Extracellular Matrix Synthesis and Remodeling by Mesenchymal Stromal Cells Is Context-Sensitive
Abstract
:1. Introduction
2. Results
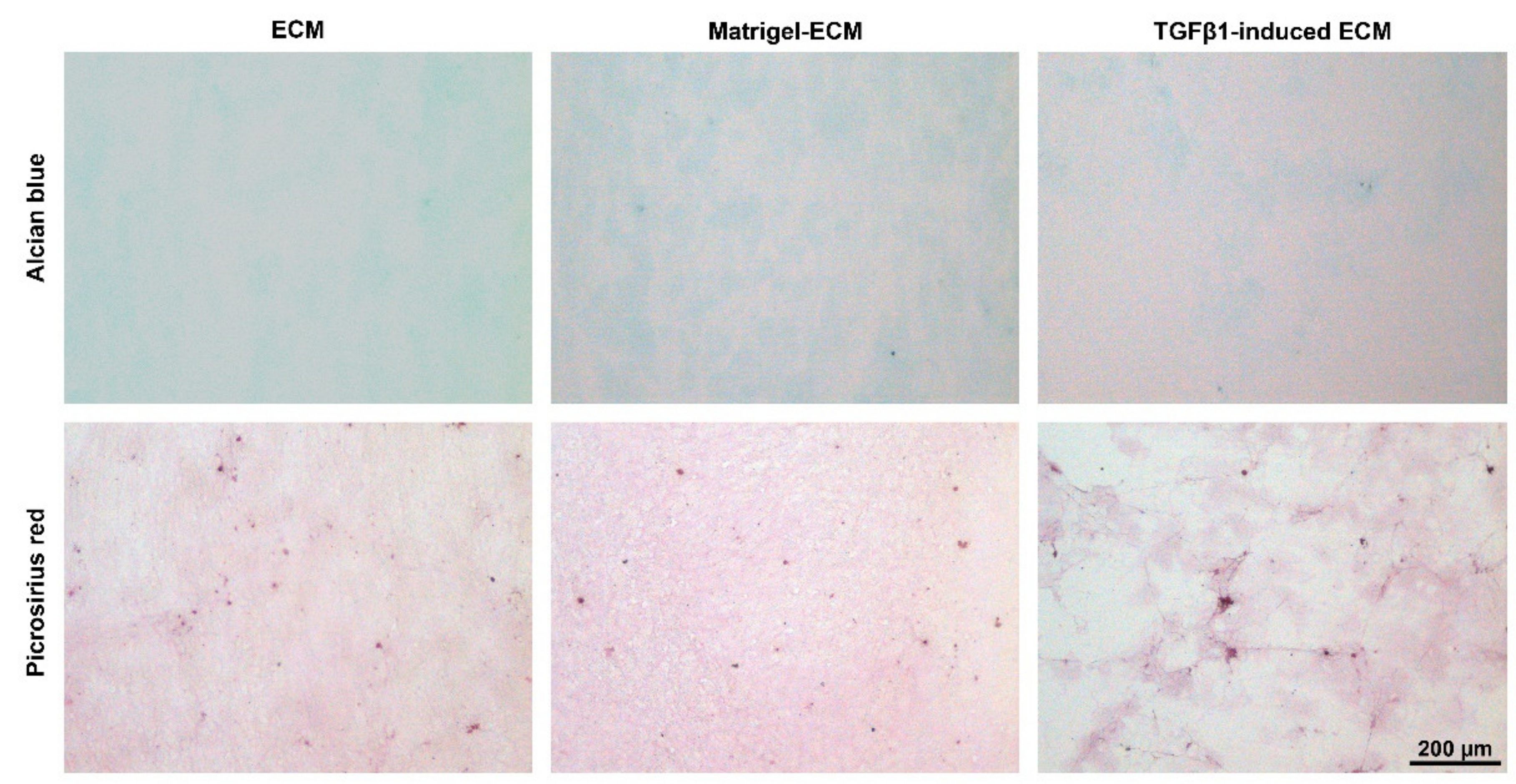
2.1. MSC-Derived ECM Culture Substrates
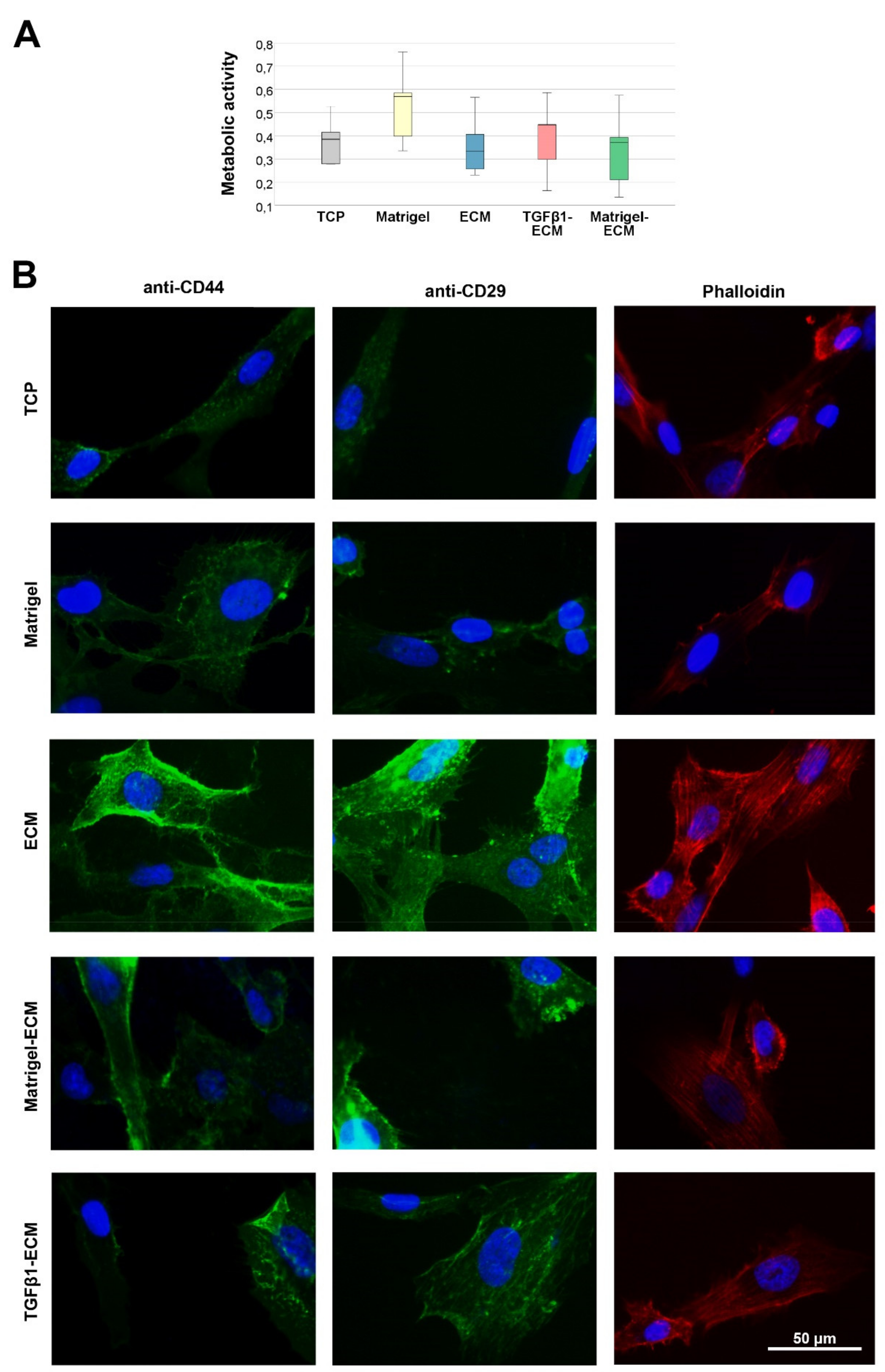
2.2. Cell Viability, Presence of ECM Receptors and Cytoskeleton Formation
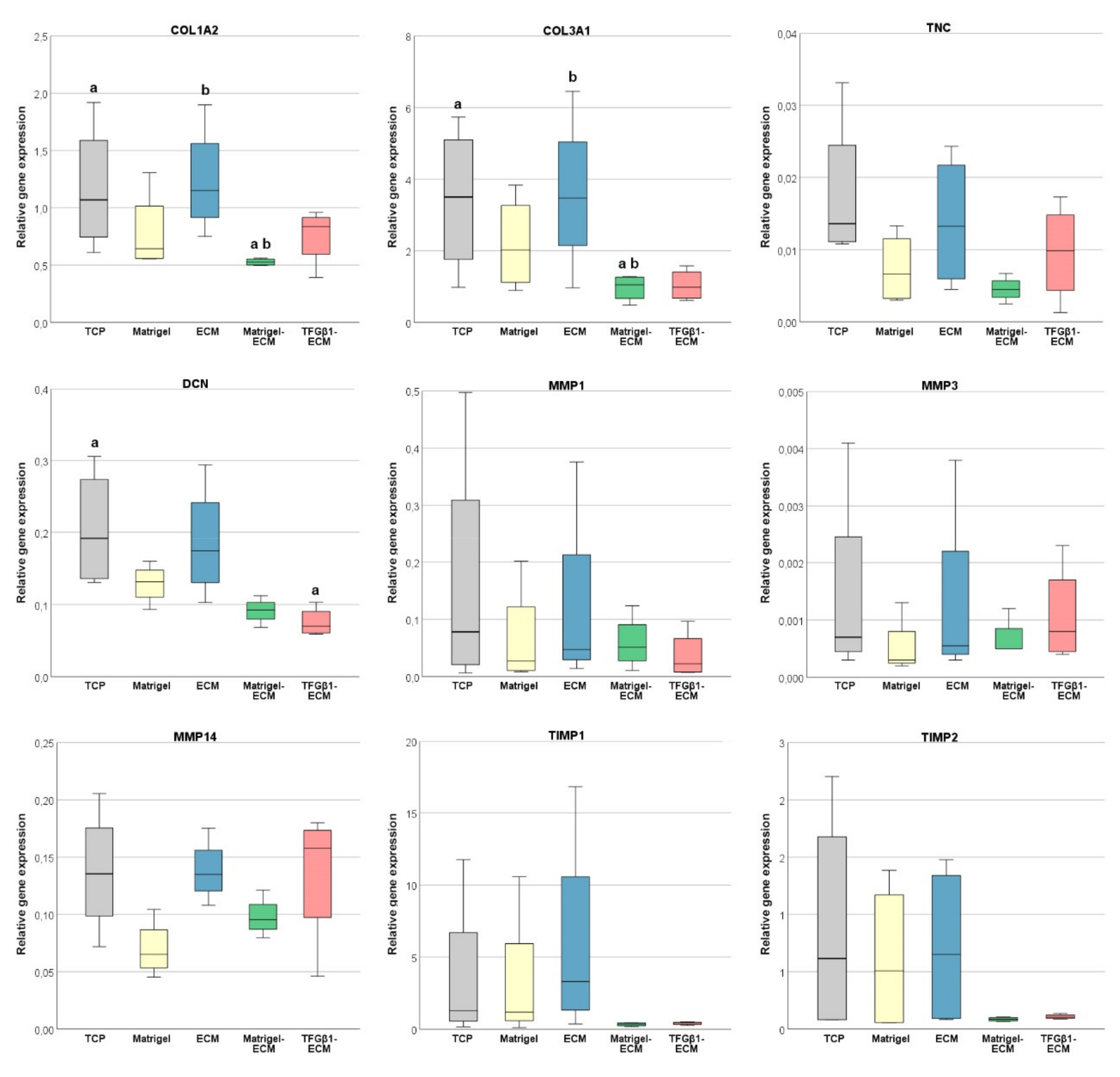
2.3. ECM, MMP and TIMP Gene Expression
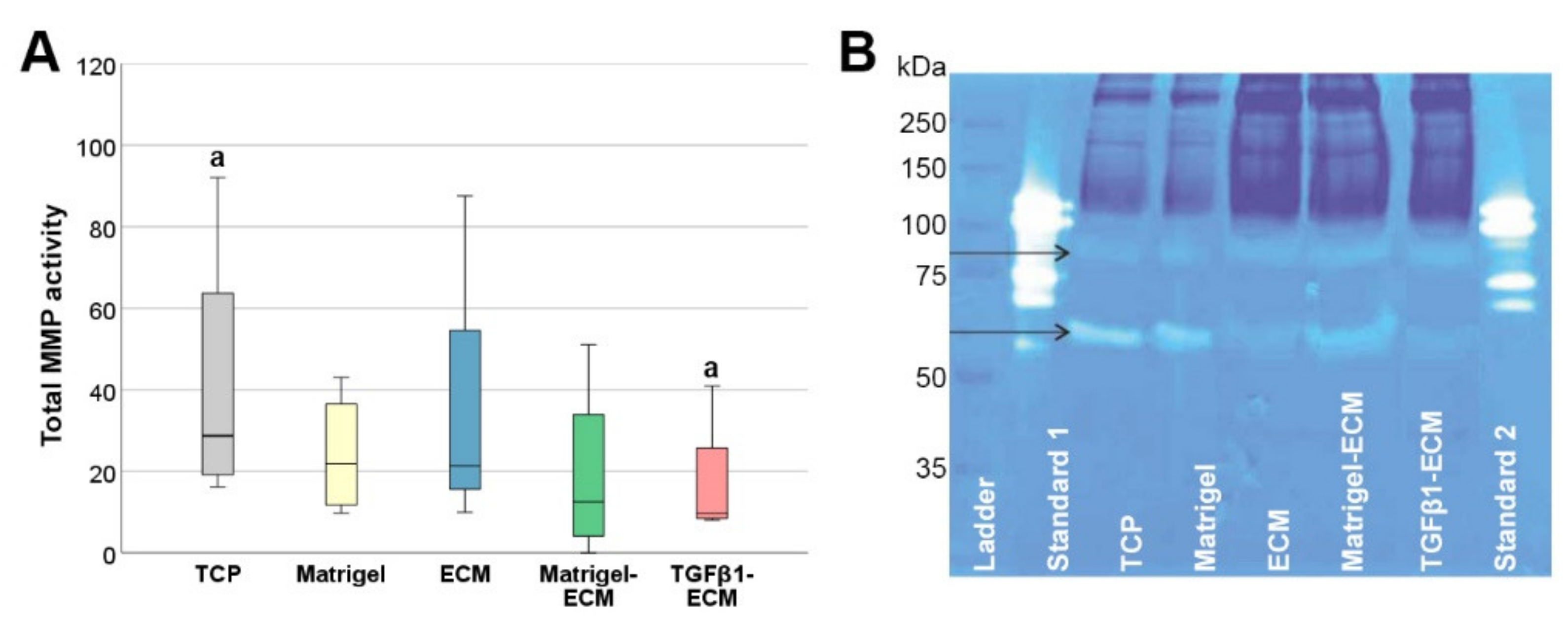
2.4. MMP Gene Expression and Activity
3. Discussion
4. Materials and Methods
4.1. MSC and Study Design
4.2. Preparation and Evaluation of Cell Culture Substrates
4.3. MSC Culture Experiments
4.4. Cell Viability Assay
4.5. (Immuno-)Fluorescence Staining
4.6. Real Time RT-PCR
4.7. MMP Activity Assay and Zymography
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matas, J.; Orrego, M.; Amenabar, D.; Infante, C.; Tapia-Limonchi, R.; Cadiz, M.I.; Alcayaga-Miranda, F.; González, P.L.; Muse, E.; Khoury, M.; et al. Umbilical Cord-Derived Mesenchymal Stromal Cells (MSCs) for Knee Osteoarthritis: Repeated MSC Dosing Is Superior to a Single MSC Dose and to Hyaluronic Acid in a Controlled Randomized Phase I/II Trial. Stem Cells Transl. Med. 2019, 8, 215–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boberg, E.; von Bahr, L.; Afram, G.; Lindström, C.; Ljungman, P.; Heldring, N.; Petzelbauer, P.; Garming Legert, K.; Kadri, N.; Le Blanc, K. Treatment of chronic GvHD with mesenchymal stromal cells induces durable responses: A phase II study. Stem Cells Transl. Med. 2020, 9, 1190–1202. [Google Scholar] [CrossRef] [PubMed]
