1. Introduction
Esophageal cancer (EC) is the sixth leading cause of cancer mortality worldwide [
1]. In 2021, in the United States alone, there is an estimated number of 19,260 new cases of EC and 15,530 deaths from EC (
www.seer.cancer.gov/statfacts, accessed on 24 January 2022). Two main types of EC are distinguished by different etiological and pathological characteristics: esophageal squamous cell carcinoma (ESCC) and adenocarcinoma (EAC). While EAC is more prevalent in the USA, ESCC predominates among Asians and male African Americans [
2]. Both cancers remain asymptomatic; therefore, patients are usually diagnosed at relatively late stages, with an overall 5-year survival rate of below 20%, according to the SEER Cancer Statistics review [
3]. The need for development of accurate and timely treatments for EC, thus, is crucial. In recent years, targeting dysregulated Ca
2+ signaling in cancer cells has become an active research area to develop new chemotherapy drugs [
4].
Ca
2+ signaling plays an important role in cell proliferation, apoptosis, autophagy, migration and cell cycle; thus, its dysregulation is associated with tumor initiation, angiogenesis, progression and metastasis [
4]. The intracellular Ca
2+ signals have different forms, such as Ca
2+ spikes, waves and oscillations [
5,
6]. They are regulated by both intracellular Ca
2+ release and extracellular Ca
2+ influx [
7]. Store-operated Ca
2+ entry (SOCE) is a ubiquitous important extracellular Ca
2+ influx, which is mainly mediated by two proteins, i.e., stromal-interacting molecule 1 (STIM1) as endoplasmic reticulum (ER) Ca
2+ storage sensor and Orai1 as plasma membrane (PM) Ca
2+ channel. During activation of SOCE, the depletion of ER Ca
2+ stores triggers translocation of STIM1 to ER-PM junctions, followed by binding and activation of Orai1 channel at PM [
8]. Accumulating evidence has shown that STIM1/Orai1-mediated SOCE actively participates in the progression of many cancers [
9], such as breast cancer [
10], pancreatic adenocarcinoma [
11] and prostate cancer [
12]. We previously reported that high expression of Orai1 in tumor tissues is associated with poor prognosis in ESCC patients, and SOCE-mediated intracellular Ca
2+ oscillations regulate cell proliferation, migration and invasion in ESCC cells [
13]. Decreased Orai1-mediated SOCE, either by gene knockdown or pharmacological channel blockers, is able to reduce the frequency of intracellular Ca
