Regulatory T Cell Apoptosis during Preeclampsia May Be Prevented by Gal-2
Abstract
1. Introduction
2. Results
2.1. The Number of Tregs Is Decreased in the Decidua of PE-affected Pregnancies
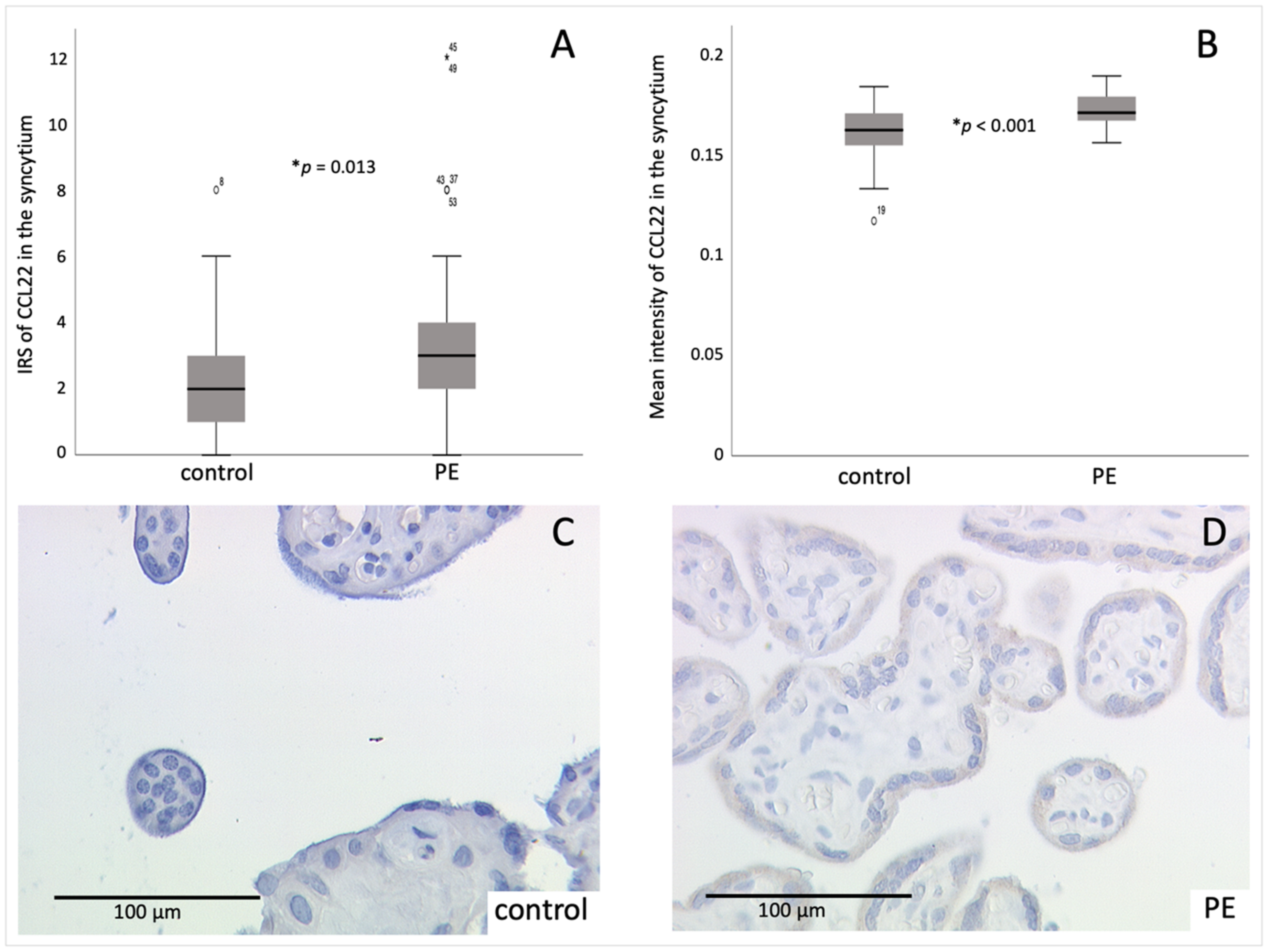
2.2. CCL22 Expression Is Increased in PE Compared to Control Placentas
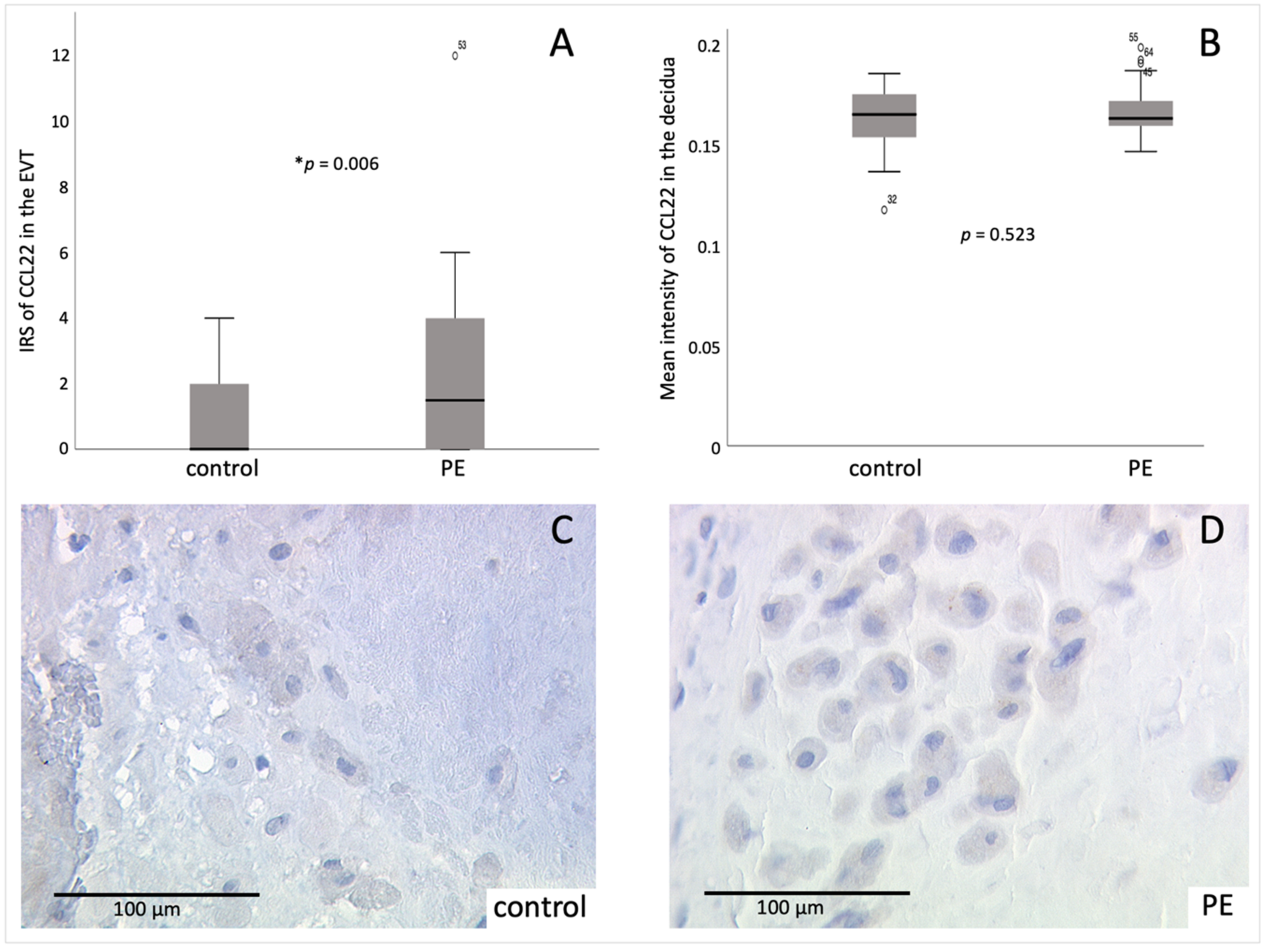
2.3. Identification of Decidual Cells Expressing CCL22 as EVT
2.4. CCL22 and FoxP3 Are Correlating Positively
2.5. Tregs Undergo Apoptosis in PE
2.6. Correlation of Gal-2 with Tregs
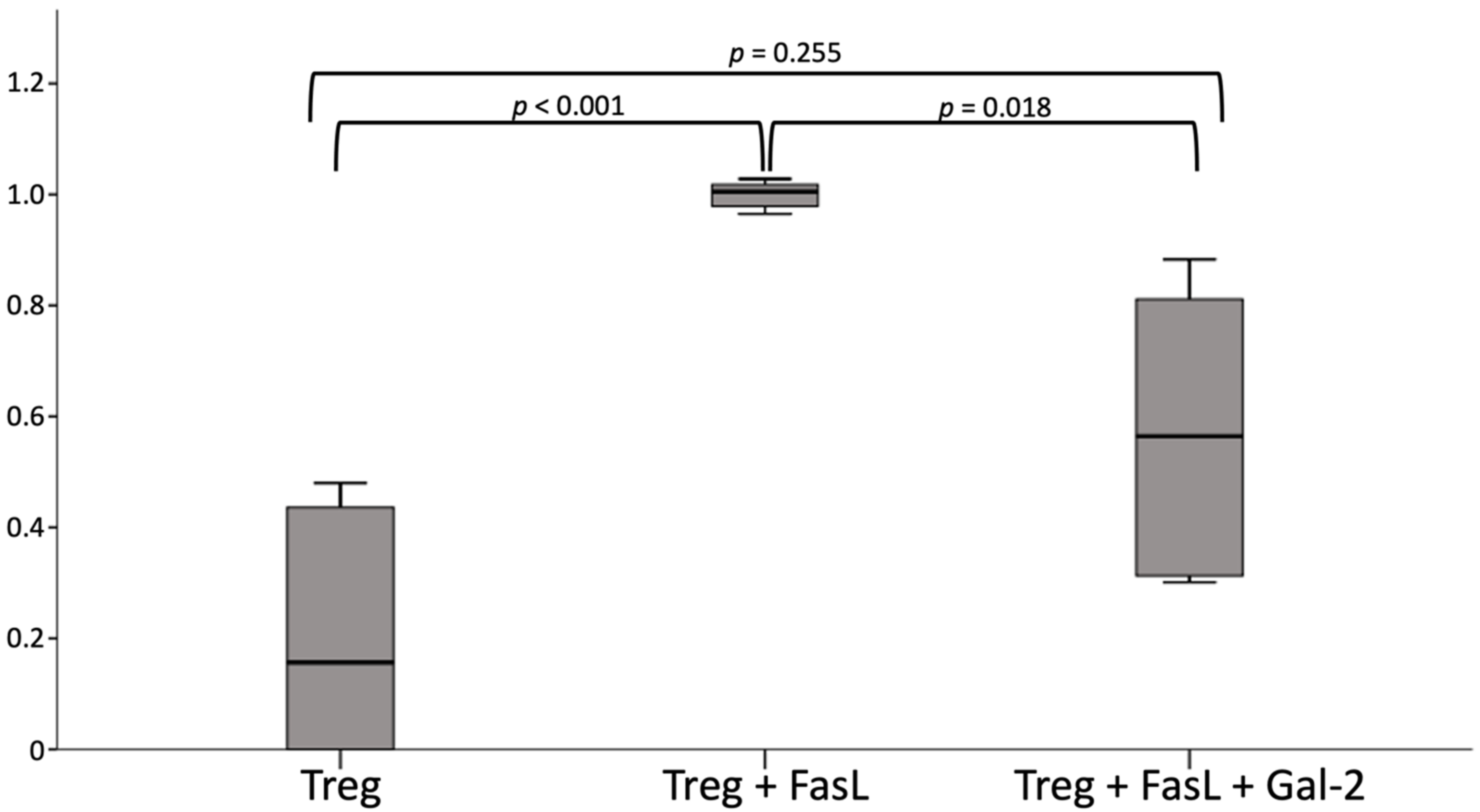
2.7. Gal 2 Protects Tregs from Apoptosis
3. Discussion
4. Materials and Methods
4.1. Sample Placental Tissue
4.2. Immunohistochemistry
4.2.1. FoxP3 Staining
4.2.2. CCL22 Staining
4.3. Immunofluorescence Staining
4.3.1. CCL22-CK7 Staining
4.3.2. FoxP3-TUNEL Staining
4.4. Evaluation of Stainings
4.5. Cell Culture of Tregs and Gal-2
4.6. Caspase 3 ELISA
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCL22 | CC-chemokine-ligand 22 |
| CCL3 | CC-chemokine-ligand 3 also known as macrophage inflammatory protein 1-alpha |
| CCL4 | CC-chemokine-ligand 4 |
| CCL5 | CC-chemokine-ligand 5 |
| CCR4 | CC-chemokine-receptor 4 |
