A Major Intestinal Catabolite of Quercetin Glycosides, 3-Hydroxyphenylacetic Acid, Protects the Hepatocytes from the Acetaldehyde-Induced Cytotoxicity through the Enhancement of the Total Aldehyde Dehydrogenase Activity
Abstract
:1. Introduction
2. Results
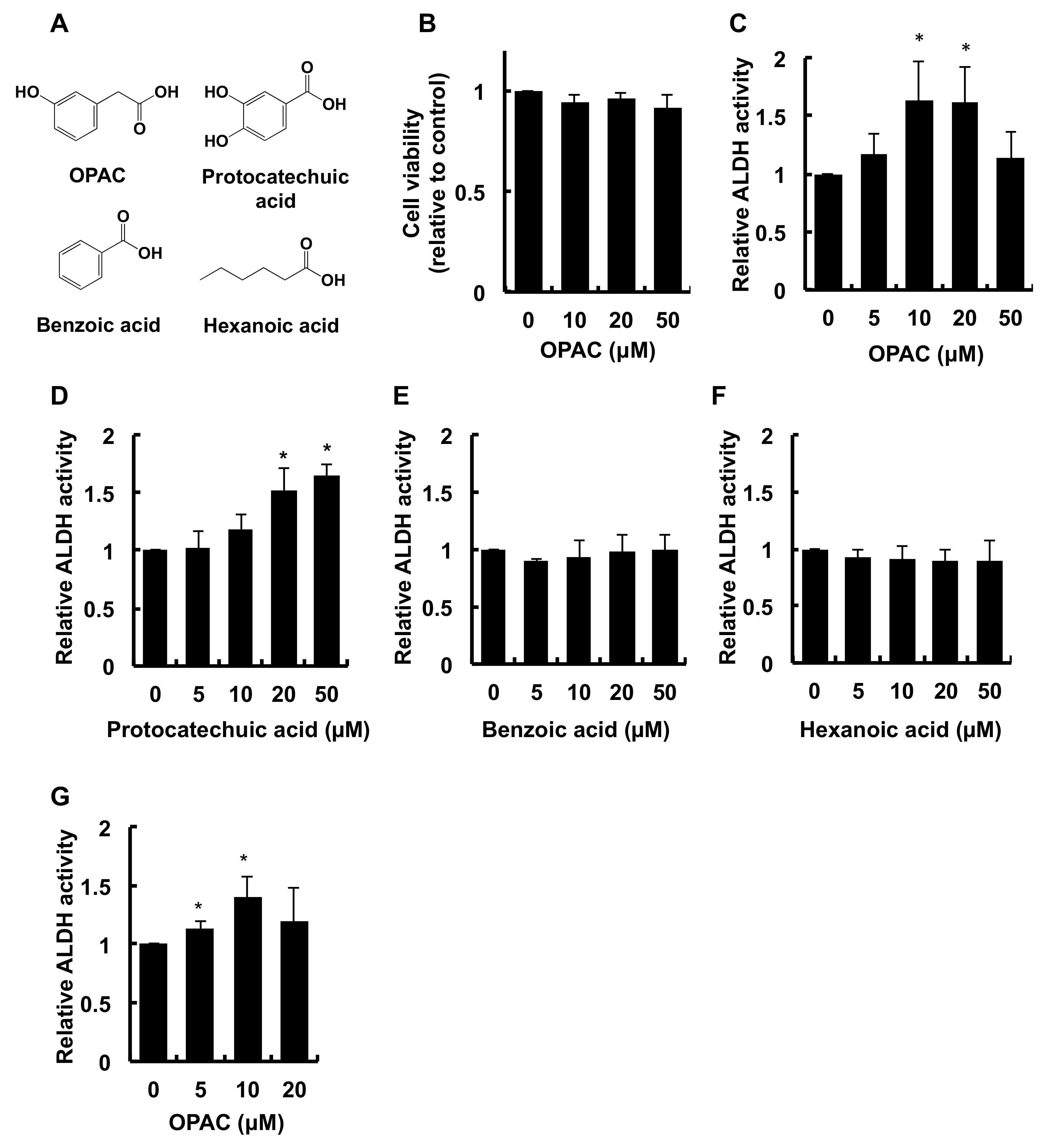
2.1. OPAC Enhanced the Total ALDH Enzyme Activity
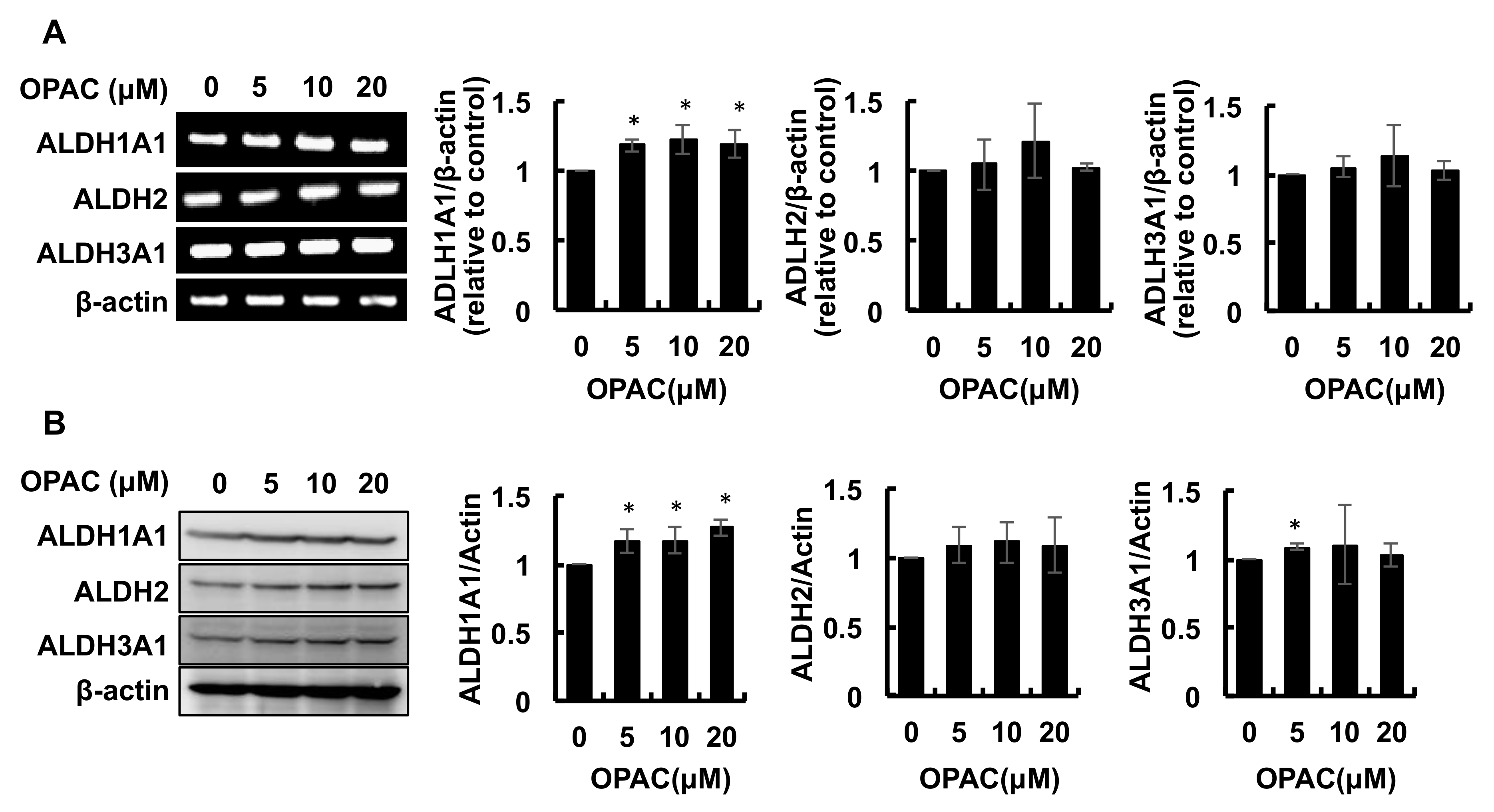
2.2. OPAC Enhanced the Protein and mRNA Levels of ALDH Isozymes
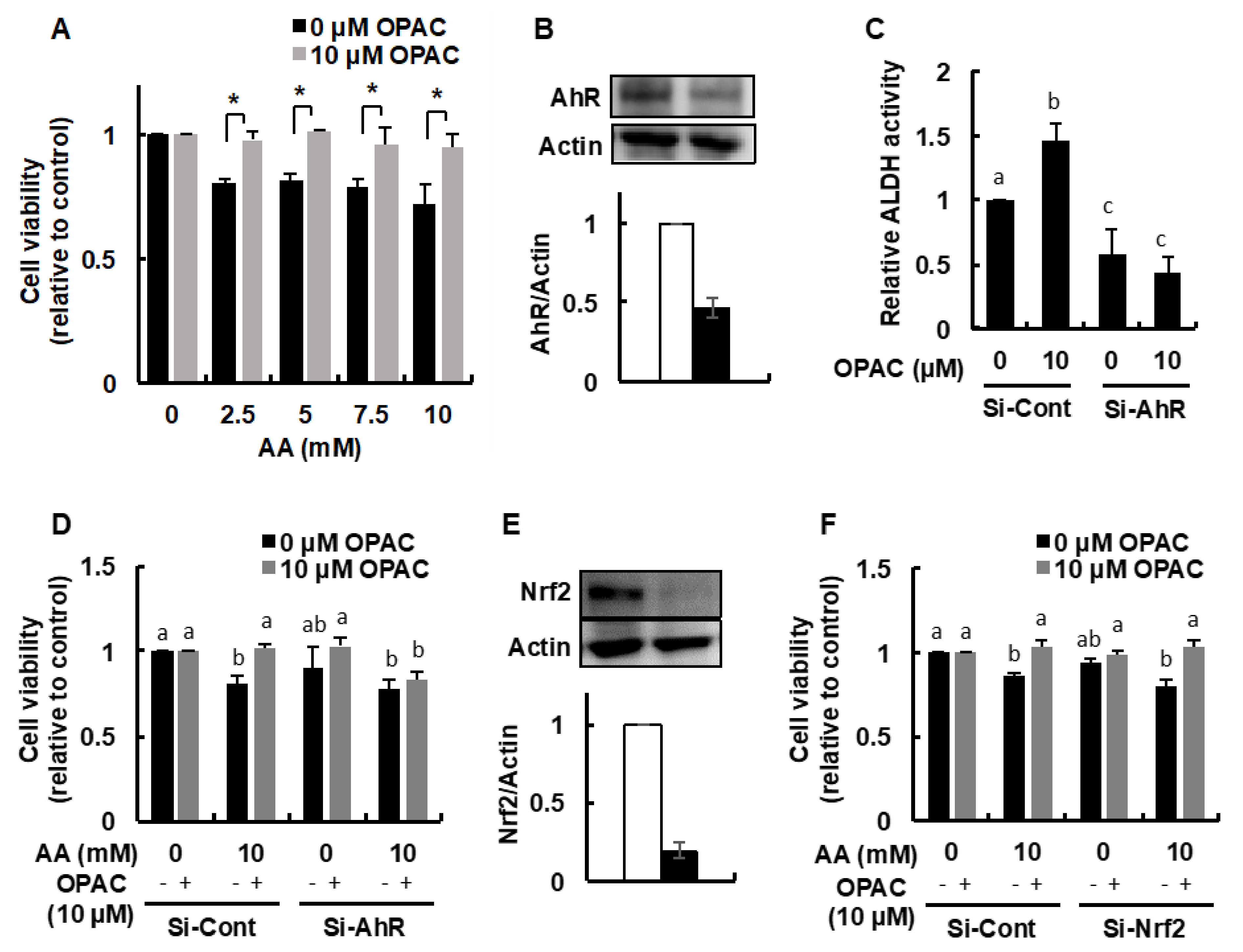
2.3. OPAC Activated the AhR Pathways
2.4. OPAC Protected the Cells from the Acetaldehyde-Induced Toxicity in an AhR-Dependent Manner
3. Discussion
4. Materials and Methods
4.1. Chemicals and Antibodies
4.2. Cell Culture
4.3. MTT Assay
4.4. ALDH Activity Assay
4.5. RT-PCR
4.6. Western Blot Analysis
4.7. Establishment of the Cell Line Stably Expressing the NanoLuc Reporter Gene
4.8. Luciferase Assay
4.9. RNA Interference
4.10. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Rocco, A.; Compare, D.; Angrisani, D.; Zamparelli, M.S.; Nardone, G. Alcoholic disease: Liver and beyond. World J. Gastroenterol. 2014, 20, 14652–14659. [Google Scholar] [CrossRef] [PubMed]
- Ceni, E.; Mello, T.; Galli, A. Pathogenesis of alcoholic liver disease: Role of oxidative metabolism. World J. Gastroenterol. 2014, 20, 17756–17772. [Google Scholar] [CrossRef] [PubMed]
- Vasiliou, V.; Nebert, D.W. Analysis and update of the human aldehyde dehydrogenase (ALDH) gene family. Hum. Genom. 2005, 2, 138–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lind, P.; Eriksson, C.J.P.; Wilhelmsen, K.C. The role of aldehyde dehydrogenase-1 (ALDH1A1) polymorphisms in harmful alcohol consumption in a Finnish population. Hum. Genom. 2008, 3, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-H.; Cruz, L.A.; Mochly-Rosen, D. Pharmacological recruitment of aldehyde dehydrogenase 3A1 (ALDH3A1) to assist ALDH2 in acetaldehyde and ethanol metabolism in vivo. Proc. Natl. Acad. Sci. USA 2015, 112, 3074–3079. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Wang, C.; Xu, H.; Gao, Y. Aldehyde Dehydrogenase, Liver Disease and Cancer. Int. J. Biol. Sci. 2020, 16, 921–934. [Google Scholar] [CrossRef]
