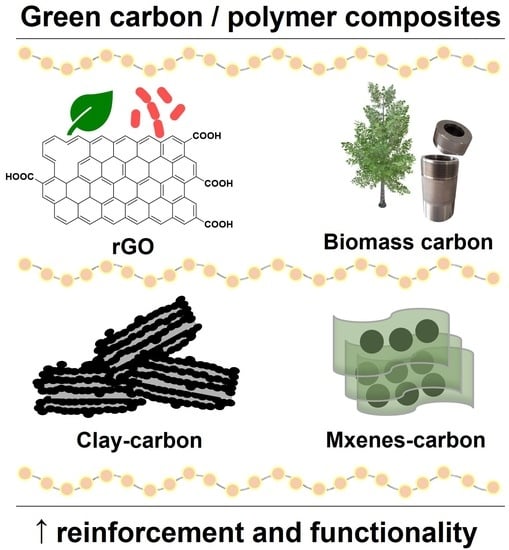
Green Carbon Nanostructures for Functional Composite Materials
Abstract
:1. Introduction
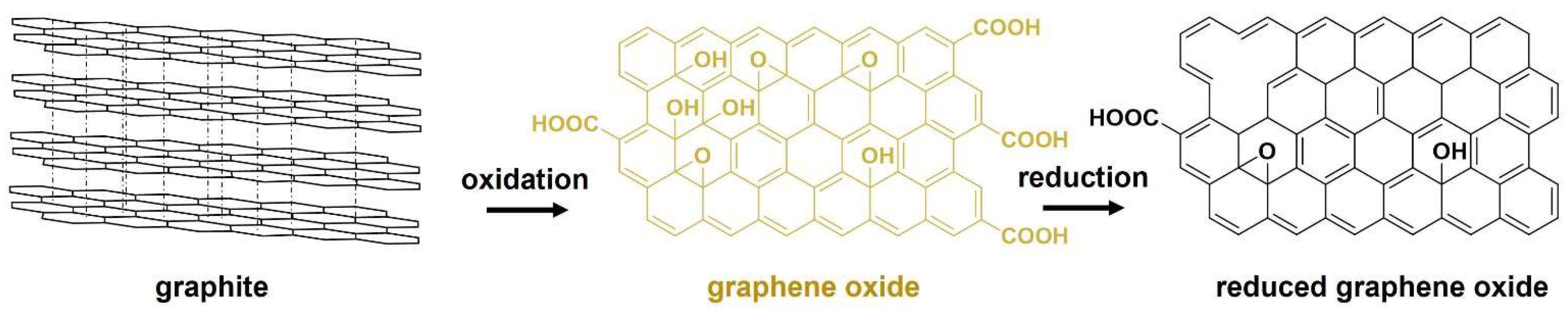
2. Chemical Reduction of Graphene Oxide
2.1. Evaluation of the Reduction Extent
2.2. Typical Reduction Methods
2.3. Green Chemical Reduction
| Reductant | Conditions | C/O ratio a | ID/IG | d-Spacing (nm) b | Ref. |
|---|---|---|---|---|---|
| Elemental sulfur | 4 h, 170 °C | 13.2 | 0.97 | 0.363 | [47] |
| POM (SiW12O405−) | 1 min | 6.1 | 1.13 | – | [48] |
| Plant Extracts | |||||
| Urtica dioica leaf | pH 12, 1 h, 90 °C | 4.8 | 1.13 | – | [29] |
| Thuja orientalis seed | 6 h, RT | – | 0.14 | 0.355 | [36] |
| Peganum harmala seed | 1 h, 90 °C | – | 0.94 | 0.355 | [49] |
| pH 12, 1 h, 90 °C | – | 0.90 | 0.380 | [50] | |
| Syzygium samarangense fruit | 40 h, 60 °C | 4.8 | 1.17 | 0.370 | [37] |
| Tridax procumbens leaf | 12 h, 95 °C | – | 1.00 | 0.360 | [45] |
| Gooseberry fruit | 3 h, 95 °C | – | 1.11 | 0.368 | [51] |
| Erythrina senegalensis leaf | 3 h, 95 °C | 6.2 | – | 0.330 | [46] |
| Bougainvillea glabra flower | 5 h, 95 °C | 4.6 | – | 0.380 | [21] |
| Eucalyptus bark | 24 h, 85 °C | 10.9 | 1.15 | 0.356 | [35] |
| Capsicum annuum fruit | 8 h, 80 °C | – | 1.30 | 0.341 | [52] |
| Camellia sinensis leaf | pH 9, 2 h, 120 °C | – | 1.14 | 0.337 | [53] |
| Bacteria | |||||
| Pseudoalteromonas sp. | 24 h | – | 1.30 | 0.335 | [39] |
| Desulfotomaculum | Few days, 37 °C | 4.5 | 1.37 | 0.370 | [41] |
| Bacillus sp. EPS | pH 8, 24 h 40 °C | 3.2 | 1.02 | 0.365 | [40] |
| G. sulfurreducens/acetate | 48 h, 30 °C | 5.5 | 1.18 | – | [38] |
| Bacillus sphaericus | 48 h, 30 °C | 2.6 | 1.17 | 0.870 | [54] |
| Combined Methods | |||||
| HTC/caffeic acid | 24 h, 180 °C | 6.0 | 1.09 | 0.343 | [20] |
| HTC/ZnO | pH 1, 24 h, 150 °C | – | 1.32 | – | [42] |
| HTC/starch | pH 9, 15 min, 120 °C | 3.6 | 1.03 | 0.378 | [25] |
| HTC/P. amboinicus leaf | 12 h, 120 °C | – | 1.30 | 0.360 | [55] |
| TVF/ascorbic acid | pH 10, 0.5 h, 95 °C | 6.2 | 1.32 | 0.390 | [43] |
| BM/Zn | 6 h, RT | 8.9 | 1.32 | – | [44] |
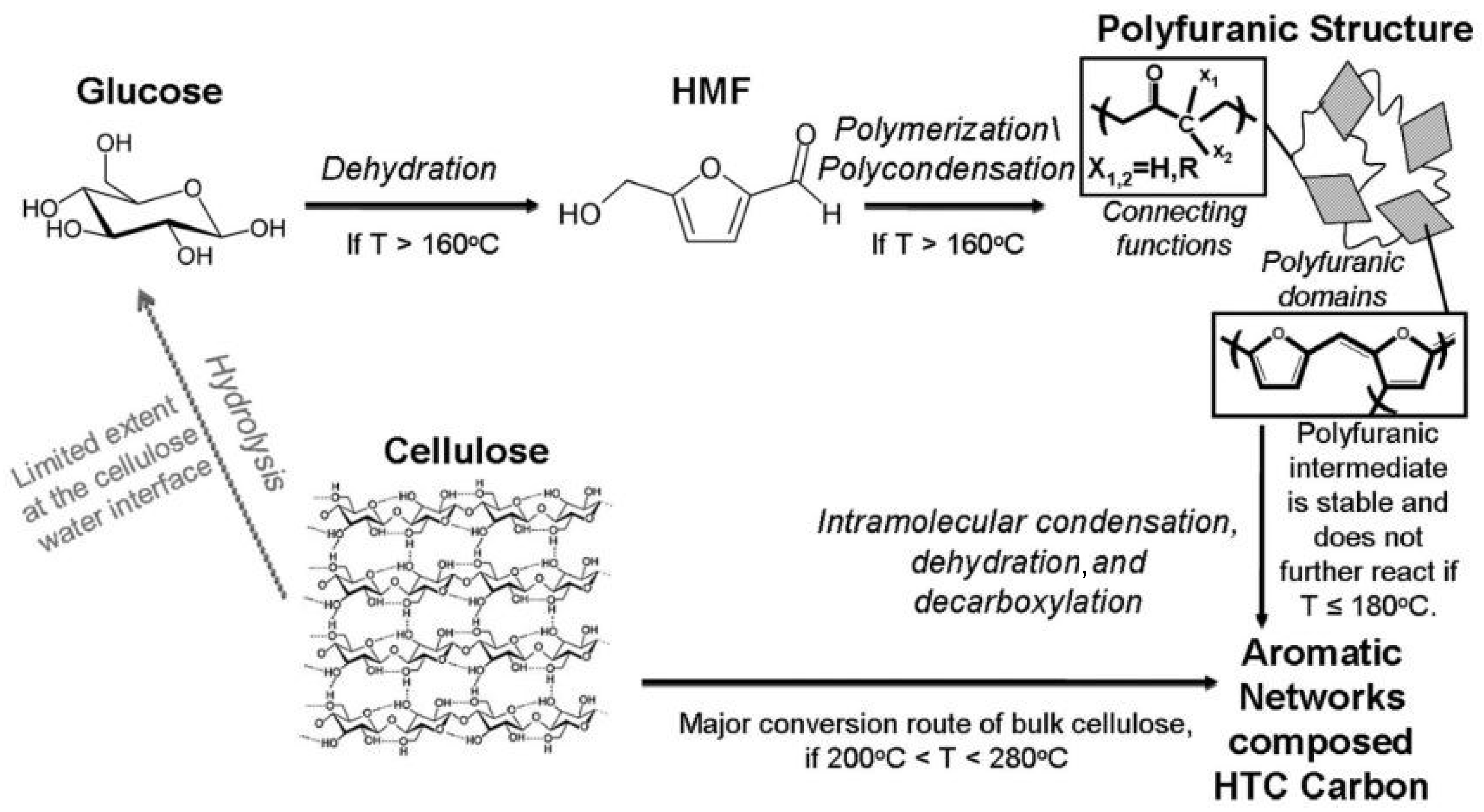
3. Carbon Structures Derived from Biomass
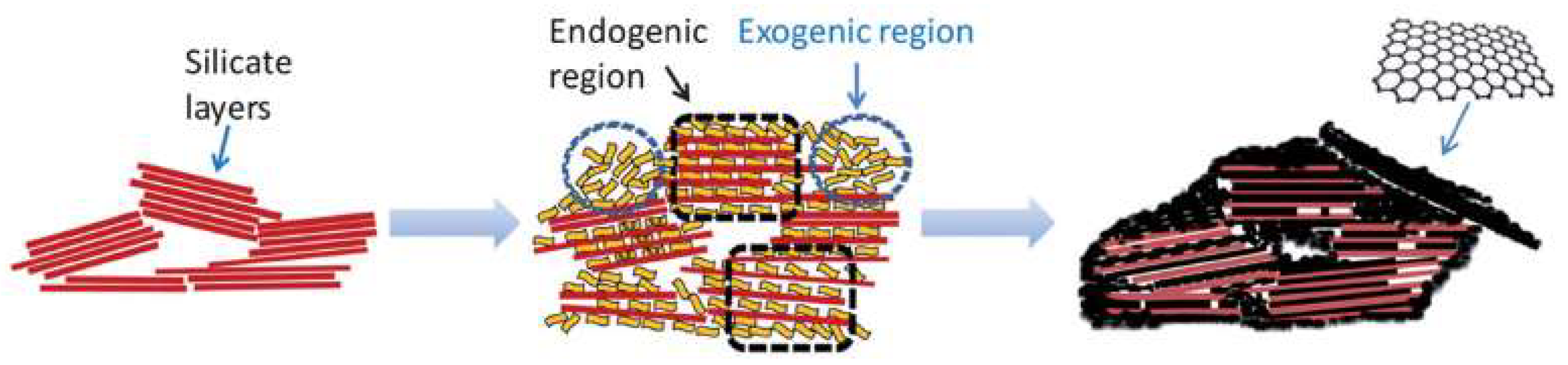
4. Graphitic Materials Supported on Lamellar Structures
5. Polymer Composites Containing rGO
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Bianco, A.; Cheng, H.M.; Enoki, T.; Gogotsi, Y.; Hurt, R.H.; Koratkar, N.; Kyotani, T.; Monthioux, M.; Park, C.R.; Tascon, J.M.D.; et al. All in the graphene family—A recommended nomenclature for two-dimensional carbon materials. Carbon 2013, 65, 1–6. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.-Y.; Narita, A.; Müllen, K. Precision synthesis versus bulk-scale fabrication of graphenes. Nat. Rev. Chem. 2018, 2, 0100. [Google Scholar] [CrossRef]
- Lin, L.; Deng, B.; Sun, J.; Peng, H.; Liu, Z. Bridging the Gap between Reality and Ideal in Chemical Vapor Deposition Growth of Graphene. Chem. Rev. 2018, 118, 9281–9343. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Yanwu, Z.; An, J.; Ruoff, R.S. Graphene-Based ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Wick, P.; Louw-Gaume, A.E.; Kucki, M.; Krug, H.F.; Kostarelos, K.; Fadeel, B.; Dawson, K.A.; Salvati, A.; Vázquez, E.; Ballerini, L.; et al. Classification Framework for Graphene-Based Materials; Wiley-VCH Verlag: New York, NY, USA, 2014; Volume 53, pp. 7714–7718. [Google Scholar]
