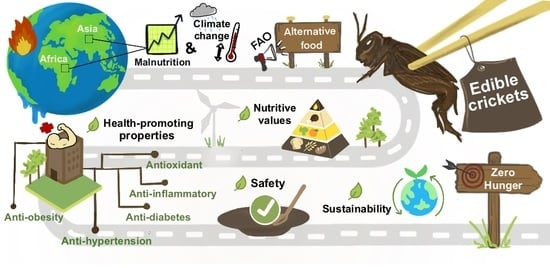
Road to The Red Carpet of Edible Crickets through Integration into the Human Food Chain with Biofunctions and Sustainability: A Review
Abstract
1. Introduction
2. Approach
3. Distribution of Edible Crickets
4. Nutritive Values of Edible Crickets
5. Health Benefits of Edible Crickets
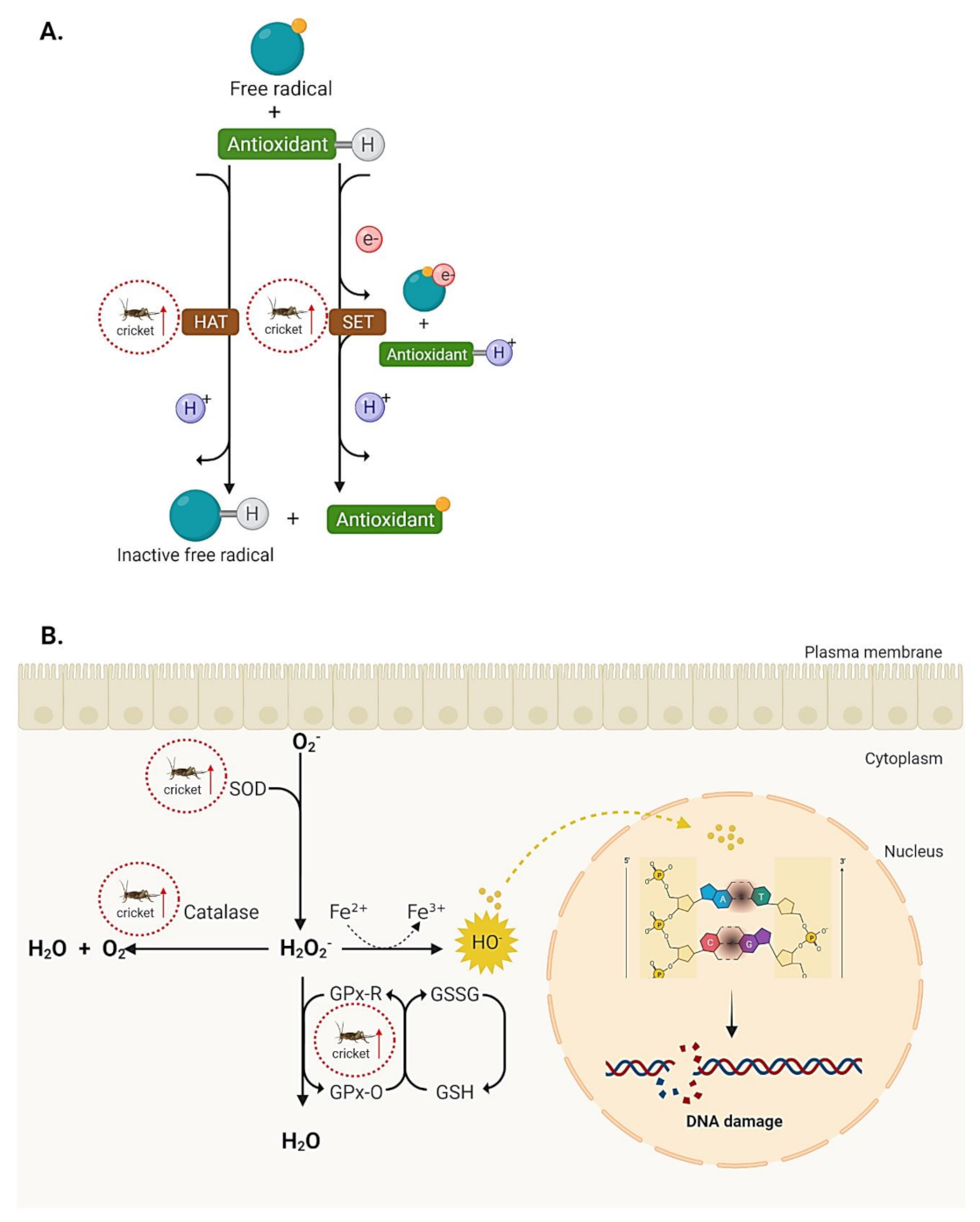
5.1. Antioxidant Activities
| Species | Extraction Methods | Antioxidant Methods | Antioxidant Activities | Ref. |
|---|---|---|---|---|
| A. domesticus | ultrasound-assisted extraction | DPPH | + | [68] |
| pressurized liquid extraction | DPPH | + | [68] | |
| enzymatic hydrolysis | DPPH | + | [70] | |
| aqueous extraction | FRAP | + | [69] | |
| aqueous extraction | ABTS | + | [69] | |
| hexane extraction | ABTS | + | [69] | |
| G. bimaculatus | ethanolic extraction | DPPH | + | [72] |
| aqueous extraction | ABTS | + | [72] | |
| protein isolate | DPPH | + | [73] | |
| subcritical water hydrolysates | DPPH | + | [77] | |
| subcritical water hydrolysates | FRAP | + | [77] | |
| fermented with probiotics | DPPH | + | [78] | |
| fermented with probiotics | FRAP | + | [78] | |
| fermented with probiotics | SOD-like activity | + | [78] | |
| aqueous extraction | Prevention of 8-OHdG formation | + | [72] | |
| ethanolic extraction | Prevention of 8-OHdG formation | + | [79] | |
| ethanolic extraction | DCFDA | + | [74] | |
| insect powder | Total free radicals | + | [76] | |
| insect powder | Catalase activity | + | [76] | |
| insect powder | GPx activity | + | [76] | |
| insect powder | SOD activity | + | [76] |
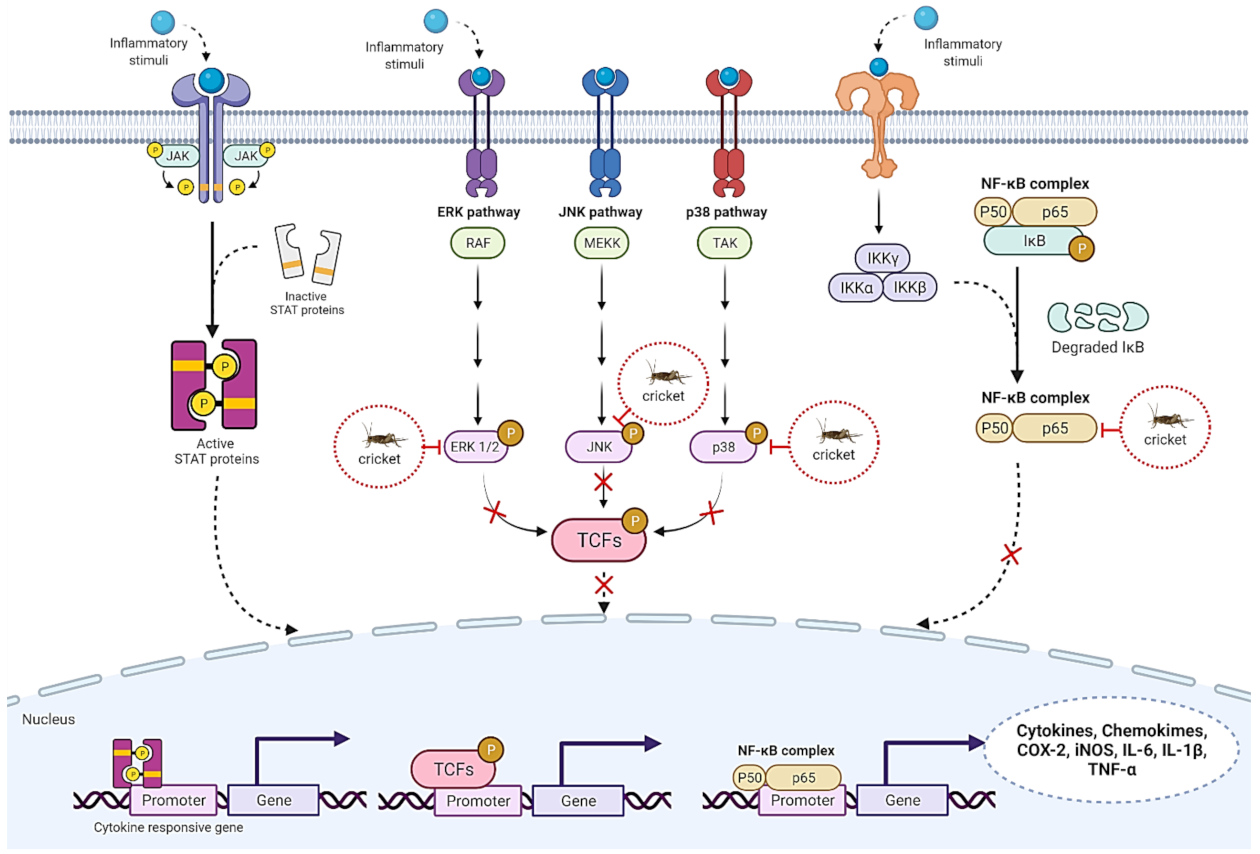
5.2. Anti-Inflammatory Properties
5.3. Anti-Diabetic Properties
5.4. Anti-Obesity Properties
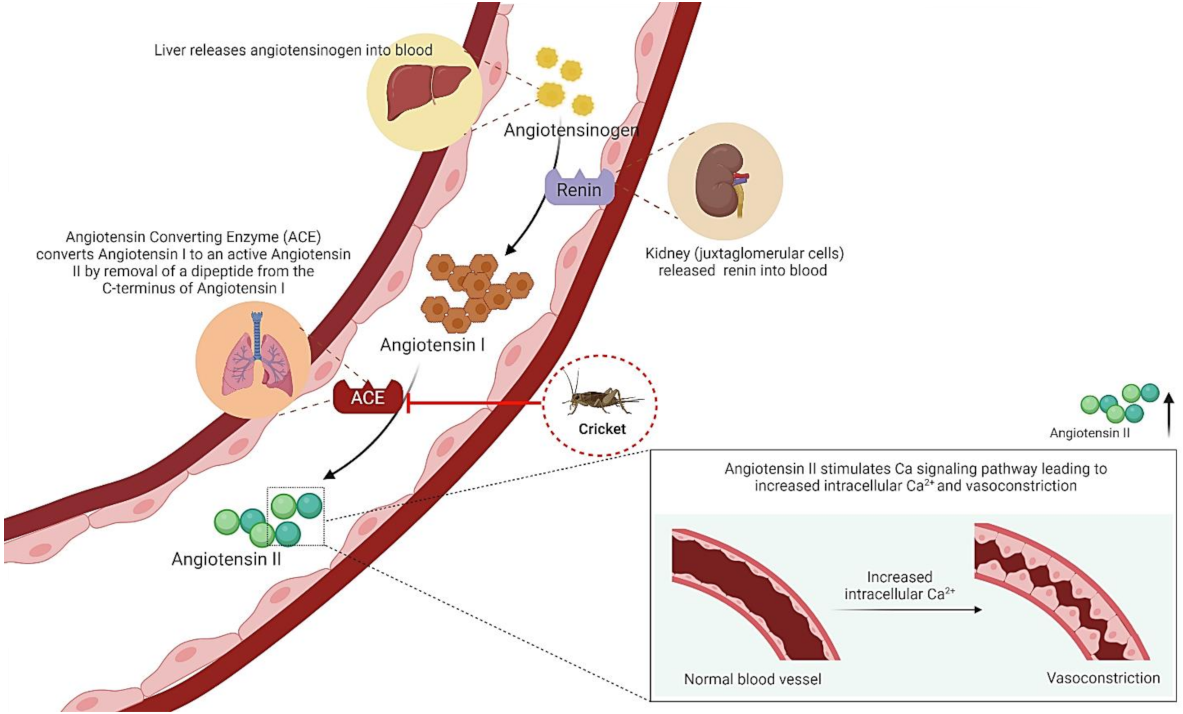
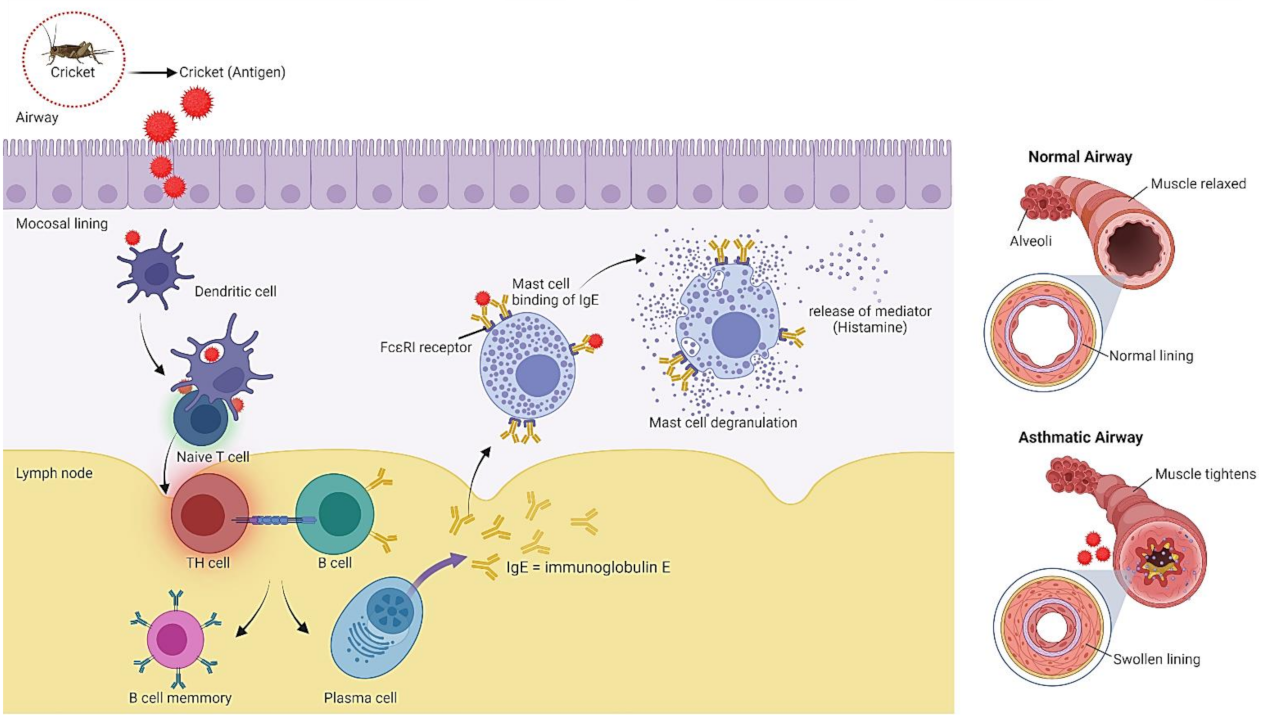
5.5. Other Biological Activities
6. Safety Aspects of Crickets