- Terao, J.; Kawai, Y.; Murota, K. Vegetable flavonoids and cardiovascular disease. Asia Pac. J. Clin. Nutr. 2008, 17. [Google Scholar]
- Galleano, M.; Calabro, V.; Prince, P.D.; Litterio, M.C.; Piotrkowski, B.; Vazquez-Prieto, M.A.; Miatello, R.M.; Oteiza, P.I.; Fraga, C.G. Flavonoids and metabolic syndrome. Ann. N Y Acad. Sci. 2012, 1259, 87–94. [Google Scholar] [CrossRef]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef] [Green Version]
- Rechner, A.R.; Kuhnle, G.; Bremner, P.; Hubbard, G.P.; Moore, K.P.; Rice-Evans, C.A. The metabolic fate of dietary polyphenols in humans. Free. Radic. Biol. Med. 2002, 33, 220–235. [Google Scholar] [CrossRef]
- Kawabata, K.; Mukai, R.; Ishisaka, A. Quercetin and related polyphenols: New insights and implications for their bioactivity and bioavailability. Food Funct. 2015, 6, 1399–1417. [Google Scholar] [CrossRef] [PubMed]
- Murota, K.; Mitsukuni, Y.; Ichikawa, M.; Tsushida, T.; Miyamoto, S.; Terao, J. Quercetin-4‘-glucoside Is More Potent than Quercetin-3-glucoside in Protection of Rat Intestinal Mucosa Homogenates against Iron Ion-Induced Lipid Peroxidation. J. Agric. Food Chem. 2004, 52, 1907–1912. [Google Scholar] [CrossRef] [PubMed]
- Mullen, W.; Edwards, C.A.; Crozier, A. Absorption, excretion and metabolite profiling of methyl-, glucuronyl-, glucosyl- and sulpho-conjugates of quercetin in human plasma and urine after ingestion of onions. Br. J. Nutr. 2006, 96, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Mullen, W.; Rouanet, J.-M.; Auger, C.; Teissedre, P.-L.; Caldwell, S.; Hartley, R.; Lean, M.E.J.; Edwards, C.A.; Crozier, A. Bioavailability of [2-14C]Quercetin-4′-glucoside in Rats. J. Agric. Food Chem. 2008, 56, 12127–12137. [Google Scholar] [CrossRef] [PubMed]
