Motility of the Zoonotic Spirochete Leptospira: Insight into Association with Pathogenicity
Abstract
1. Introduction
2. Leptospirosis
3. Morphology and Motility of Leptospira
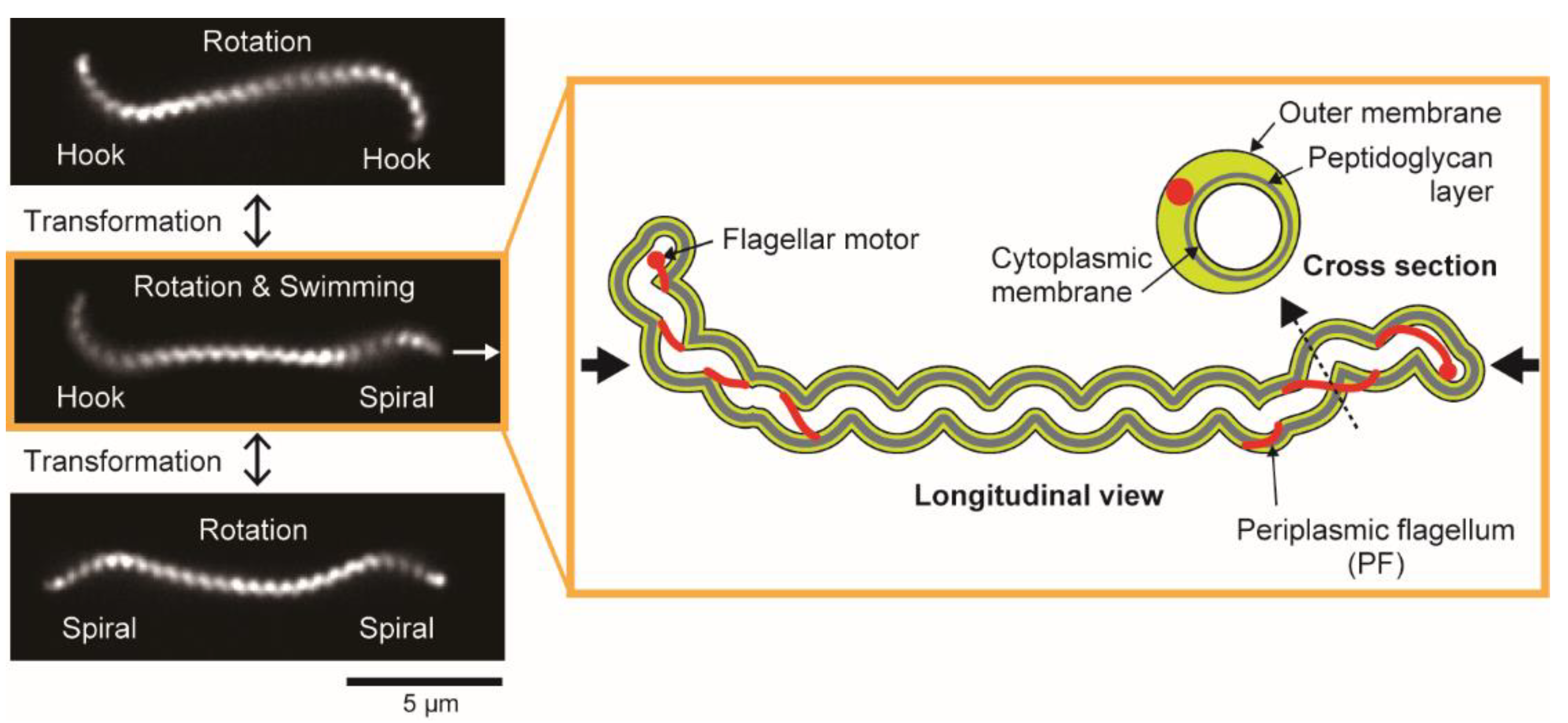
3.1. Cell Morphology
3.2. Swimming
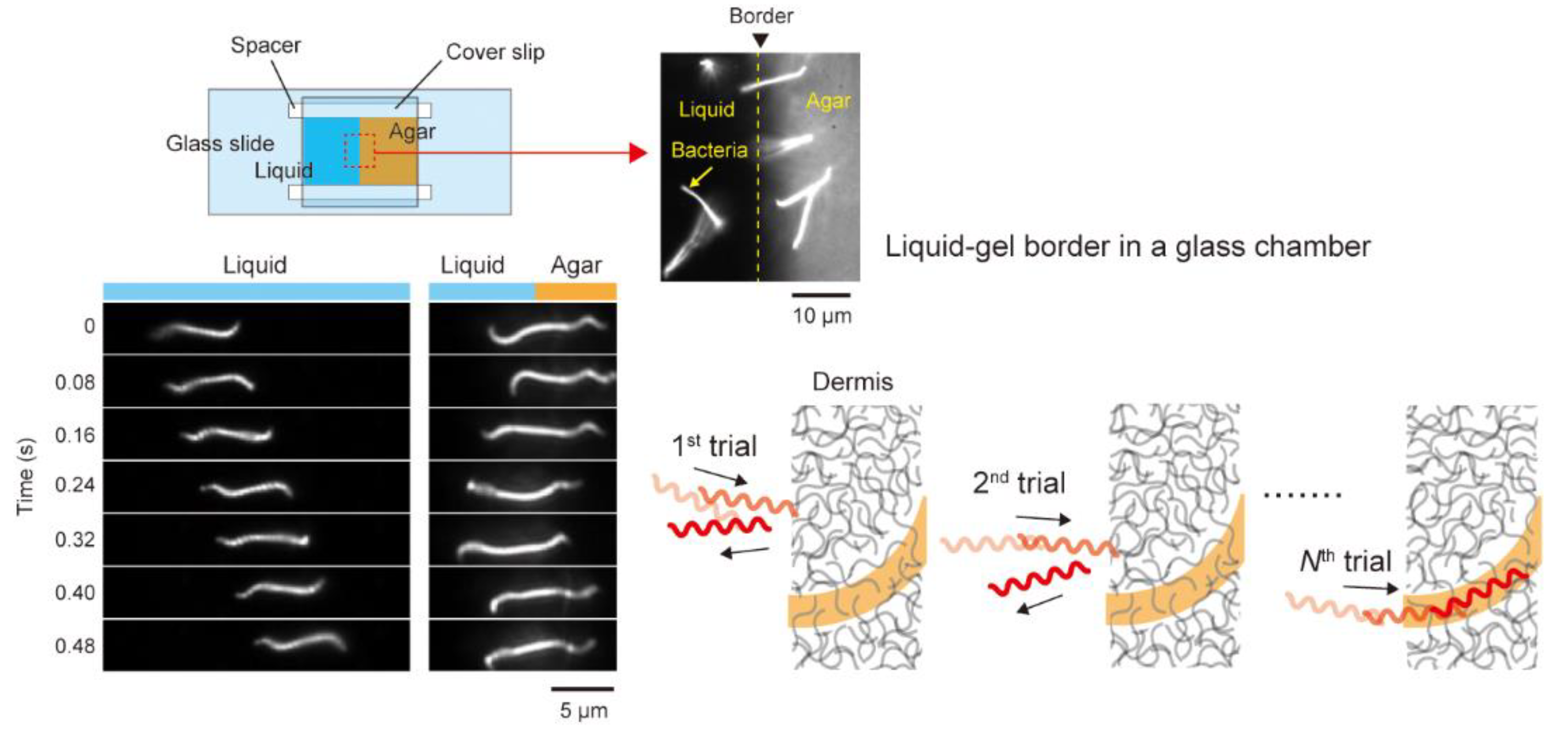
3.3. Crawling
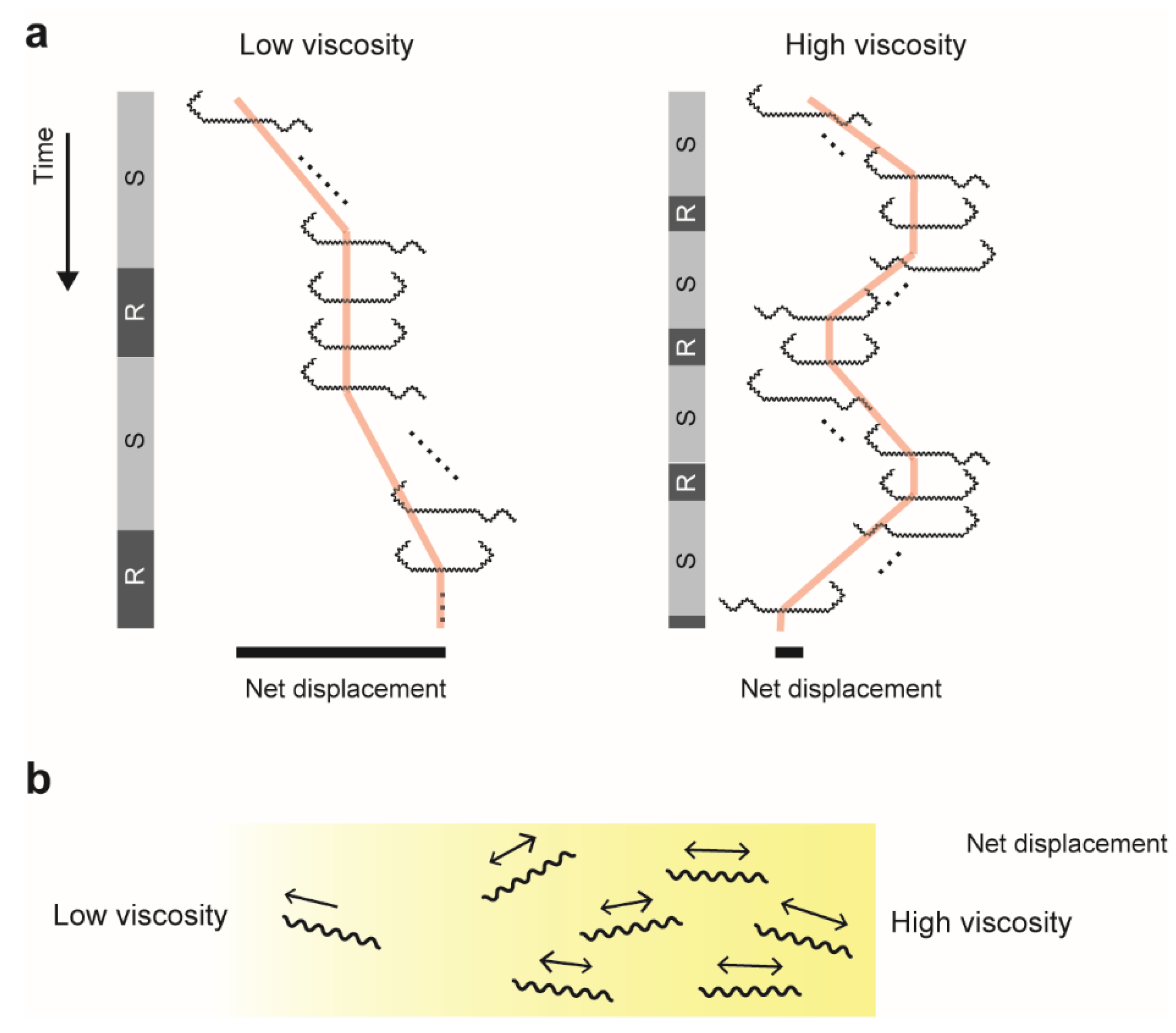
4. Swimming in a Highly Viscous Milieu
4.1. Dependence on a Type of Polymer
4.2. Back-and-Forth Motion
4.3. Trial and Error?
4.4. Interaction between PFs
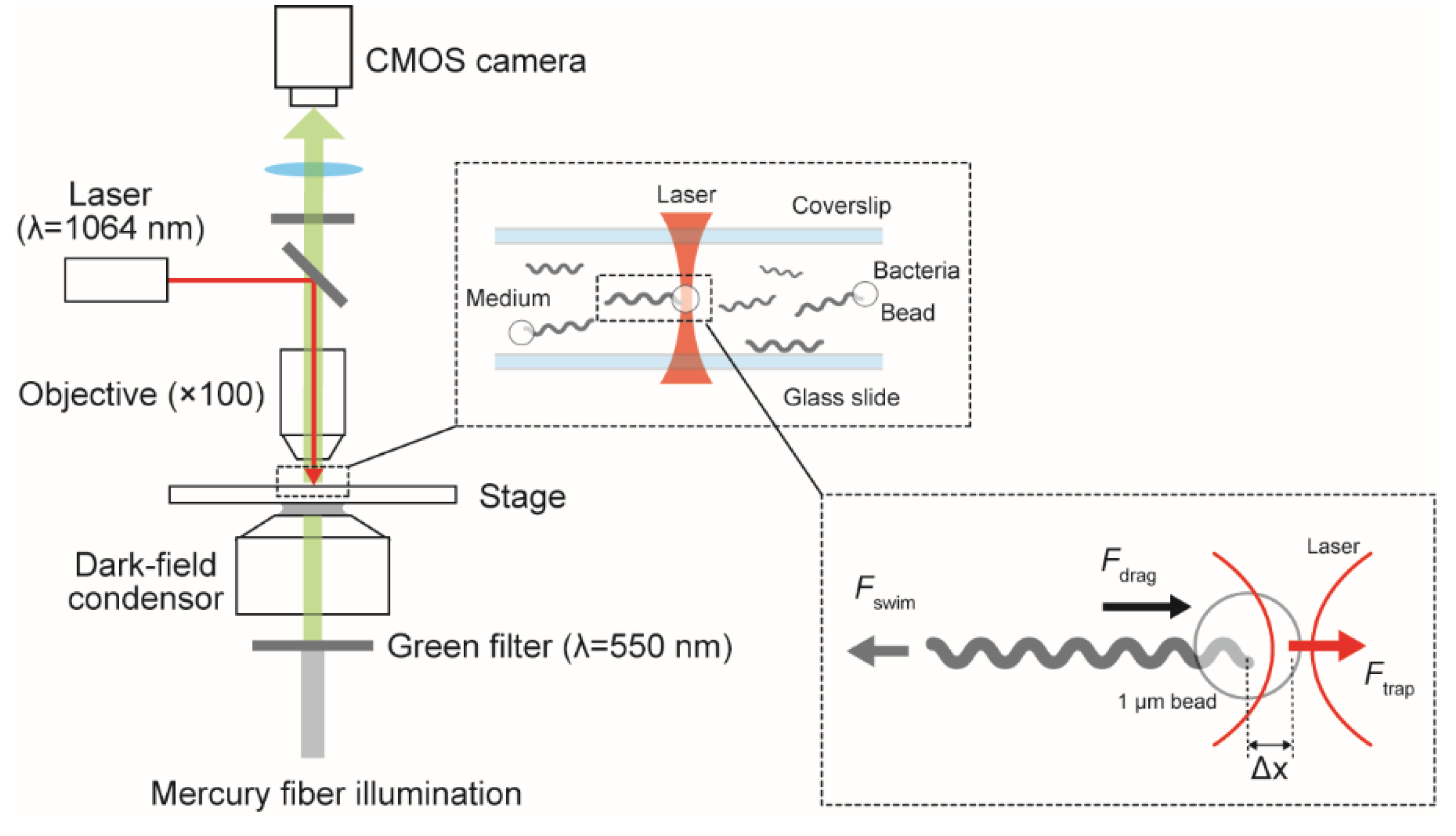
5. Swimming Force
6. Association of Crawling with Pathogenicity
6.1. Crawling on Cultured Kidney Cells
6.2. Leptospira Ligands Involved in Crawling
6.3. Host Preference
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Miyata, M.; Robinson, R.C.; Uyeda, T.Q.P.; Fukumori, Y.; Fukushima, S.; Haruta, S.; Homma, M.; Inaba, K.; Ito, M.; Kaito, C.; et al. Tree of Motility—A Proposed History of Motility Systems in the Tree of Life. Genes Cells 2020, 25, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Jarrell, K.F.; McBride, M.J. The Surprisingly Diverse Ways That Prokaryotes Move. Nat. Rev. Microbiol. 2008, 6, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Minamino, T. Flagella-Driven Motility of Bacteria. Biomolecules 2019, 9, 279. [Google Scholar] [CrossRef] [PubMed]
- Miyata, M. Unique Centipede Mechanism of Mycoplasma Gliding. Annu. Rev. Microbiol. 2010, 64, 519–537. [Google Scholar] [CrossRef]
