Minocycline Counteracts Ectopic Calcification in a Murine Model of Pseudoxanthoma Elasticum: A Proof-of-Concept Study
Abstract
:1. Introduction
2. Results
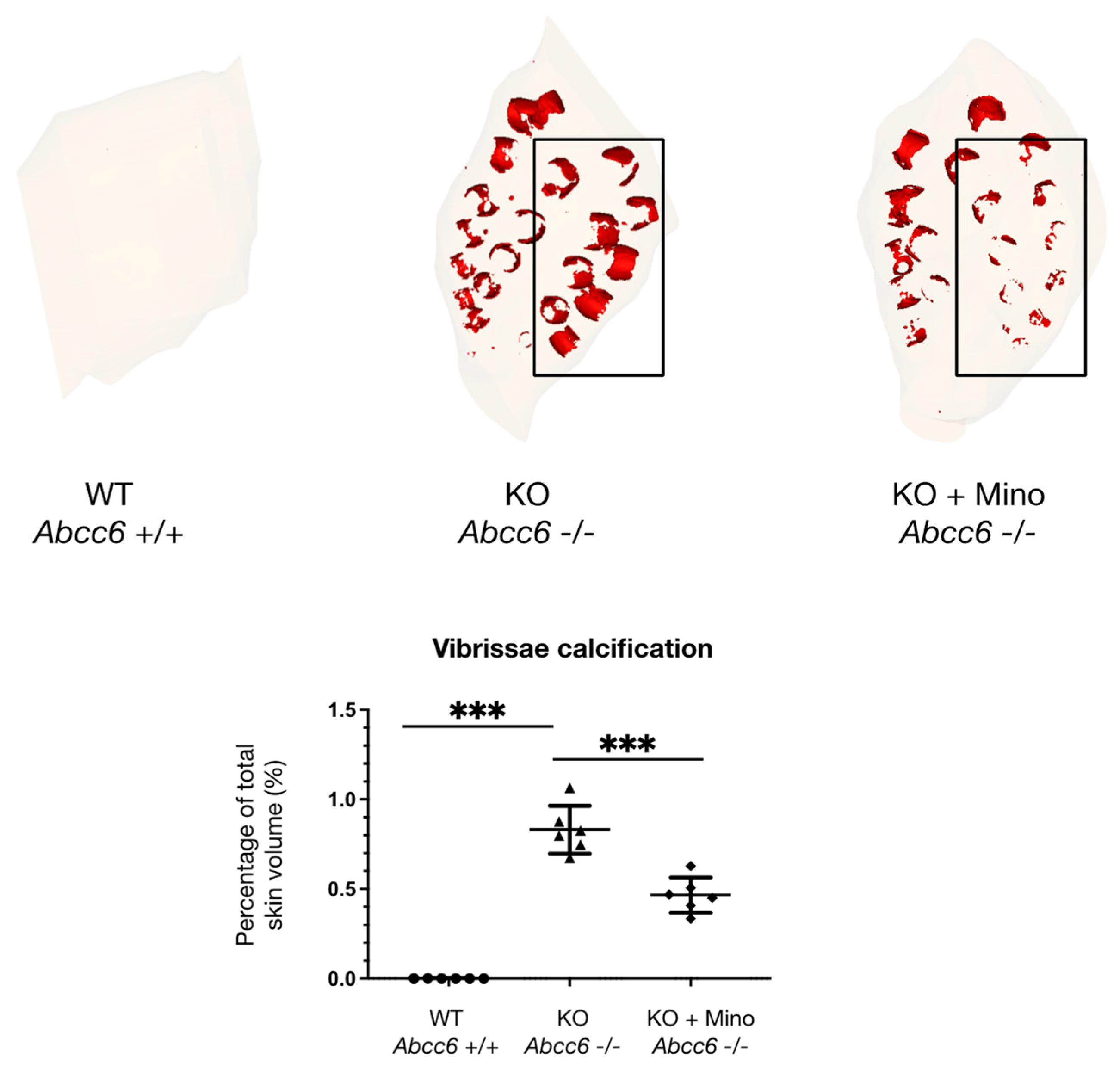
2.1. Oral Minocycline Treatment Significantly Reduces Ectopic Calcification in Abcc6−/− Mice
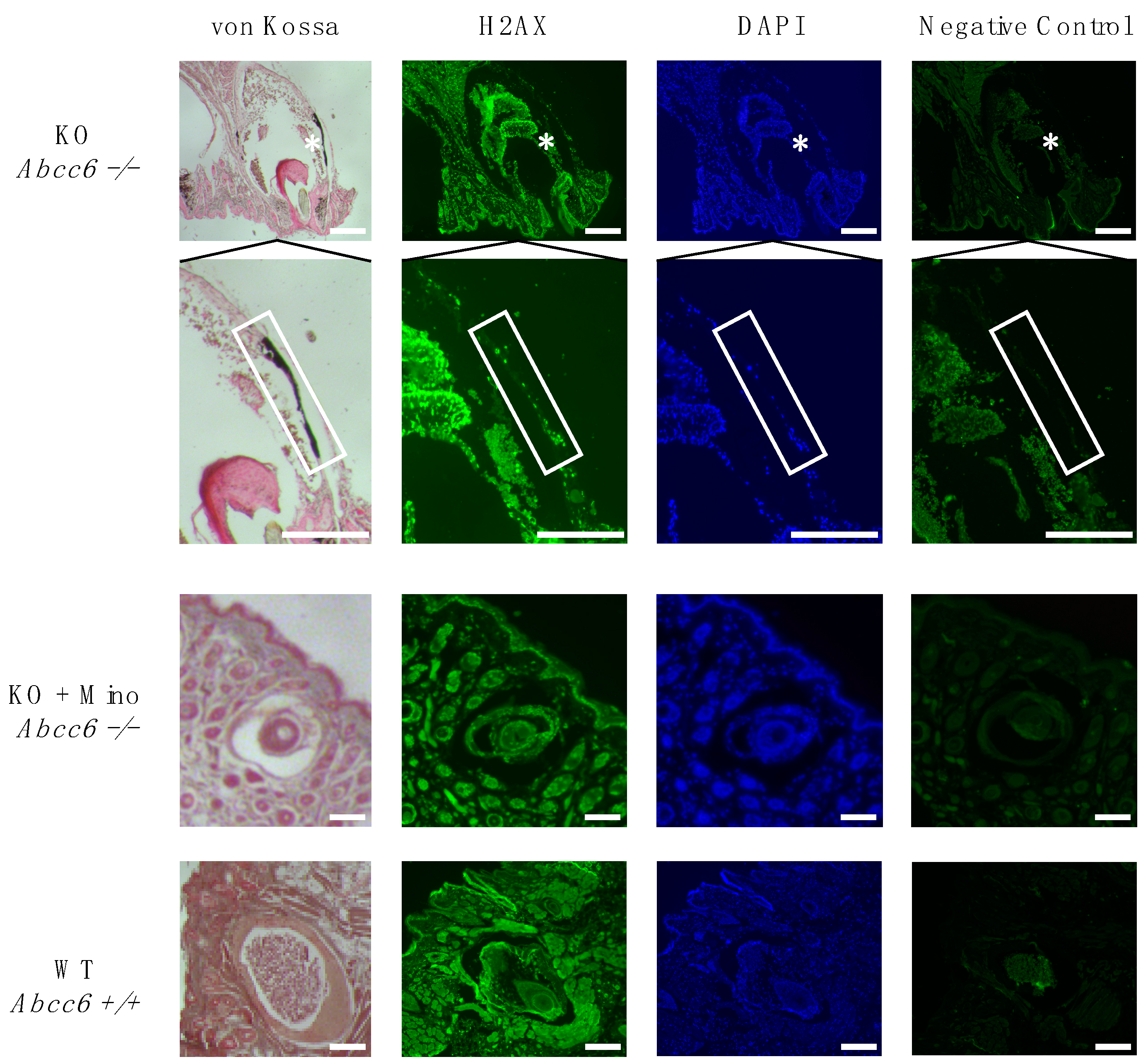
2.2. Activation of the DNA Damage Response Colocalizes with Ectopic Calcification in Abcc6-Deficient Mice
3. Discussion
4. Materials and Methods
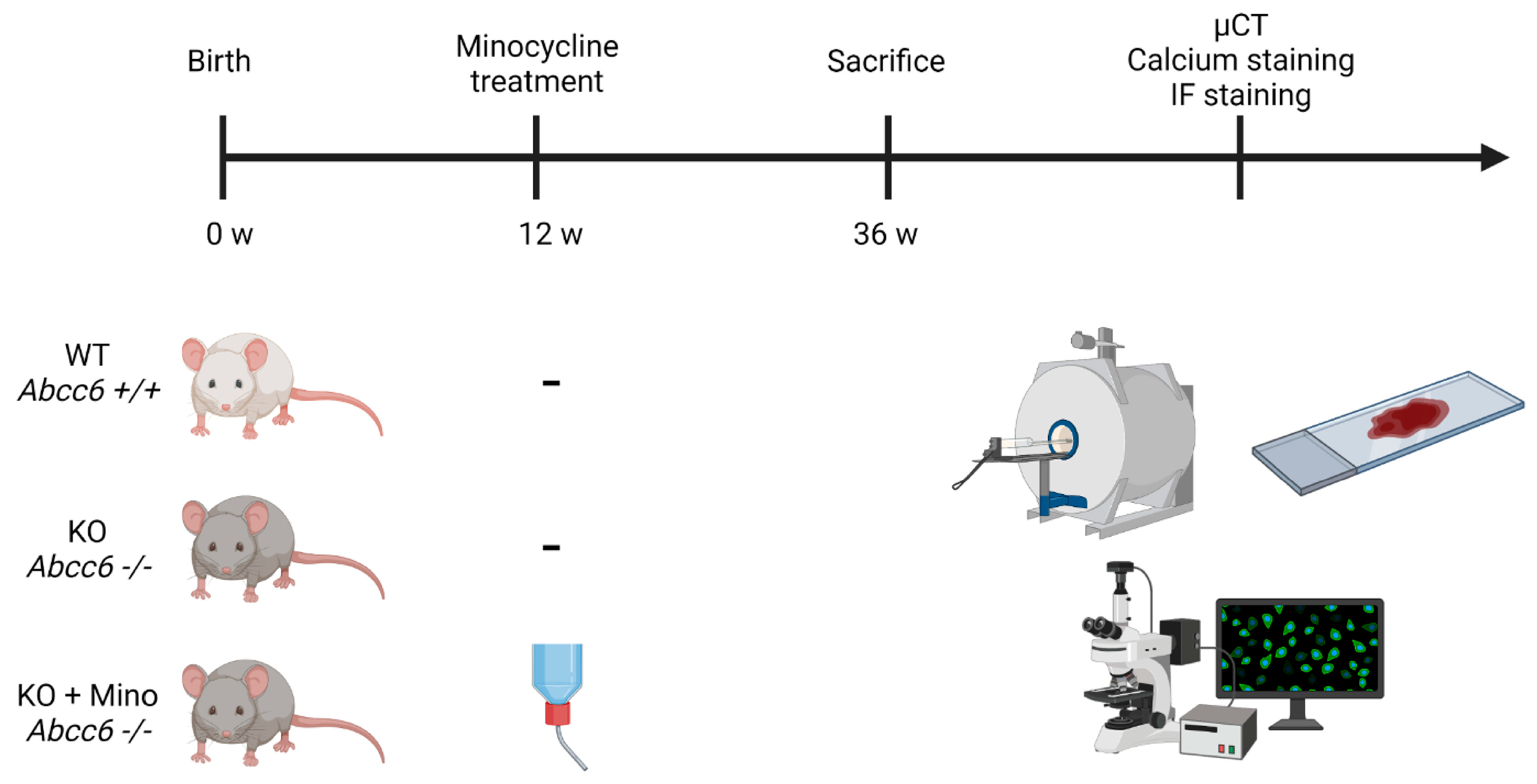
4.1. Animal Studies
4.2. Histopathology and Yasue Calcium Staining of Muzzle Skin
4.3. X-ray Microtomography and Three-Dimensional Reconstruction of Vibrissae Sheath Calcification
4.4. Immunofluorescence and Von Kossa Staining of Muzzle Skin
4.5. Statistical analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Vilder, E.Y.; Vanakker, O.M. From variome to phenome: Pathogenesis, diagnosis and management of ectopic mineralization disorders. World J. Clin. Cases 2015, 3, 556–574. [Google Scholar] [CrossRef] [PubMed]
- Nollet, L.; Van Gils, M.; Verschuere, S.; Vanakker, O. The Role of Vitamin K and Its Related Compounds in Mendelian and Acquired Ectopic Mineralization Disorders. Int. J. Mol. Sci. 2019, 20, 2142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosen, M.J.; Coucke, P.J.; Le Saux, O.; De Paepe, A.; Vanakker, O.M. Perturbation of specific pro-mineralizing signalling pathways in human and murine pseudoxanthoma elasticum. Orphanet J. Rare Dis. 2014, 9, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boraldi, F.; Annovi, G.; Vermeer, C.; Schurgers, L.J.; Trenti, T.; Tiozzo, R.; Guerra, D.; Quaglino, D. Matrix Gla Protein and Alkaline Phosphatase Are Differently Modulated in Human Dermal Fibroblasts from PXE Patients and Controls. J. Investig. Dermatol. 