Neural Crest Stem Cells in Juvenile Angiofibromas
Abstract
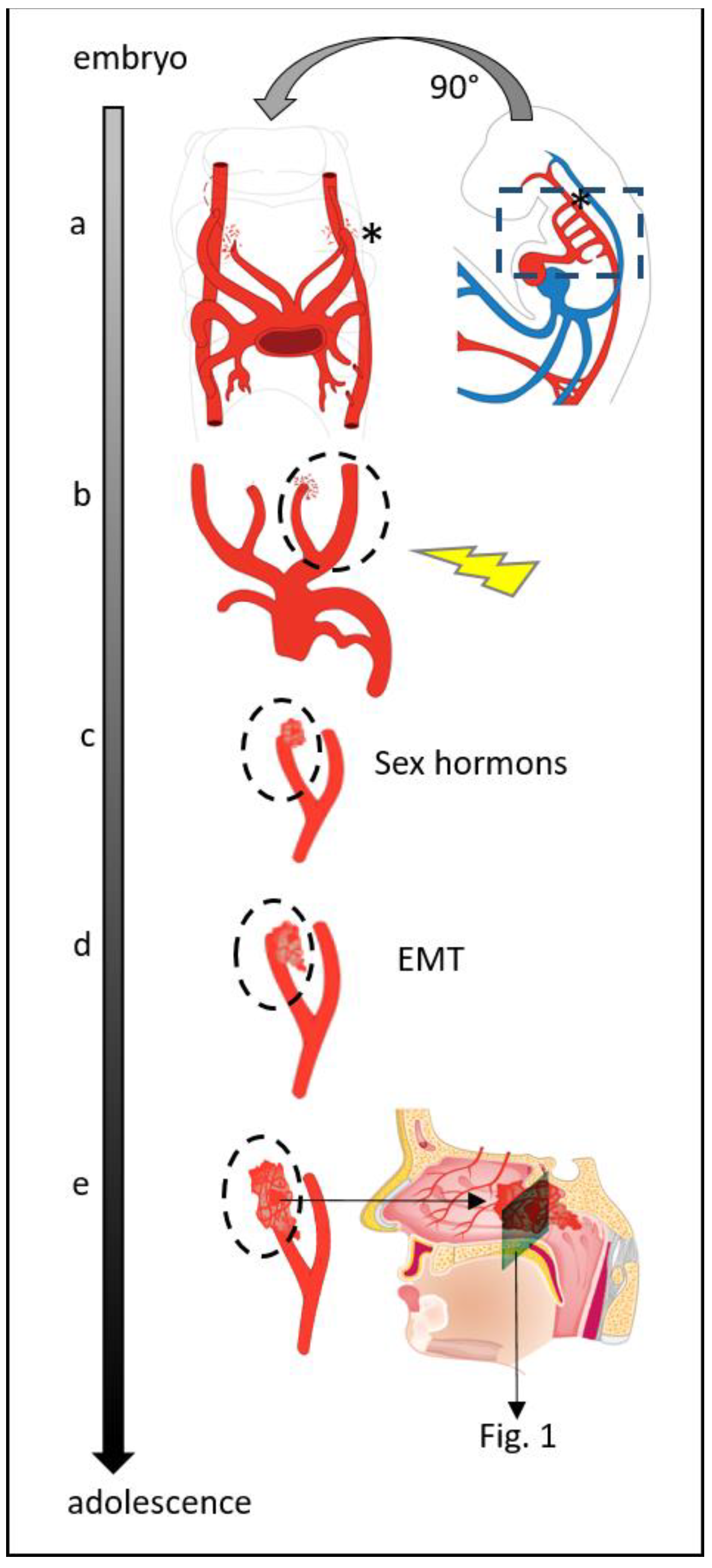
:1. Introduction
2. Results
2.1. Sample Characterization
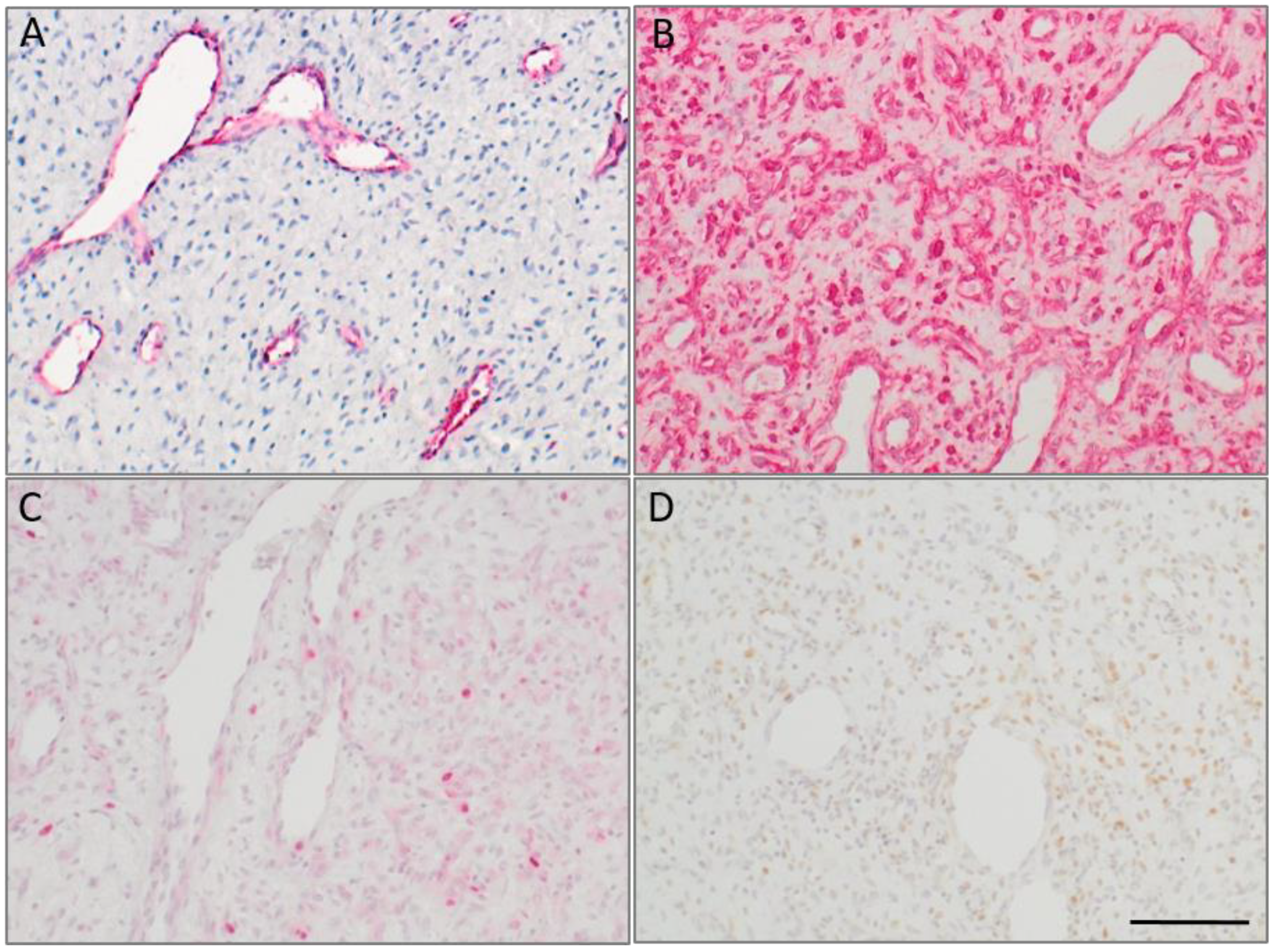
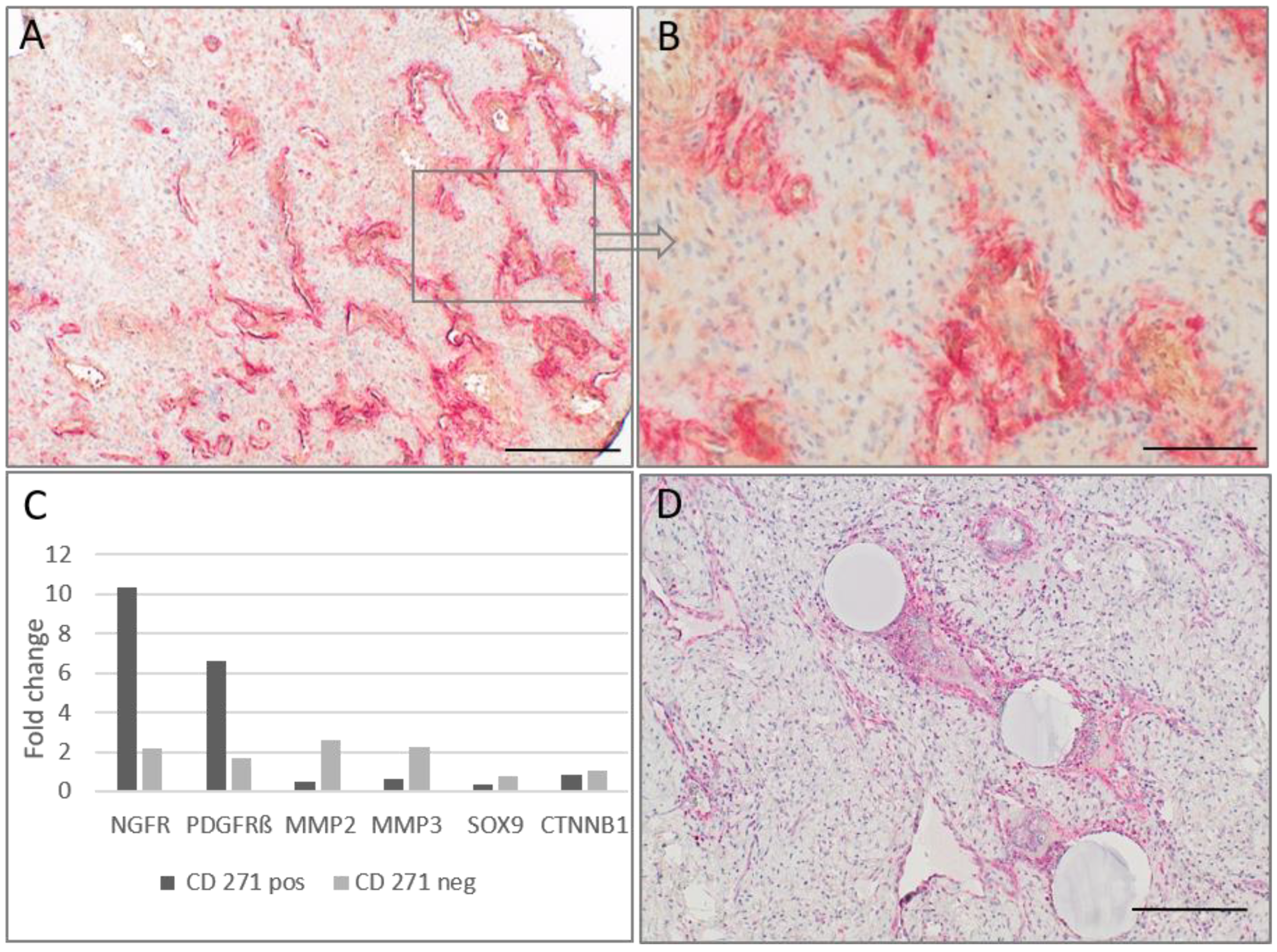
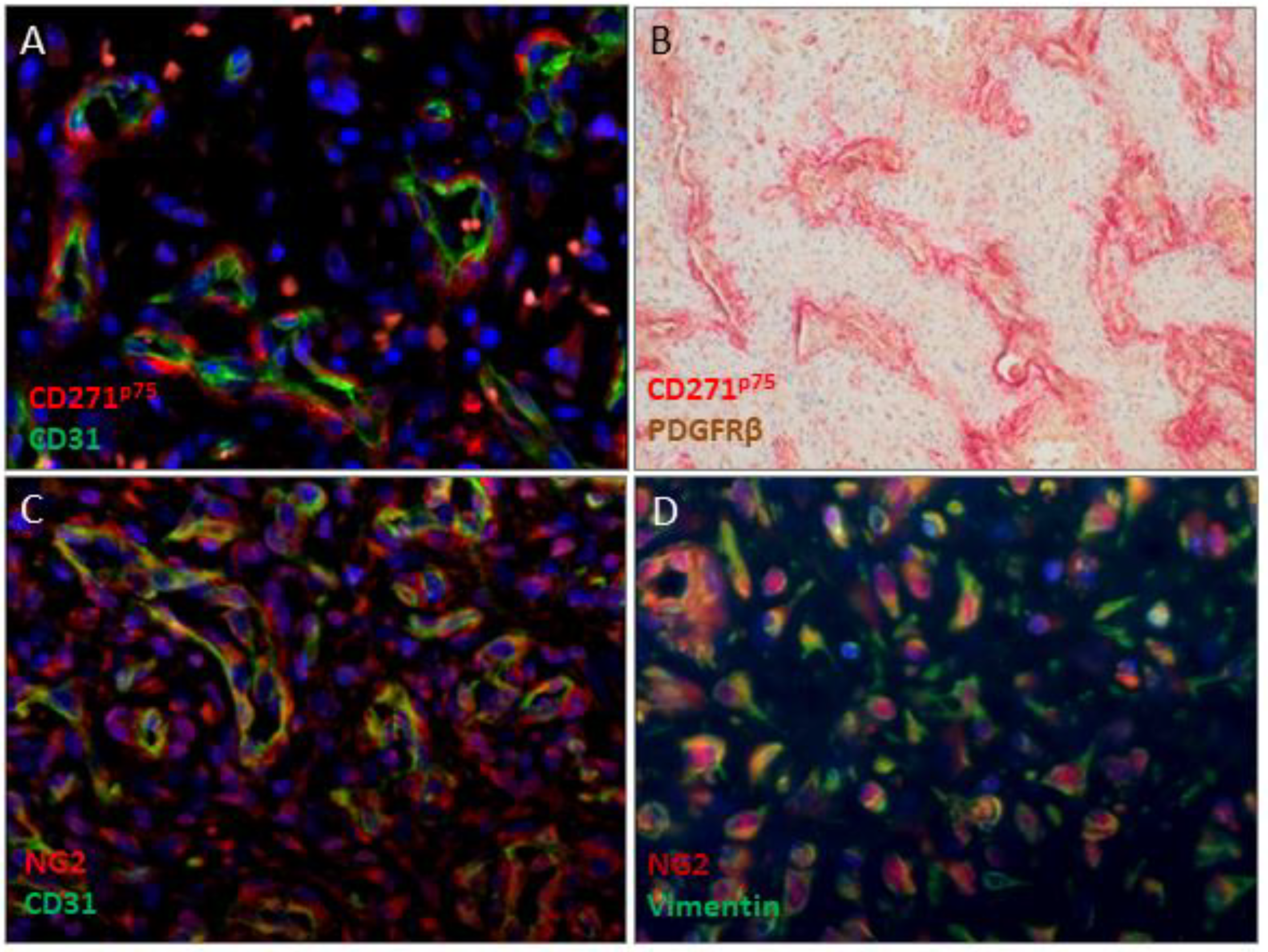
2.2. CD271p75 Analysis
2.3. Matrix Metalloproteinase-3 (MMP3) and Epithelial-Mesenchymal Transition (EMT)
3. Discussion
4. Materials and Methods
4.1. Tumor Specimens
4.2. Immunohistochemistry
4.3. Magnetic-Activated Cell Sorting (MACS) of CD271p75positive and CD271p75negative Cells
4.4. Real-Time Polymerase Chain Reaction (RT-PCR) Based Array Analysis
4.5. Real-Time PCR
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, W.; Ni, Y.; Lu, H.; Hu, L.; Wang, D. Current perspectives on the origin theory of juvenile nasopharyngeal angiofibroma. Discov. Med. 2019, 27, 245–254. [Google Scholar] [PubMed]
- Schick, B.; Urbschat, S. New aspects of pathogenesis of juvenile angiofibroma. Hosp. Med. 2004, 65, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.C.; Montgomery, E.A.; Giardiello, F.M.; Wu, T.-T. Frequent β-Catenin Mutations in Juvenile Nasopharyngeal Angiofibromas. Am. J. Pathol. 2001, 158, 1073–1078. [Google Scholar] [CrossRef]
- Wemmert, S.; Willnecker, V.; Kulas, P.; Weber, S.; Lerner, C.; Berndt, S.; Wendler, O.; Schick, B. Identification of CTNNB1 mutations, CTNNB1 amplifications, and an Axin2 splice variant in juvenile angiofibromas. Tumor Biol. 2015, 37, 5539–5549. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, U.-R.; Brieger, J.; Gosepath, J.; Wierzbicka, M.; Sokolov, M.; Roth, Y.; Szyfter, W.; Bittinger, F.; Mann, W.J. Frequent chromosomal gains in recurrent juvenile nasopharyngeal angiofibroma. Cancer Genet. Cytogenet. 