7. Sustainability of Edible Crickets
7.1. Reasons for Entomophagy
7.2. Environmental Aspects
7.3. Economics and Health
8. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | Angiotensin-converting enzyme |
| ADI | Acceptable daily intake |
| CFU | Colony forming unit |
| DM | % Dry matter |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl radicals |
| EFSA | The European Food Safety Authority |
| FAO | The Food and Agriculture Organization of the United Nations |
| GFP | Good Farming Practice |
| GMP | Good Manufacturing Practice |
| HACCP | Hazard Analysis Critical Control Points |
| IL | Interleukin |
| LPS | Lipopolysaccharide |
| LW-MWP | Low molecular weight peptides |
| MAPK | Mitogen-activated protein kinase |
| MUFA | Monounsaturated fatty acids |
| NCDs | Non-communicable diseases |
| NF-κB | Kappa-light-chain-enhancer of activated B cells |
| NO | Nitric oxide |
| NOAEL | No-observed-adverse-effect level |
| PUFA | Polyunsaturated fatty acids |
| ROS | Reactive oxygen species |
| SFA | Saturated fatty acids |
| TNF-α | Tumor necrosis factor alpha |
| UN | The United Nations |
References
- Grafton, R.Q.; Daugbjerg, C.; Qureshi, M.E. Towards food security by 2050. Food Secur. 2015, 7, 179–183. [Google Scholar] [CrossRef]
- Neto, H.R.L.; Chalfun, L.H.L. The increased consumption of animal products: Local aspirations-global opportunities. Adv. Biotech. Micro. 2018, 9, 65–68. [Google Scholar] [CrossRef]
- Kousar, S.; Ahmed, F.; Pervaiz, A.; Bojnec, Š. Food Insecuriy, Population Growth, Urbanization and Water Availability: The Role of Government Stability. Sustainability 2021, 13, 12336. [Google Scholar] [CrossRef]
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013. [Google Scholar]
- Top 5 Insects Propelling the Growth of Edible Insects Market. Available online: https://meticulousblog.org/top-5-insects-edible-insects-market/ (accessed on 14 December 2021).