- Hoshiba, T.; Chen, G.; Endo, C.; Maruyama, H.; Wakui, M.; Nemoto, E.; Kawazoe, N.; Tanaka, M. Decellularized Extracellular Matrix as an In Vitro Model to Study the Comprehensive Roles of the ECM in Stem Cell Differentiation. Stem Cells Int. 2016, 2016, 6397820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, T.R.; Erler, J.T. Remodeling and homeostasis of the extracellular matrix: Implications for fibrotic diseases and cancer. Dis. Model. Mech. 2011, 4, 165–178. [Google Scholar] [CrossRef] [Green Version]
- McKee, T.J.; Perlman, G.; Morris, M.; Komarova, S.V. Extracellular matrix composition of connective tissues: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 10542. [Google Scholar] [CrossRef] [Green Version]
- Arpino, V.; Brock, M.; Gill, S.E. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 2015, 44–46, 247–254. [Google Scholar] [CrossRef]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [Green Version]
- Lodyga, M.; Hinz, B. TGF-β1—A truly transforming growth factor in fibrosis and immunity. Semin. Cell Dev. Biol. 2020, 101, 123–139. [Google Scholar] [CrossRef]
- Weiskirchen, R.; Weiskirchen, S.; Tacke, F. Organ and tissue fibrosis: Molecular signals, cellular mechanisms and translational implications. Mol. Asp. Med. 2019, 65, 2–15. [Google Scholar] [CrossRef]