2007, 175, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Schick, B.; Wemmert, S.; Jung, V.; Steudel, W.-I.; Montenarh, M.; Urbschat, S. Genetic heterogeneity of the MYC oncogene in advanced juvenile angiofibromas. Cancer Genet. Cytogenet. 2006, 164, 25–31. [Google Scholar] [CrossRef]
- Msc, S.M.S.; Domingues, M.A.C.; Butugan, O.; Brentani, M.M.; Rogatto, S.R. Tumor microenvironmental genomic alterations in juvenile nasopharyngeal angiofibroma. Head Neck 2011, 34, 485–492. [Google Scholar] [CrossRef]
- Antonino, M.; Nicolò, M.; Renee, L.J.; Federico, M.; Chiara, V.; Stefano, S.; Maria, S.; Salvatore, C.; Antonio, B.; Calvo-Henriquez, C.; et al. Single-nucleotide polymorphism in chronic rhinosinusitis: A systematic review. Clin. Otolaryngol. 2021, 47, 14–23. [Google Scholar] [CrossRef]
- Jones, J.W.; Usman, S.; New, J.; Holcomb, A.; Gunewardena, S.; Tawfik, O.; Hoover, L.; Bruegger, D.; Thomas, S.M. Differential Gene Expression and Pathway Analysis in Juvenile Nasopharyngeal Angiofibroma Using RNA Sequencing. Otolaryngol. Neck Surg. 2018, 159, 572–575. [Google Scholar] [CrossRef]
- Schick, B.; Wemmert, S.; Willnecker, V.; Dlugaiczyk, J.; Nicolai, P.; Siwiec, H.; Thiel, C.T.; Rauch, A.; Wendler, O. Genome-wide copy number profiling using a 100K SNP array reveals novel disease-related genes BORIS and TSHZ1 in juvenile angiofibroma. Int. J. Oncol. 2011, 39, 1143–1151. [Google Scholar] [CrossRef]
- Schick, B.; Dlugaiczyk, J.; Wendler, O. Expression of sex hormone receptors in juvenile angiofibromas and antiproliferative effects of receptor modulators. Head Neck 2014, 36, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Saylam, G.; Yücel, O.T.; Sungur, A.; Önerci, M. Proliferation, angiogenesis and hormonal markers in juvenile nasopharyngeal angiofibroma. