Therapeutic Interventions to Mitigate Mitochondrial Dysfunction and Oxidative Stress–Induced Damage in Patients with Bipolar Disorder
Abstract
1. Introduction
2. BD
3. OS
4. Oxidative Damage in BD
5. Mitochondrial Dysfunction
6. DNA Damage
7. BDNF
8. Roles of DA and DAT in BD
9. Immune Dysfunction
10. Calcium Signaling Pathways
11. Nutrients, Vitamins, and Micronutrients in BD
11.1. Vitamin D
11.2. Folic Acid
11.3. Magnesium and Copper
11.4. PUFAs
12. Anti-Inflammatory Agents
Lithium Therapy
13. Antipsychotic Agents
13.1. Clozapine
13.2. Olanzapine
13.3. Risperidone
13.4. Cariprazine
13.5. Quetiapine
14. Role of Therapies
14.1. CBT
14.2. BLT
14.3. ImCT
14.4. ECT
15. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Husain, S.F.; Tang, T.-B.; Tam, W.W.; Tran, B.X.; Ho, C.S.; Ho, R.C. Cortical haemodynamic response during the verbal fluency task in patients with bipolar disorder and borderline personality disorder: A preliminary functional near—Infrared spectroscopy study. BMC Psychiatry 2021, 21, 201. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.K.; Leweke, F.M. Bipolar disorder: Clinical overview. Med. Mon. Pharm. 2016, 39, 363–369. [Google Scholar]
- McIntyre, R.; Berk, M.; Brietzke, E.; Goldstein, B.I.; López-Jaramillo, C.; Kessing, L.V.; Malhi, G.S.; Nierenberg, A.A.; Rosenblat, J.D.; Majeed, A.; et al. Bipolar disorders. Lancet 2020, 396, 1841–1856. [Google Scholar] [CrossRef]
- Moreira, F.P.; Cardoso, T.C.; Mondin, T.C.; Wiener, C.D.; de Mattos Souza, L.D.; Oses, J.P.; Jansen, K.; da Silva, R.A. Serum level of nerve growth factor is a potential biomarker of conversion to bipolar disorder in women with major depressive disorder. Psychiatry Clin. Neurosci. 2019, 73, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Milev, R. The role of electroconvulsive therapy in the treatment of bipolar disorder. In The Treatment of Bipolar Disorder: Integrative Clinical Strategies and Future Directions; Carvalho, A.F., Vieta, E., Eds.; Oxford University Press: Oxford, UK, 2017; pp. 408–422. [Google Scholar]
- Cotrena, C.; Branco, L.D.; Kochhann, R.; Shansis, F.M.; Fonseca, R.P. Quality of life, functioning and cognition in bipolar disorder and major depression: A latent profile analysis. Psychiatry Res. 2016, 241, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Smeland, O.B.; Bahrami, S.; Frei, O.; Shadrin, A.; O’Connell, K.; Savage, J.; Watanabe, K.; Krull, F.; Bettella, F.; Steen, N.E.; et al. Genome-wide analysis reveals extensive genetic overlap between schizophrenia, bipolar disorder, and intelligence. Mol. Psychiatry 2020, 25, 844–853. [Google Scholar] [CrossRef]
- Correll, C.U.; Solmi, M.; Veronese, N.; Bortolato, B.; Rosson, S.; Santonastaso, P.; Thapa-Chhetri, N.; Fornaro, M.; Gallicchio, D.; Collantoni, E.; et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: A large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry 2017, 16, 163–180. [Google Scholar] [CrossRef]
- Kim, H.K.; Andreazza, A.C.; Yeung, P.Y.; Isaacs-Trepanier, C.; Young, L.T. Oxidation and nitration in dopaminergic areas of the prefrontal cortex from patients with bipolar disorder and schizophrenia. J. Psychiatry Neurosci. 2014, 39, 276–285. [Google Scholar] [CrossRef]
- Islam, T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef]
- Kausar, S.; Wang, F.; Cui, H. The role of mitochondria in reactive oxygen species generation and its implications for neurodegenerative diseases. Cells 2018, 7, 274. [Google Scholar] [CrossRef]
- Naaldijk, Y.M.; Bittencourt, M.C.; Sack, U.; Ulrich, H. Kinins and microglial responses in bipolar disorder: A neuroinflammation hypothesis. Biol. Chem. 2016, 397, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Rege, S.; Hodgkinson, S.J. Immune dysregulation and autoimmunity in bipolar disorder: Synthesis of the evidence and its clinical application. Aust. N. Z. J. Psychiatry 2013, 47, 1136–1151. [Google Scholar] [CrossRef] [PubMed]
- Reus, G.Z.; Fries, G.R.; Stertz, L.; Badawy, M.; Passos, I.C.; Barichello, T.; Kapczinski, F.; Quevedo, J. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience 2015, 300, 141–154. [Google Scholar] [CrossRef]
- Rosenblat, J.D. Targeting the immune system in the treatment of bipolar disorder. Psychopharmacology 2019, 236, 2909–2921. [Google Scholar] [CrossRef]
- Rosenblat, J.D.; McIntyre, R.S. Bipolar disorder and inflammation. Psychiatr. Clin. N. Am. 2016, 39, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Rosenblat, J.D.; Ron Kakar, R.; Berk, M.; Kessing, L.V.; Vinberg, M.; Baune, B.T.; Mansur, R.B.; Brietzke, E.; Goldstein, B.I.; McIntyre, R.S. Anti-inflammatory agents in the treatment of bipolar depression: A systematic review and meta-analysis. Bipolar. Disord. 2016, 18, 89–101. [Google Scholar] [CrossRef]
- Young, A.H.; Juruena, M.F. The neurobiology of bipolar disorder. Curr. Top. Behav. Neurosci. 2021, 48, 1–20. [Google Scholar]
- Gao, W.; Cui, D.; Jiao, Q.; Su, L.; Yang, R.; Lu, G. Brain structural alterations in pediatric bipolar disorder patients with and without psychotic symptoms. J. Affect. Disord. 2021, 286, 87–93. [Google Scholar] [CrossRef]
- Knorr, U.; Simonsen, A.H.; Roos, P.; Weimann, A.; Henriksen, T.; Christensen, E.-M.; Vinberg, M.; Mikkelsen, R.L.; Kirkegaard, T.; Jensen, R.N.; et al. Cerebrospinal fluid oxidative stress metabolites in patients with bipolar disorder and healthy controls, a longitudinal case-control study. Transl. Psychiatry 2019, 9, 325. [Google Scholar] [CrossRef]
- Chandrasekaran, V.; Brennan-Olsen, S.L.; Stuart, A.L.; Pasco, J.A.; Berk, M.; Hodge, J.M.; Williams, L.J. Bipolar disorder and bone health: A systematic review. J. Affect. Disord. 2019, 249, 262–269. [Google Scholar] [CrossRef]
- Shen, S.-H.; Lee, S.-H. A case of lung cancer with brain metastasis following late-onset bipolar disorder. Behav. Neurol. 2021, 2021, 8880539. [Google Scholar] [CrossRef] [PubMed]
- Rantala, M.J.; Luoto, S.; Borráz-León, J.I.; Krams, I. Bipolar disorder: An evolutionary psychoneuroimmunological approach. Neurosci. Biobehav. Rev. 2021, 122, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Lucidi, L.; Pettorruso, M.; Vellante, F.; Di Carlo, F.; Ceci, F.; Santovito, M.C.; Di Muzio, I.; Fornaro, M.; Ventriglio, A.; Tomasetti, C.; et al. Gut microbiota and bipolar disorder: An overview on a novel biomarker for diagnosis and treatment. Int. J. Mol. Sci. 2021, 22, 3723. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Walder, K.R.; Berk, M.; Marx, W.; Walker, A.J.; Maes, M.; Puri, B.K. The interplay between oxidative stress and bioenergetic failure in neuropsychiatric illnesses: Can we explain it and can we treat it? Mol. Biol. Rep. 2020, 47, 5587–5620. [Google Scholar] [CrossRef]
- Vieta, E.; Berk, M.; Schulze, T.G.; Carvalho, A.F.; Suppes, T.; Calabrese, J.R.; Gao, K.; Miskowiak, K.W.; Grande, I. Bipolar disorders. Nat. Rev. Dis. Prim. 2018, 4, 18008. [Google Scholar] [CrossRef] [PubMed]
- Gordovez, F.J.A.; McMahon, F.J. The genetics of bipolar disorder. Mol. Psychiatry 2020, 25, 544–559. [Google Scholar] [CrossRef]
- Bonnín, C.D.M.; Reinares, M.; Martínez-Arán, A.; Jiménez, E.; Sánchez-Moreno, J.; Solé, B.; Montejo, L.; Vieta, E. Improving functioning, quality of life, and well-being in patients with bipolar disorder. Int. J. Neuropsychopharmacol. 2019, 22, 467–477. [Google Scholar] [CrossRef]
- Anderson, G.; Maes, M. Bipolar disorder: Role of immune-inflammatory cytokines, oxidative and nitrosative stress and tryptophan catabolites. Curr. Psychiatry Rep. 2015, 17, 8. [Google Scholar] [CrossRef]
- O’Rourke, N.; Sixsmith, A.; Kirshner, G.; Osher, Y. Perceived cognitive failures and quality of life for older adults with bipolar disorder. J. Affect. Disord. 2021, 287, 433–440. [Google Scholar] [CrossRef]
- Kessing, L.V.; Andersen, P.K. Evidence for clinical progression of unipolar and bipolar disorders. Acta Psychiatr. Scand. 2017, 135, 51–64. [Google Scholar] [CrossRef]
- Cardoso, T.; Bauer, I.E.; Meyer, T.D.; Kapczinski, F.; Soares, J.C. Neuroprogression and cognitive functioning in bipolar disorder: A systematic review. Curr. Psychiatry Rep. 2015, 17, 75. [Google Scholar] [CrossRef]
- Cyrino, L.A.R.; Delwing-de Lima, D.; Ullmann, O.M.; Maia, T.P. Concepts of neuroinflammation and their relationship with impaired mitochondrial functions in bipolar disorder. Front. Behav. Neurosci. 2021, 15. [Google Scholar] [CrossRef]
- Liu, X.; Ma, X.; Wang, W.; Zhang, J.; Sun, X.; Luo, X.; Zhang, Y. The functional impairment of different subtypes and occupational states in euthymic patients with bipolar disorder. BMC Psychiatry 2021, 21, 240. [Google Scholar] [CrossRef]
- Grande, I.; Berk, M.; Birmaher, B.; Vieta, E. Bipolar disorder. Lancet 2016, 387, 1561–1572. [Google Scholar] [CrossRef]
- Kurtz, M.; Mohring, P.; Förster, K.; Bauer, M.; Kanske, P. Deficits in explicit emotion regulation in bipolar disorder: A systematic review. Int. J. Bipolar Disord. 2021, 9, 1–23. [Google Scholar]
- Clemente, A.S.; Diniz, B.S.; Nicolato, R.; Kapczinski, F.P.; Soares, J.C.; Firmo, J.O.; Castro-Costa, E. Bipolar disorder prevalence: A systematic review and meta-analysis of the literature. Braz. J. Psychiatry 2015, 37, 155–161. [Google Scholar] [CrossRef]
- Phillips, C. Physical activity modulates common neuroplasticity substrates in major depressive and bipolar disorder. Neural. Plast. 2017, 2017, 7014146. [Google Scholar] [CrossRef]
- Gandhi, A.B.; Kaleem, I.; Alexander, J.; Hisbulla, M.; Kannichamy, V.; Antony, I.; Mishra, V.; Banerjee, A.; Khan, S. Neuroplasticity improves bipolar disorder: A review. Cureus 2020, 12, e11241. [Google Scholar] [CrossRef] [PubMed]
- Dell’Osso, B.; Dobrea, C.; Cremaschi, L.; Buoli, M.; Miller, S.; Ketter, T.A.; Altamura, A.C. Italian bipolar II vs I patients have better individual functioning, in spite of overall similar illness severity. CNS Spectr. 2017, 22, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Coryell, W.; Kriener, A.; Butcher, B.; Nurnberger, J.; McMahon, F.; Berrettini, W.; Fiedorowicz, J. Risk factors for suicide in bipolar I disorder in two prospectively studied cohorts. J. Affect. Disord. 2016, 190, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tondo, L.; Vázquez, G.H.; Baldessarini, R.J. Prevention of suicidal behavior in bipolar disorder. Bipolar Disord. 2021, 23, 14–23. [Google Scholar] [CrossRef]
- Wah, A.; Hodge, S.; Jones, S.H.; Perez Algorta, G. A qualitative exploration of how people with bipolar disorder consider risk-taking in everyday decisions. Behav. Cogn. Psychother. 2021, 49, 314–327. [Google Scholar] [CrossRef]
- Diniz, B.S.; Teixeira, A.L.; Cao, F.; Gildengers, A.; Soares, J.C.; Butters, M.A.; Reynolds, C.F. History of bipolar disorder and the risk of dementia: A systematic review and meta-analysis. Am. J. Geriatr. Psychiatry 2017, 25, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Merikangas, K.R.; Akiskal, H.S.; Angst, J.; Greenberg, P.E.; Hirschfeld, R.M.; Petukhova, M.; Kessler, R.C. Lifetime and 12–month prevalence of bipolar spectrum disorder in the national comorbidity survey replication. Arch. Gen. Psychiatry 2007, 64, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Fiedorowicz, J.G.; Palagummi, N.M.; Forman-Hoffman, V.L.; Miller, D.D.; Haynes, W.G. Elevated prevalence of obesity, metabolic syndrome, and cardiovascular risk factors in bipolar disorder. Ann. Clin. Psychiatry 2008, 20, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Schoepf, D.; Heun, R. Bipolar disorder and comorbidity: Increased prevalence and increased relevance of comorbidity for hospital-based mortality during a 12.5–year observation period in general hospital admissions. J. Affect. Disord. 2014, 169, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Hui Poon, S.; Sim, K.; Baldessarini, R.J. Pharmacological approaches for treatment-resistant bipolar disorder. Curr. Neuropharmacol. 2015, 13, 592–604. [Google Scholar] [CrossRef]