- Faure, L.M.; Fiche, J.-B.; Espinosa, L.; Ducret, A.; Anantharaman, V.; Luciano, J.; Lhospice, S.; Islam, S.T.; Tréguier, J.; Sotes, M.; et al. The Mechanism of Force Transmission at Bacterial Focal Adhesion Complexes. Nature 2016, 539, 530–535. [Google Scholar] [CrossRef]
- Burrows, L.L. Pseudomonas aeruginosa Twitching Motility: Type IV Pili in Action. Annu. Rev. Microbiol. 2012, 66, 493–520. [Google Scholar] [CrossRef]
- Wilde, A.; Mullineaux, C.W. Motility in Cyanobacteria: Polysaccharide Tracks and Type IV Pilus Motors. Mol. Microbiol. 2015, 98, 998–1001. [Google Scholar] [CrossRef]
- Haiko, J.; Westerlund-Wikström, B. The Role of the Bacterial Flagellum in Adhesion and Virulence. Biology 2013, 2, 1242–1267. [Google Scholar] [CrossRef]
- Josenhans, C.; Suerbaum, S. The Role of Motility as a Virulence Factor in Bacteria. Int. J. Med. Microbiol. 2002, 291, 605–614. [Google Scholar] [CrossRef]
- Adler, B.; de la Peña Moctezuma, A. Leptospira and Leptospirosis. Vet. Microbiol. 2010, 140, 287–296. [Google Scholar] [CrossRef]
- Picardeau, M. Virulence of the Zoonotic Agent of Leptospirosis: Still Terra Incognita? Nat. Rev. Microbiol. 2017, 15, 297–307. [Google Scholar] [CrossRef]
- Coburn, J.; Picardeau, M.; Woods, C.W.; Veldman, T.; Haake, D.A. Pathogenesis Insights from an Ancient and Ubiquitous Spirochete. PLoS Pathog. 2021, 17, e1009836. [Google Scholar] [CrossRef]