- Van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Photo, A.S., Jr. Half Hours with Insects; Estes and Lauriat: Boston, MA, USA, 1877. [Google Scholar]
- Costa-Neto, E.M.; Dunkel, F.V. Insects as Food: History, Culture, and Modern Use around the World; Dossey, A.T., Morales-Ramos, J.A., Rojas, M.G., Eds.; Academic Press, Inc.: Cambridge, MA, USA, 2016; pp. 35–36. [Google Scholar]
- Giovanni, S.; Aijun, L.; Jie, L. Understanding Edible Insects as Food in Western and Eastern Societies. In Environmental, Health, and Business Opportunities in the New Meat Alternatives Market; Diana, B., Dora, M., Talia, R., Kurt, S., Eds.; IGI Global: Hershey, PA, USA, 2019; pp. 166–181. [Google Scholar] [CrossRef][Green Version]
- Kim, T.K.; Yong, H.I.; Kim, Y.B.; Kim, H.W.; Choi, Y.S. Edible Insects as a Protein Source: A Review of Public Perception, Processing Technology, and Research Trends. Food Sci. Anim. Resour. 2019, 39, 521–540. [Google Scholar] [CrossRef] [PubMed]
- Reverberi, M. Edible insects: Cricket farming and processing as an emerging market. J. Insects Food Feed 2020, 6, 211–220. [Google Scholar] [CrossRef]
- Yen, A.L. Insects as food and feed in the Asia Pacific region: Current perspectives and future directions. J. Insects Food Feed 2015, 1, 33–55. [Google Scholar] [CrossRef]
- Madau, F.A.; Arru, B.; Furesi, R.; Pulina, P. Insect Farming for Feed and Food Production from a Circular Business Model Perspective. Sustainability 2020, 12, 5418. [Google Scholar] [CrossRef]
- Magara, H.J.O.; Niassy, S.; Ayieko, M.A.; Mukundamago, M.; Egonyu, J.P.; Tanga, C.M.; Kimathi, E.K.; Ongere, J.O.; Fiaboe, K.K.M.; Hugel, S.; et al. Edible Crickets (Orthoptera) Around the World: Distribution, Nutritional Value, and Other Benefits-A Review. Front. Nutr. 2020, 7, 537915. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Looking at Edible Insects from a Food Safety Perspective; Food and Agriculture Organization of the United Nations: Rome, Italy, 2021; 90p. [Google Scholar]
- Grabowski, N.T.; Tchibozo, S.; Abdulmawjood, A.; Acheuk, F.; Guerfali, M.M.; Sayed, W.A.A.; Plötz, M. Edible Insects in Africa in Terms of Food, Wildlife Resource, and Pest Management Legislation. Foods 2020, 9, 502. [Google Scholar] [CrossRef]
- Zielińska, E.; Baraniak, B.; Karaś, M.; Rybczyńska, K.; Jakubczyk, A. Selected species of edible insects as a source of nutrient composition. Int. Food Res. J. 2015, 77, 460–466. [Google Scholar] [CrossRef]
- Hall, F.G.; Jones, O.G.; O’Haire, M.E.; Liceaga, A.M. Functional properties of tropical banded cricket (Gryllodes sigillatus) protein hydrolysates. Food Chem. 2017, 224, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Fuah, A.M.; Siregar, H.C.H.; Apri Astuti, D. Cricket Farming in Indonesia: Challenge and Opportunity; Lambert Academic Publishing: Bogor, Indonesia, 2016. [Google Scholar]
- van Huis, A. Edible crickets, but which species? J. Insects Food Feed 2020, 6, 91–94. [Google Scholar] [CrossRef]
- Miech, P.; Berggren, Å.; Lindberg, J.E.; Chhay, T.; Khieu, B.; Jansson, A. Growth and survival of reared Cambodian field crickets (Teleogryllus testaceus) fed weeds, agricultural and food industry by-products. J. Insects Food Feed 2016, 2, 285–292. [Google Scholar] [CrossRef]
- McCluney, K.E.; Date, R.C. The effects of hydration on growth of the house cricket, Acheta domesticus. J. Insect Sci. 2008, 8, 32. [Google Scholar] [CrossRef]
- Durrant, J.; Botha, L.M.; Green, M.P.; Jones, T.M. Artificial light at night prolongs juvenile development time in the black field cricket, Teleogryllus commodus. J. Exp. Zool. Part B Mol. Dev. Evol. 2018, 330, 225–233. [Google Scholar] [CrossRef]
- Levy, K.; Wegrzyn, Y.; Efronny, R.; Barnea, A.; Ayali, A. Lifelong exposure to artificial light at night impacts stridulation and locomotion activity patterns in the cricket Gryllus bimaculatus. Proc. R. Soc. Lond. B Biol. Sci. 2021, 288, 20211626. [Google Scholar] [CrossRef]
- Ngonga, C.A.; Gor, C.O.; Okuto, E.A.; Ayieko, M.A. Growth performance of Acheta domesticus and Gryllus bimaculatus production reared under improvised cage system for increased returns and food security. J. Insects Food Feed 2021, 7, 301–310. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; Finke, M.D. Nutritional value of insects and ways to manipulate their composition. J. Insects Food Feed 2021, 7, 639–659. [Google Scholar] [CrossRef]
- Bawa, M.; Songsermpong, S.; Kaewtapee, C.; Chanput, W. Effect of Diet on the Growth Performance, Feed Conversion, and Nutrient Content of the House Cricket. J. Insect Sci. 2020, 20, 10. [Google Scholar] [CrossRef]
- Finke, M.D. Complete nutrient content of three species of wild caught insects, pallid-winged grasshopper, rhinoceros beetles and white-lined sphinx moth. J. Insects Food Feed 2015, 1, 281–292. [Google Scholar] [CrossRef]
- Poma, G.; Cuykx, M.; Amato, E.; Calaprice, C.; Focant, J.F.; Covaci, A. Evaluation of hazardous chemicals in edible insects and insect-based food intended for human consumption. Food Chem. Toxicol. 2017, 100, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.M.; Vaskou, P.; Kountouris, Y. Insect Food Products in the Western World: Assessing the Potential of a New ‘Green’ Market. Ann. Entomol. Soc. 2019, 112, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Dossey, A.T.; Tatum, J.T.; McGill, W.L. Modern Insect-Based Food Industry: Current Status, Insect Processing Technology, and Recommendations Moving Forward; Dossey, A.T., Morales-Ramos, J.A., Rojas, M.G., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 117–139. [Google Scholar]
- Hanboonsong, Y.; Jamjanya, T.; Durst, P.B. Six-Legged Livestock: Edible Insect Farming, Collection on and Market in Thailand; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Sorjonen, J.M.; Valtonen, A.; Hirvisalo, E.; Karhapää, M.; Lehtovaara, V.J.; Lindgren, J.; Marnila, P.; Mooney, P.; Mäki, M.; Siljander-Rasi, H.; et al. The plant-based by-product diets for the mass-rearing of Acheta domesticus and Gryllus bimaculatus. PLoS ONE 2019, 14, e0218830. [Google Scholar] [CrossRef]
- Udomsil, N.; Imsoonthornruksa, S.; Gosalawit, C.; Ketudat-Cairns, M. Nutritional Values and Functional Properties of House Cricket (Acheta domesticus) and Field Cricket (Gryllus bimaculatus). Food Sci. Technol. Res. 2019, 25, 597–605. [Google Scholar] [CrossRef]
- Ritvanen, T.; Pastell, H.; Welling, A.; Raatikainen, M. The nitrogen-to-protein conversion factor of two cricket species—Acheta domesticus and Gryllus bimaculatus. Agric. Food Sci. 2020, 29, 1–5. [Google Scholar] [CrossRef]
- Kulma, M.; Petříčková, D.; Kurečka, M.; Kotíková, Z.; Táborský, J.; Michlová, T.; Kouřimská, L. Effect of carrot supplementation on nutritional value of insects: A case study with Jamaican field cricket (Gryllus assimilis). J. Insects Food Feed 2021, 1–10. [Google Scholar] [CrossRef]
- ACFS. Good Agricultural Practices for Cricket Farm; National Bureau of Argricultural Commodity and Food Standards, Ed.; Royal Gazette: Bangkok, Thailand, 2017; Volume 134, Special Section 293 D. [Google Scholar]
- Ghosh, S.; Lee, S.-M.; Jung, C.; Meyer-Rochow, V.B. Nutritional composition of five commercial edible insects in South Korea. J. Asia Pac. Entomol. 2017, 20, 686–694. [Google Scholar] [CrossRef]
- Murugu, D.K.; Onyango, A.N.; Ndiritu, A.K.; Osuga, I.M.; Xavier, C.; Nakimbugwe, D.; Tanga, C.M. From Farm to Fork: Crickets as Alternative Source of Protein, Minerals, and Vitamins. Front. Nutr. 2021, 8, 704002. [Google Scholar] [CrossRef]
- Finke, M.D. Complete nutrient composition of commercially raised invertebrates used as food for insectivores. Zoo Biol. 2002, 21, 269–285. [Google Scholar] [CrossRef]
- Blásquez, J.R.-E.; Moreno, J.M.P.; Camacho, V.H.M. Could Grasshoppers Be a Nutritive Meal? Food Nutr. Sci. 2012, 03, 164–175. [Google Scholar] [CrossRef]