| CX3CL1 | C-X3-C-chemokine-ligand 1 also known as Fractalkine |
| CK7 | cytokeratin 7 |
| DAB | chromogenic 3,3′-diaminoenzidine |
| DAPI | 4′,6-Diamino-2-phenylindile |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| EVT | extravillous trophoblast |
| FasL | FAS ligand |
| FACS | fluorescence-activated cell sorting |
| FoxP3 | forkhead box protein 3 |
| Gal-2 | galectin 2 |
| hCG | human chorionic gonadotropin |
| IRS | immunoreactive Score |
| MACS | magnetic cell separation |
| MDC | macrophage derived chemokine, CCL22 |
| PBMC | peripheral blood mononuclear cell |
| PBS | phosphate buffered saline |
| PE | preeclampsia (respectively: preeclampsia placentas) |
| Treg | regulatory T cells, respectively the group of untreated regulatory T cells |
| TUNEL | TdT-mediated dUTP-biotin nick end labeling |
References
- Fox, R.; Kitt, J.; Leeson, P.; Aye, C.Y.L.; Lewandowski, A.J. Preeclampsia: Risk Factors, Diagnosis, Management, and the Cardiovascular Impact on the Offspring. J. Clin. Med. 2019, 8, 1625. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Lee, H.J.; Jung, Y.J.; Kwon, H.Y.; Kim, H.; Lee, J.; Kim, Y.H.; Maeng, Y.S.; Kwon, J.Y. CD133+/C-kit+Lin(-) endothelial progenitor cells in fetal circulation demonstrate impaired differentiation potency in severe preeclampsia. Pregnancy Hypertens. 2019, 15, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A.; Green, E.S.; Care, A.S.; Moldenhauer, L.M.; Prins, J.R.; Hull, M.L.; Barry, S.C.; Dekker, G. Therapeutic Potential of Regulatory T Cells in Preeclampsia-Opportunities and Challenges. Front. Immunol. 2019, 10, 478. [Google Scholar] [CrossRef]
- Apicella, C.; Ruano, C.S.M.; Mehats, C.; Miralles, F.; Vaiman, D. The Role of Epigenetics in Placental Development and the Etiology of Preeclampsia. Int. J. Mol. Sci. 2019, 20, 2837. [Google Scholar] [CrossRef]
- Han, C.; Han, L.; Huang, P.; Chen, Y.; Wang, Y.; Xue, F. Syncytiotrophoblast-Derived Extracellular Vesicles in Pathophysiology of Preeclampsia. Front. Physiol. 2019, 10, 1236. [Google Scholar] [CrossRef] [PubMed]
- Hypertensive Pregnancy Disorders: Diagnosis and Therapy. Guideline of the German Society of Gynecology and Obstetrics (S2k-Level, AWMF-Registery No. 015/018., March 2019). Available online: http://www.awmf.org/leitlinien/detail/II/015-018.html (accessed on 12 January 2022).