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Romero, C.; Carlos, R.; Molina, J.P.D.; Thompson, L.; De Almeida, O.P.; Piña, A.R. Nasopharyngeal Angiofibroma: A Clinical, Histopathological and Immunohistochemical Study of 42 Cases with Emphasis on Stromal Features. Head Neck Pathol. 2018, 12, 52–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.J.; Weber, R.; Liang, H.H.; Pasha, T.L.; LiVolsi, V.A. Growth factors and receptors in juvenile nasopharyngeal angi-ofibroma and nasal polyps: An immunohistochemical study. Arch. Pathol. Lab. Med. 2003, 127, 1480–1484. [Google Scholar] [CrossRef]
- Schick, B.; Kind, M.; Schwarzkopf, G.; Weber, R.; Draf, W. Das frühkindliche Angiofibrom in ungewöhnlicher Lokalisation. HNO 1997, 45, 1022–1028. [Google Scholar] [CrossRef]
- Plzák, J.; Kratochvil, V.; Kešner, A.; Šurda, P.; Vlasák, A.; Zvěřina, E. Endoscopic endonasal approach for mass resection of the pterygopalatine fossa. Clinics 2017, 72, 554–561. [Google Scholar] [CrossRef]
- Lao, W.P.; Bs, K.J.L.; Arom, G.A.; Walker, P.C.; Lee, S.C. Combined endoscopic and transoral resection of a high-staged juvenile nasopharyngeal angiofibroma: A pictorial essay. Head Neck 2021, 43, 719–724. [Google Scholar] [CrossRef]
- Maniaci, A.; Merlino, F.; Cocuzza, S.; Iannella, G.; Vicini, C.; Cammaroto, G.; Lechien, J.R.; Calvo-Henriquez, C.; La Mantia, I. Endoscopic surgical treatment for rhinogenic contact point headache: Systematic review and meta-analysis. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 1743–1753. [Google Scholar] [CrossRef]