- Finke, M.D. Complete nutrient content of four species of commercially available feeder insects fed enhanced diets during growth. Zoo Biol. 2015, 34, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Raksakantong, P.; Meeso, N.; Kubola, J.; Siriamornpun, S. Fatty acids and proximate composition of eight Thai edible terricolous insects. Int. Food Res. J. 2010, 43, 350–355. [Google Scholar] [CrossRef]
- Wang, D.; Bai, Y.; Li, J.; Zhang, C. Nutritional value of the field cricket (Gryllus testaceus Walker). Insect Sci. 2004, 11, 275–283. [Google Scholar] [CrossRef]
- Ahmed, E.; Fukuma, N.; Hanada, M.; Nishida, T. Insects as Novel Ruminant Feed and a Potential Mitigation Strategy for Methane Emissions. Animals 2021, 11, 2648. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Dalangin, R.; Kim, A.; Campbell, R.E. The Role of Amino Acids in Neurotransmission and Fluorescent Tools for Their Detection. Int. J. Mol. Sci. 2020, 21, 6197. [Google Scholar] [CrossRef]
- Kimball, S.R.; Jefferson, L.S. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J. Nutr. 2006, 136, 227s–231s. [Google Scholar] [CrossRef]
- Kulma, M.; Kouřimská, L.; Plachý, V.; Božik, M.; Adámková, A.; Vrabec, V. Effect of sex on the nutritional value of house cricket, Acheta domestica L. Food Chem. 2019, 272, 267–272. [Google Scholar] [CrossRef]
- Finke, M.D. Estimate of chitin in raw whole insects. Zoo Biol. 2007, 26, 105–115. [Google Scholar] [CrossRef]
- Feás, X.; Vázquez-Tato, M.P.; Seijas, J.A.; Pratima, G.; Nikalje, A.; Fraga-López, F. Extraction and physicochemical characterization of chitin derived from the asian hornet, Vespa velutina Lepeletier 1836 (Hym: Vespidae). Molecules 2020, 25, 384. [Google Scholar] [CrossRef] [PubMed]
- Boulos, S.; Tännler, A.; Nyström, L. Nitrogen-to-protein conversion factors for edible insects on the swiss market: T. molitor, A. domesticus, and L. migratoria. Front. Nutr. 2020, 7, 89. [Google Scholar] [CrossRef] [PubMed]
- Jonas-Levi, A.; Martinez, J.-J.I. The high level of protein content reported in insects for food and feed is overestimated. J. Food Compos. Anal. 2017, 62, 184–188. [Google Scholar] [CrossRef]
- Han, X.; Heinonen, M. Development of ultra-high performance liquid chromatographic and fluorescent method for the analysis of insect chitin. Food Chem. 2021, 334, 127577. [Google Scholar] [CrossRef]
- Bednářová, M.; Borkovcová, M.; Mlček, J.; Rop, O.; Zeman, L. Edible insects—Species suitable for entomophagy under condition of Czech Republic. Acta Univ. Agric. Silvic. Mendel. Brun. 2013, 61, 587–593. [Google Scholar] [CrossRef]
- Lewington, S.; Whitlock, G.; Clarke, R.; Sherliker, P.; Emberson, J.; Halsey, J.; Qizilbash, N.; Peto, R.; Collins, R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: A meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 2007, 370, 1829–1839. [Google Scholar] [CrossRef]
- Ramprasath, V.R.; Jones, P.J.; Buckley, D.D.; Woollett, L.A.; Heubi, J.E. Decreased plasma cholesterol concentrations after PUFA-rich diets are not due to reduced cholesterol absorption/synthesis. Lipids 2012, 47, 1063–1071. [Google Scholar] [CrossRef]
- Yanai, H.; Tada, N. Effects of Consumption of Various Fatty Acids on Serum HDL-Cholesterol Levels. J. Clin. Endocrinol. Metab. 2018, 8, 94–99. [Google Scholar] [CrossRef]
- Kang, M.J.; Shin, M.S.; Park, J.N.; Lee, S.S. The effects of polyunsaturated:saturated fatty acids ratios and peroxidisability index values of dietary fats on serum lipid profiles and hepatic enzyme activities in rats. Br. J. Nutr. 2005, 94, 526–532. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B.; Gahukar, R.T.; Ghosh, S.; Jung, C. Chemical Composition, Nutrient Quality and Acceptability of Edible Insects Are Affected by Species, Developmental Stage, Gender, Diet, and Processing Method. Foods 2021, 10, 1036. [Google Scholar] [CrossRef] [PubMed]
- Auten, R.L.; Davis, J.M. Oxygen toxicity and reactive oxygen species: The devil is in the details. Pediatr. Res. 2009, 66, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxidative Med. Cell Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef]
- Suttisansanee, U.; Thiyajai, P.; Chalermchaiwat, P.; Wongwathanarat, K.; Pruesapan, K.; Charoenkiatkul, S.; Temviriyanukul, P. Phytochemicals and In Vitro Bioactivities of Aqueous Ethanolic Extracts from Common Vegetables in Thai Food. Plants 2021, 10, 1563. [Google Scholar] [CrossRef]
- Temviriyanukul, P.; Sritalahareuthai, V.; Promyos, N.; Thangsiri, S.; Pruesapan, K.; Srinuanchai, W.; Nuchuchua, O.; Siriwan, D.; On-Nom, N.; Suttisansanee, U. The Effect of Sacred Lotus (Nelumbo nucifera) and Its Mixtures on Phenolic Profiles, Antioxidant Activities, and Inhibitions of the Key Enzymes Relevant to Alzheimer’s Disease. Molecules 2020, 25, 3713. [Google Scholar] [CrossRef] [PubMed]
- Temviriyanukul, P.; Sritalahareuthai, V.; Jom, K.N.; Jongruaysup, B.; Tabtimsri, S.; Pruesapan, K.; Thangsiri, S.; Inthachat, W.; Siriwan, D.; Charoenkiatkul, S.; et al. Comparison of Phytochemicals, Antioxidant, and In Vitro Anti-Alzheimer Properties of Twenty-Seven Morus spp. Cultivated in Thailand. Molecules 2020, 25, 2600. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Antioxidant property of coffee components: Assessment of methods that define mechanisms of action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef]
- Navarro del Hierro, J.; Gutiérrez-Docio, A.; Otero, P.; Reglero, G.; Martin, D. Characterization, antioxidant activity, and inhibitory effect on pancreatic lipase of extracts from the edible insects Acheta domesticus and Tenebrio molitor. Food Chem. 2020, 309, 125742. [Google Scholar] [CrossRef]
- Di Mattia, C.; Battista, N.; Sacchetti, G.; Serafini, M. Antioxidant Activities in vitro of Water and Liposoluble Extracts Obtained by Different Species of Edible Insects and Invertebrates. Front. Nutr. 2019, 6, 106. [Google Scholar] [CrossRef]
- Messina, C.M.; Gaglio, R.; Morghese, M.; Tolone, M.; Arena, R.; Moschetti, G.; Santulli, A.; Francesca, N.; Settanni, L. Microbiological Profile and Bioactive Properties of Insect Powders Used in Food and Feed Formulations. Foods 2019, 8, 400. [Google Scholar] [CrossRef]
- Taheri, A.; Sabeena Farvin, K.H.; Jacobsen, C.; Baron, C.P. Antioxidant activities and functional properties of protein and peptide fractions isolated from salted herring brine. Food Chem. 2014, 142, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.B.; Chang, M.H.; Lee, J.H.; Heo, W.; Kim, J.K.; Pan, J.H.; Kim, Y.J.; Kim, J.H. The Edible Insect Gryllus bimaculatus Protects against Gut-Derived Inflammatory Responses and Liver Damage in Mice after Acute Alcohol Exposure. Nutrients 2019, 11, 857. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, K.; Dutta, S.D.; Jeong, M.S.; Patel, D.K.; Cho, S.J.; Lim, K.T. Naturally-derived protein extract from Gryllus bimaculatus improves antioxidant properties and promotes osteogenic differentiation of hBMSCs. PLoS ONE 2021, 16, e0249291. [Google Scholar] [CrossRef]
- Park, W.J.; Han, J.S. Gryllus bimaculatus extract protects against lipopolysaccharide and palmitate-induced production of proinflammatory cytokines and inflammasome formation. Mol. Med. Rep. 2021, 23. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef] [PubMed]
- Karna, K.K.; Choi, N.Y.; Kim, C.Y.; Kim, H.K.; Shin, Y.S.; Park, J.K. Gui-A-Gra Attenuates Testicular Dysfunction in Varicocele-Induced Rats via Oxidative Stress, ER Stress and Mitochondrial Apoptosis Pathway. Int. J. Mol. Sci. 2020, 21, 9231. [Google Scholar] [CrossRef] [PubMed]
- Kurdi, P.; Chaowiwat, P.; Weston, J.; Hansawasdi, C. Studies on Microbial Quality, Protein Yield, and Antioxidant Properties of Some Frozen Edible Insects. Int. J. Food Sci. 2021, 2021, 5580976. [Google Scholar] [CrossRef]
- Jang, H.; Kim, M. Antidiabetic, Anticholesterol, and Antioxidant Activity of Gryllusbimaculatus Fermented by Bacillus and Lactobacillus strains. Appl. Sci. 2021, 11, 2090. [Google Scholar] [CrossRef]