- Newby, A.C. Metalloproteinase production from macrophages—A perfect storm leading to atherosclerotic plaque rupture and myocardial infarction. Exp. Physiol. 2016, 101, 1327–1337. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, Q.; Ma, R.; Yin, Y.; Lan, T.; Yu, M.; Ming, Y. Mesenchymal Stem Cells in Renal Fibrosis: The Flame of Cytotherapy. Stem Cells Int. 2019, 2019, 8387350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behnke, J.; Kremer, S.; Shahzad, T.; Chao, C.-M.; Böttcher-Friebertshäuser, E.; Morty, R.E.; Bellusci, S.; Ehrhardt, H. MSC Based Therapies-New Perspectives for the Injured Lung. J. Clin. Med. 2020, 9, 682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Yu, Y.; Hu, S.; Chen, Y.; Shen, Z. The therapeutic potential of mesenchymal stem cells for cardiovascular diseases. Cell Death Dis. 2020, 11, 349. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Ji, C.; Lu, L. Mesenchymal stem cell therapy for liver fibrosis/cirrhosis. Ann. Transl. Med. 2020, 8, 562. [Google Scholar] [CrossRef] [PubMed]
- Usunier, B.; Benderitter, M.; Tamarat, R.; Chapel, A. Management of fibrosis: The mesenchymal stromal cells breakthrough. Stem Cells Int. 2014, 2014, 340257. [Google Scholar] [CrossRef] [Green Version]
- El Agha, E.; Kramann, R.; Schneider, R.K.; Li, X.; Seeger, W.; Humphreys, B.D.; Bellusci, S. Mesenchymal Stem Cells in Fibrotic Disease. Cell Stem Cell 2017, 21, 166–177. [Google Scholar] [CrossRef]
- Lozito, T.P.; Tuan, R.S. Mesenchymal stem cells inhibit both endogenous and exogenous MMPs via secreted TIMPs. J. Cell. Physiol. 2011, 226, 385–396. [Google Scholar] [CrossRef]