- Choquet, H.; Trapani, E.; Goitre, L.; Trabalzini, L.; Akers, A.; Fontanella, M.; Hart, B.L.; Morrison, L.A.; Pawlikowska, L.; Kim, H.; et al. Cytochrome P450 and matrix metalloproteinase genetic modifiers of disease severity in Cerebral Cavernous Malformation type. Free Radic. Biol. Med. 2016, 92, 100–109. [Google Scholar] [CrossRef] [Green Version]
- Trainor, P.A. Making Headway: The Roles of Hox Genes and Neural Crest Cells in Craniofacial Development. Sci. World J. 2003, 3, 240–264. [Google Scholar] [CrossRef]
- Yakkioui, Y.; van Overbeeke, J.J.; Santegoeds, R.; van Engeland, M.; Temel, Y. Chordoma: The entity. Biochim. Biophys. Acta 2014, 1846, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Beham, A.; Beham-Schmid, C.; Regauer, S.; Auböck, L.; Stammberger, H. Nasopharyngeal Angiofibroma: True Neoplasm or Vascular Malformation? Adv. Anat. Pathol. 2000, 7, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Lumsden, A.; Keynes, R. Segmental patterns of neuronal development in the chick hindbrain. Nature 1989, 337, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-S.; Arima, Y.; Kitazawa, T.; Nishiyama, K.; Asai, R.; Uchijima, Y.; Sato, T.; Levi, G.; Kitanaka, S.; Igarashi, T.; et al. Endothelin regulates neural crest deployment and fate to form great vessels through Dlx5/Dlx6-independent mechanisms. Mech. Dev. 2013, 130, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Gramann, M.; Wendler, O.; Haeberle, L.; Schick, B. Expression of Collagen Types I, II and III in Juvenile Angiofibromas. Cells Tissues Organs 2008, 189, 403–409. [Google Scholar] [CrossRef] [Green Version]
- Starlinger, V.; Wendler, O.; Gramann, M.; Schick, B. Laminin expression in juvenile angiofibroma indicates vessel’s early developmental stage. Acta Oto-Laryngol. 2007, 127, 1310–1315. [Google Scholar] [CrossRef]