- Ahn, M.Y.; Hwang, J.S.; Yun, E.Y.; Kim, M.J.; Park, K.K. Anti-aging Effect and Gene Expression Profiling of Aged Rats Treated with G. bimaculatus Extract. Toxicol. Res. 2015, 31, 173–180. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Karna, K.K.; Choi, B.R.; Kim, M.J.; Kim, H.K.; Park, J.K. The Effect of Schisandra chinensis Baillon on Cross-Talk between Oxidative Stress, Endoplasmic Reticulum Stress, and Mitochondrial Signaling Pathway in Testes of Varicocele-Induced SD Rat. Int. J. Mol. Sci. 2019, 20, 5785. [Google Scholar] [CrossRef]
- Yoon, S.; Wong, N.A.K.; Chae, M.; Auh, J.H. Comparative Characterization of Protein Hydrolysates from Three Edible Insects: Mealworm Larvae, Adult Crickets, and Silkworm Pupae. Foods 2019, 8, 563. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.Y.; Han, J.W.; Hwang, J.S.; Yun, E.Y.; Lee, B.M. Anti-inflammatory effect of glycosaminoglycan derived from Gryllus bimaculatus (a type of cricket, insect) on adjuvant-treated chronic arthritis rat model. J. Toxicol. Environ. Health A 2014, 77, 1332–1345. [Google Scholar] [CrossRef]
- Artasensi, A.; Pedretti, A.; Vistoli, G.; Fumagalli, L. Type 2 Diabetes Mellitus: A Review of Multi-Target Drugs. Molecules 2020, 25, 1987. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Park, S.A.; Lee, G.H.; Lee, H.Y.; Hoang, T.H.; Chae, H.J. Glucose-lowering effect of Gryllus bimaculatus powder on streptozotocin-induced diabetes through the AKT/mTOR pathway. Food Sci. Nutr. 2020, 8, 402–409. [Google Scholar] [CrossRef]
- Blandino-Rosano, M.; Chen, A.Y.; Scheys, J.O.; Alejandro, E.U.; Gould, A.P.; Taranukha, T.; Elghazi, L.; Cras-Méneur, C.; Bernal-Mizrachi, E. mTORC1 signaling and regulation of pancreatic β-cell mass. Cell Cycle 2012, 11, 1892–1902. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.Y.; Kim, B.J.; Kim, H.J.; Jin, J.M.; Yoon, H.J.; Hwang, J.S.; Lee, B.M. Anti-diabetic activity of field cricket glycosaminoglycan by ameliorating oxidative stress. BMC Complement. Med. Ther. 2020, 20, 232. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Tak, Y.J.; Lee, S.Y. Anti-Obesity Drugs: Long-Term Efficacy and Safety: An Updated Review. World J. Mens Health 2021, 39, 208–221. [Google Scholar] [CrossRef]
- Zargar, V.; Asghari, M.; Dashti, A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Malm, M.; Liceaga, A.M. Physicochemical Properties of Chitosan from Two Commonly Reared Edible Cricket Species, and Its Application as a Hypolipidemic and Antimicrobial Agent. Polysaccharides 2021, 2, 339–353. [Google Scholar] [CrossRef]
- Egan, Á.M.; Sweeney, T.; Hayes, M.; O’Doherty, J.V. Prawn Shell Chitosan Has Anti-Obesogenic Properties, Influencing Both Nutrient Digestibility and Microbial Populations in a Pig Model. PLoS ONE 2015, 10, e0144127. [Google Scholar] [CrossRef]
- Ahn, M.Y.; Kim, M.J.; Kwon, R.H.; Hwang, J.S.; Park, K.K. Gene expression profiling and inhibition of adipose tissue accumulation of G. bimaculatus extract in rats on high fat diet. Lipids Health Dis. 2015, 14, 116. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.Y.; Hwang, J.S.; Kim, M.J.; Park, K.K. Antilipidemic effects and gene expression profiling of the glycosaminoglycans from cricket in rats on a high fat diet. Arch. Pharm. Res. 2016, 39, 926–936. [Google Scholar] [CrossRef]
- Im, A.R.; Yang, W.K.; Park, Y.C.; Kim, S.H.; Chae, S. Hepatoprotective Effects of Insect Extracts in an Animal Model of Nonalcoholic Fatty Liver Disease. Nutrients 2018, 10, 735. [Google Scholar] [CrossRef]
- Durack, J.; Lynch, S.V. The gut microbiome: Relationships with disease and opportunities for therapy. J. Exp. Med. 2019, 216, 20–40. [Google Scholar] [CrossRef]
- Stull, V.J.; Finer, E.; Bergmans, R.S.; Febvre, H.P.; Longhurst, C.; Manter, D.K.; Patz, J.A.; Weir, T.L. Impact of Edible Cricket Consumption on Gut Microbiota in Healthy Adults, a Double-blind, Randomized Crossover Trial. Sci. Rep. 2018, 8, 10762. [Google Scholar] [CrossRef]
- Pedret, A.; Valls, R.M.; Calderón-Pérez, L.; Llauradó, E.; Companys, J.; Pla-Pagà, L.; Moragas, A.; Martín-Luján, F.; Ortega, Y.; Giralt, M.; et al. Effects of daily consumption of the probiotic Bifidobacterium animalis subsp. lactis CECT 8145 on anthropometric adiposity biomarkers in abdominally obese subjects: A randomized controlled trial. Int. J. Obes. 2019, 43, 1863–1868. [Google Scholar] [CrossRef]
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cífková, R.; Dominiczak, A.F.; Grassi, G.; Jordan, J.; Poulter, N.R.; Rodgers, A.; et al. Hypertension. Nat. Rev. Dis. Primers 2018, 4, 18014. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Efsa Panel on Nutrition, N.F.; Food, A.; Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; et al. Safety of frozen and dried formulations from whole house crickets (Acheta domesticus) as a Novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06779. [Google Scholar] [CrossRef] [PubMed]
- SLU, Swedish University of Agricultural Sciences; Department of Biomedical Sciences and Veterinary Public Health Sweden; Fernandez-Cassi, X.; Supeanu, A.; Jansson, A.; Boqvist, S.; Vagsholm, I. Novel foods: A risk profile for the house cricket (Acheta domesticus). EFSA J. 2018, 16, e16082. [Google Scholar] [CrossRef]
- Lee, S.; Ahn, K.S.; Ryu, H.Y.; Kim, H.J.; Lee, J.K.; Cho, M.-H.; Ahn, M.Y.; Song, K.S. Safety evaluation of cricket(Gryllus bimaculatus) extract in Sprague-Dawley rats. Int. J. Indust. Entomol. 2016, 32, 12–25. [Google Scholar] [CrossRef]
- Ryu, H.Y.; Lee, S.; Ahn, K.S.; Kim, H.J.; Lee, S.S.; Ko, H.J.; Lee, J.K.; Cho, M.H.; Ahn, M.Y.; Kim, E.M.; et al. Oral Toxicity Study and Skin Sensitization Test of a Cricket. Toxicol. Res. 2016, 32, 159–173. [Google Scholar] [CrossRef]
- Ahn, M.Y.; Joo, H.J.; Kim, J.S.; Yeon, Y.; Ryu, H.Y.; Choi, B.G.; Song, K.S.; Kim, S.H.; Park, M.K.; Jo, Y.Y. Toxicity assessment of Gryllus bimaculatus (a type of cricket) glycosaminoglycan. Toxicol. Res. 2020, 36, 319–328. [Google Scholar] [CrossRef]
- Ahn, M.Y.; Bae, H.J.; Kim, I.S.; Yoo, E.J.; Kwack, S.J.; Kim, H.S.; Kim, D.H.; Ryu, K.S.; Lee, H.S.; Kim, J.W.; et al. Genotoxic evaluation of the biocomponents of the cricket, Gryllus bimaculatus, using three mutagenicity tests. J. Toxicol. Environ. Health A 2005, 68, 2111–2118. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Sampson, H.A. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef]
- Ayuso, R. Update on the diagnosis and treatment of shellfish allergy. Curr. Allergy Asthma Rep. 2011, 11, 309–316. [Google Scholar] [CrossRef]
- Piromrat, K.; Chinratanapisit, S.; Trathong, S. Anaphylaxis in an emergency department: A 2-year study in a tertiary-care hospital. Asian Pac. J. Allergy Immunol. 2008, 26, 121–128. [Google Scholar]
- Ji, K.; Chen, J.; Li, M.; Liu, Z.; Wang, C.; Zhan, Z.; Wu, X.; Xia, Q. Anaphylactic shock and lethal anaphylaxis caused by food consumption in China. Trends Food Sci. Technol. 2009, 20, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Pali-Schöll, I.; Meinlschmidt, P.; Larenas-Linnemann, D.; Purschke, B.; Hofstetter, G.; Rodríguez-Monroy, F.A.; Einhorn, L.; Mothes-Luksch, N.; Jensen-Jarolim, E.; Jäger, H. Edible insects: Cross-recognition of IgE from crustacean- and house dust mite allergic patients, and reduction of allergenicity by food processing. World Allergy Organ. J. 2019, 12, 100006. [Google Scholar] [CrossRef] [PubMed]
- Kamemura, N.; Sugimoto, M.; Tamehiro, N.; Adachi, R.; Tomonari, S.; Watanabe, T.; Mito, T. Cross-allergenicity of crustacean and the edible insect Gryllus bimaculatus in patients with shrimp allergy. Mol. Immunol. 2019, 106, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Belluco, S.; Losasso, C.; Maggioletti, M.; Alonzi, C.C.; Paoletti, M.G.; Ricci, A. Edible insects in a food safety and nutritional perspective: A critical review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 296–313. [Google Scholar] [CrossRef]