- Lambert, A.; Picardeau, M.; Haake, D.A.; Sermswan, R.W.; Srikram, A.; Adler, B.; Murray, G.A. FlaA Proteins in Leptospira Interrogans Are Essential for Motility and Virulence but Are Not Required for Formation of the Flagellum Sheath. Infect. Immun. 2012, 80, 2019–2025. [Google Scholar] [CrossRef]
- Wunder, E.A.; Figueira, C.P.; Benaroudj, N.; Hu, B.; Tong, B.A.; Trajtenberg, F.; Liu, J.; Reis, M.G.; Charon, N.W.; Buschiazzo, A.; et al. A Novel Flagellar Sheath Protein, FcpA, Determines Filament Coiling, Translational Motility and Virulence for the Leptospira Spirochete. Mol. Microbiol. 2016, 101, 457–470. [Google Scholar] [CrossRef]
- Goldstein, S.F.; Charon, N.W. Multiple-Exposure Photographic Analysis of a Motile Spirochete. Proc. Natl. Acad. Sci. USA 1990, 87, 4895–4899. [Google Scholar] [CrossRef]
- Takabe, K.; Tahara, H.; Islam, M.S.; Affroze, S.; Kudo, S.; Nakamura, S. Viscosity-Dependent Variations in the Cell Shape and Swimming Manner of Leptospira. Microbiology 2017, 163, 153–160. [Google Scholar] [CrossRef]
- Nakamura, S.; Leshansky, A.; Magariyama, Y.; Namba, K.; Kudo, S. Direct Measurement of Helical Cell Motion of the Spirochete Leptospira. Biophys. J. 2014, 106, 47–54. [Google Scholar] [CrossRef]
- Tahara, H.; Takabe, K.; Sasaki, Y.; Kasuga, K.; Kawamoto, A.; Koizumi, N.; Nakamura, S. The Mechanism of Two-Phase Motility in the Spirochete Leptospira: Swimming and Crawling. Sci. Adv. 2018, 4, eaar7975. [Google Scholar] [CrossRef]
- Takabe, K.; Nakamura, S.; Ashihara, M.; Kudo, S. Effect of Osmolarity and Viscosity on the Motility of Pathogenic and Saprophytic Leptospira. Microbiol. Immunol. 2013, 57, 236–239. [Google Scholar] [CrossRef]