- Bertulli, L.; Robert, T. Embryological development of the human cranio-facial arterial system: A pictorial review. Surg. Radiol. Anat. 2021, 43, 961–973. [Google Scholar] [CrossRef]
- Harrison, D.F.N. The Natural History, Pathogenesis, and Treatment of Juvenile Angiofibroma: Personal Experience with 44 Patients. Arch. Otolaryngol.-Head Neck Surg. 1987, 113, 936–942. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.; Singh, V.; Verma, V.; Pandey, S.; Trivedi, R.; Singh, H.P.; Kumar, S.; Dwivedi, R.C.; Mishra, S.C. Current status and clinical association of beta-catenin with juvenile nasopharyngeal angiofibroma. J. Laryngol. Otol. 2016, 130, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Nagai, M.A.; Butugan, O.; Logullo, A.; Brentani, M.M. Expression of Growth Factors, Proto-oncogenes, and p53 in Nasopharyngeal Angiofibromas. Laryngoscope 1996, 106, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Rippel, C.; Plinkert, P.K.; Schick, B. Expression von Proteinen der Cadherin- und Catenin-Proteinfamilie bei juvenilen Angiofibromen. Laryngo-Rhino-Otologie 2003, 82, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Radisky, D.C.; Levy, D.D.; Littlepage, L.E.; Liu, H.; Nelson, C.M.; Fata, J.E.; Leake, D.; Godden, E.L.; Albertson, D.G.; Nieto, M.A.; et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 2005, 436, 123–127. [Google Scholar] [CrossRef] [Green Version]
- Duerr, S.; Wendler, O.; Aigner, T.; Karosi, S.; Schick, B. Metalloproteinases in juvenile angiofibroma—a collagen rich tumor. Hum. Pathol. 2008, 39, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Gramann, M.; Wendler, O.; Haeberle, L.; Schick, B. Prominent collagen type VI expression in juvenile angiofibromas. Histochem. Cell Biol. 2009, 131, 155–164. [Google Scholar] [CrossRef]