2+ oscillations in cultured ESCC cells and to inhibit tumor growth in preclinical animal models. Among many SOCE blockers, RP4010 is a recently developed one in clinical trial phase I/IB. Our published study suggested that RP4010 is a promising chemotherapy drug targeting SOCE for ESCC patients [
14].
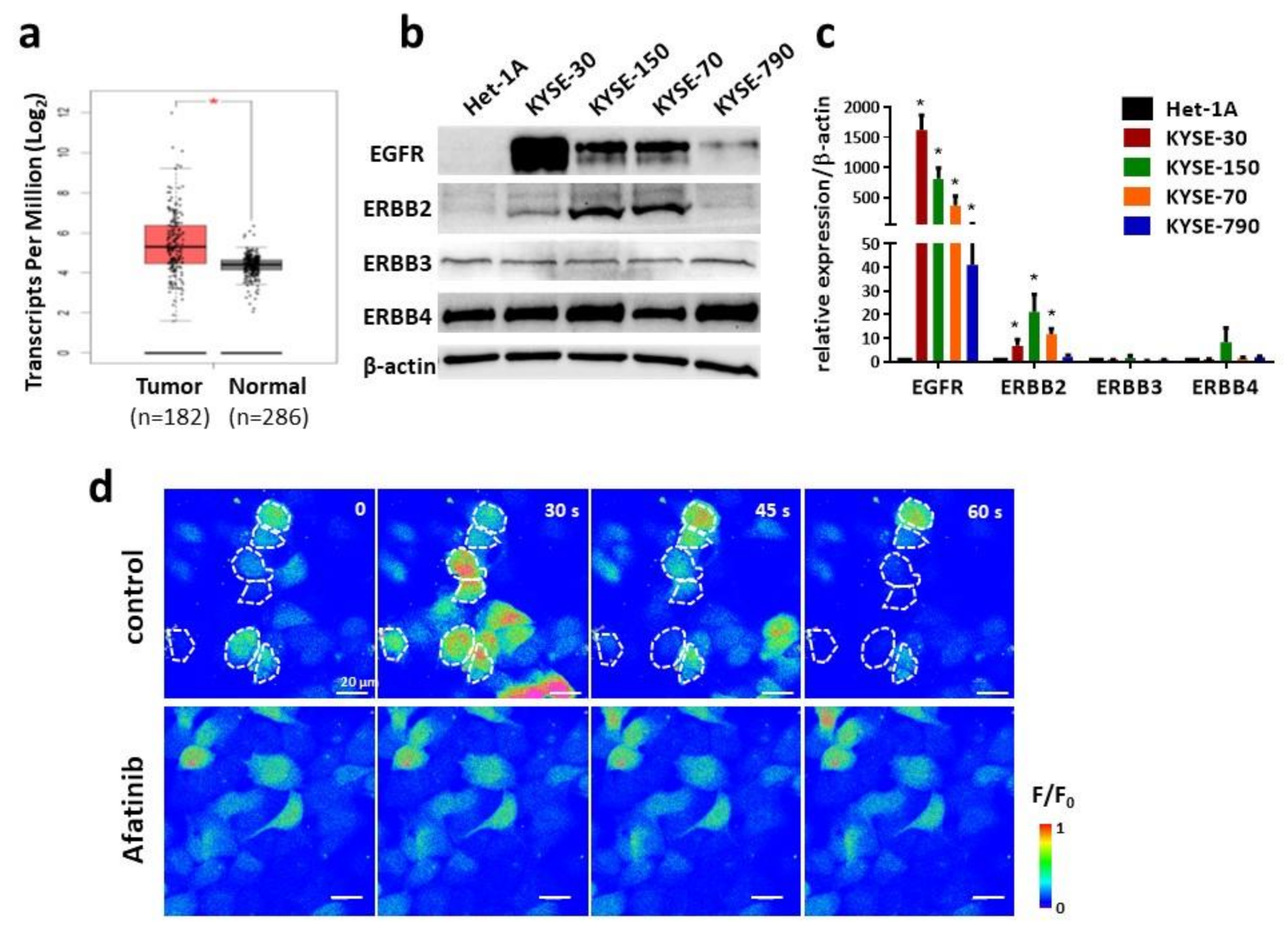
The overexpression and/or mutations of epidermal growth factor receptor (EGFR) family proteins, i.e., EGFR (HER1), ERBB2, ERBB3 and ERBB4, are often found in multiple types of cancer cells and are considered to be a significant prognostic indicator in the clinical intervention of cancer. Upon binding with the EGF or other ligands, EGFR undergoes a conformational change on the extracellular domain, followed by dimerization and trans-phosphorylation of tyrosine kinases in the intracellular domain [
15]. Activated EGFR tyrosine kinase triggers multiple signaling pathways. One is through phosphorylation of phospholipase C-γ (PLC-γ)/inositol-1,4,5-trisphosphate (IP3)/IP3 receptor (IP3R) pathway and results in intracellular Ca
2+ release from ER [
16]. Others include downstream PI3K/AKT and MEK/ERK signaling pathways, which are essential for cancer progression. Accordingly, many tyrosine kinase inhibitors (TKIs) have been developed, and more than 20 have received FDA approval as targeting cancer therapy for head and neck, lung, breast and colon cancers [
17]. Afatinib is one of the second-generation TKIs and has an irreversible inhibition on both EGFR and HER2 [
18]. As its regulatory approval for use as a treatment for non-small-cell lung cancer and squamous cell carcinoma of the lung, many preclinical and clinical studies support its benefit for patients with other cancers, including recurrent and/or metastatic ESCC [
19]. However, a challenge of using afatinib, as well as other TKIs, for chemotherapy is that most patients show TKI resistance after initial response [
20]. Consequently, combinational treatments of chemotherapy, immunotherapy or other kinds of targeting therapy have been proposed to improve the treatment effect and patient survival rate. Considering that EGFR stimulates intracellular Ca
2+ release and SOCE-mediated Ca
2+ influx regulates EGFR downstream AKT and ERK activity, we hypothesize that combined afatinib and RP4010 may achieve an enhanced anticancer effect in ESCC cells. Since the dynamic of intracellular Ca
2+ is at the intersection between the two signaling pathways and it can be rapidly simulated by a mathematical model, this study used both mathematical simulation and experimental data of the dynamics of intracellular Ca
2+ in cultured ESCC cell lines to evaluate the combined anticancer effects of afatinib and RP4010.