2013, 133, 946–954. [Google Scholar] [CrossRef] [Green Version]
- Lanzer, P.; Hannan, F.M.; Lanzer, J.D.; Janzen, J.; Raggi, P.; Furniss, D.; Schuchardt, M.; Thakker, R.; Fok, P.W.; Saez-Rodriguez, J.; et al. Medial arterial calcification: JACC state-of-the-art review. J. Am. Coll Cardiol. 2021, 78, 1145–1165. [Google Scholar] [CrossRef]
- Luscher, T.F.; Creager, M.A.; Beckman, J.A.; Cosentino, F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part II. Circulation 2003, 108, 1655–1661. [Google Scholar] [CrossRef]
- Chen, J.; Budoff, M.J.; Reilly, M.; Yang, W.; Rosas, S.E.; Rahman, M.; Zhang, X.; Roy, J.A.; Lustigova, E.; Nessel, L.; et al. Coronary Artery Calcification and Risk of Cardiovascular Disease and Death Among Patients With Chronic Kidney Disease. JAMA Cardiol. 2017, 2, 635–643. [Google Scholar] [CrossRef] [Green Version]
- Le Saux, O.; Urban, Z.; Tschuch, C.; Csiszar, K.; Bacchelli, B.; Quaglino, D.; Pasquali-Ronchetti, I.; Pope, F.M.; Richards, A.; Terry, S.F.; et al. Mutations in a gene encoding an ABC transporter cause pseudoxanthoma elasticum. Nat. Genet. 2000, 25, 223–227. [Google Scholar] [CrossRef]
- Verschuere, S.; Van Gils, M.; Nollet, L.; Vanakker, O.M. From membrane to mineralization: The curious case of the ABCC6 transporter. FEBS Lett. 2020, 594, 4109–4133. [Google Scholar] [CrossRef]
- Scheffer, G.L.; Hu, X.; Pijnenborg, A.C.; Wijnholds, J.; Bergen, A.A.; Scheper, R.J. MRP6 (ABCC6) detection in normal human tissues and tumors. Lab. Investig. 2002, 82, 515–518. [Google Scholar] [CrossRef]