- Lozito, T.P.; Jackson, W.M.; Nesti, L.J.; Tuan, R.S. Human mesenchymal stem cells generate a distinct pericellular zone of MMP activities via binding of MMPs and secretion of high levels of TIMPs. Matrix Biol. 2014, 34, 132–143. [Google Scholar] [CrossRef]
- Kasper, G.; Glaeser, J.D.; Geissler, S.; Ode, A.; Tuischer, J.; Matziolis, G.; Perka, C.; Duda, G.N. Matrix metalloprotease activity is an essential link between mechanical stimulus and mesenchymal stem cell behavior. Stem Cells 2007, 25, 1985–1994. [Google Scholar] [CrossRef]
- Ragelle, H.; Naba, A.; Larson, B.L.; Zhou, F.; Prijić, M.; Whittaker, C.A.; Del Rosario, A.; Langer, R.; Hynes, R.O.; Anderson, D.G. Comprehensive proteomic characterization of stem cell-derived extracellular matrices. Biomaterials 2017, 128, 147–159. [Google Scholar] [CrossRef]
- Harvey, A.; Yen, T.-Y.; Aizman, I.; Tate, C.; Case, C. Proteomic analysis of the extracellular matrix produced by mesenchymal stromal cells: Implications for cell therapy mechanism. PLoS ONE 2013, 8, e79283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amable, P.R.; Teixeira, M.V.T.; Carias, R.B.V.; Granjeiro, J.M.; Borojevic, R. Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton’s jelly. Stem Cell Res. Ther. 2014, 5, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, H.-C.; Jiang, L.; Geng, W.-X.; Li, J.; Zhang, R.; Dang, J.-G.; Shu, M.-G.; Li, L.-W. Growth Factor-Reinforced ECM Fabricated from Chemically Hypoxic MSC Sheet with Improved In Vivo Wound Repair Activity. Biomed Res. Int. 2017, 2017, 2578017. