Broad Spectrum Anti-Bacterial Activity and Non-Selective Toxicity of Gum Arabic Silver Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
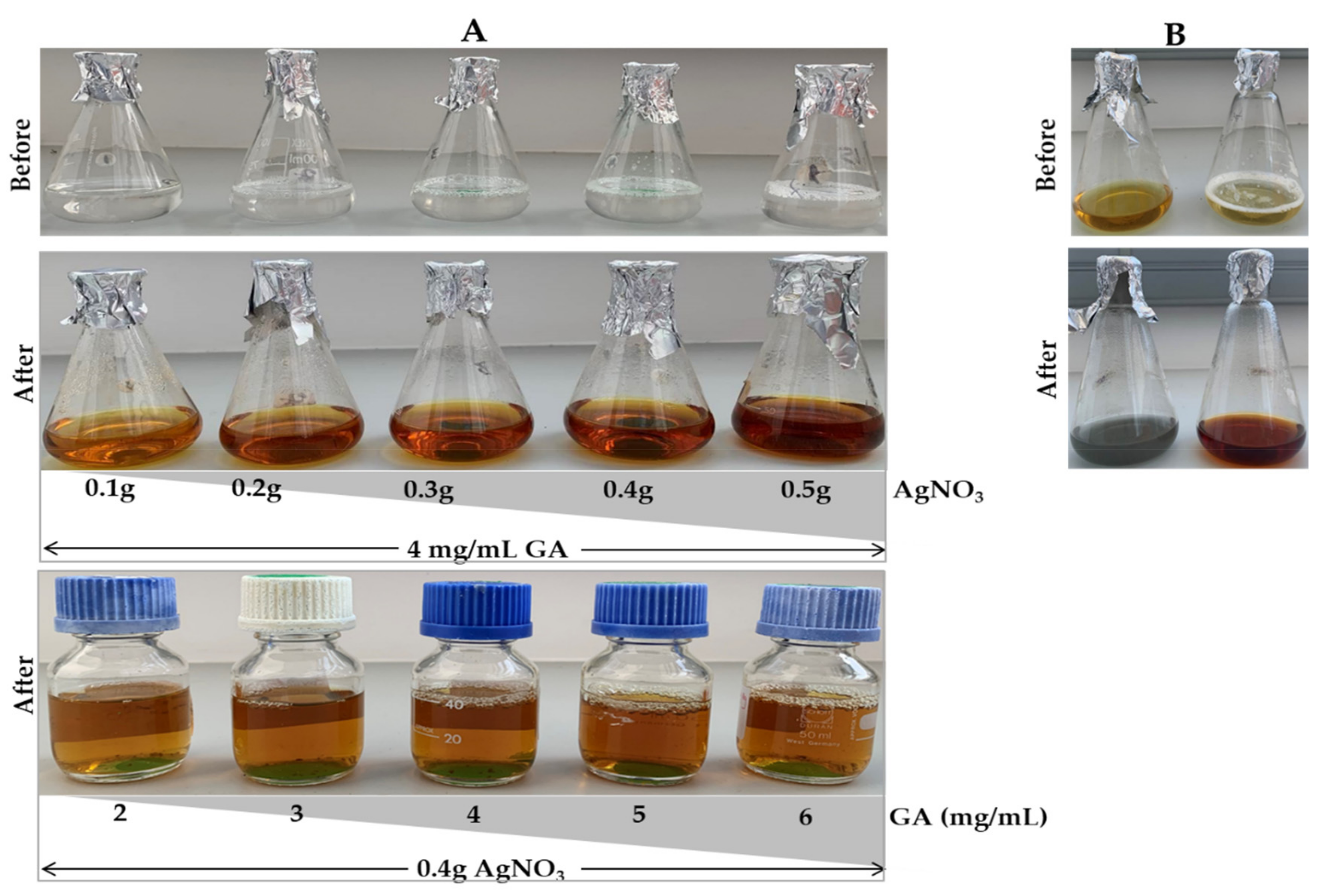
2.1. Synthesis of GA-AgNPs
2.2. Characterization of the AgNPs
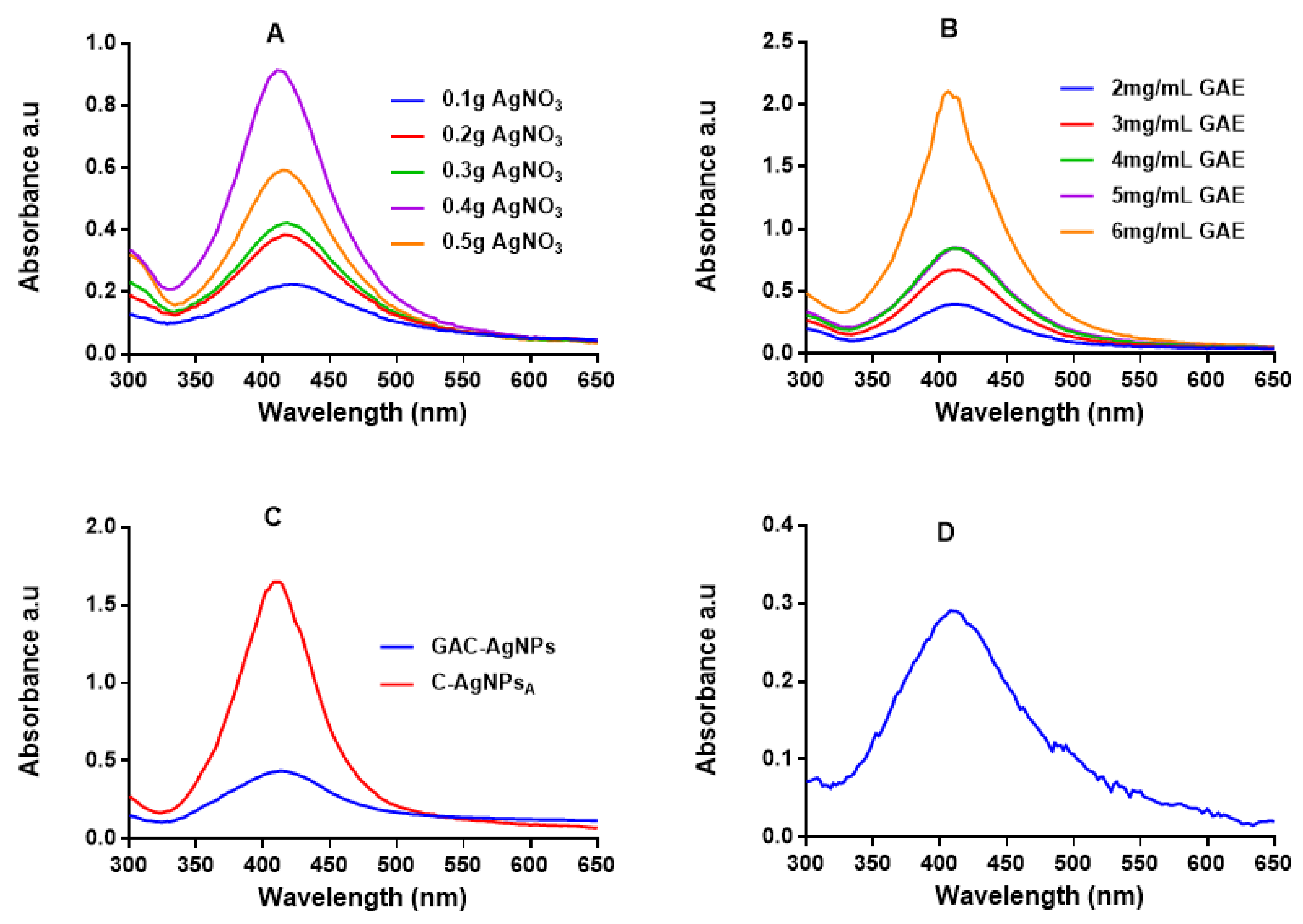
2.2.1. Optical Properties of the AgNPs
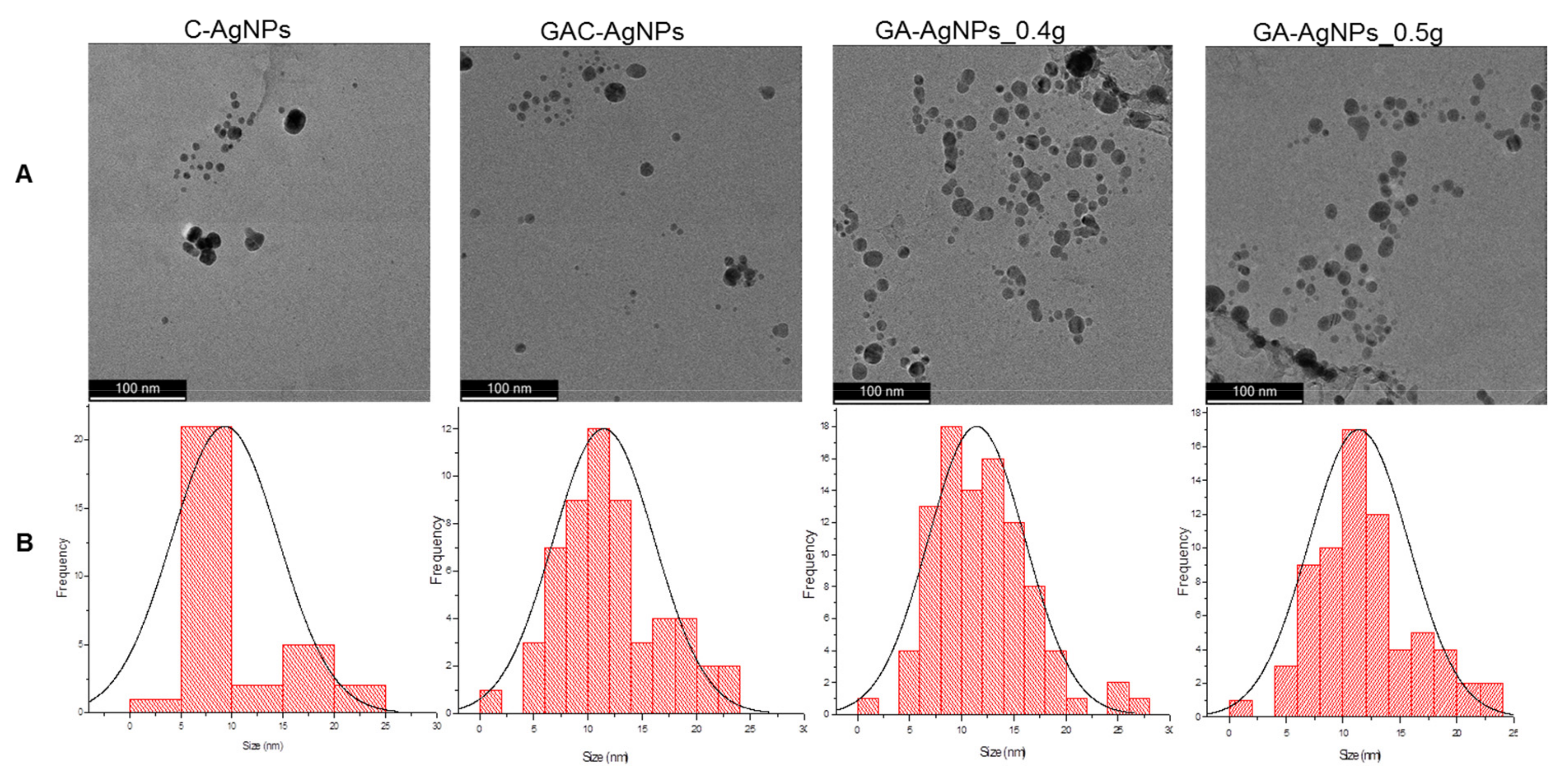
2.2.2. Morphology and Size Distribution of the AgNPs
2.2.3. FT-IR Analysis of GAE and AgNPs
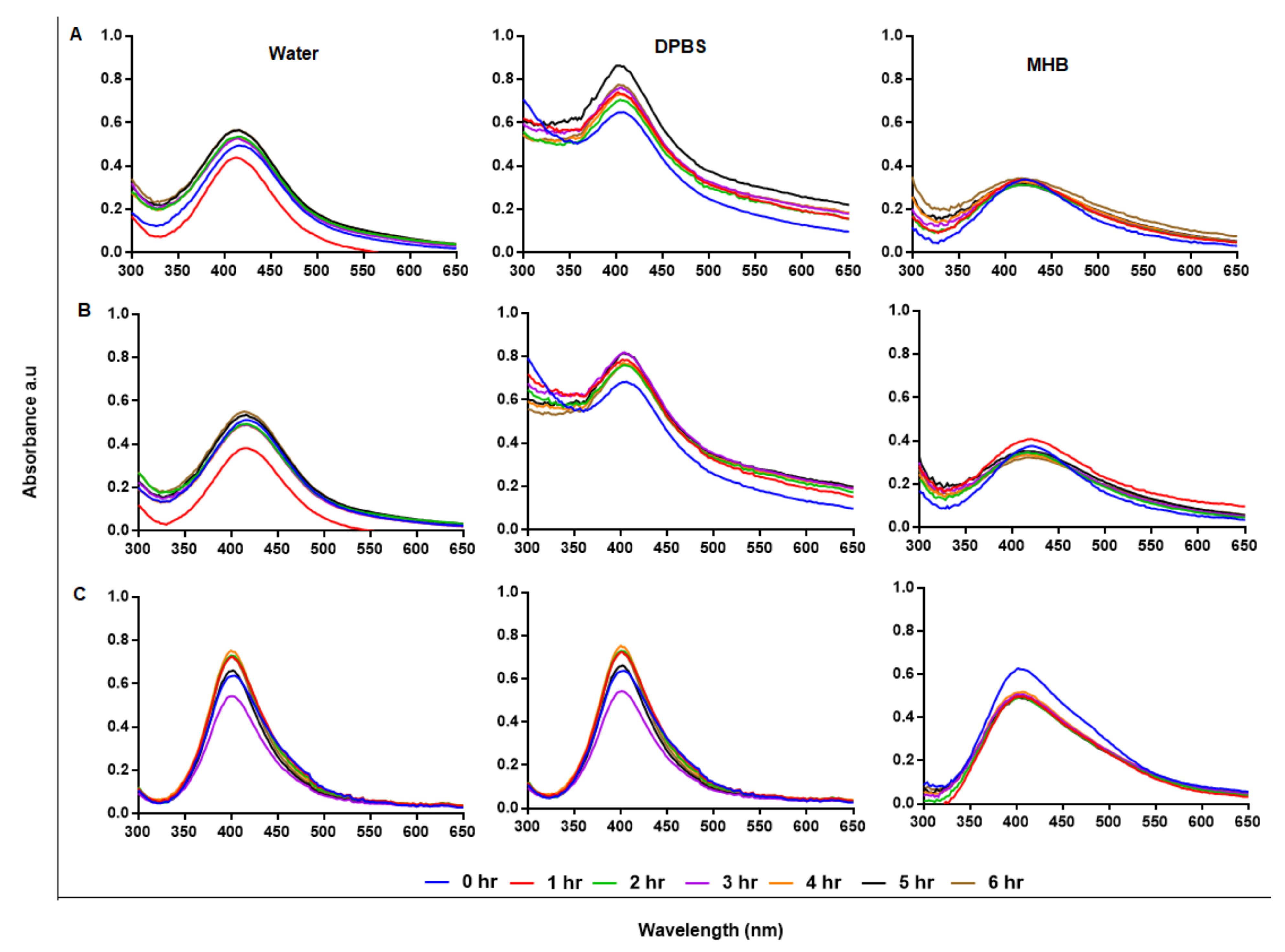
2.3. Stability of GA-AgNPs
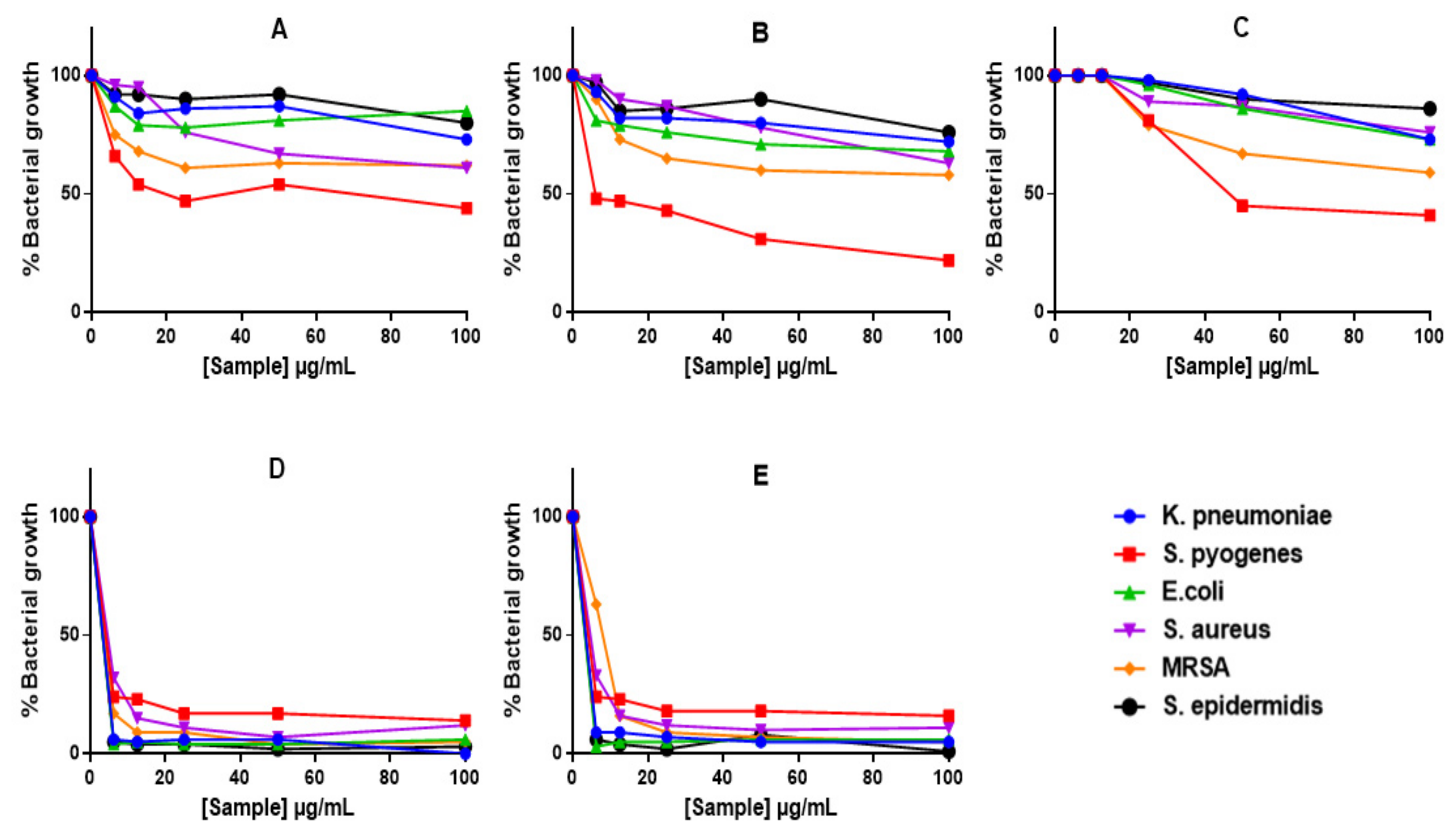
2.4. Anti-Bacterial Activity
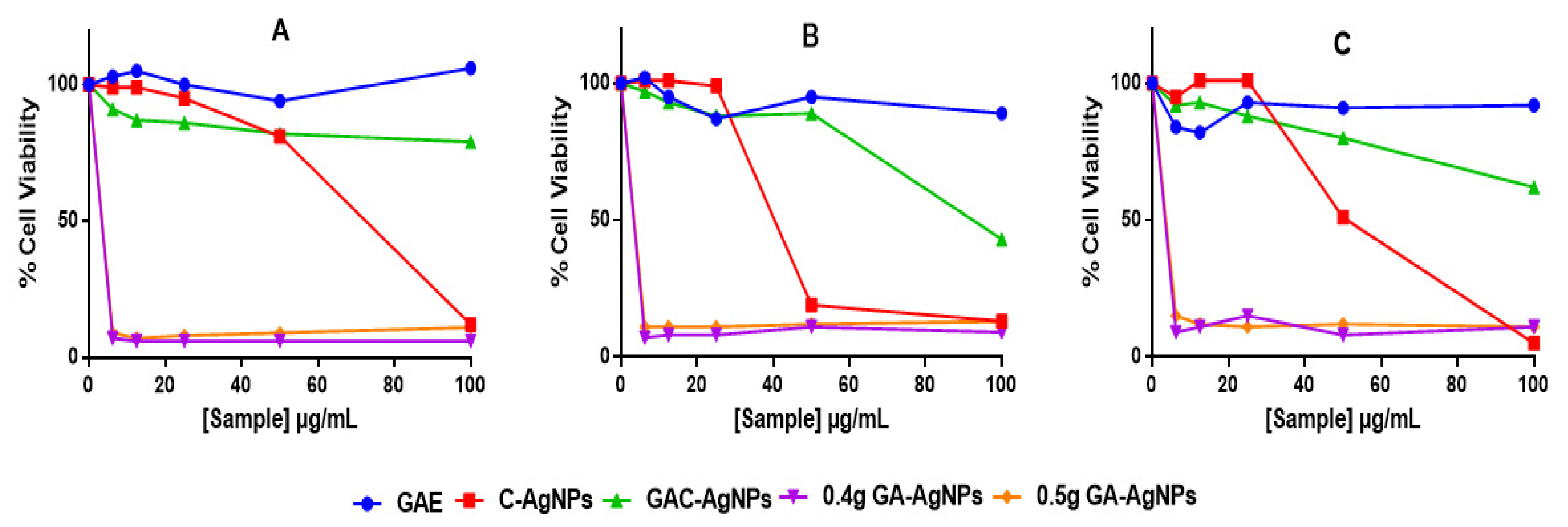
2.5. In Vitro Cytotoxicity of GA-AgNPs
3. Materials and Methods
3.1. Preparation of the GA Extracts
Phytochemical Analysis and Antioxidant Capacity
3.2. Synthesis of AgNPs
3.2.1. Chemical Synthesis
3.2.2. Green Synthesis
3.2.3. Combined Approach
3.3. Characterization of the AgNPs
3.3.1. UV-Visible Spectrophotometer
3.3.2. Dynamic Light Scattering (DLS)
3.3.3. FT-IR
3.3.4. HRTEM
3.4. Assessment of the Stability of AgNPs
3.5. Anti-Bacterial Activity of the AgNPs
3.5.1. Agar Diffusion Assay
3.5.2. Microdilution Assay
Minimum Inhibitory Concentration (MIC)
Minimum Bactericidal Concentration (MBC)
3.6. Cytotoxicity Assay of the AgNPs
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar] [PubMed]
- Rauwel, P.; Küünal, S.; Ferdov, S.; Rauwel, E. A Review on the Green Synthesis of Silver Nanoparticles and Their Morphologies Studied via TEM. Adv. Mater. Sci. Eng. 2015, 2015, 682749. [Google Scholar] [CrossRef] [Green Version]
- Greulich, C.; Braun, D.; Peetsch, A.; Diendorf, J.; Siebers, B.; Epple, M.; Köller, M. The toxic effect of silver ions and silver nanoparticles towards bacteria and human cells occurs in the same concentration range. RSC Adv. 2012, 2, 6981–6987. [Google Scholar] [CrossRef]
- Chugh, D.; Viswamalya, V.S.; Das, B. Green synthesis of silver nanoparticles with algae and the importance of capping agents in the process. J. Genet. Eng. Biotechnol. 2021, 19, 126. [Google Scholar] [CrossRef]
- Klaus-Joerger, T.; Joerger, R.; Olsson, E.; Granqvist, C.-G. Bacteria as workers in the living factory: Metal-accumulating bacteria and their potential for materials science. TRENDS Biotechnol. 2001, 19, 15–20. [Google Scholar] [CrossRef]
- Helmy, A.; El-Shazly, M.; Seleem, A.; Abdelmohsen, U.; Salem, M.A.; Samir, A.; Rabeh, M.; Elshamy, A.; Singab, A.N.B. The synergistic effect of biosynthesized silver nanoparticles from a combined extract of parsley, corn silk, and gum arabic: In vivo antioxidant, anti-inflammatory and antimicrobial activities. Mater. Res. Express 2020, 7, 025002. [Google Scholar] [CrossRef]
- Nqakala, Z.B.; Sibuyi, N.R.; Fadaka, A.O.; Meyer, M.; Onani, M.O.; Madiehe, A.M. Advances in Nanotechnology towards Development of Silver Nanoparticle-Based Wound-Healing Agents. Int. J. Mol. Sci. 2021, 22, 11272. [Google Scholar] [CrossRef]
- Aboyewa, J.A.; Sibuyi, N.R.; Meyer, M.; Oguntibeju, O.O. Green synthesis of metallic nanoparticles using some selected medicinal plants from southern africa and their biological applications. Plants 2021, 10, 1929. [Google Scholar] [CrossRef]