- Abe, K.; Kuribayashi, T.; Takabe, K.; Nakamura, S. Implications of Back-and-Forth Motion and Powerful Propulsion for Spirochetal Invasion. Sci. Rep. 2020, 10, 13937. [Google Scholar] [CrossRef]
- Cox, P.J.; Twigg, G.I. Leptospiral Motility. Nature 1974, 250, 260–261. [Google Scholar] [CrossRef]
- Charon, N.W.; Lawrence, C.W.; O’Brien, S. Movement of Antibody-Coated Latex Beads Attached to the Spirochete Leptospira Interrogans. Proc. Natl. Acad. Sci. USA 1981, 78, 7166–7170. [Google Scholar] [CrossRef]
- Schneider, W.R.; Doetsch, R.N. Effect of Viscosity on Bacterial Motility. J. Bacteriol. 1974, 117, 696–701. [Google Scholar] [CrossRef]
- Berg, H.C.; Turner, L. Movement of Microorganisms in Viscous Environments. Nature 1979, 278, 349–351. [Google Scholar] [CrossRef]
- Magariyama, Y.; Kudo, S. A Mathematical Explanation of an Increase in Bacterial Swimming Speed with Viscosity in Linear-Polymer Solutions. Biophys. J. 2002, 83, 733–739. [Google Scholar] [CrossRef]
- Nakamura, S.; Adachi, Y.; Goto, T.; Magariyama, Y. Improvement in Motion Efficiency of the Spirochete Brachyspira Pilosicoli in Viscous Environments. Biophys. J. 2006, 90, 3019–3026. [Google Scholar] [CrossRef]
- Ruby, J.D.; Charon, N.W. Effect of Temperature and Viscosity on the Motility of the Spirochete Treponema Denticola. FEMS Microbiol. Lett. 1998, 169, 251–254. [Google Scholar] [CrossRef][Green Version]