- Finger, R.P.; Charbel Issa, P.; Ladewig, M.S.; Götting, C.; Szliska, C.; Scholl, H.P.; Holz, F.G. Pseudoxanthoma Elasticum: Genetics, Clinical Manifestations and Therapeutic Approaches. Surv. Ophthalmol. 2009, 54, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Nollet, L.; Van Gils, M.; Willaert, A.; Coucke, P.J.; Vanakker, O.M. Minocycline Attenuates Excessive DNA Damage Response and Reduces Ectopic Calcification in Pseudoxanthoma Elasticum. J. Investig. Dermatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Duer, M.; Cobb, A.M.; Shanahan, C.M. DNA damage response: A molecular lynchpin in the pathobiology of arteriosclerotic calcification. Arterioscler. Thromb. Vasc. Biol. 2020, 40, e193–e202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, X.; Wang, J.-K.; Li, Y.; Cui, S.-J.; Wang, Z.; Luo, T. Phenotypic Switching of Atherosclerotic Smooth Muscle Cells is Regulated by Activated PARP1-Dependent TET1 Expression. J. Atheroscler. Thromb. 2020, 28, 55343–55729. [Google Scholar] [CrossRef]
- Wang, C.; Xu, W.; An, J.; Liang, M.; Li, Y.; Zhang, F.; Tong, Q.; Huang, K. Poly(ADP-ribose) polymerase 1 accelerates vascular calcification by upregulating Runx2. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Müller, K.H.; Hayward, R.; Rajan, R.; Whitehead, M.; Cobb, A.M.; Ahmad, S.; Sun, M.; Goldberga, I.; Li, R.; Bashtanova, U.; et al. Poly(ADP-Ribose) Links the DNA Damage Response and Biomineralization. Cell Rep. 2019, 27, 3124–3138. [Google Scholar] [CrossRef] [Green Version]