- Gold, A.K.; Otto, M.W.; Deckersbach, T.; Sylvia, L.G.; Nierenberg, A.A.; Kinrys, G. Substance use comorbidity in bipolar disorder: A qualitative review of treatment strategies and outcomes. Am. J. Addict. 2018, 27, 188–201. [Google Scholar] [CrossRef]
- Hansson, C.; Joas, E.; Pålsson, E.; Hawton, K.; Runeson, B.; Landén, M. Risk factors for suicide in bipolar disorder: A cohort study of 12 850 patients. Acta Psychiatry Scand. 2018, 138, 456–463. [Google Scholar] [CrossRef]
- Sagar, R.; Pattanayak, R.D. Potential biomarkers for bipolar disorder, Where do we stand? Indian J. Med. Res. 2017, 145, 7–16. [Google Scholar] [CrossRef]
- Munkholm, K.; Vinberg, M.; Kessing, L.V. Peripheral blood brain-derived neurotrophic factor in bipolar disorder: A comprehensive systematic review and meta-analysis. Mol. Psychiatry 2016, 21, 216–228. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Z.; Yao, L.; Ding, N.; Jiang, L.; Wu, Y. Bright light therapy in the treatment of patients with bipolar disorder: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0232798. [Google Scholar] [CrossRef] [PubMed]
- Sigitova, E.; Fišar, Z.; Hroudová, J.; Cikánková, T.; Raboch, J. Biological hypotheses and biomarkers of bipolar disorder. Psychiatry Clin. Neurosci. 2017, 71, 77–103. [Google Scholar] [CrossRef] [PubMed]
- Szepesi, Z.; Manouchehrian, O.; Bachiller, S.; Deierborg, T. Bidirectional microglia-neuron communication in health and disease. Front. Cell Neurosci. 2018, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Leff-Gelman, P.; Mancilla-Herrera, I.; Flores-Ramos, M.; Cruz-Fuentes, C.; Reyes-Grajeda, J.P.; García-Cuétara, M.D.P.; Bugnot-Pérez, M.D.; Pulido-Ascencio, D.E. The immune system and the role of inflammation in perinatal depression. Neurosci. Bull. 2016, 32, 398–420. [Google Scholar] [CrossRef] [PubMed]
- Juruena, M.F.; Jelen, L.A.; Young, A.H.; Cleare, A.J. New pharmacological interventions in bipolar disorder. Curr. Top. Behav. Neurosci. 2021, 48, 303–324. [Google Scholar] [PubMed]
- Fries, G.R.; Walss-Bass, C.; Bauer, M.E.; Teixeira, A.L. Revisiting inflammation in bipolar disorder. Pharmacol. Biochem. Behav. 2019, 177, 12–19. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression, From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef]
- Setiawan, E.; Wilson, A.A.; Mizrahi, R.; Rusjan, P.M.; Miler, L.; Rajkowska, G.; Suridjan, I.; Kennedy, J.L.; Rekkas, P.V.; Houle, S.; et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 2015, 72, 268–275. [Google Scholar] [CrossRef]
- Sayana, P.; Pinjari, O.F.; Giridharan, V.V.; Ahmad, N.; da Rosa, M.I.; de Quevedo, J.; Barichello, T. Postmortem evidence of neuroinflammation in bipolar disorder: A systematic review. J. Affect. Disord. 2019, 254, 129. [Google Scholar] [CrossRef]
- Isgren, A.; Sellgren, C.; Ekman, C.J.; Holmen-Larsson, J.; Blennow, K.; Zetterberg, H.; Jakobsson, J.; Landén, M. Markers of neuroinflammation and neuronal injury in bipolar disorder: Relation to prospective clinical outcomes. Brain Behav. Immun. 2017, 65, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Data-Franco, J.; Singh, A.; Popovic, D.; Ashton, M.; Berk, M.; Vieta, E.; Figueira, M.L.; Dean, O.M. Beyond the therapeutic shackles of the monoamines: New mechanisms in bipolar disorder biology. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 72, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Ferro, A.; Bonivento, C.; Delvecchio, G.; Bellani, M.; Perlini, C.; Dusi, N.; Marinelli, V.; Ruggeri, M.; Altamura, A.C.; Crespo-Facorro, B.; et al. Longitudinal investigation of the parietal lobe anatomy in bipolar disorder and its association with general functioning. Psychiatry Res. Neuroimaging 2017, 267, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Kapczinski, N.S.; Mwangi, B.; Cassidy, R.M.; Librenza-Garcia, D.; Bermudez, M.B.; Kauer-Sant’anna, M.; Kapczinski, F.; Passos, I.C. Neuroprogression and illness trajectories in bipolar disorder. Expert Rev. Neurother. 2017, 17, 277–285. [Google Scholar] [CrossRef] [PubMed]
- van den Ameele, S.; Fuchs, D.; Coppens, V.; de Boer, P.; Timmers, M.; Sabbe, B.; Morrens, M. Markers of inflammation and monoamine metabolism indicate accelerated aging in bipolar disorder. Front. Psychiatry 2018, 9, 250. [Google Scholar] [CrossRef] [PubMed]
- Cullen, B.; Ward, J.; Graham, N.A.; Deary, I.J.; Pell, J.P.; Smith, D.J.; Evans, J.J. Prevalence and correlates of cognitive impairment in euthymic adults with bipolar disorder: A systematic review. J. Affect. Disord. 2016, 205, 165–181. [Google Scholar] [CrossRef]
- Cao, B.; Passos, I.C.; Mwangi, B.; Bauer, I.E.; Zunta-Soares, G.B.; Kapczinski, F.; Soares, J.C. Hippocampal volume and verbal memory performance in late-stage bipolar disorder. J. Psychiatry Res. 2016, 73, 102–107. [Google Scholar] [CrossRef]
- Strakowski, S.M.; DelBello, M.P.; Zimmerman, M.E.; Getz, G.E.; Mills, N.P.; Ret, J.; Shear, P.; Adler, C.M. Ventricular and periventricular structural volumes in first-versus multiple-episode bipolar disorder. Am. J. Psychiatry 2002, 159, 1841–1847. [Google Scholar] [CrossRef]
- Hibar, D.P.; Westlye, L.T.; Doan, N.T.; Jahanshad, N.; Cheung, J.W.; Ching, C.R.K.; Versace, A.; Bilderbeck, A.C.; Uhlmann, A.; Mwangi, B.; et al. Cortical abnormalities in bipolar disorder: An MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Mol. Psychiatry 2018, 23, 932–942. [Google Scholar] [CrossRef]
- Madireddy, S.; Madireddy, S. Protection from the pathogenesis of neurodegenerative disorders, including alzheimer’s disease, amyotrophic lateral sclerosis, Huntington’s disease, and Parkinson’s diseases, through the mitigation of reactive oxygen species. J. Neurosci. Neurol. Disord. 2019, 3, 148–161. [Google Scholar] [CrossRef]
- Tunçel, O.K.; Sarısoy, G.; Bilgici, B.; Pazvantoglu, O.; Çetin, E.; Ünverdi, E.; Avcı, B.; Böke, O. Oxidative stress in bipolar and schizophrenia patients. Psychiatry Res. 2015, 228, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Yumru, M.; Savas, H.A.; Kalenderoglu, A.; Bulut, M.; Celik, H.; Erel, O. Oxidative imbalance in bipolar disorder subtypes: A comparative study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar]
- Roda, A.; Chendo, I.; Kunz, M. Biomarkers and staging of bipolar disorder: A systematic review. Trends Psychiatry Psychother. 2015, 37, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Salim, S. Oxidative stress and the central nervous system. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Bitanihirwe, B.K.; Woo, T.U. Oxidative stress in schizophrenia: An integrated approach. Neurosci. Biobehav. Rev. 2011, 35, 878–893. [Google Scholar] [CrossRef]
- Morris, G.; Puri, B.K.; Walker, A.J.; Berk, M.; Walder, K.; Bortolasci, C.C.; Marx, W.; Carvalho, A.F.; Maes, M. The compensatory antioxidant response system with a focus on neuroprogressive disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 95, 109708. [Google Scholar] [CrossRef]
- Steckert, A.V.; Valvassori, S.S.; Moretti, M.; Dal-Pizzol, F.; Quevedo, J. Role of oxidative stress in the pathophysiology of bipolar disorder. Neurochem. Res. 2010, 35, 1295–1301. [Google Scholar] [CrossRef]
- Younus, H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018, 12, 88–93. [Google Scholar]
- Collin, F. Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Wei, Y.H. Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. Int. J. Biochem. Cell Biol. 2005, 37, 822–834. [Google Scholar] [CrossRef]
- Russell, J.W.; Golovoy, D.; Vincent, A.M.; Mahendru, P.; Olzmann, J.A.; Mentzer, A.; Feldman, E.L. High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. FASEB J. 2002, 16, 1738–1748. [Google Scholar] [CrossRef]
- Wallace, D.C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: A dawn for evolutionary medicine. Annu. Rev. Genet. 2005, 39, 359–407. [Google Scholar] [CrossRef] [PubMed]
- Clay, H.; Sillivan, S.; Konradi, C. Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. Int. J. Dev. Neurosci. 2011, 29, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Madireddy, S.; Madireddy, S. Regulation of reactive oxygen species-mediated damage in the pathogenesis of schizophrenia. Brain Sci. 2020, 10, 742. [Google Scholar] [CrossRef]
- Andreazza, A.C.; Kauer-Sant’Anna, M.; Frey, B.N.; Stertz, L.; Zanotto, C.; Ribeiro, L.; Giasson, K.; Valvassori, S.S.; Réus, G.Z.; Salvador, M.; et al. Effects of mood stabilizers on DNA damage in an animal model of mania. J. Psychiatry Neurosci. 2008, 33, 516–524. [Google Scholar]
- Ben-Shachar, D. Mitochondrial dysfunction in schizophrenia: A possible linkage to dopamine. J. Neurochem. 2002, 83, 1241–1251. [Google Scholar] [CrossRef]
- Kim, H.K.; Andreazza, A.C. The relationship between oxidative stress and post-translational modification of the dopamine transporter in bipolar disorder. Expert Rev. Neurother. 2012, 12, 849–859. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef]
- Frey, B.N.; Andreazza, A.C.; Kunz, M.; Gomes, F.A.; Quevedo, J.; Salvador, M.; Gonçalves, C.A.; Kapczinski, F. Increased oxidative stress and DNA damage in bipolar disorder: A twin-case report. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 283–285. [Google Scholar] [CrossRef]
- Bengesser, S.A.; Lackner, N.; Birner, A.; Fellendorf, F.T.; Platzer, M.; Mitteregger, A.; Unterweger, R.; Reininghaus, B.; Mangge, H.; Wallner-Liebmann, S.J.; et al. Peripheral markers of oxidative stress and antioxidative defense in euthymia of bipolar disorder––Gender and obesity effects. J. Affect. Disord. 2015, 172, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Machado-Vieira, R.; Andreazza, A.C.; Viale, C.I.; Zanatto, V.; Cereser, V.; da Silva Vargas, R.; Kapczinski, F.; Portela, L.V.; Souza, D.O.; Salvador, M.; et al. Oxidative stress parameters in unmedicated and treated bipolar subjects during initial manic episode: A possible role for lithium antioxidant effects. Neurosci. Lett. 2007, 421, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Andreazza, A.C.; Wang, J.F.; Salmasi, F.; Shao, L.; Young, L.T. Specific subcellular changes in oxidative stress in prefrontal cortex from patients with bipolar disorder. J. Neurochem. 2013, 127, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Gawryluk, J.W.; Wang, J.F.; Andreazza, A.C.; Shao, L.; Young, L.T. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int. J. Neuropsychopharmacol. 2011, 14, 123–130. [Google Scholar] [CrossRef]
- Konradi, C.; Sillivan, S.E.; Clay, H.B. Mitochondria, oligodendrocytes and inflammation in bipolar disorder: Evidence from transcriptome studies points to intriguing parallels with multiple sclerosis. Neurobiol. Dis. 2012, 45, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Frey, B.N.; Andreazza, A.C.; Houenou, J.; Jamain, S.; Goldstein, B.I.; Frye, M.A.; Leboyer, M.; Berk, M.; Malhi, G.S.; Lopez-Jaramillo, C.; et al. Biomarkers in bipolar disorder: A positional paper from the International Society for Bipolar Disorders Biomarkers Task Force. Aust. N. Z. J. Psychiatry 2013, 47, 321–332. [Google Scholar] [CrossRef]
- Kapczinski, F.; Dal-Pizzol, F.; Teixeira, A.L.; Magalhaes, P.V.; Kauer-Sant’Anna, M.; Klamt, F.; Pasquali, M.A.; Quevedo, J.; Gama, C.S.; Post, R. A systemic toxicity index developed to assess peripheral changes in mood episodes. Mol. Psychiatry 2010, 15, 784–786. [Google Scholar] [CrossRef]
- Versace, A.; Andreazza, A.C.; Young, L.T.; Fournier, J.C.; Almeida, J.R.; Stiffler, R.S.; Lockovich, J.C.; Aslam, H.A.; Pollock, M.H.; Park, H.; et al. Elevated serum measures of lipid peroxidation and abnormal prefrontal white matter in euthymic bipolar adults: Toward peripheral biomarkers of bipolar disorder. Mol. Psychiatry 2014, 19, 200–208. [Google Scholar] [CrossRef]
- Andreazza, A.C.; Gildengers, A.; Rajji, T.K.; Zuzarte, P.M.L.; Mulsant, B.H.; Young, L.T. Oxidative stress in older patients with bipolar disorder. Am. J. Geriatr. Psychiatry 2015, 23, 314–319. [Google Scholar] [CrossRef]
