mice. Elife 2020, 9, e53360. [Google Scholar] [CrossRef]
- An, J.; Zhang, X.; Qin, J.; Wan, Y.; Hu, Y.; Liu, T.; Li, J.; Dong, W.; Du, E.; Pan, C.; et al. The histone methyltransferase ESET is required for the survival of spermatogonial stem/progenitor cells in mice. Cell Death Dis. 2014, 5, e1196. [Google Scholar] [CrossRef] [Green Version]
- Mu, W.; Starmer, J.; Shibata, Y.; Yee, D.; Magnuson, T. EZH1 in germ cells safeguards the function of PRC2 during spermatogenesis. Dev. Biol. 2017, 424, 198–207. [Google Scholar] [CrossRef]
- Zuo, X.; Rong, B.; Li, L.; Lv, R.; Lan, F.; Tong, M.-H. The histone methyltransferase SETD2 is required for expression of acrosin-binding protein 1 and protamines and essential for spermiogenesis in mice. J. Biol. Chem. 2018, 293, 9188–9197. [Google Scholar] [CrossRef] [Green Version]
- Cloutier, P.; Lavallée-Adam, M.; Faubert, D.; Blanchette, M.; Coulombe, B. A newly uncovered group of distantly related lysine methyltransferases preferentially interact with molecular chaperones to regulate their activity. PLoS Genet. 2013, 9, e1003210. [Google Scholar] [CrossRef] [Green Version]
- Małecki, J.; Aileni, V.K.; Ho, A.Y.; Schwarz, J.; Moen, A.; Sørensen, V.; Nilges, B.S.; Jakobsson, M.E.; Leidel, S.; Falnes, P.Ø. The novel lysine specific methyltransferase METTL21B affects mRNA translation through inducible and dynamic methylation of Lys-165 in human eukaryotic elongation factor 1 alpha (eEF1A). Nucleic Acids Res. 2017, 45, 4370–4389. [Google Scholar] [CrossRef]
- Huang, J.; Hsu, Y.; Mo, C.; Abreu, E.; Kiel, D.P.; Bonewald, L.F.; Brotto, M.; Karasik, D. METTL21C is a potential pleiotropic gene for osteoporosis and sarcopenia acting through the modulation of the NF-κB signaling pathway. J. Bone Miner. Res. 2014, 29, 1531–1540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kernstock, S.; Davydova, E.; Jakobsson, M.; Moen, A.; Pettersen, S.J.; Mælandsmo, G.M.; Egge-Jacobsen, W.; Falnes, P.Ø. Lysine methylation of VCP by a member of a novel human protein methyltransferase family. Nat. Commun. 2012, 3, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Zhang, B.; Ratliff, A.C.; Arlington, J.; Chen, J.; Xiong, Y.; Yue, F.; Nie, Y.; Hu, K.; Jin, W.; et al. Methyltransferase-like 21e inhibits 26S proteasome activity to facilitate hypertrophy of type IIb myofibers. FASEB J. 2019, 33, 9672–9684. [Google Scholar] [CrossRef] [PubMed]
- Sieber, J.; Wieder, N.; Ostrosky-Frid, M.; Dvela-Levitt, M.; Aygün, O.; Udeshi, N.D.; Carr, S.A.; Greka, A. Lysine trimethylation regulates 78-kDa glucose-regulated protein proteostasis during endoplasmic reticulum stress. J. Biol. Chem. 2017, 292, 18878–18885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiele, W.; Novac, N.; Mink, S.; Schreiber, C.; Plaumann, D.; Fritzmann, J.; Cremers, N.; Rothley, M.; Schwager, C.; Regiert, T.; et al. Discovery of a novel tumour metastasis-promoting gene, NVM-1. J. Pathol. 2011, 225, 96–105. [Google Scholar] [CrossRef]
- Ferlin, A.; Speltra, E.; Patassini, C.; Pati, M.A.; Garolla, A.; Caretta, N.; Foresta, C. Heat shock protein and heat shock factor expression in sperm: Relation to oligozoospermia and varicocele. J. Urol. 2010, 183, 1248–1252. [Google Scholar] [CrossRef]
- Ji, Z.-L.; Duan, Y.-G.; Mou, L.-S.; Allam, J.-P.; Haidl, G.; Cai, Z.-M. Association of heat shock proteins, heat shock factors and male infertility. Asian Pac. J. Reprod. 2012, 1, 76–84. [Google Scholar] [CrossRef]