- Pauli, J.; Gundelach, R.; Vanelli-Rees, A.; Rees, G.; Campbell, C.; Dubey, S.; Perry, C. Juvenile nasopharyngeal angiofibroma: An immunohistochemical characterisation of the stromal cell. Pathology 2008, 40, 396–400. [Google Scholar] [CrossRef]
- Kulas, P.; Willnecker, V.; Dlugaiczyk, J.; Laschke, M.W.; Schick, B. Mesenchymal–endothelial transition in juvenile angiofibroma? Acta Oto-Laryngol. 2015, 135, 955–961. [Google Scholar] [CrossRef]
- Dohar, J.E.; Duvall, A.J. Spontaneous Regression of Juvenile Nasopharyngeal Angiofibroma. Ann. Otol. Rhinol. Laryngol. 1992, 101, 469–471. [Google Scholar] [CrossRef]
- Weprin, L.S.; Siemers, P.T. Spontaneous Regression of Juvenile Nasopharyngeal Angiofibroma. Arch. Otolaryngol.-Head Neck Surg. 1991, 117, 796–799. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Wang, H.; Wang, D.; Hu, L.; Liu, Q.; Sun, X. Hormonal receptors and vascular endothelial growth factor in juvenile nasopharyngeal angiofibroma: Immunohistochemical and tissue microarray analysis. Acta Oto-Laryngol. 2014, 135, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.K.; Sarkar, D.K.; Weston, A.P.; De, A.; Campbell, D.R. Over expression of vascular endothelial growth factor and its receptor during the development of estrogen-induced rat pituitary tumors may mediate estrogen-initiated tumor angiogenesis. Carcinogenesis 1997, 18, 1155–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sieveking, D.P.; Lim, P.; Chow, R.W.; Dunn, L.L.; Bao, S.; McGrath, K.; Heather, A.K.; Handelsman, D.J.; Celermajer, D.; Ng, M.K. A sex-specific role for androgens in angiogenesis. J. Exp. Med. 2010, 207, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Radkowski, D.; McGill, T.; Healy, G.B.; Ohlms, L.; Jones, D.T. Angiofibroma: Changes in Staging and Treatment. Arch. Otolaryngol.-Head Neck Surg. 1996, 122, 122–129. [Google Scholar] [CrossRef] [PubMed]