- Mahadik, S.P.; Evans, D.; Lal, H. Oxidative stress and role of antioxidant and N–3 essential fatty acid supplementation in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2001, 25, 463–493. [Google Scholar] [CrossRef]
- Manji, H.; Kato, T.; DiProspero, N.A.; Ness, S.; Beal, M.F.; Krams, M.; Chen, G. Impaired mitochondrial function in psychiatric disorders. Nat. Rev. Neurosci. 2012, 13, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Grintzalis, K.; Zisimopoulos, D.; Grune, T.; Weber, D.; Georgiou, C.D. Method for the simultaneous determination of free/protein malondialdehyde and lipid/protein hydroperoxides. Free Radic. Biol. Med. 2013, 59, 27–35. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, R.T.; Zarate, C.A.; Zanetti, M.V.; Costa, A.C.; Talib, L.L.; Gattaz, W.F.; Machado-Vieira, R. Oxidative stress in early-stage bipolar disorder and the association with response to lithium. J. Psychiatry Res. 2014, 50, 36–41. [Google Scholar] [CrossRef]
- Yirun, M.C.; Ünal, K.; Şen, N.A.; Yirun, O.; Aydemir, C.; Goka, E. Evaluation of oxidative stress in bipolar disorder in terms of total oxidant status, total antioxidant status, and oxidative stress index. Noro Psikiyatr. Ars. 2016, 53, 194–198. [Google Scholar] [CrossRef]
- Siwek, M.; Sowa-Kućma, M.; Dudek, D.; Styczeń, K.; Szewczyk, B.; Kotarska, K.; Misztakk, P.; Pilc, A.; Wolak, M.; Nowak, G. Oxidative stress markers in affective disorders. Pharmacol. Rep. 2013, 65, 1558–1571. [Google Scholar] [CrossRef]
- Mohamad Kamal, N.A.; Loo, J.L.; Goon, J.A.; Damanhuri, H.A.; Sharip, S.; Saini, S.M.; Hamzah, J.; Chan, L. Oxidative stress biomarkers in bipolar disorder with suicidal behavior, A systematic review. J. Pharm. Negat. Results 2019, 10, 6–15. [Google Scholar]
- Brown, N.C.; Andreazza, A.C.; Young, L.T. An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res. 2014, 218, 61–68. [Google Scholar] [CrossRef]
- Joshi, Y.B.; Praticò, D. Lipid peroxidation in psychiatric illness: Overview of clinical evidence. Oxid. Med. Cell Longev. 2014, 2014, 828702. [Google Scholar] [CrossRef]
- Jiménez-Fernández, S.; Gurpegui, M.; Garrote-Rojas, D.A.; Gutiérrez-Rojas, L.; Carretero, M.D.; Correll, C.U. Oxidative stress parameters and antioxidants in patients with bipolar disorder: Results from a meta-analysis comparing patients, including stratification by polarity and euthymic status, with healthy controls. Bipolar Disord. 2020, 23, 11. [Google Scholar] [CrossRef] [PubMed]
- Siwek, M.; Sowa-Kucma, M.; Styczen, K.; Misztak, P.; Szewczyk, B.; Topor-Madry, R.; Nowak, G.; Dudek, D.; Rybakowski, J.K. Thiobarbituric Acid-Reactive Substances: Markers of an Acute Episode and a Late Stage of Bipolar Disorder. Neuropsychobiology 2016, 73, 116–122. [Google Scholar] [CrossRef]
- Andreazza, A.C.; Cassini, C.; Rosa, A.R.; Leite, M.C.; de Almeida, L.M.; Nardin, P.; Cunha, A.B.N.; Ceresér, K.M.; Santin, A.; Gottfried, C.; et al. Serum S100B and antioxidant enzymes in bipolar patients. J. Psychiatr. Res. 2007, 41, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Sowa-Kućma, M.; Styczeń, K.; Siwek, M.; Misztak, P.; Nowak, R.J.; Dudek, D.; Rybakowski, J.K.; Nowak, G.; Maes, M. Are there differences in lipid peroxidation and immune biomarkers between major depression and bipolar disorder: Effects of melancholia, atypical depression, severity of illness, episode number, suicidal ideation and prior suicide attempts. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 81, 372–383. [Google Scholar] [CrossRef]
- Akarsu, S.; Bolu, A.; Aydemir, E.; Zincir, S.B. The relationship between the number of manic episodes and oxidative stress indicators in bipolar disorder. Korean Neuropsychiatr. Assoc. 2018, 15, 514–519. [Google Scholar] [CrossRef]
- Andreazza, A.C.; Kapczinski, F.; Kauer-Sant’Anna, M.; Walz, J.C.; Bond, D.J.; Gonçalves, C.A.; Young, L.T.; Yatham, L.N. 3-Nitrotyrosine and glutathione antioxidant system in patients in the early and late stages of bipolar disorder. J. Psychiatry Neurosci. 2009, 34, 263–271. [Google Scholar]
- Kunz, M.; Gama, C.S.; Andreazza, A.C.; Salvador, M.; Ceresér, K.M.; Gomes, F.A.; Belmonte-de-Abreu, P.S.; Berk, M.; Kapczinski, F. Elevated serum superoxide dismutase and thiobarbituric acid reactive substances in different phases of bipolar disorder and in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1677–1681. [Google Scholar] [CrossRef]
- Andreazza, A.C.; Frey, B.N.; Erdtmann, B.; Salvador, M.; Goncalves, C.A.; Kapczinski, F. DNA damage in bipolar disorder. Psychiatry Res. 2007, 153, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Gergerlioglu, H.S.; Savas, H.A.; Bulbul, F.; Selek, S.; Uz, E.; Yumru, M. Changes in nitric oxide level and superoxide dismutase activity during antimanic treatment. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 697–702. [Google Scholar] [CrossRef]
- Kato, T. Current understanding of bipolar disorder: Toward integration of biological basis and treatment strategies. Psychiatry Clin. Neurosci. 2019, 73, 526–540. [Google Scholar] [CrossRef]
- Mansur, R.B.; Lee, Y.; McIntyre, R.S.; Brietzke, E. What is bipolar disorder? A disease model of dysregulated energy expenditure. Neurosci. Biobehav. Rev. 2020, 113, 529–545. [Google Scholar] [CrossRef]
- Singh, N.; McMahon, H.; Bilderbeck, A.; Reed, Z.E.; Tunbridge, E.; Brett, D.; Geddes, J.R.; Churchill, G.C.; Goodwin, G.M. Plasma glutathione suggests oxidative stress is equally present in early- and late-onset bipolar disorder. Bipolar Disord. 2019, 21, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, F.; Crocamo, C.; Mazza, M.G.; Clerici, M.; Carrà, G. Uric acid levels in subjects with bipolar disorder: A comparative meta-analysis. J. Psychiatr. Res. 2016, 81, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, S.; Kabekkodu, S.P.; Singh, R.P.; Thangaraj, K.; Singh, K.K.; Satyamoorthy, K. Mitochondria in health and disease. Mitochondrion 2018, 43, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Scaini, G.; Andrews, T.; Lima, C.N.C.; Benevenuto, D.; Streck, E.L.; Quevedo, J. Mitochondrial dysfunction as a critical event in the pathophysiology of bipolar disorder. Mitochondrion 2020, 57, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Kumar, S.; Vijayan, M.; Bhatti, G.K.; Reddy, P.H. Therapeutic strategies for mitochondrial dysfunction and oxidative stress in age-related metabolic disorders. Prog. Mol. Biol. Transl. Sci. 2017, 146, 13–46. [Google Scholar]
- Morris, G.; Walder, K.; McGee, S.L.; Dean, O.M.; Tye, S.J.; Maes, M.; Berk, M. A model of the mitochondrial basis of bipolar disorder. Neurosci. Biobehav. Rev. 2017, 74, 1–20. [Google Scholar] [CrossRef]
- Kim, Y.; Santos, R.; Gage, F.H.; Marchetto, M.C. Molecular mechanisms of bipolar disorder: Progress made and future challenges. Front. Cell Neurosci. 2017, 11, 30. [Google Scholar] [CrossRef]
- Grimm, A.; Eckert, A. Brain aging and neurodegeneration: From a mitochondrial point of view. J. Neurochem. 2017, 143, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Todorova, V.; Blokland, A. Mitochondria and synaptic plasticity in the mature and aging nervous system. Curr. Neuropharmacol. 2017, 15, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Niizuma, K.; Yoshioka, H.; Chen, H.; Kim, G.S.; Jung, J.E.; Katsu, M.; Okami, N.; Chan, P.H. Mitochondrial and apoptotic neuronal death signaling pathways in cerebral ischemia. Biochim. Biophys. Acta 2010, 1802, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Okamoto, K.; Hayashi, Y.; Sheng, M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell 2004, 119, 873–887. [Google Scholar] [CrossRef] [PubMed]
- Angelova, P.R.; Abramov, A.Y. Role of mitochondrial ROS in the brain: From physiology to neurodegeneration. FEBS Lett. 2018, 592, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Kuperberg, M.; Greenebaum, S.L.A.; Nierenberg, A.A. Targeting mitochondrial dysfunction for bipolar disorder. Curr. Top. Behav. Neurosci. 2021, 48, 61–99. [Google Scholar]
- Wang, J.-F.; Shao, L.; Sun, X.; Young, L.T. Increased oxidative stress in the anterior cingulate cortex of subjects with bipolar disorder and schizophrenia. Bipolar Disord. 2009, 11, 523–529. [Google Scholar] [CrossRef]
- Berk, M.; Turner, A.; Malhi, G.S.; Ng, C.H.; Cotton, S.M.; Dodd, S.; Samuni, Y.; Tanious, M.; McAulay, C.; Dowling, N.; et al. A randomised controlled trial of a mitochondrial therapeutic target for bipolar depression, mitochondrial agents: N-acetylcysteine, and placebo. BMC Med. 2019, 17, 18. [Google Scholar]
- Cikankova, T.; Sigitova, E.; Zverova, M.; Fisar, Z.; Raboch, J.; Hroudova, J. Mitochondrial dysfunctions in bipolar disorder: Effect of the disease and pharmacotherapy. CNS Neurol. Disord. Drug Targets 2017, 16, 176–186. [Google Scholar] [CrossRef]
- Scaini, G. Mitochondrial Hypothesis and Its Relationship to Bipolar Disorder. 2019. Available online: https://med.uth.edu/psychiatry/2019/09/11/mitochondrial-hypothesis-and-its-relationship-to-bipolar-disorder/ (accessed on 12 January 2021).
- Bhatti, J.S.; Bhatti, G.K.; Reddy, H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Pereira, C.; Chavarria, V.; Vian, J.; Ashton, M.M.; Berk, M.; Marx, W.; Dean, O.M. Mitochondrial agents for bipolar disorder. Int. J. Neuropsychopharmacol. 2018, 21, 550–569. [Google Scholar] [CrossRef] [PubMed]
- Scaini, G.; Rezin, G.T.; Carvalho, A.F.; Streck, E.L.; Berk, M.; Quevedo, J. Mitochondrial dysfunction in bipolar disorder: Evidence, pathophysiology and translational implications. Neurosci. Biobehav. Rev. 2016, 68, 694–713. [Google Scholar] [CrossRef] [PubMed]
- Soeiro-de-Souza, M.G.; Andreazza, A.C.; Carvalho, A.F.; Machado-Vieira, R.; Young, L.T.; Moreno, R.A. Number of manic episodes is associated with elevated DNA oxidation in bipolar I disorder. Int. J. Neuropsychopharmacol. 2013, 16, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, Z.; Liu, W.; Zhou, J.; Ma, X.; Tang, J.; Chen, X. Differential mitochondrial DNA copy number in three mood states of bipolar disorder. BMC Psychiatry 2018, 18, 149. [Google Scholar] [CrossRef]
- Munakata, K.; Fujii, K.; Nanko, S.; Kunugi, H.; Kato, T. Sequence and functional analyses of mtDNA in a maternally inherited family with bipolar disorder and depression. Mutat Res. 2007, 617, 119–124. [Google Scholar] [CrossRef]
- Chang, C.C.; Jou, S.H.; Lin, T.T.; Liu, C.S. Mitochondrial DNA variation and increased oxidative damage in euthymic patients with bipolar disorder. Psychiatry Clin. Neurosci. 2014, 68, 551–557. [Google Scholar] [CrossRef]
- Kasahara, T.; Kato, T. What can mitochondrial DNA analysis tell us about mood disorders? Biol. Psychiatry 2018, 83, 731–738. [Google Scholar] [CrossRef]
- Schulmann, A.; Ryu, E.; Goncalves, V.; Rollins, B.; Christiansen, M.; Frye, M.A.; Biernacka, V.M.P. Novel complex interactions between mitochondrial and nuclear DNA in schizophrenia and bipolar disorder. Mol. Neuropsychiatry 2019, 5, 13–27. [Google Scholar] [CrossRef]
- Cuperfain, A.B.; Zhang, Z.L.; Kennedy, J.L.; Gonçalves, V.F. The complex interaction of mitochondrial genetics and mitochondrial pathways in psychiatric disease. Mol. Neuropsychiatry 2018, 4, 52–69. [Google Scholar] [CrossRef]
- Bhatt, A.H.; Dar, K.B.; Anees, S.; Zargar, M.A.; Masood, A.; Sofi, M.A.; Ganie, S.A. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases, A mechanistic insight. Biomed. Pharmacother. 2015, 74, 101–110. [Google Scholar] [CrossRef]
- Kato, T. The role of mitochondrial dysfunction in bipolar disorder. Drug News Perspect. 2006, 19, 597–602. [Google Scholar] [CrossRef]
- Callaly, E.; Walder, K.; Morris, G.; Maes, M.; Debnath, M.; Berk, M. Mitochondrial dysfunction in the pathophysiology of bipolar disorder: Effects of pharmacotherapy. Mini Rev. Med. Chem. 2015, 15, 355–365. [Google Scholar] [CrossRef]
- Teixeira, A.L.; Barbosa, I.G.; Machado-Vieira, R.; Rizzo, L.B.; Wieck, A.; Bauer, M.E. Novel biomarkers for bipolar disorder. Expert Opin. Med. Diagn. 2013, 7, 147–159. [Google Scholar] [CrossRef]