- Weber, M.; Gohner, C.; San Martin, S.; Vattai, A.; Hutter, S.; Parraga, M.; Jeschke, U.; Schleussner, E.; Markert, U.R.; Fitzgerald, J.S. Unique trophoblast stem cell- and pluripotency marker staining patterns depending on gestational age and placenta-associated pregnancy complications. Cell Adhes. Migr. 2016, 10, 56–65. [Google Scholar] [CrossRef]
- Matthiesen, L.; Berg, G.; Ernerudh, J.; Ekerfelt, C.; Jonsson, Y.; Sharma, S. Immunology of preeclampsia. Immunol. Pregnancy 2005, 89, 49–61. [Google Scholar]
- Chakraborty, D.; Cui, W.; Rosario, G.X.; Scott, R.L.; Dhakal, P.; Renaud, S.J.; Tachibana, M.; Rumi, M.A.; Mason, C.W.; Krieg, A.J.; et al. HIF-KDM3A-MMP12 regulatory circuit ensures trophoblast plasticity and placental adaptations to hypoxia. Proc. Natl. Acad. Sci. USA 2016, 113, E7212–E7221. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Zhang, L.; Tang, Q.; Xu, Y.; Liu, S.; Li, H. Circulating levels of IFN-gamma, IL-1, IL-17 and IL-22 in pre-eclampsia: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 248, 211–221. [Google Scholar] [CrossRef]
- Pozharny, Y.; Lambertini, L.; Clunie, G.; Ferrara, L.; Lee, M.J. Epigenetics in women’s health care. Mt. Sinai J. Med. 2010, 77, 225–235. [Google Scholar] [CrossRef]
- Alijotas-Reig, J.; Esteve-Valverde, E.; Ferrer-Oliveras, R.; Llurb, E.; Gris, J.M. Tumor Necrosis Factor-Alpha and Pregnancy: Focus on Biologics. An Updated and Comprehensive Review. Clin. Rev. Allergy Immunol. 2017, 53, 40–53. [Google Scholar] [CrossRef]
- Scholz, C.; Tot, B.; Santoso, L.; Kuhn, C.; Franz, M.; Mayr, D.; Jeschke, U.; Frise, K.; Schiessl, B. Distribution and maturity of dendritic cells in diseases of insufficient placentation. Am. J. Reprod. Immunol. 2008, 60, 238–245. [Google Scholar] [CrossRef]
- Mohammadpour-Gharehbagh, A.; Jahantigh, D.; Eskandari, M.; Eskandari, F.; Rezaei, M.; Zeynali-Moghaddam, S.; Teimoori, B.; Salimi, S. The role of TNF-alpha and TLR4 polymorphisms in the placenta of pregnant women complicated by preeclampsia and in silico analysis. Int. J. Biol. Macromol. 2019, 134, 1205–1215. [Google Scholar] [CrossRef]
- Ushida, T.; Macdonald-Goodfellow, S.K.; Quadri, A.; Tse, M.Y.; Winn, L.M.; Pang, S.C.; Adams, M.A.; Kotani, T.; Kikkawa, F.; Graham, C.H. Persistence of risk factors associated with maternal cardiovascular disease following aberrant inflammation in rat pregnancy. Biol. Reprod. 2017, 97, 143–152. [Google Scholar] [CrossRef]
- Minas, V.; Mylonas, I.; Schiessl, B.; Mayr, D.; Schulze, S.; Friese, K.; Jeschke, U.; Makrigiannakis, A. Expression of the blood-group-related antigens Sialyl Lewis a, Sialyl Lewis x and Lewis y in term placentas of normal, preeclampsia, IUGR- and HELLP-complicated pregnancies. Histochem. Cell Biol. 2007, 128, 55–63. [Google Scholar] [CrossRef]
- Fantone, S.; Mazzucchelli, R.; Giannubilo, S.R.; Ciavattini, A.; Marzioni, D.; Tossetta, G. AT-rich interactive domain 1A protein expression in normal and pathological pregnancies complicated by preeclampsia. Histochem. Cell Biol. 2020, 154, 339–346. [Google Scholar] [CrossRef]
- Zhu, H.; Kong, L. LncRNA CRNDE regulates trophoblast cell proliferation, invasion, and migration via modulating miR-1277. Am. J. Transl. Res. 2019, 11, 5905–5918. [Google Scholar]
- Opichka, M.A.; Rappelt, M.W.; Guttermann, D.D.; Grobe, J.L.; McIntosh, J.J. Vascular dysfunction in preeclampsia. Cells 2021, 10, 3055. [Google Scholar] [CrossRef]
- Heim, K.R.; Mulla, M.J.; Potter, J.A.; Han, C.S.; Guller, S.; Abrahams, V.M. Excess glucose induce trophoblast inflammation and limit cell migration through HMGB1 activation of Toll-Like receptor 4. Am. J. Reprod. Immunol. 2018, 80, e13044. [Google Scholar] [CrossRef]
- Ma, Y.; Ye, Y.; Zhang, J.; Ruan, C.C.; Gao, P.J. Immune imbalance is associated with the development of preeclampsia. Medicine 2019, 98, e15080. [Google Scholar] [CrossRef]
- Weel, I.C.; Romao-Veiga, M.; Matias, M.L.; Fioratti, E.G.; Peracoli, J.C.; Borges, V.T.; Araujo, J.P., Jr.; Peracoli, M.T. Increased expression of NLRP3 inflammasome in placentas from pregnant women with severe preeclampsia. J. Reprod. Immunol. 2017, 123, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Eddy, A.C.; Chapman, H.; George, E.M. Acute Hypoxia and Chronic Ischemia Induce Differential Total Changes in Placental Epigenetic Modifications. Reprod. Sci. 2019, 26, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Sakai, M. Th1/Th2 balance in preeclampsia. J. Reprod. Immunol. 2003, 59, 161–173. [Google Scholar] [CrossRef]
- Yoshinaga, K. Two concepts on the immunological aspect of blastocyst implantation. J. Reprod. Dev. 2012, 58, 196–203. [Google Scholar] [CrossRef][Green Version]