- Bartoli-Leonard, F.; Wilkinson, F.L.; Schiro, A.; Inglott, F.S.; Alexander, M.Y.; Weston, R. Loss of SIRT1 in diabetes accelerates DNA damage-induced vascular calcification. Cardiovasc. Res. 2020, 117, 836–849. [Google Scholar] [CrossRef]
- Li, P.; Wang, Y.; Liu, X.; Liu, B.; Wang, Z.Y.; Xie, F.; Qiao, W.; Liang, E.S.; Lu, Q.H.; Zhang, M.X. Loss of PARP-1 attenuates diabetic arteriosclerotic calcification via Stat1/Runx2 axis. Cell Death Dis. 2020, 11, 22. [Google Scholar] [CrossRef]
- Gorgels, T.G.; Hu, X.; Scheffer, G.L.; van der Wal, A.; Toonstra, J.; De Jong, P.T.; Van Kuppevelt, T.H.; Levelt, C.N.; De Wolf, A.; Loves, W.J.; et al. Disruption of Abcc6 in the mouse: Novel insight in the pathogenesis of pseudoxanthoma elasticum. Hum. Mol. Genet. 2005, 14, 1763–1773. [Google Scholar] [CrossRef] [Green Version]
- Klement, J.F.; Matsuzaki, Y.; Jiang, Q.-J.; Terlizzi, J.; Choi, H.Y.; Fujimoto, N.; Li, K.; Pulkkinen, L.; Birk, D.E.; Sundberg, J.P.; et al. Targeted Ablation of the Abcc6 Gene Results in Ectopic Mineralization of Connective Tissues. Mol. Cell. Biol. 2005, 25, 8299–8310. [Google Scholar] [CrossRef] [Green Version]
- LaRusso, J.; Li, Q.; Jiang, Q.; Uitto, J. Elevated dietary magnesium prevents connective tissue mineralization in a mouse model of pseudoxanthoma elasticum (Abcc6(-/-)). J. Investig. Dermatol. 2009, 129, 1388–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letavernier, E.; Kauffenstein, G.; Huguet, L.; Navasiolava, N.; Bouderlique, E.; Tang, E.; Delaitre, L.; Bazin, D.; De Frutos, M.; Gay, C.; et al. ABCC6 Deficiency Promotes Development of Randall Plaque. J. Am. Soc. Nephrol. 2018, 29, 2337–2347. [Google Scholar] [CrossRef] [Green Version]