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, M.S.; Silva, J.C.; Cabral, J.M.S.; da Silva, C.L.; Vashishth, D. Cultured cell-derived extracellular matrices to enhance the osteogenic differentiation and angiogenic properties of human mesenchymal stem/stromal cells. J. Tissue Eng. Regen. Med. 2019, 13, 1544–1558. [Google Scholar] [CrossRef]
- Marinkovic, M.; Tran, O.N.; Block, T.J.; Rakian, R.; Gonzalez, A.O.; Dean, D.D.; Yeh, C.-K.; Chen, X.-D. Native extracellular matrix, synthesized ex vivo by bone marrow or adipose stromal cells, faithfully directs mesenchymal stem cell differentiation. Matrix Biol. Plus 2020, 8, 100044. [Google Scholar] [CrossRef] [PubMed]
- Antebi, B.; Zhang, Z.; Wang, Y.; Lu, Z.; Chen, X.-D.; Ling, J. Stromal-cell-derived extracellular matrix promotes the proliferation and retains the osteogenic differentiation capacity of mesenchymal stem cells on three-dimensional scaffolds. Tissue Eng. Part C Methods 2015, 21, 171–181. [Google Scholar] [CrossRef] [Green Version]
- García-García, A.; Martin, I. Extracellular Matrices to Modulate the Innate Immune Response and Enhance Bone Healing. Front. Immunol. 2019, 10, 2256. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Tan, J.; Hu, C.; Hou, T.; Peng, W.; Liu, J.; Yu, B.; Dai, Q.; Zhou, J.; Yang, Y.; et al. Modification of PLGA Scaffold by MSC-Derived Extracellular Matrix Combats Macrophage Inflammation to Initiate Bone Regeneration via TGF-β-Induced Protein. Adv. Healthc. Mater. 2020, 9, e2000353. [Google Scholar] [CrossRef] [PubMed]