- Shen, J.; Hu, Y.; Li, C.; Qin, C.; Ye, M. Synthesis of amphiphilic graphene nanoplatelets. Small 2009, 5, 82–85. [Google Scholar] [CrossRef]
- Loeffen, A.; Cree, D.E.; Sabzevari, M.; Wilson, L.D. Effect of Graphene Oxide as a Reinforcement in a Bio-Epoxy Composite. J. Compos. Sci. 2021, 5, 91. [Google Scholar] [CrossRef]
- Barra, A.; Ferreira, N.M.N.M.; Martins, M.A.M.A.; Lazar, O.; Pantazi, A.; Jderu, A.A.A.A.; Neumayer, S.M.S.M.; Rodriguez, B.J.B.J.; Enăchescu, M.; Ferreira, P.; et al. Eco-friendly preparation of electrically conductive chitosan-reduced graphene oxide flexible bionanocomposites for food packaging and biological applications. Compos. Sci. Technol. 2019, 173, 53–60. [Google Scholar] [CrossRef]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N. Carbon nanotubes: Properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef] [Green Version]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Xu, Y.; Bai, H.; Lu, G.; Li, C.; Shi, G. Flexible graphene films via the filtration of water-soluble noncovalent functionalized graphene sheets. J. Am. Chem. Soc. 2008, 130, 5856–5857. [Google Scholar] [CrossRef]
- Coros, M.; Pogacean, F.; Turza, A.; Dan, M.; Berghian-Grosan, C.; Pana, I.O.; Pruneanu, S. Green synthesis, characterization and potential application of reduced graphene oxide. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 119, 113971. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.B.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- De Silva, K.K.H.; Huang, H.H.; Yoshimura, M. Progress of reduction of graphene oxide by ascorbic acid. Appl. Surf. Sci. 2018, 447, 338–346. [Google Scholar] [CrossRef]
- Eigler, S.; Dotzer, C.; Hirsch, A. Visualization of defect densities in reduced graphene oxide. Carbon 2012, 50, 3666–3673. [Google Scholar] [CrossRef]
- Fujimoto, A.; Yamada, Y.; Koinuma, M.; Sato, S. Origins of sp3C peaks in C1s X-ray Photoelectron Spectra of Carbon Materials. Anal. Chem. 2016, 88, 6110–6114. [Google Scholar] [CrossRef] [Green Version]
- Hortigüela, M.J.; Machado, D.; Bdikin, I.; Neto, V.; Otero-Irurueta, G. Chemical changes of graphene oxide thin films induced by thermal treatment under vacuum conditions. Coatings 2020, 10, 113. [Google Scholar] [CrossRef] [Green Version]
- Barra, A.; Lazăr, O.; Pantazi, A.; Hortigüela, M.J.; Otero-Irurueta, G.; Enăchescu, M.; Ruiz-Hitzky, E.; Nunes, C.; Ferreira, P. Joining Caffeic Acid and Hydrothermal Treatment to Produce Environmentally Benign Highly Reduced Graphene Oxide. Nanomaterials 2021, 11, 732. [Google Scholar] [CrossRef]
- Mahendran, G.B.; Ramalingam, S.J.; Rayappan, J.B.B.; Kesavan, S.; Periathambi, T.; Nesakumar, N. Green preparation of reduced graphene oxide by Bougainvillea glabra flower extract and sensing application. J. Mater. Sci. Mater. Electron. 2020, 31, 14345–14356. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, G.Y.; Yu, C.H.; Xie, Y.; Xia, M.Y.; Lu, B.Y.; Fei, X.; Peng, Q. The UV absorption of graphene oxide is size-dependent: Possible calibration pitfalls. Microchim. Acta 2019, 186, 207. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Farivar, F.; Lay, P.; Hassan, K.; Thanh, T.; Tran, D.N.H.; Pollard, A.J.; Losic, D. Unlocking thermogravimetric analysis (TGA) in the fight against “Fake graphene” materials. Carbon 2021, 179, 505–513. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Kim, H.D.; Han, S.S. Biocompatibility and hemocompatibility of hydrothermally derived reduced graphene oxide using soluble starch as a reducing agent. Colloids Surf. B Biointerfaces 2020, 185, 110579. [Google Scholar] [CrossRef]