- Nyangena, D.N.; Mutungi, C.; Imathiu, S.; Kinyuru, J.; Affognon, H.; Ekesi, S.; Nakimbugwe, D.; Fiaboe, K.K.M. Effects of traditional processing techniques on the nutritional and microbiological quality of four edible insect species used for food and feed in East Africa. Foods 2020, 9, 574. [Google Scholar] [CrossRef] [PubMed]
- Osimani, A.; Aquilanti, L. Spore-forming bacteria in insect-based foods. Curr. Opin. Food Sci. 2021, 37, 112–117. [Google Scholar] [CrossRef]
- Fernandez-Cassi, X.; Söderqvist, K.; Bakeeva, A.; Vaga, M.; Dicksved, J.; Vagsholm, I.; Jansson, A.; Boqvist, S. Microbial communities and food safety aspects of crickets (Acheta domesticus) reared under controlled conditions. J. Insects Food Feed 2020, 6, 429–440. [Google Scholar] [CrossRef]
- Gałęcki, R.; Sokół, R. A parasitological evaluation of edible insects and their role in the transmission of parasitic diseases to humans and animals. PLoS ONE 2019, 14, e0219303. [Google Scholar] [CrossRef]
- Caparros Megido, R.; Desmedt, S.; Blecker, C.; Béra, F.; Haubruge, É.; Alabi, T.; Francis, F. Microbiological load of edible insects found in Belgium. Insects 2017, 8, 12. [Google Scholar] [CrossRef]
- Fraqueza, M.J.R.; Patarata, L. Constraints of HACCP Application on Edible Insect for Food and Feed; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Klunder, H.C.; Wolkers-Rooijackers, J.; Korpela, J.M.; Nout, M.J.R. Microbiological aspects of processing and storage of edible insects. Food Control 2012, 26, 628–631. [Google Scholar] [CrossRef]
- Machado, R.A.M.; Perry, J.J. Variability in the microbial profile of retail cricket powders in the U.S. retail market. Food Prot. Trends 2020, 40, 407–412. [Google Scholar] [CrossRef]
- Grabowski, N.T.; Klein, G. Microbiology of processed edible insect products—Results of a preliminary survey. Int. J. Food Microbiol. 2017, 243, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, N.T.; Klein, G. Microbiology of cooked and dried edible Mediterranean field crickets (Gryllus bimaculatus) and superworms (Zophobas atratus) submitted to four different heating treatments. Food Sci. Technol. Int. 2017, 23, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Vandeweyer, D.; Wynants, E.; Crauwels, S.; Verreth, C.; Viaene, N.; Claes, J.; Lievens, B.; Van Campenhout, L. Microbial dynamics during industrial rearing, processing, and storage of tropical house crickets (Gryllodes sigillatus) for human consumption. Appl. Environ. Microbiol. 2018, 84, e00255-18. [Google Scholar] [CrossRef] [PubMed]
- Gatheru, J.W.; Khamis, F.M.; Ombura, F.L.O.; Nonoh, J.; Tanga, C.M.; Maina, J.; Mohamed, S.A.; Subramanian, S.; Ekesi, S.; Fiaboe, K.K.M. Impact of processing methods on microbial load of reared and wild-caught edible crickets (Scapsipedus icipe and Gryllus bimaculatus) in Kenya. J. Insects Food Feed 2019, 5, 171–183. [Google Scholar] [CrossRef]
- De Marchi, L.; Wangorsch, A.; Zoccatelli, G. Allergens from Edible Insects: Cross-reactivity and Effects of Processing. Curr. Allergy Asthma Rep. 2021, 21, 35. [Google Scholar] [CrossRef]
- Srinroch, C.; Srisomsap, C.; Chokchaichamnankit, D.; Punyarit, P.; Phiriyangkul, P. Identification of novel allergen in edible insect, Gryllus bimaculatus and its cross-reactivity with Macrobrachium spp. allergens. Food Chem. 2015, 184, 160–166. [Google Scholar] [CrossRef]
- Gaylor, M.O.; Harvey, E.; Hale, R.C. House crickets can accumulate polybrominated diphenyl ethers (PBDEs) directly from polyurethane foam common in consumer products. Chemosphere 2012, 86, 500–505. [Google Scholar] [CrossRef]
- Kanthawongwan, T.; Wattanachaiyingcharoen, W.; Hinhumpatch, P. Acetylcholinesterase-inhibiting insecticide residues in commonly consumed fried edible insects. EnvironmentAsia 2019, 12, 68–73. [Google Scholar] [CrossRef]
- Fuller, S.; Gautam, A. A procedure for measuring microplastics using pressurized fluid extraction. Environ. Sci. Technol. 2016, 50, 5774–5780. [Google Scholar] [CrossRef]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwanga, E.; Geissen, V. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, X.; Gertsen, H.; Peters, P.; Salánki, T.; Geissen, V. A simple method for the extraction and identification of light density microplastics from soil. Sci. Total Environ. 2018, 616–617, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Akindele, E.O.; Ehlers, S.M.; Koop, J.H.E. Freshwater insects of different feeding guilds ingest microplastics in two Gulf of Guinea tributaries in Nigeria. Environ. Sci Pollut. Res. 2020, 27, 33373–33379. [Google Scholar] [CrossRef] [PubMed]
- Van Huis, A.; Oonincx, D.G.A.B. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef]
- Henchion, M.; Hayes, M.; Mullen, A.; Fenelon, M.; Tiwari, B. Future protein supply and demand: Strategies and factors influencing. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Halloran, A.; Hanboonsong, Y.; Roos, N.; Bruun, S. Life cycle assessment of cricket farming in north-eastern Thailand. J. Clean. Prod. 2017, 156, 83–94. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; de Boer, I.J.M. Environmental impact of the production of mealworms as a protein source for humans—A life cycle assessment. PLoS ONE 2012, 7, e51145. [Google Scholar] [CrossRef] [PubMed]
- Oonincx, D.G.A.B.; van Itterbeeck, J.; Heetkamp, M.J.W.; van den Brand, H.; van Loon, J.J.A.; van Huis, A. An exploration on greenhouse gas and ammonia production by insect species suitable for animal or human consumption. PLoS ONE 2010, 5, e14445. [Google Scholar] [CrossRef]
- Van Huis, A. Potential of insects as food and feed in assuring food security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef]
- Miglietta, P.; De Leo, F.; Ruberti, M.; Massari, S. Mealworms for food: A water footprint perspective. Water 2015, 7, 6190–6203. [Google Scholar] [CrossRef]
- Chang, H.-P.; Ma, C.-C.; Chen, H.-S. Climate change and consumer’s attitude toward insect food. Int. J. Environ. Res. Public Health 2019, 16, 1606. [Google Scholar] [CrossRef] [PubMed]
- Programme, U.W.W.A. The United Nations World Water Development Report 2021 Valuing Water; United Nations Educational, Scientific and Cultural Organization: Paris, France, 2021; Volume 191, p. 187. [Google Scholar]
- Boretti, A.; Rosa, L. Reassessing the projections of the world water development report. NPJ Clean Water 2019, 2, 15. [Google Scholar] [CrossRef]
- Dobermann, D.; Swift, J.A.; Field, L.M. Opportunities and hurdles of edible insects for food and feed. Nutr. Bull. 2017, 42, 293–308. [Google Scholar] [CrossRef]
- Kummu, M.; Guillaume, J.H.A.; Moel, H.D.; Eisner, S.; Flörke, M.; Porkka, M. The world’s road to water scarcity: Shortage and stress in the 20th century and pathways towards sustainability. Sci. Rep. 2016, 6, 38495. [Google Scholar] [CrossRef] [PubMed]
- Brusseau, M.L.; Ramirez-Andreotta, M.; Pepper, I.L.; Maximillian, J. Chapter 26—Environmental Impacts on Human Health and Well-Being. In Environmental and Pollution Science, 3rd ed.; Brusseau, M.L., Pepper, I.L., Gerba, C.P., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 477–499. [Google Scholar] [CrossRef]
- Berggren, Å.; Jansson, A.; Low, M. Approaching Ecological Sustainability in the Emerging Insects-as-Food Industry. Trends Ecol. Evol. 2019, 34, 132–138. [Google Scholar] [CrossRef]
- Morales-Ramos, J.A.; Rojas, M.G.; Dossey, A.T.; Berhow, M. Self-selection of food ingredients and agricultural by-products by the house cricket, Acheta domesticus (Orthoptera: Gryllidae): A holistic approach to develop optimized diets. PLoS ONE 2020, 15, e0227400. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EC) No 178/2002 of The European Parliament and of the Council of 28 January 2002 Laying Down the General Principles and Requirements of Food Law, Establishing the European Food Safety Authority and Laying Down Procedures in Matters of Food Safety. 2002.