- Watt, N.T.; Routledge, M.N.; Wild, C.P.; Hooper, N.M. Cellular prion protein protects against reactive-oxygen-species-induced DNA damage. Free Radic. Biol. Med. 2007, 43, 959–967. [Google Scholar] [CrossRef]
- Chan, H.W.; Huang, C.Y.; Feng, W.J.; Yen, Y.C. Clinical outcomes of long-acting injectable risperidone in patients with bipolar I disorder: A 1-year retrospective cohort study. J. Affect. Disord. 2016, 205, 360–364. [Google Scholar] [CrossRef]
- Wang, D.; Li, H.; Du, X.; Zhou, J.; Yuan, L.; Ren, H.; Yang, X.; Zhang, G.; Chen, X. Circulating brain-derived neurotrophic factor, antioxidant enzymes activities, and mitochondrial DNA in bipolar disorder: An exploratory report. Front. Psychiatry 2020, 11, 514658. [Google Scholar] [CrossRef]
- Song, M.; Martinowich, K.; Lee, F.S. BDNF at the synapse: Why location matters. Mol. Psychiatry 2017, 22, 1370–1375. [Google Scholar] [CrossRef]
- Fernandes, B.S.; Molendijk, M.L.; Köhler, C.A.; Soares, J.C.; Leite, C.M.; Machado-Vieira, R.; Ribeiro, T.L.; Silva, J.C.; Sales, P.M.G.; Quevedo, J.; et al. Peripheral brain-derived neurotrophic factor (BDNF) as a biomarker in bipolar disorder: A meta-analysis of 52 studies. BMC Med. 2015, 13, 289. [Google Scholar] [CrossRef]
- Wu, R.; Fan, J.; Zhao, J.; Calabrese, J.R.; Gao, K. The relationship between neurotrophins and bipolar disorder. Expert Rev. Neurother. 2014, 14, 51–65. [Google Scholar] [CrossRef]
- Polyakova, M.; Stuke, K.; Schuemberg, K.; Mueller, K.; Schoenknecht, P.; Schroeter, M.L. BDNF as a biomarker for successful treatment of mood disorders: A systematic & quantitative meta-analysis. J. Affect. Disord. 2015, 174, 432–440. [Google Scholar]
- Lin, P.Y. State-dependent decrease in levels of brain-derived neurotrophic factor in bipolar disorder: A meta-analytic study. Neurosci. Lett. 2009, 466, 139–143. [Google Scholar] [CrossRef]
- Kim, H.K.; Mendonça, K.M.; Howson, P.A.; Brotchie, J.M.; Andreazza, A.C. The link between mitochondrial complex I and brain-derived neurotrophic factor in SH-SY5Y cells—The potential of JNX1001 as a therapeutic agent. Eur. J. Pharmacol. 2015, 764, 379–384. [Google Scholar] [CrossRef]
- Mansur, R.B.; Santos, C.M.; Rizzo, L.B.; Cunha, G.R.; Asevedo, E.; Noto, M.N.; Pedrini, M.; Zeni, M.; Cordeiro, Q.; McIntyre, R.S.; et al. Inter-relation between brain-derived neurotrophic factor and antioxidant enzymes in bipolar disorder. Bipolar Disord. 2016, 18, 433–439. [Google Scholar] [CrossRef]
- Walz, J.C.; Andreazza, A.C.; Frey, B.N.; Cacilhas, A.A.; Ceresér, K.M.M.; Cunha, A.B.M.; Weyne, F.; Stertz, L.; Santin, A.; Gonçalves, C.A.; et al. Serum neurotrophin–3 is increased during manic and depressive episodes in bipolar disorder. Neurosci. Lett. 2007, 415, 87–89. [Google Scholar] [CrossRef]
- Berk, M.; Dodd, S.; Kauer-Sant’anna, M.; Malhi, G.S.; Bourin, M.; Kapczinski, F.; Norman, T. Dopamine dysregulation syndrome: Implications for a dopamine hypothesis of bipolar disorder. Acta Psychiatr. Scand. Suppl. 2007, 434, 41–49. [Google Scholar] [CrossRef]
- Haque, E.M.; Asanuma, M.; Higashi, Y.; Miyazaki, I.; Tanaka, K.; Ogawa, N. Apoptosis inducing neurotoxicity of dopamine and its metabolites via reactive quinone generation in neuroblastoma cells. Biochim. Biophys. Acta 2003, 1619, 39–52. [Google Scholar] [CrossRef]
- Benes, F.M.; Matzilevich, D.; Burke, R.E.; Walsh, J. The expression of proapoptosis genes is increased in bipolar disorder, but not in schizophrenia. Mol. Psychiatry 2006, 11, 241–251. [Google Scholar] [CrossRef]
- Quiroz, J.A.; Gray, N.A.; Kato, T.; Manji, H.K. Mitochondrially mediated plasticity in the pathophysiology and treatment of bipolar disorder. Neuropsychopharmacology 2008, 33, 2551–2565. [Google Scholar] [CrossRef]
- Valvassori, S.S.; Bavaresco, D.V.; Feier, G.; Cechinel-Recco, K.; Steckert, A.V.; Varela, R.B.; Borges, C.; Carvalho-Silva, M.; Gomes, L.M.; Streck, E.L.; et al. Increased oxidative stress in the mitochondria isolated from lymphocytes of bipolar disorder patients during depressive episodes. Psychiatry Res. 2018, 264, 192–201. [Google Scholar] [CrossRef]
- Ferrie, L.; Young, A.H.; McQuade, R. Effect of lithium and lithium withdrawal on potassium-evoked dopamine release and tyrosine hydroxylase expression in the rat. Int. J. Neuropsychopharmacol. 2006, 9, 729–735. [Google Scholar] [CrossRef]
- Himmerich, H.; Bartsch, S.; Hamer, H.; Mergl, R.; Schönherr, J.; Petersein, C.; Munzer, A.; Kirkby, K.C.; Bauer, K.; Sack, U. Modulation of cytokine production by drugs with antiepileptic or mood stabilizer properties in anti-CD3– and anti-CD40–stimulated blood in vitro. Oxid. Med. Cell Longev. 2014, 2014, 806162. [Google Scholar] [CrossRef]
- Felger, J.C. Imaging the role of inflammation in mood and anxiety-related disorders. Curr. Neuropharmacol. 2018, 16, 533–558. [Google Scholar] [CrossRef]
- Mao, R.; Zhang, C.; Chen, J.; Zhao, G.; Zhou, R.; Wang, F.; Xu, J.; Yang, T.; Su, Y.; Huang, J.; et al. Different levels of pro– and anti-inflammatory cytokines in patients with unipolar and bipolar depression. J. Affect. Disord. 2018, 237, 65–72. [Google Scholar] [CrossRef]
- Rolstad, S.; Jakobsson, J.; Sellgren, C.; Isgren, A.; Ekman, C.J.; Bjerke, M.; Blennow, K.; Zetterberg, H.; Pålsson, E.; Landén, M. CSF neuroinflammatory biomarkers in bipolar disorder are associated with cognitive impairment. Eur. Neuropsychopharmacol. 2015, 25, 1091–1098. [Google Scholar] [CrossRef]
- Sayana, P.; Colpo, G.D.; Simoes, L.R.; Giridharan, V.V.; Teixeira, A.L.; Quevedo, J.; Barichello, T. A systematic review of evidence for the role of inflammatory biomarkers in bipolar patients. J. Psychiatr. Res. 2017, 92, 160e182. [Google Scholar] [CrossRef]
- van den Ameele, S.; van Diermen, L.; Staels, W.; Coppens, V.; Dumont, G.; Sabbe, B.; Morrens, M. The effect of mood-stabilizing drugs on cytokine levels in bipolar disorder: A systematic review. J. Affect. Disord. 2016, 203, 364–373. [Google Scholar] [CrossRef]
- Barbosa, I.G.; Bauer, M.E.; Machado-Vieira, R.; Teixeira, A.L. Cytokines in bipolar disorder: Paving the way for neuroprogression. Neural. Plast. 2014, 2014, 360481. [Google Scholar] [CrossRef]
- Bauer, I.E.; Pascoe, M.C.; Wollenhaupt-Aguiar, B.; Kapczinski, F.; Soares, J.C. Inflammatory mediators of cognitive impairment in bipolar disorder. J. Psychiatr. Res. 2014, 56, 18–27. [Google Scholar] [CrossRef]
- Stertz, L.; Magalhaes, P.V.; Kapczinski, F. Is bipolar disorder an inflammatory condition? The relevance of microglial activation. Curr. Opin. Psychiatry 2013, 26, 19–26. [Google Scholar] [CrossRef]
- Jones, G.H.; Vecera, C.M.; Pinjari, O.F.; Machado-Vieira, R. Inflammatory signaling mechanisms in bipolar disorder. J. Biomed. Sci. 2021, 28, 45. [Google Scholar] [CrossRef]
- Pereira, A.C.; Oliveira, J.; Silva, S.; Madeira, N.; Pereira, C.M.F.; Cruz, M.T. Inflammation in bipolar disorder (BD): Identification of new therapeutic targets. Pharmacol. Res. 2021, 163, 105325. [Google Scholar] [CrossRef]
- Young, J.J.; Bruno, D.; Pomara, N. A review of the relationship between proinflammatory cytokines and major depressive disorder. J. Affect. Disord. 2014, 169, 15–20. [Google Scholar] [CrossRef]
- Benedetti, F.; Aggio, V.; Pratesi, M.L.; Greco, G.; Furlan, R. Neuroinflammation in bipolar depression. Front. Psychiatry 2020, 11, 71. [Google Scholar] [CrossRef]
- Kim, Y.; Jung, H.; Myint, A.; Kim, H.; Park, S. Imbalance between pro-inflammatory and anti-inflammatory cytokines in bipolar disorder. J. Affect. Disord. 2007, 104, 91–95. [Google Scholar] [CrossRef]
- Rao, J.S.; Harry, G.J.; Rapoport, S.I.; Kim, H.W. Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol. Psychiatry 2010, 15, 384–392. [Google Scholar] [CrossRef]
- Bezchlibnyk, Y.B.; Wang, J.-F.; McQueen, G.M.; Young, L.T. Gene expression differences in bipolar disorder revealed by cDNA array analysis of post-mortem frontal cortex. J. Neurochem. 2001, 79, 826–834. [Google Scholar] [CrossRef]
- Wesseling, H.; Gottschalk, M.G.; Bahn, S. Targeted multiplexed selected reaction monitoring analysis evaluates protein expression changes of molecular risk factors for major psychiatric disorders. Int. J. Neuropsychopharmacol. 2014, 18, 1. [Google Scholar] [CrossRef][Green Version]
- Kageyama, Y.; Kasahara, T.; Kato, M.; Sakai, S.; Deguchi, Y.; Tani, M.; Kuroda, K.; Hattori, K.; Yoshida, S.; Goto, Y.; et al. The relationship between circulating mitochondrial DNA and inflammatory cytokines in patients with major depression. J. Affect. Disord. 2018, 233, 15–20. [Google Scholar] [CrossRef]
- Caraci, F.; Spampinato, S.F.; Morgese, M.G.; Tascedda, F.; Salluzzo, M.G.; Giambirtone, M.C.; Caruso, G.; Munafò, A.; Torrisi, S.A.; Leggio, G.M.; et al. Neurobiological links between depression and AD: The role of TGF-beta1 signaling as a new pharmacological target. Pharmacol. Res. 2018, 130, 374–384. [Google Scholar] [CrossRef]
- Panaccione, I.; Spalletta, G.; Sani, G. Neuroinflammation and excitatory symptoms in bipolar disorder. Neuroimmunol. Neuroinflam. 2015, 2, 215–227. [Google Scholar]
- Rowland, T.; Perry, B.I.; Upthegrove, R.; Barnes, N.; Chatterjee, J.; Gallacher, D.; Marwaha, S. Neurotrophins, cytokines, oxidative stress mediators and mood state in bipolar disorder: Systematic review and meta-analyses. Br. J. Psychiatry 2018, 213, 514–525. [Google Scholar] [CrossRef]
- Mondin, T.C.; de Azevedo Cardoso, T.; Moreira, F.P.; Wiener, C.; Oses, J.P.; de Mattos Souza, L.D.; Jansen, K.; da Silva Magalhães, P.V.; Kapczinski, F.; da Silva, R.A. Circadian preferences, oxidative stress and inflammatory cytokines in bipolar disorder: A community study. J. Neuroimmunol. 2016, 301, 23–29. [Google Scholar] [CrossRef]
- Solmi, M.; Sharma, M.S.; Osimo, E.F.; Fornaro, M.; Bortolato, B.; Croatto, G.; Miola, A.; Vieta, E.; Pariante, C.M.; Smith, L.; et al. Peripheral levels of C-reactive protein, tumor necrosis factor–α, interleukin–6, and interleukin–1β across the mood spectrum in bipolar disorder: A meta-analysis of mean differences and variability. Brain Behav. Immun. 2021, 97, 193–203. [Google Scholar] [CrossRef]
- Munkholm, K.; Brauner, J.V.; Kessing, L.V.; Vinberg, M. Cytokines in bipolar disorder vs. healthy control subjects: A systematic review and meta-analysis. J. Psychiatr. Res. 2013, 47, 1119–1133. [Google Scholar] [CrossRef]
- Kohler, C.A.; Freitas, T.H.; Maes, M.; de Andrade, N.Q.; Liu, C.S.; Fernandes, B.S.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N.; et al. Peripheral cytokine and chemokine alterations in depression: A meta-analysis of 82 studies. Acta Psychiatr. Scand. 2017, 135, 373–387. [Google Scholar] [CrossRef]
- Beishuizen, A.; Thijs, L.G. Endotoxin and the hypothalamopituitary-adrenal (HPA) axis. J. Endotoxin Res. 2003, 9, 3–24. [Google Scholar]
- Harrison, P.J.; Hall, N.; Mould, A.; Al-Juffali, N.; Tunbridge, E.M. Cellular calcium in bipolar disorder: Systematic review and meta-analysis. Mol. Psychiatry 2019, 26, 4106–4116. [Google Scholar] [CrossRef]
- Belosludtsev, K.N.; Dubinin, M.V.; Belosludtseva, N.V.; Mironova, G.D. Mitochondrial Ca2+ transport: Mechanisms, molecular structures, and role in cells. Biochemistry 2019, 84, 593–607. [Google Scholar] [CrossRef]
- Giorgi, C.; Marchi, S.; Pinton, P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat. Rev. Mol. Cell Biol. 2018, 19, 713–730. [Google Scholar] [CrossRef]
- Gorlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A mutual interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef]
- Ross, W.N. Understanding calcium waves and sparks in central neurons. Nat. Rev. Neurosci. 2012, 13, 157–168. [Google Scholar] [CrossRef]
- Joshi, G.; Sultana, R.; Perluigi, M.; Butterfield, D.A. In vivo protection of synaptosomes from oxidative stress mediated by Fe2+/H2O2 or 2, 2–azobis–(2–amidinopropane) dihydrochloride by the glutathione mimetic tricyclodecan–9–yl-xanthogenate. Free Radic. Biol. Med. 2005, 38, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- van der Bliek, A.M.; Sedensky, M.M.; Morgan, P.G. Cell biology of the mitochondrion. Genetics 2017, 207, 843–871. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, D.G. Mitochondrial calcium function and dysfunction in the central nervous system. Biochim. Biophys. Acta 2009, 1787, 1416–1424. [Google Scholar] [CrossRef]