- Hebert-Schuster, M.; Rotta, B.E.; Kirkpatrick, B.; Guibourdenche, J.; Cohen, M. The interplay between glucose-regulated protein 78 (GRP78) and steroids in the reproductive system. Int. J. Mol. Sci. 2018, 19, 1842. [Google Scholar] [CrossRef] [Green Version]
- Moein-Vaziri, N.; Phillips, I.; Smith, S.; Almiňana, C.; Maside, C.; Gil, M.A.; Roca, J.; Martinez, E.A.; Holt, W.V.; Pockley, A.G.; et al. Heat-shock protein A8 restores sperm membrane integrity by increasing plasma membrane fluidity. Reproduction 2014, 147, 719–732. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.-S.; Shimazu, T.; Toyokawa, G.; Daigo, Y.; Maehara, Y.; Hayami, S.; Ito, A.; Masuda, K.; Ikawa, N.; Field, H.I.; et al. Enhanced HSP70 lysine methylation promotes proliferation of cancer cells through activation of Aurora kinase B. Nat. Commun. 2012, 3, 1–10. [Google Scholar] [CrossRef]
- Małecki, J.; Ho, A.Y.; Moen, A.; Dahl, H.-A.; Falnes, P.Ø. Human METTL20 is a mitochondrial lysine methyltransferase that targets the β subunit of electron transfer flavoprotein (ETFβ) and modulates its activity. J. Biol. Chem. 2015, 290, 423–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schultz, N.; Hamra, F.K.; Garbers, D.L. A multitude of genes expressed solely in meiotic or postmeiotic spermatogenic cells offers a myriad of contraceptive targets. Proc. Natl. Acad. Sci. USA 2003, 100, 12201–12206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyata, H.; Castaneda, J.M.; Fujihara, Y.; Yu, Z.; Archambeault, D.R.; Isotani, A.; Kiyozumi, D.; Kriseman, M.L.; Mashiko, D.; Matsumura, T.; et al. Genome engineering uncovers 54 evolutionarily conserved and testis-enriched genes that are not required for male fertility in mice. Proc. Natl. Acad. Sci. USA 2016, 113, 7704–7710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Wang, T.; Zhao, Z.; Liu, S.; Shen, C.; Li, H.; Liu, M.; Zheng, B.; Yu, J.; Huang, X. Retinoic Acid Induced Protein 14 (Rai14) is dispensable for mouse spermatogenesis. PeerJ 2021, 9, e10847. [Google Scholar] [CrossRef]
- Xu, H.; Yuan, S.-Q.; Zheng, Z.-H.; Yan, W. The cytoplasmic droplet may be indicative of sperm motility and normal spermiogenesis. Asian J. Androl. 2013, 15, 799. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Feng, S.; Ma, X.; Yuan, S.; Wang, X. METTL21A, a Non-Histone Methyltransferase, Is Dispensable for Spermatogenesis and Male Fertility in Mice. Int. J. Mol. Sci. 2022, 23, 1942. https://doi.org/10.3390/ijms23041942
Li J, Feng S, Ma X, Yuan S, Wang X. METTL21A, a Non-Histone Methyltransferase, Is Dispensable for Spermatogenesis and Male Fertility in Mice. International Journal of Molecular Sciences. 2022; 23(4):1942. https://doi.org/10.3390/ijms23041942
Chicago/Turabian StyleLi, Jinmei, Shenglei Feng, Xixiang Ma, Shuiqiao Yuan, and Xiaoli Wang. 2022. "METTL21A, a Non-Histone Methyltransferase, Is Dispensable for Spermatogenesis and Male Fertility in Mice" International Journal of Molecular Sciences 23, no. 4: 1942. https://doi.org/10.3390/ijms23041942
APA StyleLi, J., Feng, S., Ma, X., Yuan, S., & Wang, X. (2022). METTL21A, a Non-Histone Methyltransferase, Is Dispensable for Spermatogenesis and Male Fertility in Mice. International Journal of Molecular Sciences, 23(4), 1942. https://doi.org/10.3390/ijms23041942