- Regulation (EC) No 767/2009 of The European Parliament and of the Council of 13 July 2009 On the Placing on the Market and Use of Feed, Amending European Parliament and Council Regulation (EC) No 1831/2003 and Repealing Council Directive 79/373/EEC, Commission Directive 80/511/EEC, Council Directives 82/471/EEC, 83/228/EEC, 93/74/EEC, 93/113/EC and 96/25/EC and Commission Decision 2004/217/EC. 2009.
- Regulation (EC) No 1069/2009 of The European Parliament and of the Council of 21 October 2009 Laying Down Health Rules as Regards Animal By-products and Derived Products Not Intended for Human Consumption and Repealing Regulation (EC) No 1774/2002 (Animal By-products Regulation). 2009.
- Vauterin, A.; Steiner, B.; Sillman, J.; Kahiluoto, H. The potential of insect protein to reduce food-based carbon footprints in Europe: The case of broiler meat production. J. Clean. Prod. 2021, 320, 128799. [Google Scholar] [CrossRef]
- Tucker, R.; McDonald, S.; O’Keefe, M.; Craddock, T.; Galloway, J. Beef Cattle Feedlots: Waste Management and Utilisation; Meat and Livestock Australia Ltd.: North Sydney, NSW, Australia, 2015. [Google Scholar]
- Halloran, A.; Roos, N.; Hanboonsong, Y. Cricket farming as a livelihood strategy in Thailand. Geogr. J. 2017, 183, 112–124. [Google Scholar] [CrossRef]
- Peiris, J.S.M.; de Jong, M.D.; Guan, Y. Avian influenza virus (H5N1): A threat to human health. Clin. Microbiol. Rev. 2007, 20, 243–267. [Google Scholar] [CrossRef]
- Chu, D.-T.; Ngoc, T.U.; Chu-Dinh, T.; Ngoc, V.T.N.; Van Nhon, B.; Pham, V.-H.; Nghia, L.L.; Anh, L.Q.; Van Pham, T.H.; Truong, N.D. The possible zoonotic diseases transferring from pig to human in Vietnam. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, C.J.; Cardwell, D.M.; Moeller, R.B.; Gray, G.C. Humans and cattle: A review of bovine zoonoses. Vector Borne Zoonotic Dis. 2014, 14, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Li, Y.O. Edible insects as a means to address global malnutrition and food insecurity issues. Food Qual. Saf. 2018, 2, 17–26. [Google Scholar] [CrossRef]
- Orkusz, A. Edible insects versus meat-nutritional comparison: Knowledge of their composition is the key to good health. Nutrients 2021, 13, 1207. [Google Scholar] [CrossRef] [PubMed]
- Melgar-Lalanne, G.; Hernández-Álvarez, A.J.; Salinas-Castro, A. Edible insects processing: Traditional and innovative technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1166–1191. [Google Scholar] [CrossRef]
- Govorushko, S. Global status of insects as food and feed source: A review. Trends Food Sci. Technol. 2019, 91, 436–445. [Google Scholar] [CrossRef]
- Biró, B.; Sipos, M.A.; Kovács, A.; Badak-Kerti, K.; Pásztor-Huszár, K.; Gere, A. Cricket-enriched oat biscuit: Technological analysis and sensory evaluation. Foods 2020, 9, 1561. [Google Scholar] [CrossRef]
- Durst, P.B.; Hanboonsong, Y. Small-scale production of edible insects for enhanced food security and rural livelihoods: Experience from Thailand and Lao People’s Democratic Republic. J. Insects Food Feed 2015, 1, 25–31. [Google Scholar] [CrossRef]
- Hlongwane, Z.T.; Slotow, R.; Munyai, T.C. Indigenous knowledge about consumption of edible insects in South Africa. Insects 2020, 12, 22. [Google Scholar] [CrossRef]
- Guiné, R.P.F.; Florença, S.G.; Barroca, M.J.; Anjos, O. The Link between the Consumer and the Innovations in Food Product Development. Foods 2020, 9, 1317. [Google Scholar] [CrossRef]
- Köhler, R.; Kariuki, L.; Lambert, C.; Biesalski, H.K. Protein, amino acid and mineral composition of some edible insects from Thailand. J. Asia-Pac. Entomol. 2019, 22, 372–378. [Google Scholar] [CrossRef]
- Mwangi, M.N.; Oonincx, D.G.A.B.; Stouten, T.; Veenenbos, M.; Melse-Boonstra, A.; Dicke, M.; van Loon, J.J.A. Insects as sources of iron and zinc in human nutrition. Nutr. Res. Rev. 2018, 31, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Hall, F.; Johnson, P.E.; Liceaga, A. Effect of enzymatic hydrolysis on bioactive properties and allergenicity of cricket (Gryllodes sigillatus) protein. Food Chem. 2018, 262, 39–47. [Google Scholar] [CrossRef] [PubMed]
- De Matos, F.M.; de Lacerda, J.T.J.G.; Zanetti, G.; de Castro, R.J.S. Production of black cricket protein hydrolysates with α-amylase, α-glucosidase and angiotensin I-converting enzyme inhibitory activities using a mixture of proteases. Biocatal. Agric. Biotechnol. 2022, 39, 102276. [Google Scholar] [CrossRef]
- De Matos, F.M.; Novelli, P.K.; de Castro, R.J.S. Enzymatic hydrolysis of black cricket (Gryllus assimilis) proteins positively affects their antioxidant properties. J. Food Sci. 2021, 86, 571–578. [Google Scholar] [CrossRef]
- Tzompa-Sosa, D.A.; Dewettinck, K.; Provijn, P.; Brouwers, J.F.; de Meulenaer, B.; Oonincx, D.G. Lipidome of cricket species used as food. Food Chem. 2021, 349, 129077. [Google Scholar] [CrossRef]
- Mohan, K.; Ganesan, A.R.; Muralisankar, T.; Jayakumar, R.; Sathishkumar, P.; Uthayakumar, V.; Chandirasekar, R.; Revathi, N. Recent insights into the extraction, characterization, and bioactivities of chitin and chitosan from insects. Trends Food Sci. Technol. 2020, 105, 17–42. [Google Scholar] [CrossRef]
- Yu, D.; Yu, Z.; Zhao, W.; Regenstein, J.M.; Xia, W. Advances in the application of chitosan as a sustainable bioactive material in food preservation. Crit. Rev. Food Sci. Nutr. 2021, 1–36. [Google Scholar] [CrossRef]

| Search No. | First Keyword * | Second Keyword * | PubMed | ScienceDirect | Scopus |
|---|---|---|---|---|---|
| 1 | cricket | entomophagy | 34 | 24 | 91 |
| 2 | cricket | nutrition | 262 | 68 | 186 |
| 3 | cricket | bioactivity | 32 | 10 | 17 |
| 4 | cricket | safety | 78 | 42 | 144 |
| 5 | cricket | sustainability | 166 | 72 | 26 |
| Species | Sources | Nutritive Values (% Dry Matter) | Ref. | |||
|---|---|---|---|---|---|---|
| Carbohydrate | Fat | Fiber | Protein | |||
| A. domesticus | Mexico | 2.12 ± 0.3 | 24.00 ± 0.9 | 6.20 ± 1.5 | 64.10 ± 1.2 | [41] |