- Kimsey, R.B.; Spielman, A. Motility of Lyme Disease Spirochetes in Fluids as Viscous as the Extracellular Matrix. J. Infect. Dis. 1990, 162, 1205–1208. [Google Scholar] [CrossRef]
- Kaiser, G.E.; Doetsch, R.N. Enhanced Translational Motion of Leptospira in Viscous Environments. Nature 1975, 255, 656–657. [Google Scholar] [CrossRef]
- Terasawa, S.; Fukuoka, H.; Inoue, Y.; Sagawa, T.; Takahashi, H.; Ishijima, A. Coordinated Reversal of Flagellar Motors on a Single Escherichia Coli Cell. Biophys. J. 2011, 100, 2193–2200. [Google Scholar] [CrossRef]
- Charon, N.W.; Cockburn, A.; Li, C.; Liu, J.; Miller, K.A.; Miller, M.R.; Motaleb, M.A.; Wolgemuth, C.W. The Unique Paradigm of Spirochete Motility and Chemotaxis. Annu. Rev. Microbiol. 2012, 66, 349–370. [Google Scholar] [CrossRef] [PubMed]
- Vig, D.K.; Wolgemuth, C.W. Swimming Dynamics of the Lyme Disease Spirochete. Phys. Rev. Lett. 2012, 109, 218104. [Google Scholar] [CrossRef] [PubMed]
- Takabe, K.; Kawamoto, A.; Tahara, H.; Kudo, S.; Nakamura, S. Implications of Coordinated Cell-Body Rotations for Leptospira Motility. Biochem. Biophys. Res. Commun. 2017, 491, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Moldovan, R.; Yeung, C.; Wu, X.L. Swimming Efficiency of Bacterium Escherichia Coli. Proc. Natl. Acad. Sci. USA 2006, 103, 13712–13717. [Google Scholar] [CrossRef]
- Sato, K.; Nakamura, S.; Kudo, S.; Toyabe, S. Evaluation of the Duty Ratio of the Bacterial Flagellar Motor by Dynamic Load Control. Biophys. J. 2019, 116, 1952–1959. [Google Scholar] [CrossRef]