- Njagi, E.C.; Huang, H.; Stafford, L.; Genuino, H.; Galindo, H.M.; Collins, J.B.; Hoag, G.E.; Suib, S.L. Biosynthesis of iron and silver nanoparticles at room temperature using aqueous sorghum bran extracts. Langmuir 2011, 27, 264–271. [Google Scholar] [CrossRef]
- Zargar, M.; Hamid, A.A.; Bakar, F.A.; Shamsudin, M.N.; Shameli, K.; Jahanshiri, F.; Farahani, F. Green synthesis and antibacterial effect of silver nanoparticles using Vitex negundo L. Molecules 2011, 16, 6667–6676. [Google Scholar] [CrossRef]
- Arokiyaraj, S.; Arasu, M.V.; Vincent, S.; Prakash, N.U.; Choi, S.H.; Oh, Y.-K.; Choi, K.C.; Kim, K.H. Rapid green synthesis of silver nanoparticles from Chrysanthemum indicum L and its antibacterial and cytotoxic effects: An in vitro study. Int. J. Nanomed. 2014, 9, 379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murugan, K.; Senthilkumar, B.; Senbagam, D.; Al-Sohaibani, S. Biosynthesis of silver nanoparticles using Acacia leucophloea extract and their antibacterial activity. Int. J. Nanomed. 2014, 9, 2431. [Google Scholar]
- Gurunathan, S.; Raman, J.; Abd Malek, S.N.; John, P.A.; Vikineswary, S. Green synthesis of silver nanoparticles using Ganoderma neo-japonicum Imazeki: A potential cytotoxic agent against breast cancer cells. Int. J. Nanomed. 2013, 8, 4399. [Google Scholar]
- Ipe, D.S.; Kumar, P.; Love, R.M.; Hamlet, S.M. Silver nanoparticles at biocompatible dosage synergistically increases bacterial susceptibility to antibiotics. Front. Microbiol. 2020, 11, 1074. [Google Scholar] [CrossRef]
- Bilberg, K.; Hovgaard, M.B.; Besenbacher, F.; Baatrup, E. In vivo toxicity of silver nanoparticles and silver ions in zebrafish (Danio rerio). J. Toxicol. 2012, 2012, 293784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Lan, Y.; Qu, J.; Hu, X.; Wang, A. Ag/AgBr/TiO2 visible light photocatalyst for destruction of azodyes and bacteria. J. Phys. Chem. B 2006, 110, 4066–4072. [Google Scholar] [CrossRef]
- Durán, M.; Fávaro, W.J.; Islan, G.A.; Castro, G.R.; Durán, N. Silver nanoparticles for treatment of neglected diseases. In Metal Nanoparticles in Pharma; Springer: Cham, Switzerland, 2017; pp. 39–51. [Google Scholar]
- Tăbăran, A.-F.; Matea, C.T.; Mocan, T.; Tăbăran, A.; Mihaiu, M.; Iancu, C.; Mocan, L. Silver nanoparticles for the therapy of tuberculosis. Int. J. Nanomed. 2020, 15, 2231–2258. [Google Scholar] [CrossRef] [Green Version]
- Neves, P.B.A.d.; Agnelli, J.A.M.; Kurachi, C.; Souza, C.W.O.d. Addition of silver nanoparticles to composite resin: Effect on physical and bactericidal properties in vitro. Braz. Dent. J. 2014, 25, 141–145. [Google Scholar] [CrossRef] [Green Version]