- Longo, A.; Palomba, M.; Carotenuto, G. Green Solid-State Chemical Reduction of Graphene Oxide Supported on a Paper Substrate. Coatings 2020, 10, 693. [Google Scholar] [CrossRef]
- El-Hallag, I.S.; El-Nahass, M.N.; Youssry, S.M.; Kumar, R.; Abdel-Galeil, M.M.; Matsuda, A. Facile in-situ simultaneous electrochemical reduction and deposition of reduced graphene oxide embedded palladium nanoparticles as high performance electrode materials for supercapacitor with excellent rate capability. Electrochim. Acta 2019, 314, 124–134. [Google Scholar] [CrossRef]
- Mascarenhas, F.C.; Sykam, N.; Selvakumar, M.; Mahesha, M.G. Green reduction of graphene oxide using Indian gooseberry (amla) extract for gas sensing applications. J. Environ. Chem. Eng. 2020, 8, 103712. [Google Scholar] [CrossRef]
- Mahmudzadeh, M.; Yari, H.; Ramezanzadeh, B.; Mahdavian, M. Urtica dioica extract as a facile green reductant of graphene oxide for UV resistant and corrosion protective polyurethane coating fabrication. J. Ind. Eng. Chem. 2019, 78, 125–136. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Yang, Y.; Wen, Y.; Wang, M. Reduction of graphene oxide by thiourea. J. Nanosci. Nanotechnol. 2011, 11, 10082–10086. [Google Scholar] [CrossRef]
- Amarnath, C.A.; Hong, C.E.; Kim, N.H.; Ku, B.C.; Kuila, T.; Lee, J.H. Efficient synthesis of graphene sheets using pyrrole as a reducing agent. Carbon 2011, 49, 3497–3502. [Google Scholar] [CrossRef]
- Wang, J.; Fei, G.; Pan, Y.; Zhang, K.; Hao, S.; Zheng, Z.; Xia, H. Simultaneous reduction and surface functionalization of graphene oxide by cystamine dihydrochloride for rubber composites. Compos. Part A Appl. Sci. Manuf. 2019, 122, 18–26. [Google Scholar] [CrossRef]
- Zhang, H.; Kuila, T.; Kim, N.H.; Yu, D.S.; Lee, J.H. Simultaneous reduction, exfoliation, and nitrogen doping of graphene oxide via a hydrothermal reaction for energy storage electrode materials. Carbon 2014, 69, 66–78. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Shen, G.; Cheng, P.; Zhang, J.; Guo, S. Reduction of graphene oxide via L-ascorbic acid. Chem. Commun. 2010, 46, 1112–1114. [Google Scholar] [CrossRef] [PubMed]
- Manchala, S.; Tandava, V.S.R.K.; Jampaiah, D.; Bhargava, S.K.; Shanker, V. Novel and Highly Efficient Strategy for the Green Synthesis of Soluble Graphene by Aqueous Polyphenol Extracts of Eucalyptus Bark and Its Applications in High-Performance Supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 11612–11620. [Google Scholar] [CrossRef]
- Kumar, P.; Harish; Andersson, G.; Subhedar, K.M.; Dhami, H.S.; Gupta, G.; Mukhopadhyay, A.K.; Joshi, R.P. Utilization of green reductant Thuja Orientalis for reduction of GO to RGO. Ceram. Int. 2021, 47, 14862–14878. [Google Scholar] [CrossRef]
- Kindalkar, V.S.; Kumara, K.; Bhat, S.; Dharmaprakash, S.M. An eco-friendly approach for the reduction of graphene oxide using Syzygium samarangense fruit extract. Mater. Chem. Phys. 2021, 261, 124224. [Google Scholar] [CrossRef]
- Kalathil, S.; Katuri, K.P.; Alazmi, A.S.; Pedireddy, S.; Kornienko, N.; Costa, P.M.F.J.; Saikaly, P.E. Bioinspired Synthesis of Reduced Graphene Oxide-Wrapped Geobacter sulfurreducens as a Hybrid Electrocatalyst for Efficient Oxygen Evolution Reaction. Chem. Mater. 2019, 31, 3686–3693. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Cheng, S.; Han, M.; Li, Q.; Chen, W.; Zhou, W. The characteristic and performance of reduced graphene oxide by marine bacterium Pseudoalteromonas sp. CF10-13. Ceram. Int. 2020, 46, 21699–21706. [Google Scholar] [CrossRef]