- Redman, C.; Sargent, I. Pre-eclampsia, the placenta and the maternal systemic inflammatory response—A review. Placenta 2003, 24, S21–S27. [Google Scholar] [CrossRef]
- Perez-Sepulveda, A.; Torres, M.J.; Khoury, M.; Illanes, S.E. Innate immune system and preeclampsia. Front. Immunol. 2014, 5, 244. [Google Scholar] [CrossRef]
- Schiessl, B. Inflammatory response in preeclampsia. Mol. Asp. Med. 2007, 28, 210–219. [Google Scholar] [CrossRef]
- Steinborn, A.; Sohn, C.; Sayehli, C.; Niederhut, A.; Schmitt, E.; Kaufmann, M. Preeclampsia, a pregnancy-specific disease, is associated with fetal monocyte activation. Clin. Immunol. 2001, 100, 305–313. [Google Scholar] [CrossRef]
- Kingsley, C.I.; Karim, M.; Bushell, A.R.; Wood, K.J. CD25+ CD4+ regulatory T cells prevent graft rejection: CTLA-4-and IL-10-dependent immunoregulation of alloresponses. J. Immunol. 2002, 168, 1080–1086. [Google Scholar] [CrossRef]
- Ghiringhelli, F.; Ménard, C.; Terme, M.; Flament, C.; Taieb, J.; Chaput, N.; Puig, P.E.; Novault, S.; Escudier, B.; Vivier, E.; et al. CD4+ CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor–β–dependent manner. J. Exp. Med. 2005, 202, 1075–1085. [Google Scholar] [CrossRef]
- Baecher-Allan, C.; Wolf, E.; Hafler, D.A. Functional analysis of highly defined, FACS-isolated populations of human regulatory CD4+ CD25+ T cells. Clin. Immunol. 2005, 115, 10–18. [Google Scholar] [CrossRef]
- Figueiredo, A.S.; Schumacher, A. The T helper type 17/regulatory T cell paradigm in pregnancy. Immunology 2016, 148, 13–21. [Google Scholar] [CrossRef]
- Paeschke, S.; Chen, F.; Horn, N.; Fotopoulou, C.; Zambon-Bertoja, A.; Sollwedel, A.; Zenclussen, M.L.; Casalis, P.A.; Dudenhausen, J.W.; Volk, H.D.; et al. Pre-eclampsia is not associated with changes in the levels of regulatory T cells in peripheral blood. Am. J. Reprod. Immunol. 2005, 54, 384–389. [Google Scholar] [CrossRef]
- Toldi, G.; Švec, P.; Vásárhelyi, B.; Mészáros, G.; Rigó, J.; Tulassay, T.; Treszl, A. Decreased number of FoxP3+ regulatory T cells in preeclampsia. Acta Obstet. Gynecol. Scand. 2008, 87, 1229–1233. [Google Scholar] [CrossRef]
- Jung, Y.J.; Park, Y.; Kim, H.S.; Lee, H.J.; Kim, Y.N.; Lee, J.H.; Kim, Y.H.; Maeng, Y.S.; Kwon, J.Y. Abnormal lymphatic vessel development is associated with decreased decidual regulatory T cells in severe preeclampsia. Am. J. Reprod. Immunol. 2018, 80, e12970. [Google Scholar] [CrossRef]
- Gobert, M.; Treilleux, I.; Bendriss-Vermare, N.; Bachelot, T.; Goddard-Leon, S.; Arfi, V.; Biota, C.; Doffin, A.C.; Durand, I.; Olive, D.; et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009, 69, 2000–2009. [Google Scholar] [CrossRef]
- Faget, J.; Biota, C.; Bachelot, T.; Gobert, M.; Treilleux, I.; Goutagny, N.; Durand, I.; Léon-Goddard, S.; Blay, J.Y.; Caux, C.; et al. Early detection of tumor cells by innate immune cells leads to Treg recruitment through CCL22 production by tumor cells. Cancer Res. 2011, 71, 6143–6152. [Google Scholar] [CrossRef]
- Ushio, A.; Arakaki, R.; Otsuka, K.; Yamada, A.; Tsunematsu, T.; Kudo, Y.; Aota, K.; Azuma, M.; Ishimaru, N. CCL22-Producing Resident Macrophages Enhance T Cell Response in Sjögren’s Syndrome. Front. Immunol. 2018, 9, 2594. [Google Scholar] [CrossRef]
- Riezu-Boj, J.-I.; Larrea, E.; Aldabe, R.; Guembe, L.; Casares, N.; Galeano, E.; Echeverria, I.; Sarobe, P.; Herrero, I.; Sangro, B.; et al. Hepatitis C virus induces the expression of CCL17 and CCL22 chemokines that attract regulatory T cells to the site of infection. J. Hepatol. 2011, 54, 422–431. [Google Scholar] [CrossRef]
- Higuchi, T.; Matsuo, K.; Hashida, Y.; Kitahata, K.; Ujihara, T.; Taniguchi, A.; Yoshie, O.; Nakayama, T.; Daibata, M. Epstein–Barr virus-positive pyothorax-associated lymphoma expresses CCL17 and CCL22 chemokines that attract CCR4-expressing regulatory T cells. Cancer Lett. 2019, 453, 184–192. [Google Scholar] [CrossRef]
- Freier, C.P.; Kuhn, C.; Rapp, M.; Endres, S.; Mayr, D.; Friese, K.; Anz, D.; Jeschke, U. Expression of CCL 22 and Infiltration by Regulatory T Cells are Increased in the Decidua of Human Miscarriage Placentas. Am. J. Reprod. Immunol. 2015, 74, 216–227. [Google Scholar] [CrossRef]
- Jones, R.L.; Hannan, N.J.; Kaitu’u, T.u.J.; Zhang, J.; Salamonsen, L.A. Identification of chemokines important for leukocyte recruitment to the human endometrium at the times of embryo implantation and menstruation. J. Clin. Endocrinol. Metab. 2004, 89, 6155–6167. [Google Scholar] [CrossRef] [PubMed]