- Mahross, H.Z.; Baroudi, K. Effect of silver nanoparticles incorporation on viscoelastic properties of acrylic resin denture base material. Eur. J. Dent. 2015, 9, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.Y.U.; Gallego, J.; Assunção, W.G.; Briso, A.L.F.; Dos Santos, P.H. Influence of silver nanoparticle solution on the mechanical properties of resin cements and intrarradicular dentin. PLoS ONE 2019, 14, e0217750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Rafie, H.; El-Rafie, M.; Zahran, M. Green synthesis of silver nanoparticles using polysaccharides extracted from marine macro algae. Carbohydr. Polym. 2013, 96, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Maziero, J.S.; Thipe, V.C.; Rogero, S.O.; Cavalcante, A.K.; Damasceno, K.C.; Ormenio, M.B.; Martini, G.A.; Batista, J.G.; Viveiros, W.; Katti, K.K. Species-Specific in vitro and in vivo Evaluation of Toxicity of Silver Nanoparticles Stabilized with Gum Arabic Protein. Int. J. Nanomed. 2020, 15, 7359. [Google Scholar] [CrossRef] [PubMed]
- Rezazadeh, N.H.; Buazar, F.; Matroodi, S. Synergistic effects of combinatorial chitosan and polyphenol biomolecules on enhanced antibacterial activity of biofunctionalized silver nanoparticles. Sci. Rep. 2020, 10, 19615. [Google Scholar]
- Johnson, W. Final report of the safety assessment of Acacia catechu gum, Acacia concinna fruit extract, Acacia dealbata leaf extract, Acacia dealbata leaf wax, Acacia decurrens extract, Acacia farnesiana extract, Acacia farnesiana flower wax, Acacia farnesiana gum, Acacia senegal extract, Acacia senegal gum, and Acacia senegal gum extract. Int. J. Toxicol. 2005, 24, 75–118. [Google Scholar]
- Ali, B.H.; Ziada, A.; Blunden, G. Biological effects of gum arabic: A review of some recent research. Food Chem. Toxicol. 2009, 47, 1–8. [Google Scholar] [CrossRef]
- Roque, A.C.A.; Bicho, A.; Batalha, I.L.; Cardoso, A.S.; Hussain, A. Biocompatible and bioactive gum Arabic coated iron oxide magnetic nanoparticles. J. Biotechnol. 2009, 144, 313–320. [Google Scholar] [CrossRef]
- Williams, D.N.; Gold, K.A.; Holoman, T.R.P.; Ehrman, S.H.; Wilson, O.C. Surface Modification of Magnetic Nanoparticles Using Gum Arabic. J. Nanoparticle Res. 2006, 8, 749–753. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, F.; Cole, A.J.; Chertok, B.; David, A.E.; Wang, J.; Yang, V.C. Gum arabic-coated magnetic nanoparticles for potential application in simultaneous magnetic targeting and tumor imaging. AAPS J. 2009, 11, 693–699. [Google Scholar] [CrossRef] [Green Version]
- Wilson, O.C., Jr.; Blair, E.; Kennedy, S.; Rivera, G.; Mehl, P. Surface modification of magnetic nanoparticles with oleylamine and gum Arabic. Mater. Sci. Eng. C 2008, 28, 438–442. [Google Scholar] [CrossRef]
- Banerjee, S.S.; Chen, D.-H. Magnetic nanoparticles grafted with cyclodextrin for hydrophobic drug delivery. Chem. Mater. 2007, 19, 6345–6349. [Google Scholar] [CrossRef]
- Kannan, R.; Rahing, V.; Cutler, C.; Pandrapragada, R.; Katti, K.K.; Kattumuri, V.; Robertson, J.D.; Casteel, S.J.; Jurisson, S.; Smith, C. Nanocompatible chemistry toward fabrication of target-specific gold nanoparticles. J. Am. Chem. Soc. 2006, 128, 11342–11343. [Google Scholar] [CrossRef] [PubMed]
- Kattumuri, V.; Katti, K.; Bhaskaran, S.; Boote, E.J.; Casteel, S.W.; Fent, G.M.; Robertson, D.J.; Chandrasekhar, M.; Kannan, R.; Katti, K.V. Gum arabic as a phytochemical construct for the stabilization of gold nanoparticles: In vivo pharmacokinetics and X-ray-contrast-imaging studies. Small 2007, 3, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Aboyewa, J.A.; Sibuyi, N.R.; Meyer, M.; Oguntibeju, O.O. Gold Nanoparticles Synthesized Using Extracts of Cyclopia intermedia, Commonly Known as Honeybush, Amplify the Cytotoxic Effects of Doxorubicin. Nanomaterials 2021, 11, 132. [Google Scholar] [CrossRef]