- Wang, H.; Huang, W.; Huang, S.; Xia, L.; Liu, X.; Li, Y.; Song, S.; Yang, L. A Green Method toward Graphene Oxide Reduction by Extracellular Polymeric Substances Assisted with NH4+. Arab. J. Sci. Eng. 2021, 46, 485–494. [Google Scholar] [CrossRef]
- Dong, S.; Wang, Z.; Wang, J.; Asif, M.; Yao, Y.; Xiao, F.; Liu, H. Sulfate-reducing bacteria respiration approach to fabricating flexible N,S-reduced graphene oxide thin film electrode for in situ cancer biomarker detection. J. Electroanal. Chem. 2020, 859, 113867. [Google Scholar] [CrossRef]
- Alves, Z.; Nunes, C.; Ferreira, P. Unravelling the Role of Synthesis Conditions on the Structure of Zinc Oxide-Reduced Graphene Oxide Nanofillers. Nanomaterials 2021, 11, 2149. [Google Scholar] [CrossRef]
- Nam, K.H.; Jung Kim, U.; Hee Jeon, M.; Lee, T.R.; Yu, J.; You, N.H.; Kim, Y.K.; Won Suk, J.; Ku, B.C. Green, fast, and scalable production of reduced graphene oxide via Taylor vortex flow. Chem. Eng. J. 2020, 391, 123482. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Nimbalkar, A.S.; Hong, C.K.; Ha, S.K. A Green Route for Quick and Kilogram Production of Reduced Graphene Oxide and Their Applications at Low Loadings in Epoxy Resins. ChemistrySelect 2019, 4, 1266–1274. [Google Scholar] [CrossRef]
- Thiyagarajulu, N.; Arumugam, S.; Narayanan, A.L.; Mathivanan, T.; Renuka, R.R. Green synthesis of reduced graphene nanosheets using leaf extract of tridax procumbens and its potential in vitro biological activities. Biointerface Res. Appl. Chem. 2020, 11, 9975–9984. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, S.; Xie, C.; Liu, Q.; Yang, S. Fabrication of Erythrina senegalensis leaf extract mediated reduced graphene oxide for cardiac repair applications in the nursing care. Inorg. Nano-Metal Chem. 2021, 51, 143–149. [Google Scholar] [CrossRef]
- Nam, K.H.; Kim, K.; Kim, S.G.; Lee, H.S.; Jung, H.; Yu, J.; Jang, S.G.; Ku, B.C.; Moon, B.; You, N.H. Sustainable production of reduced graphene oxide using elemental sulfur for multifunctional composites. Compos. Part B Eng. 2019, 176, 107236. [Google Scholar] [CrossRef]
- Debiemme-Chouvy, C.; Thomas, B.; Lucas, I.T.; Maï Tran, T.T.; Heintz, J.M.; Veillère, A.; Silvain, J.F. Facile and Green Reduction of Graphene Oxide by a Reduced Polyoxometalate and Formation of a Nanohybrid. Chempluschem 2017, 82, 186–189. [Google Scholar] [CrossRef]
- Mohammadkhani, R.; Ramezanzadeh, M.; Akbarzadeh, S.; Bahlakeh, G.; Ramezanzadeh, B. Graphene oxide nanoplatforms reduction by green plant-sourced organic compounds for construction of an active anti-corrosion coating; experimental/electronic-scale DFT-D modeling studies. Chem. Eng. J. 2020, 397, 125433. [Google Scholar] [CrossRef]
- Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Green synthesis of reduced graphene oxide nanosheets decorated with zinc-centered metal-organic film for epoxy-ester composite coating reinforcement: DFT-D modeling and experimental explorations. J. Taiwan Inst. Chem. Eng. 2020, 114, 311–330. [Google Scholar] [CrossRef]
- Ansari, M.Z.; Johari, R.; Siddiqi, W.A. Novel and green synthesis of chemically reduced graphene sheets using Phyllanthus emblica (Indian Gooseberry) and its photovoltaic activity. Mater. Res. Express 2019, 6, 055027. [Google Scholar] [CrossRef]