- Brakebusch, C.; Fässler, R. The integrin-actin connection, an eternal love affair. EMBO J. 2003, 22, 2324–2333. [Google Scholar] [CrossRef]
- Doll, C.U.; Niebert, S.; Burk, J. Mesenchymal Stromal Cells Adapt to Chronic Tendon Disease Environment with an Initial Reduction in Matrix Remodeling. Int. J. Mol. Sci. 2021, 22, 12798. [Google Scholar] [CrossRef]
- Fukushima, K.; Itaba, N.; Kono, Y.; Okazaki, S.; Enokida, S.; Kuranobu, N.; Murakami, J.; Enokida, M.; Nagashima, H.; Kanzaki, S.; et al. Secreted matrix metalloproteinase-14 is a predictor for antifibrotic effect of IC-2-engineered mesenchymal stem cell sheets on liver fibrosis in mice. Regen. Ther. 2021, 18, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Burk, J.; Holland, H.; Lauermann, A.F.; May, T.; Siedlaczek, P.; Charwat, V.; Kasper, C. Generation and characterization of a functional human adipose-derived multipotent mesenchymal stromal cell line. Biotechnol. Bioeng. 2019, 116, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primer Sequences | Accession Number | PCR Product |
|---|---|---|---|
| GAPDH | CCACTCCTCCACCTTTGACG CCCTGTTGCTGTAGCCAAATTC | NM_002046.7 | 101 bp |
| COL1A2 | CAAGGACAAGAAACACGTCTGG CAAGGACAAGAAACACGTCTGG | NM_000089.4 | 101 bp |
| COL3A1 | TGAATATCGAACACGCAAGGC AAAGCAAACAGGGCCAACG | NM_000090.4 | 109 bp |
| DCN | GGAGATACAGCCATCCACCTTC CCAGGTTATAAAAATGAGGGCTTTC | NM_001920.5 | 102 bp |
| TNC | CTCTGGAAGACACCGTTGGC GAAGTGGTGTTTCTTGGAAGCTG | NM_002160.4 | 101 bp |