- Islam, M.S.; Morimoto, Y.V.; Kudo, S.; Nakamura, S. H+ and Na+ Are Involved in Flagellar Rotation of the Spirochete Leptospira. Biochem. Biophys. Res. Commun. 2015, 466, 196–200. [Google Scholar] [CrossRef]
- Beeby, M.; Ribardo, D.A.; Brennan, C.A.; Ruby, E.G.; Jensen, G.J.; Hendrixson, D.R. Diverse High-Torque Bacterial Flagellar Motors Assemble Wider Stator Rings Using a Conserved Protein Scaffold. Proc. Natl. Acad. Sci. USA 2016, 113, E1917–E1926. [Google Scholar] [CrossRef]
- Xu, J.; Koizumi, N.; Nakamura, S. Crawling Motility on the Host Tissue Surfaces Is Associated with the Pathogenicity of the Zoonotic Spirochete Leptospira. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Sebastián, I.; Okura, N.; Humbel, B.M.; Xu, J.; Hermawan, I.; Matsuura, C.; Hall, M.; Takayama, C.; Yamashiro, T.; Nakamura, S.; et al. Disassembly of the Apical Junctional Complex during the Transmigration of Leptospira Interrogans across Polarized Renal Proximal Tubule Epithelial Cells. Cell. Microbiol. 2021, 23, e13343. [Google Scholar] [CrossRef]
- Haake, D.A.; Matsunaga, J. Leptospira: A Spirochaete with a Hybrid Outer Membrane. Mol. Microbiol. 2010, 77, 805–814. [Google Scholar] [CrossRef]
- Matsunaga, J.; Barocchi, M.A.; Croda, J.; Young, T.A.; Sanchez, Y.; Siqueira, I.; Bolin, C.A.; Reis, M.G.; Riley, L.W.; Haake, D.A.; et al. Pathogenic Leptospira Species Express Surface-Exposed Proteins Belonging to the Bacterial Immunoglobulin Superfamily. Mol. Microbiol. 2003, 49, 929–946. [Google Scholar] [CrossRef]