- Hashmi, A.; Singh, A.K.; Khan, A.A.P.; Asiri, A.M. Novel and Green Reduction of Graphene Oxide by Capsicum Annuum: Its Photo Catalytic Activity. J. Nat. Fibers 2020, 1–16. [Google Scholar] [CrossRef]
- Fatima, F.; Singh, H.R.; Jha, S.K. Assessment of antioxidant and cytotoxicity activities against A-549 lung cancer cell line by synthesized reduced graphene oxide nanoparticles mediated by Camellia sinensis. 3 Biotech 2021, 11, 494. [Google Scholar] [CrossRef]
- Xu, Q.; Lin, X.; Gan, L.; Owens, G.; Chen, Z. Green reduction of graphene oxide using Bacillus sphaericus. J. Colloid Interface Sci. 2022, 605, 881–887. [Google Scholar] [CrossRef]
- Dominic, R.M.; Punniyakotti, P.; Balan, B.; Angaiah, S. Green synthesis of reduced graphene oxide using Plectranthus amboinicus leaf extract and its supercapacitive performance. Bull. Mater. Sci. 2021, 45, 2. [Google Scholar] [CrossRef]
- Titirici, M.M.; White, R.J.; Brun, N.; Budarin, V.L.; Su, D.S.; Del Monte, F.; Clark, J.H.; MacLachlan, M.J. Sustainable carbon materials. Chem. Soc. Rev. 2015, 44, 250–290. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Li, M.; Wang, Y. Biomass-derived carbon: Synthesis and applications in energy storage and conversion. Green Chem. 2016, 18, 4824–4854. [Google Scholar] [CrossRef]
- Foong, S.Y.; Liew, R.K.; Yang, Y.; Cheng, Y.W.; Yek, P.N.Y.; Wan Mahari, W.A.; Lee, X.Y.; Han, C.S.; Vo, D.V.N.; Van Le, Q.; et al. Valorization of biomass waste to engineered activated biochar by microwave pyrolysis: Progress, challenges, and future directions. Chem. Eng. J. 2020, 389, 124401. [Google Scholar] [CrossRef]
- Kazmierczak-Razna, J.; Nowicki, P.; Wiśniewska, M.; Nosal-Wiercińska, A.; Pietrzak, R. Thermal and physicochemical properties of phosphorus-containing activated carbons obtained from biomass. J. Taiwan Inst. Chem. Eng. 2017, 80, 1006–1013. [Google Scholar] [CrossRef]
- Budarin, V.L.; Clark, J.H.; Luque, R.; Macquarrie, D.J.; Milkowski, K.; White, R.J. Carbonaceous Materials. U.S. Patent 8790548B2, 29 July 2014. [Google Scholar]
- Budarin, V.; Clark, J.H.; Hardy, J.J.E.; Luque, R.; Milkowski, K.; Tavener, S.J.; Wilson, A.J. Starbons: New Starch-Derived Mesoporous Carbonaceous Materials with Tunable Properties. Angew. Chem. Int. Ed. 2006, 45, 3782–3786. [Google Scholar] [CrossRef]
- White, R.J.; Antonio, C.; Budarin, V.L.; Bergström, E.; Thomas-Oates, J.; Clark, J.H. Polysaccharide-derived carbons for polar analyte separations. Adv. Funct. Mater. 2010, 20, 1834–1841. [Google Scholar] [CrossRef]
- Jung, A.; Han, S.; Kim, T.; Cho, W.J.; Lee, K. Synthesis of high carbon content microspheres using 2-step microwave carbonization, and the influence of nitrogen doping on catalytic activity. Carbon N. Y. 2013, 60, 307–316. [Google Scholar] [CrossRef]
- Falco, C.; Baccile, N.; Titirici, M.-M. Morphological and structural differences between glucose, cellulose and lignocellulosic biomass derived hydrothermal carbons. Green Chem. 2011, 13, 3273. [Google Scholar] [CrossRef] [Green Version]
- Titirici, M.M.; Antonietti, M.; Baccile, N. Hydrothermal carbon from biomass: A comparison of the local structure from poly- to monosaccharides and pentoses/hexoses. Green Chem. 2008, 10, 1204–1212. [Google Scholar] [CrossRef] [Green Version]