- Rios, L.Y.; Gonthier, M.P.; Rémésy, C.; Mila, I.; Lapierre, C.; Lazarus, S.A.; Williamson, G.; Scalbert, A. Chocolate intake increases urinary excretion of polyphenol-derived phenolic acids in healthy human subjects. Am. J. Clin. Nutr. 2003, 77, 912–918. [Google Scholar] [CrossRef] [Green Version]
- Aura, A.-M.; O’Leary, K.A.; Williamson, G.; Ojala, M.; Bailey, M.; Puupponen-Pimiä, R.; Nuutila, A.M.; Oksman-Caldentey, K.-M.; Poutanen, K. Quercetin Derivatives Are Deconjugated and Converted to Hydroxyphenylacetic Acids but Not Methylated by Human Fecal Flora in Vitro. J. Agric. Food Chem. 2002, 50, 1725–1730. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Qian, D.; Guo, J.; Jiang, S.; Shang, E.X.; Duan, J.A.; Xu, J. Identification of the major metabolites of hyperoside produced by the human intestinal bacteria using the ultra performance liquid chromatography/quadrupole-time-of-flight mass spectrometry. J. Ethnopharmacol. 2013, 147, 174–179. [Google Scholar] [CrossRef]
- Tang, Y.; Nakashima, S.; Saiki, S.; Myoi, Y.; Abe, N.; Kuwazuru, S.; Zhu, B.; Ashida, H.; Murata, Y.; Nakamura, Y. 3,4-Dihydroxyphenylacetic acid is a predominant biologically-active catabolite of quercetin glycosides. Food Res. Int. 2016, 89, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Verzelloni, E.; Pellacani, C.; Tagliazucchi, D.; Tagliaferri, S.; Calani, L.; Costa, L.G.; Brighenti, F.; Borges, G.; Crozier, A.; Conte-Junior, C.; et al. Antiglycative and neuroprotective activity of colon-derived polyphenol catabolites. Mol. Nutr. Food Res. 2011, 55 (Suppl. S1), S35–S43. [Google Scholar] [CrossRef]
- Zhu, M.; Zhou, X.; Zhao, J. Quercetin prevents alcohol-induced liver injury through targeting of PI3K/Akt/nuclear factor-kappaB and STAT3 signaling pathway. Exp. Ther. Med. 2017, 14, 6169–6175. [Google Scholar]