- Choy, H.A.; Kelley, M.M.; Chen, T.L.; Møller, A.K.; Matsunaga, J.; Haake, D.A. Physiological Osmotic Induction of Leptospira Interrogans Adhesion: LigA and LigB Bind Extracellular Matrix Proteins and Fibrinogen. Infect. Immun. 2007, 75, 2441–2450. [Google Scholar] [CrossRef]
- Stevenson, B.; Choy, H.A.; Pinne, M.; Rotondi, M.L.; Miller, M.C.; DeMoll, E.; Kraiczy, P.; Cooley, A.E.; Creamer, T.P.; Suchard, M.A.; et al. Leptospira Interrogans Endostatin-like Outer Membrane Proteins Bind Host Fibronectin, Laminin and Regulators of Complement. PLoS ONE 2007, 2, e1188. [Google Scholar] [CrossRef]
- Verma, A.; Hellwage, J.; Artiushin, S.; Zipfel, P.F.; Kraiczy, P.; Timoney, J.F.; Stevenson, B. LfhA, a Novel Factor H-Binding Protein of Leptospira Interrogans. Infect. Immun. 2006, 74, 2659–2666. [Google Scholar] [CrossRef]
- Barbosa, A.S.; Abreu, P.A.E.; Neves, F.O.; Atzingen, M.V.; Watanabe, M.M.; Vieira, M.L.; Morais, Z.M.; Vasconcellos, S.A.; Nascimento, A.L.T.O. A Newly Identified Leptospiral Adhesin Mediates Attachment to Laminin. Infect. Immun. 2006, 74, 6356–6364. [Google Scholar] [CrossRef][Green Version]
- Daroz, B.B.; Fernandes, L.G.V.; Cavenague, M.F.; Kochi, L.T.; Passalia, F.J.; Takahashi, M.B.; Nascimento Filho, E.G.; Teixeira, A.F.; Nascimento, A.L.T.O. A Review on Host-Leptospira Interactions: What We Know and Future Expectations. Front. Cell. Infect. Microbiol. 2021, 11, 1179. [Google Scholar] [CrossRef]