- Bandyopadhyaya, R.; Nativ-Roth, E.; Regev, O.; Yerushalmi-Rozen, R. Stabilization of individual carbon nanotubes in aqueous solutions. Nano Lett. 2002, 2, 25–28. [Google Scholar] [CrossRef]
- Park, C.; Lim, K.H.; Kwon, D.; Yoon, T.H. Biocompatible quantum dot nanocolloids stabilized by gum Arabic. Bull. -Korean Chem. Soc. 2008, 29, 1277. [Google Scholar]
- Ibekwe, C.A.; Oyatogun, G.M.; Esan, T.A.; Oluwasegun, K.M. Synthesis and characterization of chitosan/gum arabic nanoparticles for bone regeneration. Am. J. Mater. Sci. Eng. 2017, 5, 28–36. [Google Scholar]
- Venkatesham, M.; Ayodhya, D.; Madhusudhan, A.; Veerabhadram, G. Synthesis of stable silver nanoparticles using gum acacia as reducing and stabilizing agent and study of its microbial properties: A novel green approach. Int. J. Green Nanotechnol. 2012, 4, 199–206. [Google Scholar] [CrossRef]
- Solomon, M.M.; Gerengi, H.; Umoren, S.A.; Essien, N.B.; Essien, U.B.; Kaya, E. Gum Arabic-silver nanoparticles composite as a green anticorrosive formulation for steel corrosion in strong acid media. Carbohydr. Polym. 2018, 181, 43–55. [Google Scholar] [CrossRef]
- Babiker, R.; Merghani, T.H.; Elmusharaf, K.; Badi, R.M.; Lang, F.; Saeed, A.M. Effects of gum Arabic ingestion on body mass index and body fat percentage in healthy adult females: Two-arm randomized, placebo controlled, double-blind trial. Nutr. J. 2012, 11, 1–7. [Google Scholar] [CrossRef]
- Patel, S.; Goyal, A. Applications of natural polymer gum arabic: A review. Int. J. Food Prop. 2015, 18, 986–998. [Google Scholar] [CrossRef]
- Musa, H.H.; Ahmed, A.A.; Musa, T.H. Chemistry, biological, and pharmacological properties of gum Arabic. In Bioactive Molecules in Food; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 1–18. [Google Scholar]
- Ansari, M.A.; Khan, H.M.; Khan, A.A.; Cameotra, S.S.; Saquib, Q.; Musarrat, J. Gum arabic capped-silver nanoparticles inhibit biofilm formation by multi-drug resistant strains of Pseudomonas aeruginosa. J. Basic Microbiol. 2014, 54, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Kora, A.J.; Arunachalam, J. Green fabrication of silver nanoparticles by gum tragacanth (Astragalus gummifer): A dual functional reductant and stabilizer. J. Nanomater. 2012, 2012, 69. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, M.Z.; Chowdhury, A.R.; Singh, B.R.; Maurya, S.; Prasad, N. Synthesis, Characterization and Antimicrobial Evaluation of Piyar Gum-Induced Silver Nanoparticles. Natl. Acad. Sci. Lett. 2021, 44, 203–208. [Google Scholar] [CrossRef]
- Majoumouo, M.S.; Sibuyi, N.R.S.; Tincho, M.B.; Mbekou, M.; Boyom, F.F.; Meyer, M. Enhanced anti-bacterial activity of biogenic silver nanoparticles synthesized from Terminalia mantaly Extracts. Int. J. Nanomed. 2019, 14, 9031. [Google Scholar] [CrossRef] [Green Version]