- Falco, C.; Caballero, F.P.; Babonneau, F.; Gervais, C.; Laurent, G.; Titirici, M.-M.; Baccile, N. Hydrothermal Carbon from Biomass: Structural Differences between Hydrothermal and Pyrolyzed Carbons via 13C Solid State NMR. Langmuir 2011, 27, 14460–14471. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, D.; Raidongia, K.; Shao, J.; Huang, J. Graphene oxide assisted hydrothermal carbonization of carbon hydrates. ACS Nano 2014, 8, 449–457. [Google Scholar] [CrossRef]
- Li, Y.Q.; Samad, Y.A.; Polychronopoulou, K.; Liao, K. Lightweight and Highly Conductive Aerogel-like Carbon from Sugarcane with Superior Mechanical and EMI Shielding Properties. ACS Sustain. Chem. Eng. 2015, 3, 1419–1427. [Google Scholar] [CrossRef]
- Zhao, L.; Baccile, N.; Gross, S.; Zhang, Y.; Wei, W.; Sun, Y.; Antonietti, M.; Titirici, M.M. Sustainable nitrogen-doped carbonaceous materials from biomass derivatives. Carbon 2010, 48, 3778–3787. [Google Scholar] [CrossRef] [Green Version]
- Zhong, R.; Liao, Y.; Shu, R.; Ma, L.; Sels, B.F. Vapor-phase assisted hydrothermal carbon from sucrose and its application in acid catalysis. Green Chem. 2018, 20, 1345–1353. [Google Scholar] [CrossRef]
- Yu, L.; Falco, C.; Weber, J.; White, R.J.; Howe, J.Y.; Titirici, M.M. Carbohydrate-derived hydrothermal carbons: A thorough characterization study. Langmuir 2012, 28, 12373–12383. [Google Scholar] [CrossRef]
- Olszewski, M.P.; Nicolae, S.A.; Arauzo, P.J.; Titirici, M.; Kruse, A. Wet and dry? Influence of hydrothermal carbonization on the pyrolysis of spent grains. J. Clean. Prod. 2020, 260, 121101. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Aranda, P.; Serratosa, J.M. Clay-organic Interactions: Organoclay Complexes and Polymer-clay Nanocomposite. In Handbook of Layered Materials; Aucherbach, S., Carrado, K.A., Dutta, P., Eds.; Taylor & Francis: New York, NY, USA, 2004; p. 19. [Google Scholar]
- Bergaya, F.; Theng, B.K.G.; Lagaly, G. Handbook of Clay Science, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2006; ISBN 9780080441832. [Google Scholar]
- Faustini, M.; Nicole, L.; Ruiz-Hitzky, E.; Sanchez, C. History of Organic–Inorganic Hybrid Materials: Prehistory, Art, Science, and Advanced Applications. Adv. Funct. Mater. 2018, 28, 1704158. [Google Scholar] [CrossRef]
- Kyotani, T.; Sonobe, N.; Tomita, A. Formation of highly orientated graphite from polyacrylonitrile by using a two-dimensional space between montmorillonite lamellae. Nature 1988, 331, 331–333. [Google Scholar] [CrossRef]
- Fernández-Saavedra, R.; Aranda, P.; Ruiz-Hitzky, E. Templated synthesis of carbon nanofibers from polyacrylonitrile using sepiolite. Adv. Funct. Mater. 2004, 14, 77–82. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Darder, M.; Fernandes, F.M.; Zatile, E.; Palomares, F.J.; Aranda, P. Supported graphene from natural resources: Easy preparation and applications. Adv. Mater. 2011, 23, 5250–5255. [Google Scholar] [CrossRef] [Green Version]
- Darder, M.; Ruiz-Hitzky, E. Caramel-clay nanocomposites. J. Mater. Chem. 2005, 15, 3913–3918. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E. Molecular access to intracrystalline tunnels of sepiolite. J. Mater. Chem. 2001, 11, 86–91. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Q.; Liu, C.; Zhang, X.; Chung, D.D.L. Carbon-coated sepiolite clay fibers with acid pre-treatment as low-cost organic adsorbents. Carbon 2017, 123, 259–272. [Google Scholar] [CrossRef]