3. Discussion
Targeting SOCE-mediated Ca
2+ signaling in cancer cells is an emerging chemotherapy approach, and several SOCE blockers, including RP4010, are currently being evaluated in clinical trials [
23]. As a second-generation TKI, afatinib received FDA approval to be used in targeted therapy for patients with EGFR mutation-positive cancers, but not for ESCC. To improve the treatment response and patient survival rate, it is a common practice to combine several treatments or several chemotherapeutic drugs. In this study, we examined whether a combination of afatinib and RP4010 could achieve better anticancer effects in ESCC cells. Using both mathematical simulation and live-cell intracellular Ca
2+ measurement, we evaluated the inhibitory effect of afatinib and RP4010 on intracellular Ca
2+ oscillations in KYSE-150 cells. This computer-aided mathematical model successfully predicted the frequency of intracellular Ca
2+ oscillations in KYSE-150 cells responding to RP4010 and afatinib either individually or in combination. Both experimental and mathematical simulation data showed that a combination of afatinib and RP4010 could synergistically reduce the frequency of intracellular Ca
2+ oscillations and helped to predict their effects on cell viability in KYSE-150 cells. This intracellular Ca
2+ dynamic-based mathematical simulation approach could be used as a rapid and cost-effective evaluation of combined targeting therapy drugs.
The anticancer function of TKI has been mainly studied in the aspect of inhibiting downstream PI3K/Akt and MEK/ERK pathways; however, its PLC-γ axis has received less attention. During the preparation of this article, Kim et al. reported that gefitinib, a first-generation reversible TKI, inhibits EGF-stimulated intracellular Ca
2+ oscillations in non-small-cell lung cancer cells and that restricting extracellular Ca
2+ can consequently enhance gefitinib sensitivity [
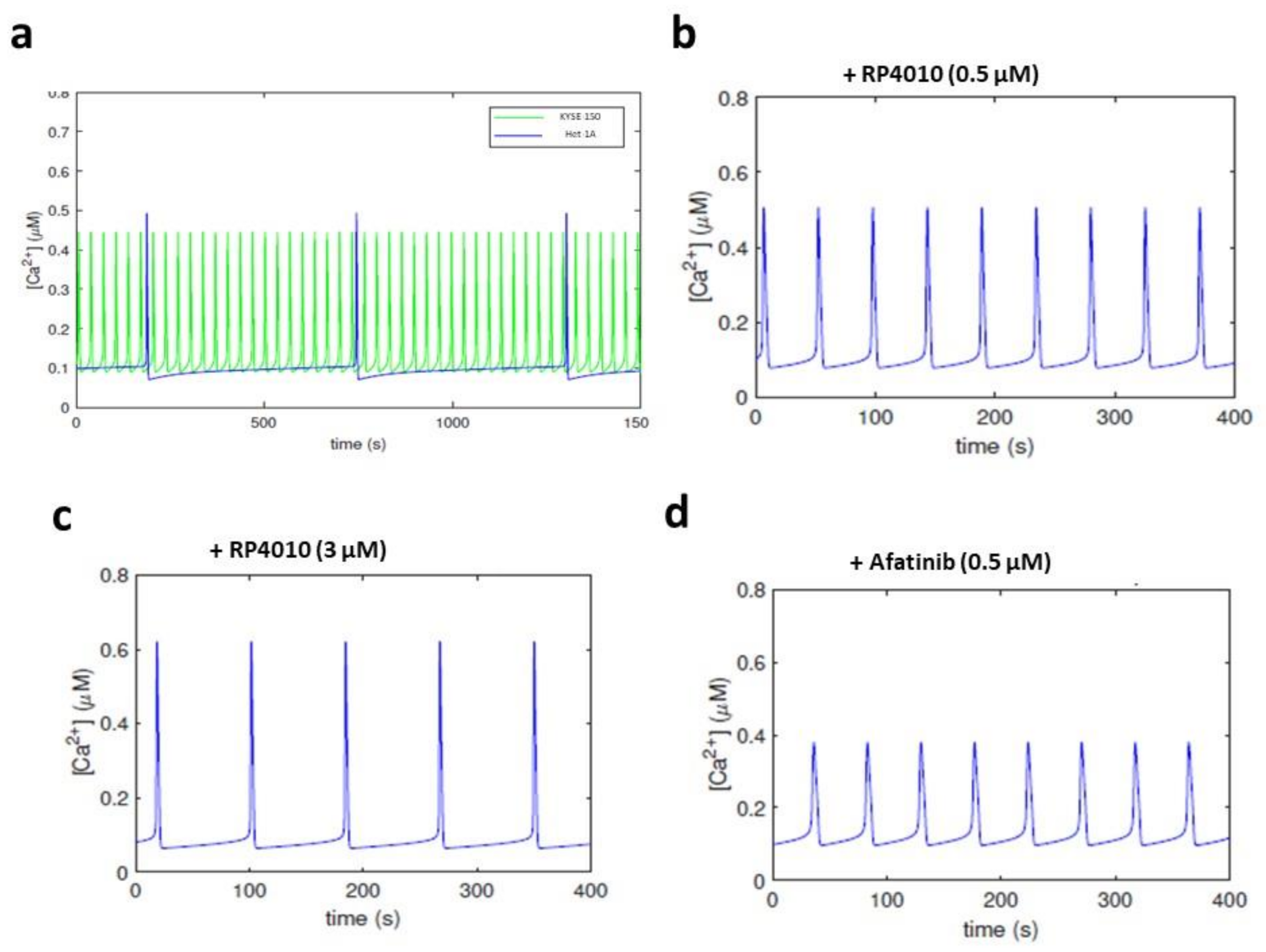
24]. As an irreversible TKI, afatinib potently inhibits signaling from all EGFR family receptor homodimers and heterodimers and the downstream events. Our experimental data showed that afatinib could reduce the frequency of intracellular Ca
2+ oscillations in a dose-dependent manner (
Figure 1c,
Figure 2d and
Figure 4c). The working model is that afatinib can regulate the Ca
2+ release from ER through the EGFR–PLC–PIP2–IP3–IP3R axis and, thus, reshape the intracellular Ca
2+ oscillations (illustration in
Figure 6). Another major factor to control the intracellular Ca
2+ oscillations is extracellular Ca
2+ influx through the SOCE pathway, which is supported by our earlier reports [
13,
14]. This notion was further confirmed by this study. The data showed that RP4010 could potently inhibit the frequency of intracellular Ca
2+ oscillations in ESCC cells in a dose-dependent manner (