- Simon, S.; Sibuyi, N.R.S.; Fadaka, A.O.; Meyer, M.; Madiehe, A.M.; du Preez, M.G. The antimicrobial activity of biogenic silver nanoparticles synthesized from extracts of Red and Green European pear cultivars. Artif. Cells Nanomed. Biotechnol. 2021, 49, 614–625. [Google Scholar] [CrossRef]
- Dube, P.; Meyer, S.; Madiehe, A.; Meyer, M. Antibacterial activity of biogenic silver and gold nanoparticles synthesized from Salvia africana-lutea and Sutherlandia frutescens. Nanotechnology 2020, 31, 505607. [Google Scholar] [CrossRef]
- Mamza, P.A.; Arthur, D.M.; Aliyu, M.J.; Desk, S. Purification, characterization and modification of gum arabic for possible use as additive for poly (vinyl chloride). SDRP J. Comput. Chem. Mol. Model. 2015, 1, 1–8. [Google Scholar]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons Ltd.: Chichester, UK, 2000. [Google Scholar]
- Adam, H.; Siddig, M.A.; Siddig, A.A.; Eltahir, N.A. Electrical and optical properties of two types of Gum Arabic. Sudan Med. Monit. 2013, 8, 174. [Google Scholar]
- Park, K.; Lee, Y. The stability of citrate-capped silver nanoparticles in isotonic glucose solution for intravenous injection. J. Toxicol. Environ. Health Part A 2013, 76, 1236–1245. [Google Scholar] [CrossRef]
- Sim, W.; Barnard, R.T.; Blaskovich, M.; Ziora, Z.M. Antimicrobial silver in medicinal and consumer applications: A patent review of the past decade (2007–2017). Antibiotics 2018, 7, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, M.; Guo, H.; Liu, L.; Liu, Y.; Xie, L. Size-dependent cellular uptake and localization profiles of silver nanoparticles. Int. J. Nanomed. 2019, 14, 4247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siritongsuk, P.; Hongsing, N.; Thammawithan, S.; Daduang, S.; Klaynongsruang, S.; Tuanyok, A.; Patramanon, R. Two-phase bactericidal mechanism of silver nanoparticles against Burkholderia pseudomallei. PLoS ONE 2016, 11, e0168098. [Google Scholar] [CrossRef] [PubMed]
- Tyavambiza, C.; Elbagory, A.M.; Madiehe, A.M.; Meyer, M.; Meyer, S. The Antimicrobial and Anti-Inflammatory Effects of Silver Nanoparticles Synthesised from Cotyledon orbiculata Aqueous Extract. Nanomaterials 2021, 11, 1343. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Muñoz, R.; Bogdanchikova, N.; Huerta-Saquero, A. Beyond the nanomaterials approach: Influence of culture conditions on the stability and antimicrobial activity of silver nanoparticles. ACS Omega 2020, 5, 28441–28451. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, H.; Song, X.; Wei, C.; Xiong, Z.; Yu, F.; Li, C.; Ai, F.; Guo, G.; Wang, X. NaCl: For the safer in vivo use of antibacterial silver based nanoparticles. Int. J. Nanomed. 2018, 13, 1737. [Google Scholar] [CrossRef] [Green Version]
- Fadaka, A.O.; Sibuyi, N.R.S.; Madiehe, A.M.; Meyer, M. Nanotechnology-based delivery systems for antimicrobial peptides. Pharmaceutics 2021, 13, 1795. [Google Scholar] [CrossRef]
- Bakare, O.O.; Fadaka, A.O.; Klein, A.; Pretorius, A. Dietary effects of antimicrobial peptides in therapeutics. All Life 2020, 13, 78–91. [Google Scholar] [CrossRef] [Green Version]