- Bouderlique, E.; Tang, E.; Perez, J.; Coudert, A.; Bazin, D.; Verpont, M.-C.; Duranton, C.; Rubera, I.; Haymann, J.-P.; Leftheriotis, G.; et al. Vitamin D and Calcium Supplementation Accelerates Randall’s Plaque Formation in a Murine Model. Am. J. Pathol. 2019, 189, 2171–2180. [Google Scholar] [CrossRef]
- Garrido-Mesa, N.; Zarzuelo, A.; Galvez, J. Minocycline: Far beyond an antibiotic. J. Cereb. Blood Flow Metab. 2013, 169, 337–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mungrue, I.N.; Zhao, P.; Yao, Y.; Meng, H.; Rau, C.; Havel, J.V.; Gorgels, T.G.; Bergen, A.A.; MacLellan, W.R.; Drake, T.A.; et al. Abcc6 Deficiency Causes Increased Infarct Size and Apoptosis in a Mouse Cardiac Ischemia-Reperfusion Model. Arter. Thromb. Vasc. Biol. 2011, 31, 2806–2812. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.-J.; Seo, I.; Jha, B.K.; Suh, S.-I.; Suh, M.-H.; Baek, W.-K. Minocycline inhibits angiogenesis in vitro through the translational suppression of HIF-1α. Arch. Biochem. Biophys. 2014, 545, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Finger, R.P.; Fenwick, E.; Marella, M.; Issa, P.C.; Scholl, H.P.; Holz, F.G.; Lamoureux, E.L. The relative impact of vision impairment and cardiovascular disease on quality of life: The example of pseudoxanthoma elasticum. Health Qual. Life Outcomes 2011, 9, 113. [Google Scholar] [CrossRef] [Green Version]
- Klein, N.C.; Cunha, B.A. Tetracyclines. Med. Clin. N. Am. 1995, 79, 789–801. [Google Scholar] [CrossRef]