- Yebra-Rodríguez, A.; Martín-Ramos, J.D.; Del Rey, F.; Viseras, C.; López-Galindo, A. Effect of acid treatment on the structure of sepiolite. Clay Miner. 2003, 38, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Aznar, A.J.; Sanz, J.; Ruiz-Hitzky, E. Mechanism of the grafting of organosilanes on mineral surfaces. IV. Phenylderivatives of sepiolite and poly (organosiloxanes). Colloid Polym. Sci. 1992, 270, 165–176. [Google Scholar] [CrossRef]
- Gozález, L.; Ibarra, L.M.; Rodríguez, A.; Moya, J.S.; Valle, F.J. Fibrous silica gel obtained from sepiolite by HCl attack. Clay Miner. 1984, 19, 93–98. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Darder, M.; Aranda, P.; Del Burgo, M.Á.M.; Real, G. Del Bionanocomposites as new carriers for influenza vaccines. Adv. Mater. 2009, 21, 4167–4171. [Google Scholar] [CrossRef]
- Alcântara, A.C.S.; Darder, M.; Aranda, P.; Tateyama, S.; Okajima, M.K.; Kaneko, T.; Ogawa, M.; Ruiz-Hitzky, E. Clay-bionanocomposites with sacran megamolecules for the selective uptake of neodymium. J. Mater. Chem. A 2014, 2, 1391–1399. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-García, C.; Pérez-Carvajal, J.; Berenguer-Murcia, A.; Darder, M.; Aranda, P.; Cazorla-Amorós, D.; Ruiz-Hitzky, E. Clay-supported graphene materials: Application to hydrogen storage. Phys. Chem. Chem. Phys. 2013, 15, 18635–18641. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-García, C.; Darder, M.; Aranda, P.; Ruiz-Hitzky, E. Toward a green way for the chemical production of supported graphenes using porous solids. J. Mater. Chem. A 2017, 2009–2017. [Google Scholar] [CrossRef]
- Liu, C.; Cai, W.; Liu, L. Applied Clay Science Hydrothermal carbonization synthesis of Al-pillared montmorillonite@carbon composites as high performing toluene adsorbents. Appl. Clay Sci. 2018, 162, 113–120. [Google Scholar] [CrossRef]
- Gabriel, R.; Gonçalves, L.; De Carvalho, M.; Morais, R.; Andrade, E.; Frigi, G.; Macedo, S.; Augusto, M.; Regina, V.; Constantino, L.; et al. Mesoporous carbon derived from a biopolymer and a clay: Preparation, characterization and application for an organochlorine pesticide adsorption. Microporous Mesoporous Mater. 2016, 225, 342–354. [Google Scholar] [CrossRef]
- Benucci, I.; Liburdi, K.; Cacciotti, I.; Lombardelli, C.; Zappino, M.; Nanni, F.; Esti, M. Chitosan/clay nanocomposite films as supports for enzyme immobilization: An innovative green approach for winemaking applications. Food Hydrocoll. 2018, 74, 124–131. [Google Scholar] [CrossRef]
- Hantanasirisakul, K.; Gogotsi, Y. Electronic and Optical Properties of 2D Transition Metal Carbides and Nitrides (MXenes). Adv. Mater. 2018, 30, 1804779. [Google Scholar] [CrossRef]
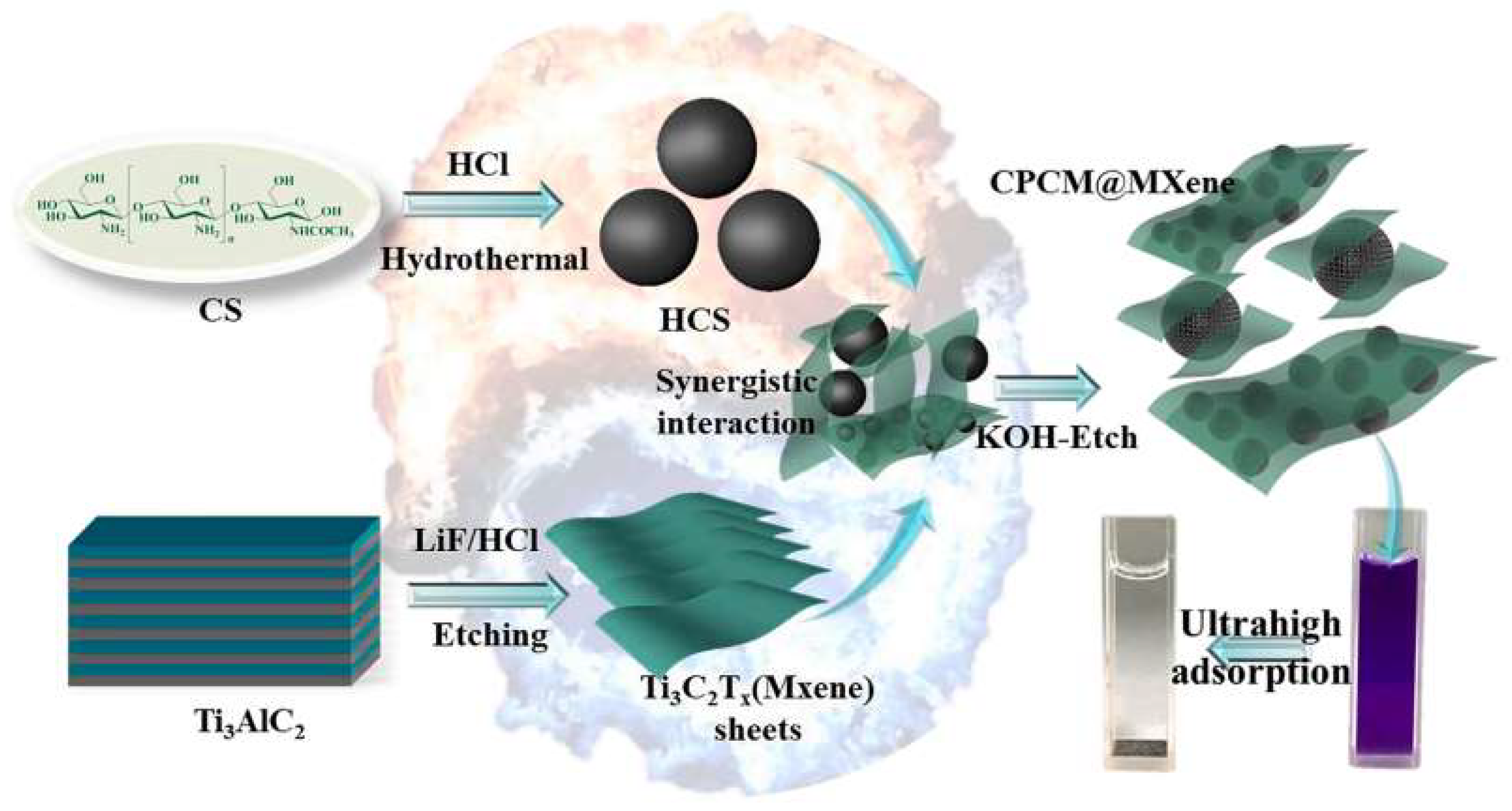
- Wu, Z.; Deng, W.; Tang, S.; Ruiz-Hitzky, E.; Luo, J.; Wang, X. Pod-inspired MXene/porous carbon microspheres with ultrahigh adsorption capacity towards crystal violet. Chem. Eng. J. 2021, 426, 130776. [Google Scholar] [CrossRef]
- Alves, Z.; Ferreira, N.M.; Mendo, S.; Ferreira, P.; Nunes, C. Design of Alginate-Based Bionanocomposites with Electrical Conductivity for Active Food Packaging. Int. J. Mol. Sci. 2021, 22, 9943. [Google Scholar] [CrossRef]
- Barra, A.; Santos, J.D.C.; Silva, M.R.F.; Nunes, C.; Ruiz-Hitzky, E.; Gonçalves, I.; Yildirim, S.; Ferreira, P.; Marques, P.A.A.P. Graphene Derivatives in Biopolymer-Based Composites for Food Packaging Applications. Nanomaterials 2020, 10, 2077. [Google Scholar] [CrossRef] [PubMed]