- Fišar, Z.; Hroudová, J.; Singh, N.; Kopřivová, A.; Macecková, D. Effect of simvastatin, coenzyme Q10, resveratrol, acetylcysteine and acetylcarnitine on mitochondrial respiration. Folia Biol. 2016, 62, 53–66. [Google Scholar]
- Madireddy, S.; Madireddy, S. The role of diet in maintaining strong brain health by taking the advantage of the gut-brain axis. J. Food Nutr. Res. 2019, 7, 41–50. [Google Scholar]
- Olagunju, A.T.; Morgan, J.A.; Aftab, A.; Gatchel, J.R.; Chen, P.; Dols, A.; Sajatovic, M.; Regenold, W.T. A review of the evidence base for nutrition and nutritional supplements in older adults with bipolar disorder: A report from the OABD task force. J. Frailty Aging 2021, 10, 241–246. [Google Scholar]
- Ashton, M.M.; Kavanagh, B.E.; Marx, W.; Berk, M.; Sarris, J.; Ng, C.H.; Hopwood, M.; Williams, L.J.; Dean, O.M. A systematic review of nutraceuticals for the treatment of bipolar disorder. Can. J. Psychiatry 2020, 66, 262–273. [Google Scholar] [CrossRef]
- Sarris, J.; Murphy, J.; Mischoulon, D.; Papakostas, G.I.; Fava, M.; Berk, M.; Ng, C.H. Adjunctive nutraceuticals for depression: A systematic review and meta-analyses. Am. J. Psychiatry 2016, 173, 575–587. [Google Scholar] [CrossRef]
- Madireddy, S.; Madireddy, S. Most effective combination of nutraceuticals for improved memory and cognitive performance in the house cricket, Acheta domesticus. Nutrients 2021, 13, 362. [Google Scholar] [CrossRef]
- Ng, F.; Berk, M.; Dean, O.; Bush, A.I. Oxidative stress in psychiatric disorders: Evidence base and therapeutic implications. Int. J. Neuropsychopharmacol. 2008, 21, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Pandya, C.D.; Howell, K.R.; Pillai, A.K. Antioxidants as potential therapeutics for neuropsychiatric disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 46, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Murrough, J.W.; Huryk, K.M.; Mao, X.; Iacoviello, B.; Collins, K.; Nierenberg, A.A.; Kang, G.; Shungu, D.C.; Iosifescu, D.V. A pilot study of minocycline for the treatment of bipolar depression: Effects on cortical glutathione and oxidative stress in vivo. J. Affect. Disord. 2018, 230, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Ashton, M.M.; Berk, M.; Ng, C.H.; Hopwood, M.; Kavanagh, B.; Williams, L.J.; Sarris, J.; Dean, O.M. Nutraceuticals and nutritional supplements for the treatment of bipolar disorder: Protocol for a systematic review. BMJ Open 2019, 9, e025640. [Google Scholar] [CrossRef] [PubMed]
- Iddir, M.; Brito, A.; Dingeo, G.; Fernandez Del Campo, S.S.; Samouda, H.; La Frano, M.R.; Bohn, T. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: Considerations during the COVID-19 crisis. Nutrients 2020, 12, 1562. [Google Scholar] [CrossRef] [PubMed]
- Boerman, R.; Cohen, D.; Schulte, P.F.; Nugter, A. Prevalence of vitamin D deficiency in adult outpatients with bipolar disorder or schizophrenia. J. Clin. Psychopharmacol. 2016, 36, 588–592. [Google Scholar] [CrossRef]
- Naifar, M.; Bouali, M.; Guidara, W.; Ellouze, A.S.; Jmal, K.; Omri, S.; Messedi, M.; Zouari, L.; Elleuch, A.; Maalej, M.; et al. Bipolar disorder vulnerability: The vitamin D path. Can. J. Psychiatry 2020, 65, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Cereda, G.; Enrico, P.; Ciappolino, V.; Delvecchio, G.; Brambilla, P. The role of vitamin D in bipolar disorder: Epidemiology and influence on disease activity. J. Affect. Disord. 2021, 278, 209–217. [Google Scholar] [CrossRef]
- Scorza, F.A.; Almeida, A.C.G.; Scorza, C.A.; Moret, M.A.; Finsterer, J. Bipolar disorder: The vitamin D debate. J. Affect. Disord. 2021, 286, 338–339. [Google Scholar] [CrossRef]
- Marsh, W.K.; Penny, J.L.; Rothschild, A.J. Vitamin D supplementation in bipolar depression: A double blind placebo-controlled trial. J. Psychiatr. Res. 2017, 95, 48–53. [Google Scholar] [CrossRef]
- Geddes, J.R.; Gardiner, A.; Rendell, J.; Voysey, M.; Tunbridge, E.; Hinds, C.; Yu, L.; Hainsworth, J.; Attenburrow, M.; Simon, J.; et al. Comparative evaluation of quetiapine plus lamotrigine combination versus quetiapine monotherapy (and folic acid versus placebo) in bipolar depression (cequel): A 2 × 2 factorial randomised trial. Lancet Psychiatry 2016, 3, 31–39. [Google Scholar] [CrossRef]
- Crider, K.S.; Yang, T.P.; Berry, R.J.; Bailey, L.B. Folate and DNA methylation: A review of molecular mechanisms and the evidence for folate’s role. Adv. Nutr. 2012, 3, 21–38. [Google Scholar] [CrossRef]
- Morris, D.W.; Trivedi, M.H.; Rush, A.J. Folate and unipolar depression. J. Altern. Complement. Med. 2008, 14, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Łojko, D.; Stelmach, M.; Suwalska, A. Is diet important in bipolar disorder? Psychiatry Pol. 2018, 52, 783–795. [Google Scholar] [CrossRef]
- Behzadi, A.H.; Omrani, Z.; Chalian, M.; Asadi, S.; Ghadiri, M. Folic acid efficacy as an alternative drug added to sodium valproate in the treatment of acute phase of mania in bipolar disorder: A double-blind randomized controlled trial. Acta Psychiatr. Scand. 2009, 120, 441–445. [Google Scholar] [CrossRef]
- Nierenberg, A.A.; Montana, R.; Kinrys, G.; Deckersbach, T.; Dufour, S.; Baek, J.H. L-Methylfolate for bipolar I depressive episodes: An open trial proof-of-concept registry. J. Affect. Disord. 2017, 207, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Siwek, M.; Styczeń, K.; Sowa-Kućma, M.; Dudek, D.; Reczyński, W.; Szewczyk, B.; Misztak, P.; Opoka, W.; Topór-Mądry, R.; Nowak, G. The serum concentration of magnesium as a potential state marker in patients with diagnosis of bipolar disorder. Psychiatr. Pol. 2015, 49, 1277–1287. [Google Scholar] [CrossRef]
- Siwek, M.; Styczeń, K.; Sowa-Kućma, M.; Dudek, D.; Reczyński, W.; Szewczyk, B.; Misztak, P.; Opoka, W.; Topór-Mądry, R.; Nowak, G.; et al. The serum concentration of copper in bipolar disorder. Psychiatr. Pol. 2017, 51, 469–481. [Google Scholar] [CrossRef]
- Koga, N.; Ogura, J.; Yoshida, F.; Hattori, K.; Hori, H.; Aizawa, E.; Ishida, I.; Kunugi, H. Altered polyunsaturated fatty acid levels in relation to proinflammatory cytokines, fatty acid desaturase genotype, and diet in bipolar disorder. Transl. Psychiatry 2019, 9, 208. [Google Scholar] [CrossRef]
- McNamara, R.K.; Welge, J.A. Meta-analysis of erythrocyte polyunsaturated fatty acid biostatus in bipolar disorder. Bipolar Disord. 2016, 18, 300–306. [Google Scholar] [CrossRef]
- Fusar-Poli, L.; Surace, T.; Vanella, A.; Meo, V.; Patania, F.; Furnari, R.; Signorelli, M.S.; Aguglia, E. The effect of adjunctive nutraceuticals in bipolar disorder: A systematic review of randomized placebo-controlled trials. J. Affect. Disord. 2019, 252, 334–349. [Google Scholar] [CrossRef]
- Rakofsky, J.J.; Dunlop, B.W. Review of nutritional supplements for the treatment of bipolar depression. Depress. Anxiety 2014, 31, 379–390. [Google Scholar] [CrossRef]
- Balanza-Martinez, V.; Fries, G.R.; Colpo, G.D.; Silveira, P.P.; Portella, A.K.; Tabares-Seisdedos, R.; Kapczinski, F. Therapeutic use of omega–3 fatty acids in bipolar disorder. Expert Rev. Neurother. 2011, 11, 1029–1047. [Google Scholar] [CrossRef]
- Saunders, E.F.H.; Ramsden, C.E.; Sherazy, M.S.; Gelenberg, A.J.; Davis, J.M.; Rapoport, S.I. Omega–3 and omega–6 polyun-saturated fatty acids in bipolar disorder: A review of biomarker and treatment studies. J. Clin. Psychiatry 2016, 77, e1301–e1308. [Google Scholar] [CrossRef]
- Lange, K.W. Omega–3 fatty acids and mental health. Glob. Health J. 2020, 4, 18–30. [Google Scholar] [CrossRef]
- Husain, M.I.; Strawbridge, R.; Stokes, P.R.A.; Young, A.H. Anti-inflammatory treatments for mood disorders: Systematic review and meta-analysis. J. Psychopharmacol. 2017, 31, 1137–1148. [Google Scholar] [CrossRef]
- Bloch, M.H.; Hannestad, J. Omega–3 fatty acids for the treatment of depression: Systematic review and meta-analysis. Mol. Psychiatry 2012, 17, 1272–1282. [Google Scholar] [CrossRef]
- Rosenblat, J.D.; McIntyre, R.S. Bipolar disorder and immune dysfunction: Epidemiological findings, proposed pathophysiology and clinical implications. Brain Sci. 2017, 7, 144. [Google Scholar] [CrossRef]
- Wilczynska, A. Fatty acids in treatment and prevention of depression. Psychiatr. Pol. 2013, 47, 657–666. [Google Scholar]
- Beyer, J.L.; Payne, M.E. Nutrition and bipolar depression. Psychiatr. Clin. N. Am. 2016, 39, 75–86. [Google Scholar] [CrossRef]
- Sarris, J. Clinical use of nutraceuticals in the adjunctive treatment of depression in mood disorders. Australas. Psychiatry 2017, 25, 369–372. [Google Scholar] [CrossRef]
- McPhilemy, G.; Byrne, F.; Waldron, M.; Hibbeln, J.R.; Davis, J.; McDonald, C.; Hallahan, B. A 52-week prophylactic randomised control trial of omega-3 polyunsaturated fatty acids in bipolar disorder. Bipolar Disord. 2020, 23, 697–706. [Google Scholar] [CrossRef]
- Ciappolino, V.; DelVecchio, G.; Prunas, C.; Andreella, A.; Finos, L.; Caletti, E.; Siri, F.; Mazzocchi, A.; Botturi, A.; Turolo, S.; et al. The effect of DHA supplementation on cognition in patients with bipolar disorder: An exploratory randomized control trial. Nutrients 2020, 12, 708. [Google Scholar] [CrossRef]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress-implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef]
- Rubin, L.P.; Ross, A.C.; Stephensen, C.B.; Bohn, T.; Tanumihardjo, S.A. Metabolic effects of inflammation on vitamin A and carotenoids in humans and animal models. Adv. Nutr. 2017, 8, 197–212. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Lowe, G.D.; Rumley, A.; Bruckdorfer, K.R.; Whincup, P.H. Associations of vitamin C status, fruit and vegetable intakes, and markers of inflammation and hemostasis. Am. J. Clin. Nutr. 2006, 83, 567–574. [Google Scholar] [CrossRef]
- Bozonet, S.M.; Carr, A.C.; Pullar, J.M.; Vissers, M.C. Enhanced human neutrophil vitamin C status, chemotaxis and oxidant generation following dietary supplementation with vitamin C-rich SunGold kiwifruit. Nutrients 2015, 7, 2574–2588. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and immune function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Rosenblat, J.D.; Gregory, J.M.; McIntyre, R.S. Pharmacologic implications of inflammatory comorbidity in bipolar disorder. Curr. Opin. Pharmacol. 2016, 29, 63–69. [Google Scholar] [CrossRef]
- Berk, M.; Dean, O.M.; Cotton, S.M.; Gama, C.S.; Kapczinski, F.; Fernandes, B.; Kohlmann, K.; Jeavons, S.; Hewitt, K.; Moss, K.; et al. Maintenance N-acetyl cysteine treatment for bipolar disorder: A double-blind randomized placebo-controlled trial. BMC Med. 2012, 10, 91. [Google Scholar] [CrossRef]
- Berk, M.; Copolov, D.L.; Dean, O.; Lu, K.; Jeavons, S.; Schapkaitz, I.; Anderson-Hunt, M.; Bush, A.I. Nacetyl cysteine for depressive symptoms in bipolar disorder—A double-blind randomized placebocontrolled trial. Biol. Psychiatry 2008, 64, 468–475. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Z.Y.; Wu, Y.; Schluesener, H.J. Valproic acid ameliorates inflammation in experimental autoimmune encephalomyelitis rats. Neuroscience 2012, 221, 140–150. [Google Scholar] [CrossRef]
- Amirzargar, M.A.; Yaghubi, F.; Hosseinipanah, M.; Jafari, M.; Pourjafar, M.; Rezaeepoor, M.; Rezaei, H.; Roshanaei, G.; Hajilooi, M.; Solgi, G. Anti-inflammatory effects of valproic acid in a rat model of renal ischemia/reperfusion injury: Alteration in cytokine profile. Inflammation 2017, 40, 1310–1318. [Google Scholar] [CrossRef]
- Fountoulakis, K.N.; Grunze, H.; Vieta, E.; Young, A.; Yatham, L.; Blier, P.; Kasper, S.; Moeller, H.J. The international college of neuro-psychopharmacology (CINP) treatment guidelines for bipolar disorder in adults (CINP-BD-2017), Part 3, the clinical guidelines. Int. J. Neuropsychopharmacol. 2017, 20, 180–195. [Google Scholar] [CrossRef]