- Sánchez, A.R.; Rogers, R.S., 3rd; Sheridan, P.J. Tetracycline and other tetracycline-derivative staining of the teeth and oral cavity. Int J. Dermatol. 2004, 43, 709–715. [Google Scholar] [CrossRef]
- Berger, N.A.; Besson, V.C.; Boulares, A.H.; Bürkle, A.; Chiarugi, A.; Clark, R.S.; Curtin, N.J.; Cuzzocrea, S.; Dawson, T.M.; Dawson, V.L.; et al. Opportunities for the repurposing of PARP inhibitors for the therapy of non-oncological diseases. Br. J. Pharmacol. 2018, 175, 192–222. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouderlique, E.; Nollet, L.; Letavernier, E.; Vanakker, O.M. Minocycline Counteracts Ectopic Calcification in a Murine Model of Pseudoxanthoma Elasticum: A Proof-of-Concept Study. Int. J. Mol. Sci. 2022, 23, 1838. https://doi.org/10.3390/ijms23031838
Bouderlique E, Nollet L, Letavernier E, Vanakker OM. Minocycline Counteracts Ectopic Calcification in a Murine Model of Pseudoxanthoma Elasticum: A Proof-of-Concept Study. International Journal of Molecular Sciences. 2022; 23(3):1838. https://doi.org/10.3390/ijms23031838
Chicago/Turabian StyleBouderlique, Elise, Lukas Nollet, Emmanuel Letavernier, and Olivier M. Vanakker. 2022. "Minocycline Counteracts Ectopic Calcification in a Murine Model of Pseudoxanthoma Elasticum: A Proof-of-Concept Study" International Journal of Molecular Sciences 23, no. 3: 1838. https://doi.org/10.3390/ijms23031838
APA StyleBouderlique, E., Nollet, L., Letavernier, E., & Vanakker, O. M. (2022). Minocycline Counteracts Ectopic Calcification in a Murine Model of Pseudoxanthoma Elasticum: A Proof-of-Concept Study. International Journal of Molecular Sciences, 23(3), 1838. https://doi.org/10.3390/ijms23031838