- Iellem, A.; Mariani, M.; Lang, R.; Recalde, H.; Panina-Bordignon, P.; Sinigaglia, F.; D’Ambrosio, D. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4+ CD25+ regulatory T cells. J. Exp. Med. 2001, 194, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Nakamura, K.; Oyama, N.; Kaneko, F.; Tsunemi, Y.; Saeki, H.; Tamaki, K. Macrophage-derived chemokine (MDC)/CCL22 produced by monocyte derived dendritic cells reflects the disease activity in patients with atopic dermatitis. J. Dermatol. Sci. 2006, 44, 93–99. [Google Scholar] [CrossRef]
- Layseca-Espinosa, E.; Korniotis, S.; Montandon, R.; Gras, C.; Bouillié, M.; Gonzalez-Amaro, R.; Dy, M.; Zavala, F. CCL22-producing CD8α−myeloid dendritic cells mediate regulatory T cell recruitment in response to G-CSF treatment. J. Immunol. 2013, 191, 2266–2272. [Google Scholar] [CrossRef] [PubMed]
- Godiska, R.; Chantry, D.; Raport, C.J.; Sozzani, S.; Allavena, P.; Leviten, D.; Manovani, A.; Gray, P.W. Human macrophage–derived chemokine (MDC), a novel chemoattractant for monocytes, monocyte-derived dendritic cells, and natural killer cells. J. Exp. Med. 1997, 185, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.-Y.; Rabinovich, G.A.; Liu, F.-T. Galectins: Structure, function and therapeutic potential. Expert Rev. Mol. Med. 2008, 10, e17. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Toscano, M.A. Turning ’sweet’ on immunity: Galectin–glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 2009, 9, 338–352. [Google Scholar] [CrossRef]
- Liu, F.T.; Yang, R.Y.; Hsu, D.K. Galectins in acute and chronic inflammation. Ann. N. Y. Acad. Sci. 2012, 1253, 80–91. [Google Scholar] [CrossRef]
- Liu, F.T.; Rabinovich, G.A. Galectins: Regulators of acute and chronic inflammation. Ann. N. Y. Acad. Sci. 2010, 1183, 158–182. [Google Scholar] [CrossRef]
- Sturm, A.; Lensch, M.; André, S.; Kaltner, H.; Wiedenmann, B.; Rosewicz, S.; Dignass, A.U.; Gabius, H.J. Human galectin-2: Novel inducer of T cell apoptosis with distinct profile of caspase activation. J. Immunol. 2004, 173, 3825–3837. [Google Scholar] [CrossRef]
- Hutter, S.; Martin, N.; Von Schonfeldt, V.; Messner, J.; Kuhn, C.; Hofmann, S.; Andergassen, U.; Knabl, J.; Jeschke, U. Galectin 2 (gal-2) expression is downregulated on protein and mRNA level in placentas of preeclamptic (PE) patients. Placenta 2015, 36, 438–445. [Google Scholar] [CrossRef]
- Unverdorben, L.; Haufe, T.; Santoso, L.; Hofmann, S.; Jeschke, U.; Hutter, S. Prototype and chimera-type galectins in placentas with spontaneous and recurrent miscarriages. Int. J. Mol. Sci. 2016, 17, 644. [Google Scholar] [CrossRef]
- Paclik, D.; Lohse, K.; Wiedenmann, B.; Dignass, A.U.; Sturm, A. Galectin-2 and-4, but not galectin-1, promote intestinal epithelial wound healing in vitro through a TGF-beta-independent mechanism. Inflamm. Bowel Dis. 2008, 14, 1366–1372. [Google Scholar] [CrossRef]
- Terness, P.; Kallikourdis, M.; Betz, A.G.; Rabinovich, G.A.; Saito, S.; Clark, D.A. Tolerance signaling molecules and pregnancy: IDO, galectins, and the renaissance of regulatory T cells. Am. J. Reprod. Immunol. 2007, 58, 238–254. [Google Scholar] [CrossRef]
- Yang, L.T.; Shu, Q.M.; Luo, X.Q.; Liu, Z.Q.; Qiu, S.Q.; Liu, J.Q.; Guo, H.J.; Li, L.J.; Li, D.B.; Xia, L.X.; et al. Long-term effects: Galectin-1 and specific immunotherapy for allergic responses in the intestine. Allergy 2018, 73, 106–114. [Google Scholar] [CrossRef]
- Hutter, S.; Knabl, J.; Andergassen, U.; Hofmann, S.; Kuhn, C.; Mahner, S.; Arck, P.; Jeschke, U. Placental expression patterns of galectin-1, galectin-2, galectin-3 and galectin-13 in cases of intrauterine growth restriction (IUGR). Int. J. Mol. Sci. 2016, 17, 523. [Google Scholar] [CrossRef]
- Charkiewicz, K.; Goscik, J.; Raba, G.; Laudanski, P. Syndecan 4, galectin 2, and death receptor 3 (DR3) as novel proteins in pathophysiology of preeclampsia. J. Matern.-Fetal Neonatal Med. 2021, 34, 2965–2970. [Google Scholar] [CrossRef]
- Huppertz, B.; Schleußner, E. Die Plazenta; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Hokama, A.; Mizoguchi, E.; Mizoguchi, A. Roles of galectins in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 5133–5137. [Google Scholar] [CrossRef]
- Loser, K.; Sturm, A.; Voskort, M.; Kupas, V.; Balkow, S.; Auriemma, M.; Sternemann, C.; Dignass, A.U.; Luger, T.A.; Beissert, S. Galectin-2 suppresses contact allergy by inducing apoptosis in activated CD8+ T cells. J. Immunol. 2009, 182, 5419–5429. [Google Scholar] [CrossRef]
- Many, A.; Hubel, C.A.; Fisher, S.J.; Roberts, J.M.; Zhou, Y. Invasive cytotrophoblasts manifest evidence of oxidative stress in preeclampsia. Am. J. Pathol. 2000, 156, 321–331. [Google Scholar] [CrossRef]
- Roberts, J.M.; Escudero, C. The placenta in preeclampsia. Pregnancy Hypertens. Int. J. Women’s Cardiovasc. Health 2012, 2, 72–83. [Google Scholar] [CrossRef]