- Kokubu, E.; Kikuchi, Y.; Okamoto-Shibayama, K.; Nakamura, S.; Ishihara, K. Crawling Motility of Treponema denticola Modulated by Outer Sheath Protein. Microbiol. Immunol. 2021, 65, 551–558. [Google Scholar] [CrossRef]
- Harman, M.W.; Dunham-Ems, S.M.; Caimano, M.J.; Belperron, A.A.; Bockenstedt, L.K.; Fu, H.C.; Radolf, J.D.; Wolgemuth, C.W. The Heterogeneous Motility of the Lyme Disease Spirochete in Gelatin Mimics Dissemination through Tissue. Proc. Natl. Acad. Sci. USA 2012, 109, 3059–3064. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, S. Motility of the Zoonotic Spirochete Leptospira: Insight into Association with Pathogenicity. Int. J. Mol. Sci. 2022, 23, 1859. https://doi.org/10.3390/ijms23031859
Nakamura S. Motility of the Zoonotic Spirochete Leptospira: Insight into Association with Pathogenicity. International Journal of Molecular Sciences. 2022; 23(3):1859. https://doi.org/10.3390/ijms23031859
Chicago/Turabian StyleNakamura, Shuichi. 2022. "Motility of the Zoonotic Spirochete Leptospira: Insight into Association with Pathogenicity" International Journal of Molecular Sciences 23, no. 3: 1859. https://doi.org/10.3390/ijms23031859
APA StyleNakamura, S. (2022). Motility of the Zoonotic Spirochete Leptospira: Insight into Association with Pathogenicity. International Journal of Molecular Sciences, 23(3), 1859. https://doi.org/10.3390/ijms23031859