- Liu, Y.; Kurita, A.; Nakashima, S.; Zhu, B.; Munemasa, S.; Nakamura, T.; Murata, Y.; Nakamura, Y. 3,4-Dihydroxyphenylacetic acid is a potential aldehyde dehydrogenase inducer in murine hepatoma Hepa1c1c7 cells. Biosci. Biotechnol. Biochem. 2017, 81, 1978–1983. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yamanaka, M.; Abe-Kanoh, N.; Liu, X.; Zhu, B.; Munemasa, S.; Nakamura, T.; Murata, Y.; Nakamura, Y. Benzyl isothiocyanate ameliorates acetaldehyde-induced cytotoxicity by enhancing aldehyde dehydrogenase activity in murine hepatoma Hepa1c1c7 cells. Food Chem Toxicol. 2017, 108, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Ushida, Y.; Talalay, P. Sulforaphane Accelerates Acetaldehyde Metabolism by Inducing Aldehyde Dehydrogenases: Relevance to Ethanol Intolerance. Alcohol Alcohol. 2013, 48, 526–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reisdorph, R.; Lindahl, R. Constitutive and 3-Methylcholanthrene-Induced Rat ALDH3A1 Expression Is Mediated by Multiple Xenobiotic Response Elements. Drug Metab. Dispos. 2006, 35, 386–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alnouti, Y.; Klaassen, C.D. Tissue Distribution, Ontogeny, and Regulation of Aldehyde Dehydrogenase (Aldh) Enzymes mRNA by Prototypical Microsomal Enzyme Inducers in Mice. Toxicol. Sci. 2008, 101, 51–64. [Google Scholar] [CrossRef] [Green Version]
- Shao, C.; Sullivan, J.P.; Girard, L.; Augustyn, A.; Yenerall, P.; Rodriguez-Canales, J.; Liu, H.; Behrens, C.; Shay, J.W.; Wistuba, I.I.; et al. Essential Role of Aldehyde Dehydrogenase 1A3 for the Maintenance of Non–Small Cell Lung Cancer Stem Cells Is Associated with the STAT3 Pathway. Clin. Cancer Res. 2014, 20, 4154–4166. [Google Scholar] [CrossRef] [Green Version]
- Oraldi, M.; Saracino, S.; Maggiora, M.; Chiaravalloti, A.; Buemi, C.; Martinasso, G.; Paiuzzi, E.; Thompson, D.; Vasiliou, V.; Canuto, R.A. Importance of inverse correlation between ALDH3A1 and PPARgamma in tumor cells and tissue regeneration. Chem. Biol. Interact. 2011, 191, 171–176. [Google Scholar] [CrossRef]