- Darder, M.; Aranda, P.; Ruiz-García, C.; Fernandes, F.M.; Ruiz-Hitzky, E. The Meeting Point of Carbonaceous Materials and Clays: Toward a New Generation of Functional Composites. Adv. Funct. Mater. 2017, 28, 1704323. [Google Scholar] [CrossRef]
| Application | Carbon Nanostructure | Polymer Composite | Results | Ref. |
|---|---|---|---|---|
| Corrosion protection | rGO (Urtica dioica leaf) | Polyurethane/rGO (0.15 wt%) coatings (tested on mild steel) | Resistance against accelerated weathering condition; improved UV shielding and corrosion protection efficiency. | [29] |
| Corrosion protection | rGO (Peganum harmala seed) | Epoxy resin/rGO-Zn (0.15 wt%) coatings (tested on steel) | Dual active and barrier corrosion protection. | [49] |
| Corrosion protection | rGO (Peganum harmala seed) | Epoxy ester resin/rGO-Zn (0.15 wt%) coating (tested on steel) | Improved tensile strength (78%), Young’s modulus (102%) and fracture energy (83%); improved thermal stability (62%). | [50] |
| Gas diffusion barrier | rGO (elemental sulfur) | Polyimide/rGO (0.5–5 wt%) films | Improved tensile strength and Young’s modulus; 95% reduction of oxygen permeability. | [47] |
| Sensing | rGO (Bougainvillea glabra flower) | Nafion/rGO solution drop-casted on a carbon working electrode | Sensor electrode used for Pb2+ detection; improved sensitivity and ultralow limit of detection. | [21] |
| Supercapacitors | rGO (eucalyptus bark) | Nafion/rGO solution drop-casted on a glassy carbon electrode | High specific capacitance (239 F g−1) and high energy density (71 W h kg−1) at a current density of 2 A g−1. | [35] |
| Environmental remediation | rGO (Pseudoalteromonas sp.) | Sodium alginate/rGO solution dripped into CaCl2 solution to obtain spheres | MB and CR dye adsorption from water. Reusable absorbent with adsorption efficiency of the MB and CR 77.91% and 68.27% after 4 adsorption–desorption cycles. | [39] |
| Food packaging | rGO (HTC/caffeic acid) | Chitosan/rGO (50%) film | Electrically conductive film to sterilize food by in-pack PEF; electrical conductivity of 0.7 S m−1 and 2.1 × 10−5 S m−1 in-plane and through-plane, respectively. | [9] |
| Food packaging | rGO (HTC/ZnO) | Alginate/sepiolite/ZnO-rGO (50%) | Antimicrobial and electrically conductive film for food packaging. E. coli and S. Inhibition of aureus growth; electrical conductivity of 0.1 S m−1 and 7.5 × 10−5 S m−1 in-plane and through-plane, respectively. | [94] |
| Not mentioned | rGO (BM/Zn) | Epoxy resin/rGO (0.1–0.3%) composites | Improvement of thermomechanical properties. | [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barra, A.; Nunes, C.; Ruiz-Hitzky, E.; Ferreira, P. Green Carbon Nanostructures for Functional Composite Materials. Int. J. Mol. Sci. 2022, 23, 1848. https://doi.org/10.3390/ijms23031848
Barra A, Nunes C, Ruiz-Hitzky E, Ferreira P. Green Carbon Nanostructures for Functional Composite Materials. International Journal of Molecular Sciences. 2022; 23(3):1848. https://doi.org/10.3390/ijms23031848
Chicago/Turabian StyleBarra, Ana, Cláudia Nunes, Eduardo Ruiz-Hitzky, and Paula Ferreira. 2022. "Green Carbon Nanostructures for Functional Composite Materials" International Journal of Molecular Sciences 23, no. 3: 1848. https://doi.org/10.3390/ijms23031848
APA StyleBarra, A., Nunes, C., Ruiz-Hitzky, E., & Ferreira, P. (2022). Green Carbon Nanostructures for Functional Composite Materials. International Journal of Molecular Sciences, 23(3), 1848. https://doi.org/10.3390/ijms23031848