- Loo, Y.Y.; Rukayadi, Y.; Nor-Khaizura, M.-A.-R.; Kuan, C.H.; Chieng, B.W.; Nishibuchi, M.; Radu, S. In vitro antimicrobial activity of green synthesized silver nanoparticles against selected gram-negative foodborne pathogens. Front. Microbiol. 2018, 9, 1555. [Google Scholar] [CrossRef]
- Singh, P.; Garg, A.; Pandit, S.; Mokkapati, V.; Mijakovic, I. Antimicrobial effects of biogenic nanoparticles. Nanomaterials 2018, 8, 1009. [Google Scholar] [CrossRef] [Green Version]
- Ojo, O.A.; Oyinloye, B.E.; Ojo, A.B.; Afolabi, O.B.; Peters, O.A.; Olaiya, O.; Fadaka, A.; Jonathan, J.; Osunlana, O. Green synthesis of silver nanoparticles (AgNPs) using Talinum triangulare (Jacq.) Willd. leaf extract and monitoring their antimicrobial activity. J. Bionanosci. 2017, 11, 292–296. [Google Scholar] [CrossRef]
- Baien, S.H.; Seele, J.; Henneck, T.; Freibrodt, C.; Szura, G.; Moubasher, H.; Nau, R.; Brogden, G.; Mörgelin, M.; Singh, M. Antimicrobial and immunomodulatory effect of gum arabic on human and bovine granulocytes against Staphylococcus aureus and Escherichia coli. Front. Immunol. 2020, 10, 3119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Alawi, S.M.; Hossain, M.A.; Abusham, A.A. Antimicrobial and cytotoxic comparative study of different extracts of Omani and Sudanese Gum acacia. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 22–26. [Google Scholar] [CrossRef]
- Al-Ansari, M.M.; Al-Dahmash, N.D.; Ranjitsingh, A. Synthesis of silver nanoparticles using gum Arabic: Evaluation of its inhibitory action on Streptococcus mutans causing dental caries and endocarditis. J. Infect. Public Health 2021, 14, 324–330. [Google Scholar] [CrossRef] [PubMed]
- El-Adawy, M.M.; Eissa, A.E.; Shaalan, M.; Ahmed, A.A.; Younis, N.A.; Ismail, M.M.; Abdelsalam, M. Green synthesis and physical properties of Gum Arabic-silver nanoparticles and its antibacterial efficacy against fish bacterial pathogens. Aquac. Res. 2021, 52, 1247–1254. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Zhang, Z.; Wang, Z.; Zhao, Y.; Sun, L. A facile method to prepare size-tunable silver nanoparticles and its antibacterial mechanism. Adv. Powder Technol. 2018, 29, 407–415. [Google Scholar] [CrossRef]
- Rampersad, S.N. Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors 2012, 12, 12347–12360. [Google Scholar] [CrossRef] [PubMed]
- Rai, Y.; Pathak, R.; Kumari, N.; Sah, D.K.; Pandey, S.; Kalra, N.; Soni, R.; Dwarakanath, B.; Bhatt, A.N. Mitochondrial biogenesis and metabolic hyperactivation limits the application of MTT assay in the estimation of radiation induced growth inhibition. Sci. Rep. 2018, 8, 1531. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and cytotoxic properties of silver nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef] [Green Version]
- Molina-Hernandez, A.I.; Diaz-Gonzalez, J.M.; Saeb-Lima, M.; Dominguez-Cherit, J. Argyria after silver nitrate intake: Case report and brief review of literature. Indian J. Dermatol. 2015, 60, 520. [Google Scholar] [CrossRef]