| MMP1 | CACAGCTTCCCAGCGACTCT TTCAGCATCTGGTTTCCCAGT | NM_002421.4 | 195 bp |
| MMP3 | CCCGAGGTTGGACCTACAAG ATAGGCTGAGCAAACTGCCA | NM_002422.4 | 132 bp |
| MMP14 | GGCGGGTGAGGAATAACCAA ACGCCTCATCAAACACCCAA | NM_004995.4 | 156 bp |
| TIMP1 | GCTGGAAAACTGCAGGATGG TCCGTCCACAAGCAATGAGT | NM_003254.3 | 191 bp |
| TIMP2 | TCCAAAGCCACCTTAGCCTG GTACAGCATGAAAACGCCCG | NM_003255.5 | 196 bp |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burk, J.; Sassmann, A.; Kasper, C.; Nimptsch, A.; Schubert, S. Extracellular Matrix Synthesis and Remodeling by Mesenchymal Stromal Cells Is Context-Sensitive. Int. J. Mol. Sci. 2022, 23, 1758. https://doi.org/10.3390/ijms23031758
Burk J, Sassmann A, Kasper C, Nimptsch A, Schubert S. Extracellular Matrix Synthesis and Remodeling by Mesenchymal Stromal Cells Is Context-Sensitive. International Journal of Molecular Sciences. 2022; 23(3):1758. https://doi.org/10.3390/ijms23031758
Chicago/Turabian StyleBurk, Janina, Anna Sassmann, Cornelia Kasper, Ariane Nimptsch, and Susanna Schubert. 2022. "Extracellular Matrix Synthesis and Remodeling by Mesenchymal Stromal Cells Is Context-Sensitive" International Journal of Molecular Sciences 23, no. 3: 1758. https://doi.org/10.3390/ijms23031758
APA StyleBurk, J., Sassmann, A., Kasper, C., Nimptsch, A., & Schubert, S. (2022). Extracellular Matrix Synthesis and Remodeling by Mesenchymal Stromal Cells Is Context-Sensitive. International Journal of Molecular Sciences, 23(3), 1758. https://doi.org/10.3390/ijms23031758