- Elizondo, G.; Corchero, J.; Sterneck, E.; Gonzalez, F.J. Feedback inhibition of the retinaldehyde dehydrogenase gene ALDH1 by retinoic acid through retinoic acid receptor alpha and CCAAT/enhancer-binding protein beta. J. Biol. Chem. 2000, 275, 39747–39753. [Google Scholar] [CrossRef] [Green Version]
- Ni, R.; Leo, M.A.; Zhao, J.; Lieber, C.S. Toxicity of beta-carotene and its exacerbation by acetaldehyde in HepG2 cells. Alcohol Alcohol. 2001, 36, 281–285. [Google Scholar] [CrossRef]
- Van Breemen, R.B.; Li, Y. Caco-2 cell permeability assays to measure drug absorption. Expert Opin. Drug Metab. Toxicol. 2005, 1, 175–185. [Google Scholar] [CrossRef]
- Yan, T.; Zhao, Y.; Zhang, X. Acetaldehyde Induces Cytotoxicity of SH-SY5Y Cells via Inhibition of Akt Activation and Induction of Oxidative Stress. Oxidative Med. Cell. Longev. 2015, 2016, 4512309. [Google Scholar] [CrossRef] [Green Version]
- Sastre, J.; Serviddio, G.; Pereda, J.; Minana, J.B.; Arduini, A.; Vendemiale, G.; Poli, G.; Pallardo, F.V.; Vina, J. Mitochondrial function in liver disease. Front. Biosci. 2007, 12, 1200–1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, Y.; Miyoshi, N. Electrophiles in Foods: The Current Status of Isothiocyanates and Their Chemical Biology. Biosci. Biotechnol. Biochem. 2010, 74, 242–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koli, R.; Erlund, I.; Jula, A.; Marniemi, J.; Mattila, P.; Alfthan, G. Bioavailability of Various Polyphenols from a Diet Containing Moderate Amounts of Berries. J. Agric. Food Chem. 2010, 58, 3927–3932. [Google Scholar] [CrossRef] [PubMed]