- Yatham, L.N.; Kennedy, S.H.; Parikh, S.V.; Schaffer, A.; Bond, D.J.; Frey, B.N.; Sharma, V.; Goldstein, B.I.; Rej, S.; Beaulieu, S.; et al. Canadian network for mood and anxiety treatments (CANMAT) and international society for bipolar disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018, 20, 97–170. [Google Scholar] [CrossRef]
- Baldessarini, R.J.; Vazquez, G.H.; Tondo, L. Bipolar depression, A major unsolved challenge. Int. J. Bipolar Disord. 2020, 8, 1. [Google Scholar] [CrossRef]
- Malhi, G.S.; Gessler, D.; Outhred, T. Use of lithium for treatment of bipolar disorder: Recommendations from clinical practice guidelines. J. Affect. Disord. 2017, 217, 266–280. [Google Scholar] [CrossRef]
- Benard, V.; Vaiva, G.; Masson, M.; Geoffroy, P.A. Lithium and suicide prevention in bipolar disorder. Encephale 2016, 42, 234–241. [Google Scholar] [CrossRef]
- Cipriani, A.; Hawton, K.; Stockton, S.; Geddes, J.R. Lithium in the prevention of suicide in mood disorders: Updated systematic review and meta-analysis. BMJ 2013, 346, f3646. [Google Scholar] [CrossRef]
- Sani, G.; Fiorillo, A. The use of lithium in mixed states. CNS Spectr. 2019, 25, 449–451. [Google Scholar] [CrossRef]
- Kapur, V.; Nadella, R.K.; Raghuraman, B.S.; Saraf, G.; Mishra, S.; Srinivasmurthy, N.; Jain, S.; Zompo, M.D.; Viswanath, B. Clinical factors associated with lithium treatment response in bipolar disorder patients from India. Asian J. Psychiatr. 2019, 39, 165–168. [Google Scholar] [CrossRef]
- Paul, P.; Iyer, S.; Nadella, R.K.; Nayak, R.; Chellappa, A.S.; Ambardar, S.; Sud, R.; Sukumaran, S.K.; Purushottam, M.; Jain, S.; et al. Lithium response in bipolar disorder correlates with improved cell viability of patient derived cell lines. Sci. Rep. 2020, 10, 7428. [Google Scholar] [CrossRef]
- Jauhar, S.; Young, A.H. Controversies in bipolar disorder; role of second-generation antipsychotic for maintenance therapy. Int. J. Bipolar Disord. 2019, 7, 10. [Google Scholar] [CrossRef]
- Severus, E.; Bauer, M.; Geddes, J. Efficacy and effectiveness of lithium in the long-term treatment of bipolar disorders: An update 2018. Pharmacopsychiatry 2018, 51, 173–176. [Google Scholar] [CrossRef]
- Sato, K. Why is lithium effective in alleviating bipolar disorder? Med. Hypotheses 2021, 147, 110484. [Google Scholar] [CrossRef]
- Luu, B.; Rodway, G. Lithium therapy for bipolar disorder. J. Nurse Pract. 2018, 14, 93–99. [Google Scholar] [CrossRef]
- Girardi, P.; Brugnoli, R.; Manfredi, G.; Sani, G. Lithium in bipolar disorder: Optimizing therapy using prolonged-release formulations. Drugs R&D 2016, 16, 293–302. [Google Scholar]
- Carvalho, A.; Firth, J.; Vieta, E. Bipolar disorder. N. Engl. J. Med. 2020, 383, 58–66. [Google Scholar] [CrossRef]
- de Mendiola, P.X.; Hidalgo-Mazzei, D.; Vieta, E.; González-Pinto, A. Overview of lithium’s use: A nationwide survey. Int. J. Bipolar Disord. 2021, 9, 10. [Google Scholar] [CrossRef]
- Parabiaghi, A.; Barbato, A.; Risso, P.; Fortino, I.; Bortolotti, A.; Merlino, L.; D’Avanzo, B. Lithium use from 2000 to 2010 in Italy: A population-based study. Pharmacopsychiatry 2015, 48, 89–94. [Google Scholar] [CrossRef]
- Renes, J.W.; Regeer, E.J.; Hoogendoorn, A.W.; Nolen, W.A.; Kupka, R.W. A nationwide study on concordance with multimodal treatment guidelines in bipolar disorder. Int. J. Bipolar Disord. 2018, 6, 22. [Google Scholar] [CrossRef]
- Rhee, T.G.; Olfson, M.; Nierenberg, A.A.; Wilkinson, S.T. 20–Year trends in the pharmacologic treatment of bipolar disorder by psychiatrists in outpatient care settings. Am. J. Psychiatry 2020, 177, 706–715. [Google Scholar] [CrossRef]
- Lazzara, C.A.; Kim, Y.H. Potential application of lithium in Parkinson’s and other neurodegenerative diseases. Front. Neurosci. 2015, 9, 403. [Google Scholar] [CrossRef]
- Morris, G.; Berk, M. The putative use of lithium in Alzheimer’s disease. Curr. Alzheimer Res. 2016, 13, 853–861. [Google Scholar] [CrossRef]
- Murru, A.; Manchia, M.; Hajek, T.; Nielsen, R.E.; Rybakowski, J.K.; Sani, G.; Schulze, T.G.; Tondo, L.; Bauer, M. Lithium’s antiviral effects: A potential drug for COVID-19 disease? Int. J. Bipolar Disord. 2020, 8, 21. [Google Scholar] [CrossRef]
- van Gestel, H.; Franke, K.; Petite, J.; Slaney, C.; Garnham, J.; Helmick, C.; Johnson, K.; Uher, R.; Alda, M.; Hajek, T. Brain age in bipolar disorders: Effects of lithium treatment. Aust. N. Z. J. Psychiatry 2019, 53, 1179–1188. [Google Scholar] [CrossRef]
- Alqahtani, S.; Aljuma’ah, N.; Aydan, N.B.; Alsultan, A.; Alsarhani, E.; Asiri, Y. Estimation of lithium clearance in patients with bipolar disorder. Int. Clin. Psychopharmacol. 2020, 35, 157–162. [Google Scholar] [CrossRef]
- Andrade, C. Lithium levels and treatment efficacy. Bipolar Disord. 2020, 22, 89–90. [Google Scholar] [CrossRef]
- Ommati, M.M.; Niknahad, H.; Farshad, O.; Azarpira, N.; Heidari, R. In vitro and in vivo evidence on the role of mitochondrial impairment as a mechanism of lithium-induced nephrotoxicity. Biol. Trace Elem. Res. 2021, 199, 1908–1918. [Google Scholar] [CrossRef]
- Morris, G.; Berk, M. The many roads to mitochondrial dysfunction in neuroimmune and neuropsychiatric disorders. BMC Med. 2015, 13, 68. [Google Scholar] [CrossRef]
- Elvsåshagen, T.; Vera, E.; Bøen, E.; Bratlie, J.; Andreassen, O.A.; Josefsen, D.; Malt, U.F.; Blasco, M.A.; Boye, B. The load of short telomeres is increased and associated with lifetime number of depressive episodes in bipolar II disorder. J. Affect. Disord. 2011, 135, 43–50. [Google Scholar] [CrossRef]
- Khairova, R.; Pawar, R.; Salvadore, G.; Juruena, M.F.; de Sousa, R.T.; Soeiro-de-Souza, M.G.; Salvador, M.; Zarate, C.A.; Gattaz, W.F.; Machado-Vieira, R. Effects of lithium on oxidative stress parameters in healthy subjects. Mol. Med. Rep. 2012, 5, 680–682. [Google Scholar]
- Lundberg, M.; Millischer, V.; Backlund, L.; Martinsson, L.; Stenvinkel, P.; Sellgren, C.M.; Lavebratt, C.; Schalling, M. Lithium and the interplay between telomeres and mitochondria in bipolar disorder. Front. Psychiatry 2020, 11, 586083. [Google Scholar] [CrossRef]
- Barjasteh-Askari, F.; Davoudi, M.; Amini, H.; Ghorbani, M.; Yaseri, M.; Yunesian, M.; Mahvi, A.H.; Lester, D. Relationship between suicide mortality and lithium in drinking water: A systematic review and meta-analysis. J. Affect. Disord. 2020, 264, 234–241. [Google Scholar] [CrossRef]
- Lv, Q.; Guo, Y.; Zhu, M.; Geng, R.; Cheng, X.; Bao, C.; Wang, Y.; Huang, X.; Zhang, C.; Hao, Y.; et al. Predicting individual responses to lithium with oxidative stress markers in drug-free bipolar disorder. World J. Biol. Psychiatry 2019, 20, 778–789. [Google Scholar] [CrossRef]
- Smith, K.A.; Cipriani, A. Lithium and suicide in mood disorders: Updated meta-review of the scientific literature. Bipolar Disord. 2017, 19, 575–586. [Google Scholar] [CrossRef]
- Song, J.; Sjölander, A.; Joas, E.; Bergen, S.E.; Runeson, B.; Larsson, H.; Landén, M.; Lichtenstein, P. Suicidal behavior during lithium and valproate treatment: A within-individual 8–year prospective study of 50,000 patients with bipolar disorder. Am. J. Psychiatry 2017, 174, 795–802. [Google Scholar] [CrossRef]
- Post, R.M. The new news about lithium: An underutilized treatment in the United States. Neuropsychopharmacology 2018, 43, 1174–1179. [Google Scholar] [CrossRef]
- Rybakowski, J.K. Challenging the negative perception of lithium and optimizing its long-term administration. Front. Mol. Neurosci. 2018, 11, 349. [Google Scholar] [CrossRef]
- Machado-Vieira, R. Lithium, stress, and resilience in bipolar disorder: Deciphering this key homeostatic synaptic plasticity regulator. J. Affect. Disord. 2018, 233, 92–99. [Google Scholar] [CrossRef]
- Morsel, A.M.; Morrens, M.; Sabbe, B. An overview of pharmacotherapy for bipolar I disorder. Expert Opin. Pharmacother. 2018, 19, 203–222. [Google Scholar] [CrossRef]
- Vasudev, A.; Chaudhari, S.; Sethi, R.; Fu, R.; Sandieson, R.M.; Forester, B.P. A review of the pharmacological and clinical profile of newer atypical antipsychotics as treatments for bipolar disorder: Considerations for use in older patients. Drugs Aging 2018, 35, 887–895. [Google Scholar] [CrossRef]
- Maddu, N.; Raghavendra, P.B. Review of lithium effects on immune cells. Immunopharmacol. Immunotoxicol. 2015, 37, 111–125. [Google Scholar] [CrossRef]
- Nassar, A.; Azab, A.N. Effects of lithium on inflammation. ACS Chem. Neurosci. 2014, 5, 451–458. [Google Scholar] [CrossRef]
- Valvassori, S.S.; Resende, W.R.; Dal-Pont, G.; Sangaletti-Pereira, H.; Gava, F.F.; Peterle, B.R.; Carvalho, A.F.; Varela, R.B.; Dal-Pizzol, F.; Quevedo, J. Lithium ameliorates sleep deprivation-induced mania-like behavior, hypothalamic-pituitary-adrenal (HPA) axis alterations, oxidative stress and elevations of cytokine concentrations in the brain and serum of mice. Bipolar Disord. 2017, 19, 246–258. [Google Scholar] [CrossRef]
- Muneer, A. Bipolar disorder: Role of inflammation and the development of disease biomarkers. Psychiatry Investig. 2016, 13, 18–33. [Google Scholar] [CrossRef]
- Nahman, S.; Belmaker, R.H.; Azab, A.N. Effects of lithium on lipopolysacchride-induced inflammation in rat primary glial cells. Innate Immun. 2012, 18, 447–458. [Google Scholar] [CrossRef]
- Kang, K.; Kim, Y.J.; Kim, Y.H.; Roh, J.N.; Nam, J.M.; Kim, P.Y.; Ryu, W.-S.; Lee, S.-H.; Yoon, B.-W. Lithium pretreatment reduces brain injury after intracerebral hemorrhage in rats. Neurol. Res. 2012, 34, 447–454. [Google Scholar] [CrossRef]
- Won, E.; Kim, Y.K. An oldie but goodie: Lithium in the treatment of bipolar disorder through neuroprotective and neurotrophic mechanisms. Int. J. Mol. Sci. 2017, 18, 2679. [Google Scholar] [CrossRef]
- Machado-Vieira, R.; Manji, H.K.; Zarate, C.A. The role of lithium in the treatment of bipolar disorder: Convergent evidence for neurotrophic effects as a unifying hypothesis. Bipolar Disord. 2009, 11, 92–109. [Google Scholar] [CrossRef]
- Najt, P.; Bayer, U.; Hausmann, M. Right fronto-parietal dysfunction underlying spatial attention in bipolar disorder. Psychiatry Res. 2013, 210, 479–484. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maurer, I.C.; Schippel, P.; Volz, H.P. Lithium-induced enhancement of mitochondrial oxidative phosphorylation in human brain tissue. Bipolar Disord. 2009, 11, 515–522. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, R.T.; Streck, E.L.; Zanetti, M.V.; Ferreira, G.K.; Diniz, B.S.; Brunoni, A.R.; Busatto, G.F.; Gattaz, W.F.; Machado-Vieira, R. Lithium increases leukocyte mitochondrial complex I activity in bipolar disorder during depressive episodes. Psychopharmacology 2015, 232, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Adams, W.K.; Levesque, D.L.; Cocker, P.J.; Kaur, S.; Bodnar, T.S.; Young, A.H.; Winstanley, C.A. Decreased motor impulsivity following chronic lithium treatment in male rats is associated with reduced levels of pro-inflammatory cytokines in the orbitofrontal cortex. Brain Behav. Immun. 2020, 89, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Berk, M.; Dandash, O.; Daglas, R.; Cotton, S.M.; Allott, K.; Fornito, A.; Suo, C.; Klauser, P.; Liberg, B.; Henry, L.; et al. Neuroprotection after a first episode of mania: A randomized controlled maintenance trial comparing the effects of lithium and quetiapine on grey and white matter volume. Transl. Psychiatry 2017, 7, e1011. [Google Scholar] [CrossRef] [PubMed]
- González-Pinto, A.; López-Peña, P.; Bermúdez-Ampudia, C.; Vieta, E.; Martinez-Cengotitabengoa, M. Can lithium salts prevent depressive episodes in the real world? Eur. Neuropsychopharmacol. 2018, 28, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Kessing, L.V.; Bauer, M.; Nolen, W.A.; Severus, E.; Goodwin, G.M.; Geddes, J. Effectiveness of maintenance therapy of lithium vs. other mood stabilizers in monotherapy and in combinations: A systematic review of evidence from observational studies. Bipolar Disord. 2018, 20, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Lahteenvuo, M.; Tanskanen, A.; Taipale, H.; Hoti, F.; Vattulainen, P.; Vieta, E.; Tiihonen, J. Real-world effectiveness of pharmacologic treatments for the prevention of rehospitalization in a finnish nationwide cohort of patients with bipolar disorder. JAMA Psychiatry 2018, 75, 347–355. [Google Scholar] [CrossRef]