| Case | Grading | Age | Vimentin | CD31 | CD271 | PDGFRß | MMP3 | NG2 | PCNA | Ki67 | HNK1 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3a | 13 | 3 | 3 | 2 | 2 | 1–2 | 1–2 | 2 | - | - |
| 1a | rec | 15 | - | 3 | 2 | 2 | 1–2 | 0–1 | - | 1 | - |
| 2 | rec | 15 | 3 | 3 | 2 | 2 | 2 | 2 | 2 | 1 | 1 |
| 3 | 3a | 12 | 3 | 3 | 1 | 2 | 3 | 1 | 2 | 1 | - |
| 4 | 1 | 15 | 3 | 3 | 2 | 2 | 2–3 | 1 | - | 1 | - |
| 5 | unk | 17 | 3 | 3 | 2 | 1 | 2 | 3 | - | 1 | - |
| 6 | 3a | 11 | 3 | 3 | 2 | 2 | 3 | 2 | 2 | 1 | - |
| 7 | 3b | 14 | 3 | 3 | 1 | 2 | 3 | 2 | 2 | 2 | 1 |
| 8 | rec | 18 | - | - | 2 | 2 | 3 | - | - | - | - |
| 8a | rec | 19 | 3 | 3 | 2 | 2 | 3 | - | 2 | 1 | 1 |
| 9 | rec | 13 | 3 | 3 | 2 | 2 | 2 | 2 | 2 | 3 | 1 |
| 10 | unk | 17 | 3 | 3 | 1 | 1 | 2–3 | 2 | 2 | 1 | 1 |
| 11 | 3a | 17 | 3 | 3 | 3 | 3 | 2–3 | 1 | 2 | 2 | 0–1 |
| 12 | 4 | 13 | 3 | 3 | 2 | 2 | 1 | 0–1 | - | 0–1 | 1 |
| 13 | 2 | 18 | 3 | 3 | 1 | 1 | 2 | 1 | - | 0 | - |
| 14 | 3a | 12 | 3 | 3 | 2 | 2 | 3 | 2 | - | - | 1 |
| 15 | unk | 20 | 3 | 3 | 3 | 2 | 3 | 2 | - | - | 1 |
| 16 | unk | 24 | 3 | 3 | 2 | 2 | 3 | 1–2 | - | - | - |
| 17 * | unk | 20 | 3 | 3 | 3 | 3 | 3 | - | - | - | 1 |
| 18 | unk | 13 | - | 3 | 2 | 3 | 3 | 0 | - | - | 1 |
| 19 | 3a | 21 | 3 | 3 | 1 | - | - | 2 | - | 2 | - |
| 20 | 3b rec | 46 | 3 | 3 | 2 | 2 | 2 | 0 | - | 1 | - |
| 21 | 3a | unk | 3 | 3 | 2 | 1 | 1 | 0 | - | 0 | - |
| 22 | unk | 17 | 3 | 3 | 1 | 2 | 1 | - | - | 0 | - |
| Primary Antibody | Concentration | Reference No, Company |
|---|---|---|