- Lyall, F.; Robson, S.C.; Bulmer, J.N. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: Relationship to clinical outcome. Hypertension 2013, 62, 1046–1054. [Google Scholar] [CrossRef]
- Geusens, N.; Hering, L.; Verlohren, S.; Luyten, C.; Drijkoningen, K.; Taube, M.; Vercruysse, L.; Hanssens, M.; Dechend, R.; Pijnenborg, R. Changes in endovascular trophoblast invasion and spiral artery remodelling at term in a transgenic preeclamptic rat model. Placenta 2010, 31, 320–326. [Google Scholar] [CrossRef]
- Zenclussen, A.C.; Gerlof, K.; Zenclussen, M.L.; Sollwedel, A.; Bertoja, A.Z.; Ritter, T.; Kotsch, K.; Leber, J.; Volk, H.-D. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: Adoptive transfer of pregnancy-induced CD4+ CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am. J. Pathol. 2005, 166, 811–822. [Google Scholar] [CrossRef]
- Quinn, K.H.; Lacoursiere, D.Y.; Cui, L.; Bui, J.; Parast, M.M. The unique pathophysiology of early-onset severe preeclampsia: Role of decidual T regulatory cells. J. Reprod. Immunol. 2011, 91, 76–82. [Google Scholar] [CrossRef]
- Hosseini, A.; Dolati, S.; Hashemi, V.; Abdollahpour-Alitappeh, M.; Yousefi, M. Regulatory T and T helper 17 cells: Their roles in preeclampsia. J. Cell. Physiol. 2018, 233, 6561–6573. [Google Scholar] [CrossRef]
- Huang, N.; Chi, H.; Qiao, J. Role of regulatory t cells in regulating fetal-maternal immune tolerance in healthy pregnancies and reproductive diseases. Front. Immunol. 2020, 11, 1023. [Google Scholar] [CrossRef]
- Zhang, N.N.; Chen, J.N.; Xiao, L.; Tang, F.; Zhang, Z.G.; Zhang, Y.W.; Feng, Z.Y.; Jiang, Y.; Shao, C.K. Accumulation Mechanisms of CD4(+)CD25(+)FOXP3(+) Regulatory T Cells in EBV-associated Gastric Carcinoma. Sci. Rep. 2015, 5, 18057. [Google Scholar] [CrossRef]
- Williams, P.; Searle, R.; Robson, S.; Innes, B.; Bulmer, J. Decidual leucocyte populations in early to late gestation normal human pregnancy. J. Reprod. Immunol. 2009, 82, 24–31. [Google Scholar] [CrossRef]
- Jaiswal, M.K.; Mallers, T.M.; Larsen, B.; Kwak-Kim, J.; Chaouat, G.; Gilman-Sachs, A.; Beaman, K.D. V-ATPase upregulation during early pregnancy: A possible link to establishment of an inflammatory response during preimplantation period of pregnancy. Reproduction 2012, 143, 713. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Xu, X.-H.; Jin, L. Macrophage polarization in physiological and pathological pregnancy. Front. Immunol. 2019, 10, 792. [Google Scholar] [CrossRef]
- Svensson, J.; Jenmalm, M.C.; Matussek, A.; Geffers, R.; Berg, G.; Ernerudh, J. Macrophages at the fetal–maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. J. Immunol. 2011, 187, 3671–3682. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, C.; Mjösberg, J.; Matussek, A.; Geffers, R.; Matthiesen, L.; Berg, G.; Sharma, S.; Ernerudh, J. Gene expression profiling of human decidual macrophages: Evidence for immunosuppressive phenotype. PLoS ONE 2008, 3, e2078. [Google Scholar] [CrossRef] [PubMed]
- Stefańska, K.; Zieliński, M.; Jankowiak, M.; Zamkowska, D.; Sakowska, J.; Adamski, P.; Jassem-Bobowicz, J.; Piekarska, K.; Leszczyńska, K.; Świątkowska-Stodulska, R.; et al. Cytokine imprint in preeclampsia. Front. Immunol. 2021, 12, 2337. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.M.; Lundberg, K.; Özenci, V.; Banham, A.H.; Hellström, M.; Egevad, L.; Pisa, P. CD4+ CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J. Immunol. 2006, 177, 7398–7405. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, Y.; Kono, K.; Kawaguchi, Y.; Akaike, H.; Kamimura, K.; Sugai, H.; Fujii, H. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. Int. J. Cancer 2008, 122, 2286–2293. [Google Scholar] [CrossRef]
- Maruyama, T.; Kono, K.; Izawa, S.; Mizukami, Y.; Kawaguchi, Y.; Mimura, K.; Watanabe, M.; Fujii, H. CCL17 and CCL22 chemokines within tumor microenvironment are related to infiltration of regulatory T cells in esophageal squamous cell carcinoma. Dis. Esophagus 2010, 23, 422–429. [Google Scholar] [CrossRef]
- Ramhorst, R.; Fraccaroli, L.; Aldo, P.; Alvero, A.B.; Cardenas, I.; Leirós, C.P.; Mor, G. Modulation and recruitment of inducible regulatory T cells by first trimester trophoblast cells. Am. J. Reprod. Immunol. 2012, 67, 17–27. [Google Scholar] [CrossRef]
- Leber, A.; Teles, A.; Zenclussen, A.C. Regulatory T cells and their role in pregnancy. Am. J. Reprod. Immunol. 2010, 63, 445–459. [Google Scholar] [CrossRef]
- Schumacher, A.; Brachwitz, N.; Sohr, S.; Engeland, K.; Langwisch, S.; Dolaptchieva, M.; Alexander, T.; Taran, A.; Malfertheiner, S.F.; Costa, S.D.; et al. Human chorionic gonadotropin attracts regulatory T cells into the fetal-maternal interface during early human pregnancy. J. Immunol. 2009, 182, 5488–5497. [Google Scholar] [CrossRef]