- Miura, T.; Noma, H.; Furukawa, T.A.; Mitsuyasu, H.; Tanaka, S.; Stockton, S.; Salanti, G.; Motomura, K.; Shimano-Katsuki, S.; Leucht, S.; et al. Comparative efficacy and tolerability of pharmacological treatments in the maintenance treatment of bipolar disorder: A systematic review and network meta-analysis. Lancet Psychiatry 2014, 1, 351–359. [Google Scholar] [CrossRef]
- Severus, E.; Taylor, M.J.; Sauer, C.; Pfennig, A.; Ritter, P.; Bauer, M.; Geddes, J.R. Lithium for prevention of mood episodes in bipolar disorders: Systematic review and meta-analysis. Int. J. Bipolar Disord. 2014, 2, 15. [Google Scholar] [CrossRef]
- Hjerde, E.; Dahl, S.G.; Sylte, I. Atypical and typical antipsychotic drug interactions with the dopamine D2 receptor. Eur. J. Med. Chem. 2005, 40, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Vacheron-Trystram, M.N.; Braitman, A.; Cheref, S.; Auffray, L. Antipsychotics in bipolar disorders. Encephale 2004, 30, 417–424. [Google Scholar] [CrossRef]
- Bessonova, L.; Velligan, D.I.; Weiden, P.J.; O’Sullivan, A.K.; Yarlas, A.; Bayliss, M.; Baranwal, N.; Rychlec, K.; Carpenter-Conlin, J.; Doane, M.J.; et al. Antipsychotic treatment experiences of people with bipolar I disorder: Patient perspectives from an online survey. BMC Psychiatry 2020, 20, 354. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, A.; Rendell, J.; Geddes, J.R. Olanzapine in the long-term treatment of bipolar disorder: A systematic review and meta-analysis. J. Psychopharmacol. 2010, 24, 1729–1738. [Google Scholar] [CrossRef]
- Malhi, G.S.; Bassett, D.; Boyce, P.; Bryant, R.; Fitzgerald, P.B.; Fritz, K.; Hopwood, M.; Lyndon, B.; Mulder, R.; Murray, G.; et al. Royal Australian and New Zealand college of psychiatrists clinical practice guidelines for mood disorders. Aust. N. Z. J. Psychiatry 2015, 49, 1087–1206. [Google Scholar] [CrossRef]
- López-Muñoz, F.; Shen, W.W.; D’Ocon, P.; Romero, A.; Álamo, C. A history of the pharmacological treatment of bipolar disorder. Int. J. Mol. Sci. 2018, 19, 2143. [Google Scholar] [CrossRef]
- Derry, S.; Moore, R.A. Atypical antipsychotics in bipolar disorder: Systematic review of randomized trials. BMC Psychiatry 2007, 7, 40. [Google Scholar] [CrossRef]
- Earley, W.; Burgess, M.V.; Rekeda, L.; Dickinson, R.; Szatmári, B.; Németh, G.; McIntyre, R.S.; Sachs, G.S.; Yatham, L.N. Cariprazine treatment of bipolar depression: A randomized double-blind placebo-controlled phase 3 study. Am. J. Psychiatry 2019, 176, 439–448. [Google Scholar] [CrossRef]
- Cichoń, L.; Janas-Kozik, M.; Siwiec, A.; Rybakowski, J.K. Clinical picture and treatment of bipolar affective disorder in children and adolescents. Psychiatry Pol. 2020, 54, 35–50. [Google Scholar] [CrossRef]
- Gammon, D.; Cheng, C.; Volkovinskaia, A.; Baker, G.B.; Dursun, S.M. Clozapine: Why is it so uniquely effective in the treatment of a range of neuropsychiatric disorders? Biomolecules 2021, 11, 1030. [Google Scholar] [CrossRef]
- Renzenbrink, M.; Wand, A.P.F. A systematic review of clozapine’s effectiveness for primary psychotic and bipolar disorders in older adults. Int. Psychogeriatr. 2021, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.; Velosa, J.; Zhang, J.; Dursun, S.M.; Kapczinski, F.; de Azevedo Cardoso, T. Clozapine in bipolar disorder: A systematic review and meta-analysis. J. Psychiatr. Res. 2020, 125, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Li, X.B.; Tang, Y.L.; Wang, C.Y.; Leon, J. Clozapine for treatment-resistant bipolar disorder, A systematic review. Bipolar Disord. 2015, 17, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Ifteni, P.; Teodorescu, A.; Moga, M.A.; Pascu, A.M.; Miclaus, R.S. Switching bipolar disorder patients treated with clozapine to another antipsychotic medication: A mirror image study. Neuropsychiatr. Dis. Treat. 2017, 13, 201–204. [Google Scholar] [CrossRef]
- Wilkowska, A.; Wiglusz, M.S.; Cubała, W.J. Clozapine in treatment-resistant bipolar disorder with suicidality. Three case reports. Front. Psychiatry 2019, 10, 520. [Google Scholar] [CrossRef]
- Benazzi, F.; Berk, M.; Frye, M.A.; Wang, W.; Barraco, A.; Tohen, M. Olanzapine/fluoxetine combination for the treatment of mixed depression in bipolar I disorder: A post hoc analysis. J. Clin. Psychiatry 2009, 70, 1424–1431. [Google Scholar] [CrossRef]
- Macfadden, W.; Adler, C.M.; Turkoz, I.; Haskins, J.T.; Turner, N.; Alphs, L. Adjunctive long-acting risperidone in patients with bipolar disorder who relapse frequently and have active mood symptoms. BMC Psychiatry 2011, 11, 171. [Google Scholar] [CrossRef]
- Saxena, K.; Chang, K.; Steiner, H. Treatment of aggression with risperidone in children and adolescents with bipolar disorder: A case series. Bipolar Disord. 2006, 8, 405–410. [Google Scholar] [CrossRef]
- Biederman, J.; Mick, E.; Wozniak, J.; Aleardi, M.; Spencer, T.; Faraone, S.V. An open-label trial of risperidone in children and adolescents with bipolar disorder. J. Child Adolesc. Psychopharmacol. 2005, 15, 311–317. [Google Scholar] [CrossRef]
- Stahl, S.M. Mechanism of action of cariprazine. CNS Spectr. 2016, 21, 123–127. [Google Scholar] [CrossRef]
- Citrome, L. Cariprazine in bipolar disorder: Clinical efficacy, tolerability, and place in therapy. Adv. Ther. 2013, 30, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.J.; Findlay, L.J.; El-Mallakh, P.L.; El-Mallakh, R.S. Update on schizophrenia and bipolar disorder: Focus on cariprazine. Neuropsychiatr. Dis. Treat 2016, 12, 1837–1842. [Google Scholar] [PubMed]
- Edinoff, A.; Ruoff, M.T.; Ghaffar, Y.T.; Rezayev, A.; Jani, D.; Kaye, A.M.; Cornett, E.M.; Kaye, A.D.; Viswanath, O.; Urits, I. Cariprazine to treat schizophrenia and bipolar disorder in adults. Psychopharmacol. Bull. 2020, 50, 83–117. [Google Scholar] [PubMed]
- Silfstein, M.; Abi-Dargham, A.; D’Souza, D.C.; Carson, R.E.; Laszlovszky, I.; Durgam, S.; Adham, N.; Kiss, B. Cariprazine demonstrates high dopamine D3 and D2 receptor occupancy in patients with schizophrenia: A clinical PET study with [11C]–(+)–PHNO. Neuropharmacology 2013, 38, S520. [Google Scholar]
- Kiss, B.; Horti, F.; Bobok, A. In vitro and in vivo comparison of [3H](+)–PHNO and [3H]raclopride binding to rat striatum and lobes 9 and 10 of the cerebellum: A method to distinguish dopamine D3 from D2 receptor sites. Synapse 2011, 65, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Roza, T.H.; Lopes, S.L.S.; Passos, I.C. Cariprazine for acute mood episodes in bipolar disorder. Bipolar Disord. 2020, 22, 759–760. [Google Scholar] [CrossRef] [PubMed]
- Findlay, L.J.; El-Mallakh, P.L.; El-Mallakh, R.S. Cariprazine for the treatment of bipolar disorder. Perspect. Psychiatr. Care 2017, 53, 148–155. [Google Scholar] [CrossRef]
- Poweleit, E.A.; Colestock, M.; Kantemneni, E.C.; Strawn, J.R.; Patino, L.R.; DelBello, M.P.; Ramsey, L.B. Cariprazine in youth with bipolar and psychotic disorders: A retrospective chart review. J. Child Adolesc. Psychopharmacol. 2020, 30, 267–272. [Google Scholar] [CrossRef]
- Earley, W.; Durgam, S.; Lu, K.; Debelle, M.; Laszlovszky, I.; Vieta, V.; Yatham, L.N. Tolerability of cariprazine in the treatment of acute bipolar I mania: A pooled post hoc analysis of 3 phase II/III studies. J. Affect. Disord. 2017, 215, 205–212. [Google Scholar] [CrossRef]
- Ragguett, R.; McIntyre, R.S. Cariprazine for the treatment of bipolar depression: A review. Expert Rev. Neurother. 2019, 19, 317–323. [Google Scholar] [CrossRef]
- Chhatlani, A.; Farheen, S.A.; Setty, M.J.; Tampi, R. Use of cariprazine in psychiatric disorders: A systematic review. Ann. Clin. Psychiatry 2018, 30, 326–334. [Google Scholar] [PubMed]
- Earley, W.; Durgam, S.; Lu, K.; Ruth, A.; Németh, G.; Laszlovszky, I.; Yatham, L.N. Clinically relevant response and remission outcomes in cariprazine-treated patients with bipolar I disorder. J. Affect. Disord. 2018, 226, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Yatham, L.N.; Vieta, E.; McIntyre, R.S.; Jain, R.; Patel, M.; Earley, W. Broad efficacy of cariprazine on depressive symptoms in bipolar disorder and the clinical implications. Prim. Care Companion CNS Disord. 2020, 22, 24841. [Google Scholar] [CrossRef] [PubMed]
- Saraf, G.; Pinto, J.V.; Yatham, L.N. Efficacy and safety of cariprazine in the treatment of bipolar disorder. Expert Opin. Pharmacother. 2019, 20, 2063–2072. [Google Scholar] [CrossRef] [PubMed]
- Vieta, E.; Calabrese, J.R.; Whelan, J.; Tohen, M.; Earley, W.R. The efficacy of cariprazine on function in patients with bipolar depression: A post hoc analysis of a randomized controlled trial. Curr. Med. Res. Opin. 2021, 37, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Durgam, S.; Earley, W.; Lipschitz, A.; Guo, H.; Laszlovszky, I.; Németh, G.; Vieta, E.; Calabrese, J.R.; Yatham, L.N. An 8–week randomized, double-blind, placebo-controlled evaluation of the safety and efficacy of cariprazine in patients with bipolar I depression. Am. J. Psychiatry 2016, 173, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.V.; Saraf, G.; Vigo, V.; Keramatian, K.; Chakrabarty, T.; Yatham, L.N. Cariprazine in the treatment of bipolar disorder: A systematic review and meta-analysis. Bipolar Disord. 2020, 22, 360–371. [Google Scholar] [CrossRef]
- Sanford, M.; Keating, G.M. Quetiapine: A review of its use in the management of bipolar depression. CNS Drugs 2012, 26, 435–460. [Google Scholar] [CrossRef]
- Lei, D.; Li, W.; Tallman, M.J.; Patino, L.R.; McNamara, R.K.; Strawn, J.R.; Klein, C.C.; Nery, F.G.; Fleck, D.E.; Qin, K.; et al. Changes in the brain structural connectome after a prospective randomized clinical trial of lithium and quetiapine treatment in youth with bipolar disorder. Neuropsychopharmacology 2021, 46, 1315–1323. [Google Scholar] [CrossRef]
- Lai, J.; Lu, Q.; Huang, T.; Hu, S.; Xu, Y. Convulsive syncope related to a small dose of quetiapine in an adolescent with bipolar disorder. Neuropsychiatr. Dis. Treat. 2017, 13, 1905–1908. [Google Scholar] [CrossRef][Green Version]
- Masi, G.; Milone, A.; Stawinoga, A.; Veltri, S.; Pisano, S. Efficacy and safety of risperidone and quetiapine in adolescents with bipolar II disorder comorbid with conduct disorder. J. Clin. Psychopharmacol. 2015, 35, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Muneer, A. Pharmacotherapy of bipolar disorder with quetiapine: A recent literature review and an update. Clin. Psychopharmacol. Neurosci. 2015, 13, 25–35. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gonzalez-Pinto, A.; Gonzalez, C.; Enjuto, S.; Fernandez de Corres, B.; Lopez, P.; Palomo, J.; Gutierrez, M.; Mosquera, F.; de Heredia, J.L.P. Psychoeducation and cognitive-behavioral therapy in bipolar disorder: An update. Acta Psychiatr. Scand. 2004, 109, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Henken, H.T.; Kupka, R.W.; Draisma, S.; Lobbestael, J.; van den Berg, K.; Demacker, S.M.A.; Regeer, E.J. A cognitive behavioural group therapy for bipolar disorder using daily mood monitoring. Behav. Cogn. Psychother. 2020, 48, 515–529. [Google Scholar] [CrossRef]
- Chiang, K.J.; Tsai, J.C.; Liu, D.; Lin, C.H.; Chiu, H.L.; Chou, K.R. Efficacy of cognitive-behavioral therapy in patients with bipolar disorder: A meta-analysis of randomized controlled trials. PLoS ONE 2017, 12, e0176849. [Google Scholar] [CrossRef]
- Deckersbach, T.; Eisner, L.; Sylvia, L. Cognitive behavioral therapy for bipolar disorder. In The Massachusetts General Hospital Handbook of Cognitive Behavioral Therapy; Petersen, T.J., Sprich, S.E., Wilhelm, S., Eds.; Humana Press: Totowa, NJ, USA; Springer Nature: Berlin, Germany, 2016; pp. 87–103. [Google Scholar]