- Jabir, M.S.; Saleh, Y.M.; Sulaiman, G.M.; Yaseen, N.Y.; Sahib, U.I.; Dewir, Y.H.; Alwahibi, M.S.; Soliman, D.A. Green Synthesis of Silver Nanoparticles Using Annona muricata Extract as an Inducer of Apoptosis in Cancer Cells and Inhibitor for NLRP3 Inflammasome via Enhanced Autophagy. Nanomaterials 2021, 11, 384. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, S.; Luo, J.; Wang, R.; Ding, W. Enhancement of the antibacterial activity of silver nanoparticles against phytopathogenic bacterium Ralstonia solanacearum by stabilization. J. Nanomater. 2016, 2016, 7135852. [Google Scholar] [CrossRef] [Green Version]
- Van der Zande, M.; Undas, A.K.; Kramer, E.; Monopoli, M.P.; Peters, R.J.; Garry, D.; Antunes Fernandes, E.C.; Hendriksen, P.J.; Marvin, H.J.; Peijnenburg, A.A. Different responses of Caco-2 and MCF-7 cells to silver nanoparticles are based on highly similar mechanisms of action. Nanotoxicology 2016, 10, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Recordati, C.; De Maglie, M.; Bianchessi, S.; Argentiere, S.; Cella, C.; Mattiello, S.; Cubadda, F.; Aureli, F.; D’Amato, M.; Raggi, A. Tissue distribution and acute toxicity of silver after single intravenous administration in mice: Nano-specific and size-dependent effects. Part. Fibre Toxicol. 2015, 13, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Dube, P.; Meyer, S.; Marnewick, J.L. Antimicrobial and antioxidant activities of different solvent extracts from fermented and green honeybush (Cyclopia intermedia) plant material. South Afr. J. Bot. 2017, 110, 184–193. [Google Scholar] [CrossRef]
- Mulfinger, L.; Solomon, S.D.; Bahadory, M.; Jeyarajasingam, A.V.; Rutkowsky, S.A.; Boritz, C. Synthesis and study of silver nanoparticles. J. Chem. Educ. 2007, 84, 322. [Google Scholar] [CrossRef]
- Sibuyi, N.R.S.; Thipe, V.C.; Panjtan-Amiri, K.; Meyer, M.; Katti, K.V. Green synthesis of gold nanoparticles using Acai berry and Elderberry extracts and investigation of their effect on prostate and pancreatic cancer cells. Nanobiomedicine 2021, 8, 1849543521995310. [Google Scholar] [CrossRef]
- Simo, A.; Drah, M.; Sibuyi, N.; Nkosi, M.; Meyer, M.; Maaza, M. Hydrothermal synthesis of cobalt-doped vanadium oxides: Antimicrobial activity study. Ceram. Int. 2018, 44, 7716–7722. [Google Scholar] [CrossRef]
- Radhakrishnan, V.S.; Mudiam, M.K.R.; Kumar, M.; Dwivedi, S.P.; Singh, S.P.; Prasad, T. Silver nanoparticles induced alterations in multiple cellular targets, which are critical for drug susceptibilities and pathogenicity in fungal pathogen (Candida albicans). Int. J. Nanomed. 2018, 13, 2647. [Google Scholar] [CrossRef] [Green Version]
- Tshweu, L.L.; Shemis, M.A.; Abdelghany, A.; Gouda, A.; Pilcher, L.A.; Sibuyi, N.R.; Meyer, M.; Dube, A.; Balogun, M.O. Synthesis, physicochemical characterization, toxicity and efficacy of a PEG conjugate and a hybrid PEG conjugate nanoparticle formulation of the antibiotic moxifloxacin. RSC Adv. 2020, 10, 19770–19780. [Google Scholar] [CrossRef]

| Phytochemical Content | 4 mg/mL GAE |
|---|---|
| Flavanols (mg/g) | 0.0187 |
| Flavonols (mg/g) | 0.0019 |
| TPC (mgGAE/g) | 0.0003 |
| DPPH (µmolTE/g) | 0.0000 |
| ORAC (µmolTE/g) | 0.0000 |
| FRAP (µmolAAE/g) | 0.0000 |