- Darmochwal-Kolarz, D.; Kludka-Sternik, M.; Tabarkiewicz, J.; Kolarz, B.; Rolinski, J.; Leszczynska-Gorzelak, B.; Oleszczuk, J. The predominance of Th17 lymphocytes and decreased number and function of Treg cells in preeclampsia. J. Reprod. Immunol. 2012, 93, 75–81. [Google Scholar] [CrossRef]
- Steinborn, A.; Schmitt, E.; Kisielewicz, A.; Rechenberg, S.; Seissler, N.; Mahnke, K.; Zeier, M.; Sohn, C. Pregnancy-associated diseases are characterized by the composition of the systemic regulatory T cell (Treg) pool with distinct subsets of Tregs. Clin. Exp. Immunol. 2012, 167, 84–98. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, S.; Zhao, M.; Yang, T.; Quan, S.; Yang, Q.; Song, L.; Yang, X. SUV39H1-DNMT3A-mediated epigenetic regulation of Tim-3 and galectin-9 in the cervical cancer. Cancer Cell Int. 2020, 20, 325. [Google Scholar] [CrossRef]
- Watanabe, M.; Shimoya, K.; Zhang, Q.; Temma-Asano, K.; Kimura, T.; Murata, Y. The expression of fractalkine in the endometrium during the menstrual cycle. Int. J. Gynecol. Obstet. 2006, 92, 242–247. [Google Scholar] [CrossRef]
- Red-Horse, K.; Drake, P.M.; Gunn, M.D.; Fisher, S.J. Chemokine ligand and receptor expression in the pregnant uterus: Reciprocal patterns in complementary cell subsets suggest functional roles. Am. J. Pathol. 2001, 159, 2199–2213. [Google Scholar] [CrossRef]
- Akiyama, M.; Okabe, H.; Takakura, K.; Fujiyama, Y.; Noda, Y. Expression of macrophage inflammatory protein-1α (MIP-1α) in human endometrium throughout the menstrual cycle. BJOG Int. J. Obstet. Gynaecol. 1999, 106, 725–730. [Google Scholar] [CrossRef]
- Caballero-Campo, P.; Domínguez, F.; Coloma, J.; Meseguer, M.; Remohí, J.; Pellicer, A.; Simón, C. Hormonal and embryonic regulation of chemokines IL-8, MCP-1 and RANTES in the human endometrium during the window of implantation. Mol. Hum. Reprod. 2002, 8, 375–384. [Google Scholar] [CrossRef]
- Daikoku, N.; Kitaya, K.; Nakayama, T.; Fushiki, S.; Honjo, H. Expression of macrophage inflammatory protein-3β in human endometrium throughout the menstrual cycle. Fertil. Steril. 2004, 81, 876–881. [Google Scholar] [CrossRef]
- Fontenot, J.D.; Rasmussen, J.P.; Williams, L.M.; Dooley, J.L.; Farr, A.G.; Rudensky, A.Y. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity 2005, 22, 329–341. [Google Scholar] [CrossRef] [PubMed]
- LaMarca, B.; Cornelius, D.C.; Harmon, A.C.; Amaral, L.M.; Cunningham, M.W.; Faulkner, J.L.; Wallace, K. Identifying immune mechanisms mediating the hypertension during preeclampsia. Am. J. Physiol.—Regul. Integr. Comp. Physiol. 2016, 311, R1–R9. [Google Scholar] [CrossRef] [PubMed]
- Tilburgs, T.; Roelen, D.L.; Van Der Mast, B.J.; Van Schip, J.J.; Kleijburg, C.; De Groot-Swings, G.M.; Kanhai, H.H.H.; Claas, F.H.J.; Scherjon, S.A. Differential distribution of CD4+ CD25bright and CD8+ CD28− T-cells in decidua and maternal blood during human pregnancy. Placenta 2006, 27, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Salvany-Celades, M.; Van der Zwan, A.; Benner, M.; Setrajcic-Dragos, V.; Gomes, H.A.B.; Iyer, V.; Norwitz, E.R.; Strominger, J.L.; Tilburgs, T. Three types of functional regulatory T cells control T cell responses at the human maternal-fetal interface. Cell Rep. 2019, 27, 2537–2547. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: New York, NY, USA, 1977. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meister, S.; Hahn, L.; Beyer, S.; Mannewitz, M.; Perleberg, C.; Schnell, K.; Anz, D.; Corradini, S.; Schmoeckel, E.; Mayr, D.; et al. Regulatory T Cell Apoptosis during Preeclampsia May Be Prevented by Gal-2. Int. J. Mol. Sci. 2022, 23, 1880. https://doi.org/10.3390/ijms23031880
Meister S, Hahn L, Beyer S, Mannewitz M, Perleberg C, Schnell K, Anz D, Corradini S, Schmoeckel E, Mayr D, et al. Regulatory T Cell Apoptosis during Preeclampsia May Be Prevented by Gal-2. International Journal of Molecular Sciences. 2022; 23(3):1880. https://doi.org/10.3390/ijms23031880
Chicago/Turabian StyleMeister, Sarah, Laura Hahn, Susanne Beyer, Mareike Mannewitz, Carolin Perleberg, Konstantin Schnell, David Anz, Stefanie Corradini, Elisa Schmoeckel, Doris Mayr, and et al. 2022. "Regulatory T Cell Apoptosis during Preeclampsia May Be Prevented by Gal-2" International Journal of Molecular Sciences 23, no. 3: 1880. https://doi.org/10.3390/ijms23031880
APA StyleMeister, S., Hahn, L., Beyer, S., Mannewitz, M., Perleberg, C., Schnell, K., Anz, D., Corradini, S., Schmoeckel, E., Mayr, D., Hasbargen, U., Zati Zehni, A., Mahner, S., Jeschke, U., & Kolben, T. (2022). Regulatory T Cell Apoptosis during Preeclampsia May Be Prevented by Gal-2. International Journal of Molecular Sciences, 23(3), 1880. https://doi.org/10.3390/ijms23031880