- Lam, D.H.; Hayward, P.; Watkins, E.R.; Wright, K.; Sham, P. Relapse prevention in patients with bipolar disorder: Cognitive therapy outcome after 2 years. Am. J. Psychiatry 2005, 162, 324–329. [Google Scholar] [CrossRef]
- Ye, B.; Jiang, Z.; Li, X.; Cao, B.; Cao, L.; Lin, Y.; Xu, G.; Miao, G. Effectiveness of cognitive behavioral therapy in treating bipolar disorder: An updated meta-analysis with randomized controlled trials. Psychiatry Clin. Neurosci. 2016, 70, 351–361. [Google Scholar] [CrossRef]
- Scott, J.; Colom, F. Gaps and limitations of psychological interventions for bipolar disorders. Psychother. Psychosom. 2008, 22, 4–11. [Google Scholar] [CrossRef]
- Scott, J.; Paykel, E.; Morriss, R.; Bentall, R.; Kinderman, P.; Johnson, T.; Abbott, R.; Hayhurst, H. Cognitive-behavioral therapy for severe and recurrent bipolar disorders: Randomized controlled trial. Br. J. Psychiatry 2006, 188, 313–320. [Google Scholar] [CrossRef]
- Gregory, V. Cognitive-Behavioral therapy for depression in bipolar disorder: A meta-analysis. J. Evid. Based Soc. Work 2010, 7, 269–279. [Google Scholar] [CrossRef]
- Cuijpers, P.; Noma, H.; Karyotaki, E.; Cipriani, A.; Furukawa, T.A. Effectiveness and acceptability of cognitive behavior therapy delivery formats in adults with depression. JAMA Psychiatry 2019, 76, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Hamatani, S.; Matsumoto, K.; Shimizu, E. Cognitive behavioral therapy for three patients with bipolar II disorder during depressive episodes. Case Rep. Psychiatry 2020, 2020, 3892024. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.T.; Cheniaux, E.; Rosaes, P.A.; Carvalho, M.R.; Freire, R.C.; Versiani, M.; Rangé, B.P.; Nardi, A.E. The effectiveness of cognitive behavioral group therapy in treating bipolar disorder: A randomized controlled study. Braz. J. Psychiatry 2011, 33, 144–149. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pjrek, E.; Friedrich, M.E.; Cambioli, L.; Dold, M.; Jäger, F.; Komorowski, A.; Lanzenberger, R.; Kasper, S.; Winkler, D. The efficacy of light therapy in the treatment of seasonal affective disorder: A metaanalysis of randomized controlled trials. Psychother. Psychosom. 2020, 89, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Bais, B.; Kamperman, A.M.; Bijma, H.H.; Hoogendijk, W.J.; Souman, J.L.; Knijff, E.; Lambregtse-van den Berg, M.P. Effects of bright light therapy for depression during pregnancy: A randomised, double-blind controlled trial. BMJ Open 2020, 10, e038030. [Google Scholar] [CrossRef] [PubMed]
- Onega, L.L.; Pierce, T.W. Use of bright light therapy for older adults with dementia. BJPsych. Adv. 2020, 26, 221–228. [Google Scholar] [CrossRef]
- Johansson, V.; Kuja-Halkola, R.; Cannon, T.D.; Hultman, C.M.; Hedman, A.M. A population-based heritability estimate of bipolar disorder—In a Swedish twin sample. Psychiatry Res. 2019, 278, 180–187. [Google Scholar] [CrossRef]
- McGuffin, P.; Rijsdijk, F.; Andrew, M.; Sham, P.; Katz, R.; Cardno, A. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch. Gen. Psychiatry 2003, 60, 497–502. [Google Scholar] [CrossRef]
- Evans, M.; Rohan, K.J.; Sitnikov, L.; Mahon, J.N.; Nillni, Y.I.; Lindsey, K.T.; Vacekc, P.M. Cognitive change across cognitive-behavioral and light therapy treatments for seasonal affective disorder: What accounts for clinical status the next winter? Cognit. Ther. Res. 2013, 37, 1201–1213. [Google Scholar] [CrossRef]
- Knapen, S.E.; van de Werken, M.; Gordijn, M.C.M.; Meesters, Y. The duration of light treatment and therapy outcome in seasonal affective disorder. J. Affect. Disord. 2014, 166, 343–346. [Google Scholar] [CrossRef]
- Maruani, J.; Geoffroy, P.A. Bright light as a personalized precision treatment of mood disorders. Front. Psychiatry 2019, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, M.; Utsumi, T.; Aoki, Y.; Wang, Z.; Suzuki, M.; Okajima, I.; Watanabe, N.; Watanabe, K.; Takaesu, Y. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: A systematic review and meta-analysis. Psychiatry Clin. Neurosci. 2020, 74, 247–256. [Google Scholar] [CrossRef]
- Nasr, S.J.; Elmaadawi, A.Z.; Patel, R. Bright light therapy for bipolar depression. Curr. Psychiatry 2018, 17, 28–32. [Google Scholar]
- Geoffroy, P.A.; Schroder, C.M.; Bourgin, P. Light treatment in depression: An antique treatment with new insights. Sleep Med. Rev. 2018, 40, 218–219. [Google Scholar] [CrossRef] [PubMed]
- Al-Karawi, D.; Jubair, L. Bright light therapy for nonseasonal depression: Meta-analysis of clinical trials. J. Affect. Disord. 2016, 198, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Mårtensson, B.; Pettersson, A.; Berglund, L.; Ekselius, L. Bright white light therapy in depression: A critical review of the evidence. J. Affect. Disord. 2015, 182, 1–7. [Google Scholar] [CrossRef]
- Perera, S.; Eisen, R.; Bhatt, M.; Bhatnagar, N.; de Souza, R.; Thabane, L.; Samaan, Z. Light therapy for non-seasonal depression: Systematic review and meta-analysis. BJPsych. Open 2016, 2, 116–126. [Google Scholar] [CrossRef]
- Tseng, P.T.; Chen, Y.W.; Tu, K.Y.; Chung, W.; Wang, H.Y.; Wu, C.K.; Lin, P.Y. Light therapy in the treatment of patients with bipolar depression: A meta-analytic study. Eur. Neuropsychopharmacol. 2016, 26, 1037–1047. [Google Scholar] [CrossRef]
- Fries, G.R.; Zamzow, M.J.; Andrews, T.; Pink, O.; Scaini, G.; Quevedo, J. Accelerated aging in bipolar disorder: A comprehensive review of molecular findings and their clinical implications. Neurosci. Biobehav. Rev. 2020, 112, 107–116. [Google Scholar] [CrossRef]
- Sit, D.; McGowan, J.; Wiltrout, C.; Weingarden, J.; Diler, R.S.; Dills, J.L.; Luther, J.F.; Seltman, H.; Terman, M.; Wisniewski, S.; et al. A randomized, placebo-controlled trial of light therapy for bipolar depression: Antidepressant efficacy, side effects, changes in suicidality and sleep. Biol. Psychiatry 2015, 77, 66S. [Google Scholar]
- Sit, D.K.; McGowan, J.; Wiltrout, C.; Diler, R.S.; Dills, J.; Luther, J.; Yang, A.; Ciolino, J.D.; Seltman, H.; Wisniewski, S.; et al. Adjunctive bright light therapy for bipolar depression: A randomized double-blind placebo-controlled trial. Am. J. Psychiatry 2018, 175, 131–139. [Google Scholar] [CrossRef]
- Desautels, C.; Savard, J.; Ivers, H. Moderators of cognitive therapy and bright light therapy effects on depressive symptoms in patients with breast cancer. Int. J. Behav. Med. 2019, 26, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Lam, R.W.; Teng, M.Y.; Jung, Y.E.; Evans, V.C.; Gottlieb, J.F.; Chakrabarty, T.; Michalak, E.E.; Murphy, J.K.; Yatham, L.N.; Sit, D.K. Light therapy for patients with bipolar depression: Systematic review and meta-analysis of randomized controlled trials. Can. J. Psychiatry 2020, 65, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Geoffroy, P.A.; Schroder, C.M.; Reynaud, E.; Bourgin, P. Efficacy of light therapy versus antidepressant drugs, and of the combination versus monotherapy, in major depressive episodes: A systematic review and meta-analysis. Sleep Med. Rev. 2019, 48, 101213. [Google Scholar] [CrossRef]
- Kupeli, N.Y.; Bulut, N.S.; Bulut, G.C.; Kurt, E.; Kora, K. Efficacy of bright light therapy in bipolar depression. Psychiatry Res. 2018, 260, 432–438. [Google Scholar] [CrossRef]
- Benedetti, F.; Avery, D.H.; Bauer, M.; Bunney, W.E.; Caliyurt, O.; Camardese, G.; Colombo, C.; Dallaspezia, S.; Henriksen, T.E.; Kasper, S.; et al. Evidence for the efficacy of bright light therapy for bipolar depression. Am. J. Psychiatry 2018, 175, 905–906. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, H.; Terao, T.; Muronaga, M.; Ishii, N. Adjunctive bright light therapy for treating bipolar depression: A systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Zhou, T.H.; Dang, W.M.; Ma, Y.T.; Hu, C.Q.; Wang, N.; Zhang, G.Y.; Wang, G.; Shi, C.; Zhang, H.; Guo, B.; et al. Clinical efficacy, onset time and safety of bright light therapy in acute bipolar depression as an adjunctive therapy: A randomized controlled trial. J. Affect. Disord. 2018, 227, 90–96. [Google Scholar] [CrossRef]
- Holmes, E.A.; Hales, S.A.; Young, K.; Di Simplicio, M. Imagery-Based Cognitive Therapy for Bipolar Disorder and Mood Instability; Guilford Press: New York, NY, USA, 2019. [Google Scholar]
- Hales, S.A.; Di Simplicio, M.; Iyadurai, L.; Blackwell, S.E.; Young, K.; Fairburn, C.G.; Geddes, J.R.; Goodwin, G.M.; Holmes, E.A. Imagery-focused cognitive therapy (ImCT) for mood instability and anxiety in a small sample of patients with bipolar disorder: A pilot clinical audit. Behav. Cogn. Psychother. 2018, 46, 706–725. [Google Scholar] [CrossRef] [PubMed]
- Versiani, M.; Cheniaux, E.; Landeira-Fernandez, J. Efficacy and safety of electroconvulsive therapy in the treatment of bipolar disorder: A systematic review. J. ECT 2011, 27, 153–164. [Google Scholar] [CrossRef]
- Liebman, L.S.; Ahle, G.M.; Briggs, M.C.; Kellner, C.H. Electroconvulsive therapy and bipolar disorder. In The Bipolar Book: History, Neurobiology, and Treatment; Yildiz, A., Ruiz, P., Nemeroff, C.B., Eds.; Oxford University Press: Oxford, UK, 2015; pp. 367–375. [Google Scholar]
- Lv, Q.; Hu, Q.; Zhang, W.; Huang, X.; Zhu, M.; Geng, R.; Cheng, X.; Bao, C.; Wang, Y.; Zhang, C.; et al. Disturbance of oxidative stress parameters in treatment-resistant bipolar disorder and their association with electroconvulsive therapy Response. Int. J. Neuropsychopharmacol. 2020, 23, 207–216. [Google Scholar] [CrossRef]
- Başgül, Ş.S.; Luş, M.G.; Hashimov, A. Electroconvulsive therapy in an adolescent with bipolar disorder, substance use, and body dysmorphic disorder comorbidity: Case report. Neurocase 2020, 26, 51–54. [Google Scholar] [CrossRef]
- Schoeyen, H.K.; Kessler, U.; Andreassen, O.A.; Auestad, B.H.; Bergsholm, P.; Malt, U.F.; Morken, G.; Oedegaard, K.J.; Vaaler, A. Treatment-resistant bipolar depression: A randomized controlled trial of electroconvulsive therapy versus algorithm based pharmacological treatment. Am. J. Psychiatry 2015, 172, 41–51. [Google Scholar] [CrossRef]
- Perugi, G.; Medda, P.; Toni, C.; Mariani, M.G.; Socci, C.; Mauri, M. The role of Electroconvulsive Therapy (ECT) in bipolar disorder: Effectiveness in 522 patients with bipolar depression, mixed-state, mania and catatonic features. Curr. Neuropharmacol. 2017, 15, 359–371. [Google Scholar] [CrossRef]
- Fink, M.; Kellner, C.H.; McCall, W.V. The role of ECT in suicide prevention. J. ECT 2014, 30, 5–9. [Google Scholar] [CrossRef]
- Liang, C.; Chung, C.; Ho, P.; Tsai, C.; Chien, W. Superior anti-suicidal effects of electroconvulsive therapy in unipolar disorder and bipolar depression. Bipolar Disord. 2018, 20, 539–546. [Google Scholar] [CrossRef]
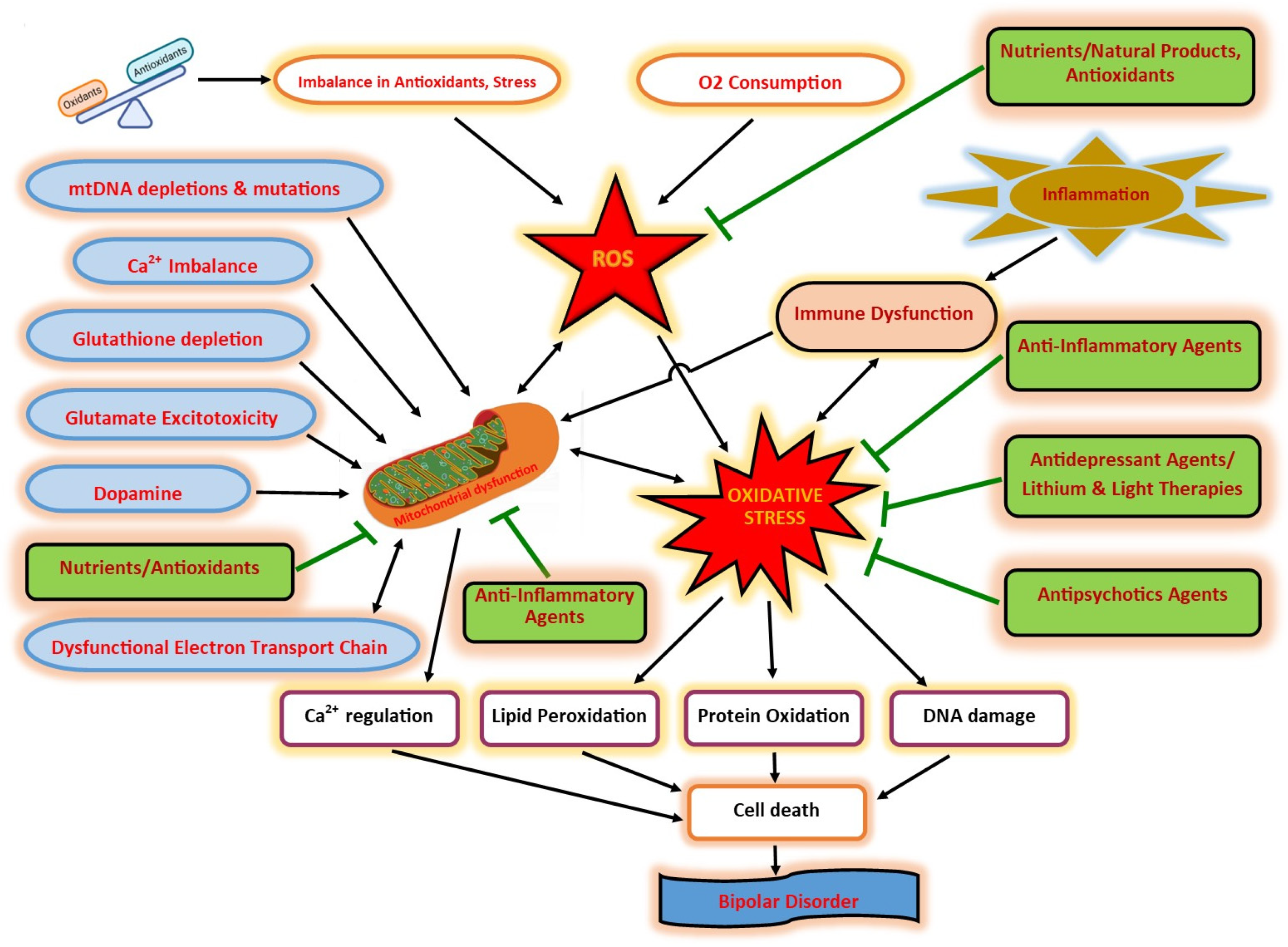
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madireddy, S.; Madireddy, S. Therapeutic Interventions to Mitigate Mitochondrial Dysfunction and Oxidative Stress–Induced Damage in Patients with Bipolar Disorder. Int. J. Mol. Sci. 2022, 23, 1844. https://doi.org/10.3390/ijms23031844
Madireddy S, Madireddy S. Therapeutic Interventions to Mitigate Mitochondrial Dysfunction and Oxidative Stress–Induced Damage in Patients with Bipolar Disorder. International Journal of Molecular Sciences. 2022; 23(3):1844. https://doi.org/10.3390/ijms23031844
Chicago/Turabian StyleMadireddy, Sahithi, and Samskruthi Madireddy. 2022. "Therapeutic Interventions to Mitigate Mitochondrial Dysfunction and Oxidative Stress–Induced Damage in Patients with Bipolar Disorder" International Journal of Molecular Sciences 23, no. 3: 1844. https://doi.org/10.3390/ijms23031844
APA StyleMadireddy, S., & Madireddy, S. (2022). Therapeutic Interventions to Mitigate Mitochondrial Dysfunction and Oxidative Stress–Induced Damage in Patients with Bipolar Disorder. International Journal of Molecular Sciences, 23(3), 1844. https://doi.org/10.3390/ijms23031844