- Selvam, K.P.; Nagahata, T.; Kato, K.; Koreishi, M.; Nakamura, T.; Nakamura, Y.; Nishikawa, T.; Satoh, A.; Hayashi, Y. Synthesis and characterization of conductive flexible cellulose carbon nanohorn sheets for human tissue applications. Biomater. Res. 2020, 24, 18. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Myojin, T.; Li, K.; Kurita, A.; Seto, M.; Motoyama, A.; Liu, X.; Satoh, A.; Munemasa, S.; Murata, Y.; et al. A Major Intestinal Catabolite of Quercetin Glycosides, 3-Hydroxyphenylacetic Acid, Protects the Hepatocytes from the Acetaldehyde-Induced Cytotoxicity through the Enhancement of the Total Aldehyde Dehydrogenase Activity. Int. J. Mol. Sci. 2022, 23, 1762. https://doi.org/10.3390/ijms23031762
Liu Y, Myojin T, Li K, Kurita A, Seto M, Motoyama A, Liu X, Satoh A, Munemasa S, Murata Y, et al. A Major Intestinal Catabolite of Quercetin Glycosides, 3-Hydroxyphenylacetic Acid, Protects the Hepatocytes from the Acetaldehyde-Induced Cytotoxicity through the Enhancement of the Total Aldehyde Dehydrogenase Activity. International Journal of Molecular Sciences. 2022; 23(3):1762. https://doi.org/10.3390/ijms23031762
Chicago/Turabian StyleLiu, Yujia, Takumi Myojin, Kexin Li, Ayuki Kurita, Masayuki Seto, Ayano Motoyama, Xiaoyang Liu, Ayano Satoh, Shintaro Munemasa, Yoshiyuki Murata, and et al. 2022. "A Major Intestinal Catabolite of Quercetin Glycosides, 3-Hydroxyphenylacetic Acid, Protects the Hepatocytes from the Acetaldehyde-Induced Cytotoxicity through the Enhancement of the Total Aldehyde Dehydrogenase Activity" International Journal of Molecular Sciences 23, no. 3: 1762. https://doi.org/10.3390/ijms23031762
APA StyleLiu, Y., Myojin, T., Li, K., Kurita, A., Seto, M., Motoyama, A., Liu, X., Satoh, A., Munemasa, S., Murata, Y., Nakamura, T., & Nakamura, Y. (2022). A Major Intestinal Catabolite of Quercetin Glycosides, 3-Hydroxyphenylacetic Acid, Protects the Hepatocytes from the Acetaldehyde-Induced Cytotoxicity through the Enhancement of the Total Aldehyde Dehydrogenase Activity. International Journal of Molecular Sciences, 23(3), 1762. https://doi.org/10.3390/ijms23031762