| AgNPs | λmax/SPR (nm) | Core Size (nm) | Hydrodynamic Size (nm) | ζ-Potential (mV) | Pdi |
|---|---|---|---|---|---|
| C-AgNPs | 408 | 10 ± 1.69 | 87.22 ± 5.94 | −30.50 ± 4.63 | 0.30 ± 0.03 |
| GAC-AgNPs | 414 | 12 ± 0.61 | 144.39 ± 4.99 | +9.33 ± 17.23 | 0.55 ± 0.01 |
| GA-AgNPs_0.4g | 416 | 12 ± 0.47 | 76.21 ± 6.35 | −29.60 ± 1.90 | 0.28 ± 0.03 |
| GA-AgNPs_0.5g | 414 | 12 ± 0.25 | 94.62 ± 10.06 | −27.07 ± 3.71 | 0.23 ± 0.06 |
| Treatments | ZOI (mm) | ||||
|---|---|---|---|---|---|
| S. aureus | MRSA | S. epidermidis | K. pneumoniae | E. coli | |
| MHB | 0 | 0 | 0 | 0 | 0 |
| GAE | 0 | 0 | 0 | 0 | 0 |
| C-AgNPs | 9.8 | 9.8 | 8.4 | 11 | 6.2 |
| GAC-AgNPs | 0 | 0 | 0 | 0 | 0 |
| GA-AgNPs_0.4g | 14.2 | 13.8 | 20 | 13.6 | 11.2 |
| GA-AgNPs_0.5g | 13 | 9.8 | 19 | 14.6 | 10.2 |
| Treatments | MIC (µg/mL) | |||||
|---|---|---|---|---|---|---|
| S. aureus | MRSA | S. epidermidis | S. pyogenes | K. pneumoniae | E. coli | |
| GAE | >100 | >100 | >100 | >100 | >100 | >100 |
| C-AgNPs | >100 | >100 | >100 | >100 | >100 | >100 |
| GAC-AgNPs | >100 | >100 | >100 | >100 | >100 | >100 |
| GA-AgNPs_0.4g | 6.25 | 6.25 | 6.25 | 6.25 | 6.25 | 25 |
| GA-AgNPs_0.5g | 6.25 | 6.25 | 6.25 | 6.25 | 6.25 | 6.25 |
| Treatments | MBC (µg/mL) | |||||
|---|---|---|---|---|---|---|
| S. aureus | MRSA | S. epidermidis | S. pyogenes | K. pneumoniae | E. coli | |
| GAE | >100 | >100 | >100 | >100 | >100 | >100 |
| C-AgNPs | >100 | >100 | >100 | >100 | >100 | >100 |
| GAC-AgNPs | >100 | >100 | >100 | >100 | >100 | >100 |
| GA-AgNPs_0.4g | >100 | 12.5 | 100 | >100 | 25 | 12.5 |
| GA-AgNPs_0.5g | 100 | 12.5 | 25 | >100 | 12.5 | 12.5 |
| Cell-Lines | IC50 (µg/mL) | ||||
|---|---|---|---|---|---|
| GAE | C-AgNPs | GAC-AgNPs | GA-AgNPs_0.4g | GA-AgNPs_0.5g | |
| KMST-6 | >100 | 87.40 | >100 | 0.67 | 0.90 |
| Caco-2 | >100 | 41.67 | 92.00 | 0.82 | 1.26 |
| HT-29 | >100 | 50.54 | >100 | 1.16 | 1.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadaka, A.O.; Meyer, S.; Ahmed, O.; Geerts, G.; Madiehe, M.A.; Meyer, M.; Sibuyi, N.R.S. Broad Spectrum Anti-Bacterial Activity and Non-Selective Toxicity of Gum Arabic Silver Nanoparticles. Int. J. Mol. Sci. 2022, 23, 1799. https://doi.org/10.3390/ijms23031799
Fadaka AO, Meyer S, Ahmed O, Geerts G, Madiehe MA, Meyer M, Sibuyi NRS. Broad Spectrum Anti-Bacterial Activity and Non-Selective Toxicity of Gum Arabic Silver Nanoparticles. International Journal of Molecular Sciences. 2022; 23(3):1799. https://doi.org/10.3390/ijms23031799
Chicago/Turabian StyleFadaka, Adewale O., Samantha Meyer, Omnia Ahmed, Greta Geerts, Madimabe A. Madiehe, Mervin Meyer, and Nicole R. S. Sibuyi. 2022. "Broad Spectrum Anti-Bacterial Activity and Non-Selective Toxicity of Gum Arabic Silver Nanoparticles" International Journal of Molecular Sciences 23, no. 3: 1799. https://doi.org/10.3390/ijms23031799
APA StyleFadaka, A. O., Meyer, S., Ahmed, O., Geerts, G., Madiehe, M. A., Meyer, M., & Sibuyi, N. R. S. (2022). Broad Spectrum Anti-Bacterial Activity and Non-Selective Toxicity of Gum Arabic Silver Nanoparticles. International Journal of Molecular Sciences, 23(3), 1799. https://doi.org/10.3390/ijms23031799