| CD271p75-NGFR | 1:1000 | MA5-31968, Thermo Fisher Scientific, Dreieich, Germany |
| MMP3 | 1:300 | ab52915, Abcam, Cambridge, UK |
| CD31 | 1:40 | M0823; Dako Agilent, Santa Clara, CA, USA |
| Vimentin | 1:700 | M0725, Dako Agilent, Santa Clara, CA, USA |
| NG2 | 1:500 | ab129051, Abcam, Cambridge, UK |
| PDGFRβ | 1:1000 | TA506230, Thermo Fisher Scientific, Dreieich, Germany |
| HNK1 (CD57) | 1:100 | MA5-11605, Thermo Fisher Scientific, Dreieich, Germany |
| Ki67 | 1:50 | ab833, Abcam, Cambridge, UK |
| PCNA | 1:10.000 | ab29, Abcam, Cambridge, UK |
| Gene | RefSeq Accession | Sequence |
|---|---|---|
| CTNNB1 | NM_001098209.1 | Hs00355045_m1 |
| MMP2 | NM_001127891.3 | Hs00234422_m1 |
| MMP3 | NM_002422.4 | Hs00968305_m1 |
| NGFR (p75) | NM_002507.3 | Hs00609976_m1 |
| PDGFRβ | NM_002609.3 | Hs01019589_m1 |
| SOX9 | NM_000346.3 | Hs00165814_m1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schick, B.; Pillong, L.; Wenzel, G.; Wemmert, S. Neural Crest Stem Cells in Juvenile Angiofibromas. Int. J. Mol. Sci. 2022, 23, 1932. https://doi.org/10.3390/ijms23041932
Schick B, Pillong L, Wenzel G, Wemmert S. Neural Crest Stem Cells in Juvenile Angiofibromas. International Journal of Molecular Sciences. 2022; 23(4):1932. https://doi.org/10.3390/ijms23041932
Chicago/Turabian StyleSchick, Bernhard, Lukas Pillong, Gentiana Wenzel, and Silke Wemmert. 2022. "Neural Crest Stem Cells in Juvenile Angiofibromas" International Journal of Molecular Sciences 23, no. 4: 1932. https://doi.org/10.3390/ijms23041932
APA StyleSchick, B., Pillong, L., Wenzel, G., & Wemmert, S. (2022). Neural Crest Stem Cells in Juvenile Angiofibromas. International Journal of Molecular Sciences, 23(4), 1932. https://doi.org/10.3390/ijms23041932