| A. domesticus | Thailand | 1.60 ± 0.1 | 10.40 ± 0.1 | 4.60 ± 0.2 | 71.70 ± 0.5 | [34] |
| A. domesticus | USA | nr | 22.80 | 19.10 | 64.38 | [42] |
| B. portentosus | Thailand | 9.74 ± 0.5 | 20.60 ± 0.6 | 11.61 ± 0.2 | 48.69 ± 0.3 | [43] |
| G. bimaculatus | Korea | nr | 11.88 ± 0.2 | 9.53 ± 0.5 | 58.32 ± 0.3 | [38] |
| G. bimaculatus | Thailand | 0.10 ± 0.01 | 23.40 ± 0.1 | 10.00 ± 0.3 | 60.70 ± 0.4 | [34] |
| G. testaceus | China | nr | 10.30 ± 0.3 | nr | 58.30 ± 0.9 | [44] |
| Amino Acids | Crickets | |||
|---|---|---|---|---|
| A. domesticus | G. assimillis | G. bimaculatus | G. testaceus | |
| Essential | ||||
| Histidine | 1.72 ± 0.02 | 1.32 | 2.50 ± 0.08 | 1.94 ± 0.01 |
| Isoleucine | 2.90 ± 0.10 | 2.12 | 2.16 ± 0.04 | 3.09 ± 0.00 |
| Leucine | 3.80 ± 0.14 | 4.96 | 3.97 ± 0.05 | 5.52 ± 0.10 |
| Lysine | 3.22 ± 0.08 | 7.91 | 2.42 ± 0.01 | 4.79 ± 0.10 |
| Methionine | 0.98 ± 0.03 | 0.63 | 0.27 ± 0.01 | 1.93 ± 0.06 |
| Phenylalanine | 2.38 ± 0.00 | 0.72 | 1.83 ± 0.01 | 2.86 ± 0.06 |
| Threonine | 1.65 ± 0.05 | 3.55 | 2.00 ± 0.04 | 2.75 ± 0.10 |
| Tryptophan | 0.43 ± 0.03 | 0.95 | nr | nr |
| Valine | 4.50 ± 0.03 | 4.62 | 3.20 ± 0.03 | 4.42 ± 0.00 |
| Non-essential | ||||
| Alanine | 3.67 ± 0.05 | 4.02 | 5.64 ± 0.01 | 5.55 ± 0.09 |
| Arginine | 3.92 ± 0.05 | 8.64 | 3.60 ± 0.04 | 3.68 ± 0.12 |
| Asparagine | 4.61 ± 0.23 * | nr | nr | 6.29 ± 0.29 |
| Aspartic acid | nr | 3.02 | nr | nr |
| Cystine/cysteine | 0.40 ± 0.00 | 0.74 | 5.10 ± 0.00 | 1.01 ± 0.02 |
| Glutamic acid | nr | 3.64 | 6.39 ± 0.07 | 9.07 ± 0.31 |
| Glutamine | 6.45 ± 0.05 ** | nr | nr | nr |
| Glycine | 2.60 ± 0.15 | 2.41 | 3.32 ± 0.01 | 3.62 ± 0.11 |
| Proline | 3.04 ± 0.03 | 1.26 | 1.99 ± 0.01 | 4.50 ± 0.08 |
| Serine | 1.59 ± 0.09 | 0.61 | 2.73 ± 0.01 | 3.72 ± 0.07 |
| Tyrosine | 2.71 ± 0.10 | 5.44 | 2.73 ± 0.02 | 3.94 ± 0.02 |
| Total amino acids | 50.55 | 56.56 + | 53.83 | 68.68 + |
| Ref. | [34] | [55] | [38] | [44] |
| Fatty Acids | Crickets | |||
|---|---|---|---|---|
| A. domesticus | B. portentosus | G. bimaculatus | G. testaceus | |
| C10:0 Capric acid | 0.011 ± 0.00 | nr | 0.008 ± 0.00 | nr |
| C11:0 Undecylic acid | 0.003 ± 0.00 | nr | 0.004 ± 0.00 | nr |
| C12:0 Lauric acid | 0.028 ± 0.00 | nr | 0.045 ± 0.00 | 0.540 ± 0.04 |
| C14:0 Myristic acid | 0.107 ± 0 00 | nd | 0.271 ± 0.02 | 0.390 ± 0.02 |
| C15:0 Pentadecanoic acid | 0.009 ± 0.00 | nd | 0.036 ± 0.00 | nr |
| C16:0 Palmitic acid | 5.870 ± 0.31 | 0.021 ± 0.00 | 9.260 ± 0.77 | 10.180 ± 0.20 |
| C17:0 Heptadecanoic acid | 0.078 ± 0.00 | 0.002 ± 0.00 | 0.101 ± 0.01 | nr |
| C18:0 Stearic acid | 1.830 ± 0.09 | 0.473 ± 0.01 | 2.730 ± 0.25 | 2.630 ± 0.09 |
| C20:0 Arachidic acid | 0.125 ± 0.01 | nd | 0.212 ± 0.01 | nr |
| C21:0 Heneicosanoic acid | 0.005 ± 0.00 | nr | 0.008 ± 0.00 | nr |
| C22:0 Behenic acid | 0.064 ± 0.01 | nr | 0.049 ± 0.01 | nr |
| C24:0 Lignoceric acid | 0.024 ± 0.01 | nr | 0.037 ± 0.02 | nr |
| C14:1 Myristoleic acid | 0.019 ± 0. 02 | nr | 0.007 ± 0.00 | nr |
| C16:1 Palmitoleic acid | 0.153 ± 0. 01 | 0.009 ± 0.00 | 0.295 ± 0.07 | 3.110 ± 0.10 |
| C18:1n-9 trans Elaidic acid | 0.031 ± 0. 00 | nr | 0.044 ± 0.01 | nr |
| C18:1n-9 cis Oleic acid | 3.900 ± 0. 24 | nr | 9.400 ± 2.20 | 29.580 ± 0.20 |
| C20:1 cis-11-Eicosenoic acid | 0.024 ± 0. 01 | nr | 0.084 ± 0.05 | nr |
| C22:1n-9 Erucic acid | 0.014 ± 0. 01 | nr | 0.020 ± 0.01 | nr |
| C18:2n-6 trans Linolelaidic acid | nd | 0.045 ± 0.01 | 0.011 ± 0.01 | nr |
| C18:2n-6 cis Linoleic acid | 1.170 ± 0.36 | nr | 1.390 ± 0.73 | 37.820 ± 0.20 |
| C20:2 cis-11,14-Eicosadienoic acid | 0.194 ± 0.04 | nr | 0.173 ± 0.07 | nr |
| C22:6n-3 Docosahexaenoic acid | nd | nd | 0 | nr |
| C18:3n-6 cis Linolenic acid | 0.007 ± 0.00 | nr | 0.062 ± 0.01 | 10.120 ± 0.10 |
| C18:3n-3 Linolenic acid | 0 011 ± 0.00 | nd | 0.014 ± 0.01 | nr |
| C20:3n-6 cis-8,11,14-Eicosatrienoic acid | 0.011 ± 0. 00 | 0.105 ± 0.00 | 0.077 ± 0.08 | nr |
| C20:3n-3 cis-11,14,17-Eicosatrienoic acid | 0.006 ± 0.00 | nr | 0 | nr |
| C20:4n-6 Arachidonic acid | nd | 0.667 ± 0.00 | 0.005 ± 0.00 | nr |
| C20:5n-3 cis-5,8,11,14,17-Eicosapentaenoic acid | 0.057 ± 0.01 | nd | 0.070 ± 0.05 | nr |
| Total | 13.742 ± 0.76 | 1.321 | 24.413 ± 2.77 | 94.370 |
| SFA (saturated fatty acids) | 8.145 ± 0.35 | 0.496 ± 0.01 | 12.761 ± 1.07 | 13.740 |
| MUFA (monounsaturated fatty acids) | 4.141 ± 0.25 | 0.054 ± 0.01 | 9.850 ± 2.35 | 32.690 |
| PUFA (polyunsaturated fatty acids) | 1.456 ± 0.33 | 0.771 ± 0.00 | 1.802 ± 0.53 | 47.940 |
| PUFA:SFA | 0.178 | 1.554 | 0.141 | 3.480 |
| OMEGA 3 | 0.074 ± 0.01 | nr | 0.084 ± 0.04 | nr |
| OMEGA 6 | 1.125 ± 0.35 | nr | 1.545 ± 0.88 | nr |
| Ref. | [34] | [43] | [34] | [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kemsawasd, V.; Inthachat, W.; Suttisansanee, U.; Temviriyanukul, P. Road to The Red Carpet of Edible Crickets through Integration into the Human Food Chain with Biofunctions and Sustainability: A Review. Int. J. Mol. Sci. 2022, 23, 1801. https://doi.org/10.3390/ijms23031801
Kemsawasd V, Inthachat W, Suttisansanee U, Temviriyanukul P. Road to The Red Carpet of Edible Crickets through Integration into the Human Food Chain with Biofunctions and Sustainability: A Review. International Journal of Molecular Sciences. 2022; 23(3):1801. https://doi.org/10.3390/ijms23031801
Chicago/Turabian StyleKemsawasd, Varongsiri, Woorawee Inthachat, Uthaiwan Suttisansanee, and Piya Temviriyanukul. 2022. "Road to The Red Carpet of Edible Crickets through Integration into the Human Food Chain with Biofunctions and Sustainability: A Review" International Journal of Molecular Sciences 23, no. 3: 1801. https://doi.org/10.3390/ijms23031801
APA StyleKemsawasd, V., Inthachat, W., Suttisansanee, U., & Temviriyanukul, P. (2022). Road to The Red Carpet of Edible Crickets through Integration into the Human Food Chain with Biofunctions and Sustainability: A Review. International Journal of Molecular Sciences, 23(3), 1801. https://doi.org/10.3390/ijms23031801