Figure 4c). It is worthwhile to note that SOCE-mediated Ca
2+ influx regulates AKT and ERK1/2, two key molecules in downstream signaling pathways of EGFR, and activation of EGFR tyrosine kinase triggers the phosphorylation of STIM1 at ERK1/2 target sites to active SOCE [
25]. Therefore, besides intracellular Ca
2+, the crosstalk between EGFR and SOCE signaling pathways may have other interacting points, and these points require further investigation.
The intracellular Ca
2+ oscillations are the event downstream of both EGFR and SOCE signaling pathways and control consequent gene transcription through Ca
2+-dependent enzymes, including NF-κB, MAPK/ERK, CAMKII, etc. [
26] Therefore, the intracellular Ca
2+ oscillations could be used as a readout to evaluate any chemotherapeutic drug targeting EGFR or SOCE pathways. Fluorescence-based live-cell imaging can conveniently record the dynamics of intracellular Ca
2+ measurement and can be easily up scaled as high-throughput analysis for drug screening. Another advantage using intracellular Ca
2+ oscillations as a readout for drug evaluation is that a mathematical-modeling-based toolbox can be established to simulate [Ca
2+]cyto. While the experimental data on multiple drugs are difficult to obtain and many of the drugs are expensive to acquire, the computer-aid mathematical simulation could greatly facilitate the mechanistic understanding of cross-talk between two pathways and provide rapid cost-effective test on hundreds of drugs and countless combinations with different ratios and concentrations. In this study, we established a mathematical model to simulate the intracellular Ca
2+ oscillations by using a set of parameters obtained from experimental data (
Figure 3 and
Figure 4). The frequency of intracellular Ca
2+ oscillations was the focus to adjust the parameters, using experimental data from a single drug, either RP4010 or afatinib. Then the mathematical model successfully predicted the synergetic action when both drugs were included into consideration, suggesting that this mathematical simulation system could provide reliable evaluation on the anticancer effects of combined TKIs and SOCE blockers.
Both the intracellular Ca
2+ oscillations and cell-viability analysis showed that there is a synergetic effect between RP4010 and afatinib (
Figure 4 and
Figure 5c). This could be explained by the crosstalk between EGFR and SOCE signaling pathways. The aforementioned issue in many failed TKI clinical trials in ESCC is due to short-term adaptive responses and long-term acquired resistant of cancer cells to TKIs. Targeting both EGFR and SOCE simultaneously will provide synergetic anticancer effects to kill the cancer cells and effectively avoid their adaptive responses. Further studies are urgently needed to explore the combined TKIs and SOCE blockers for better chemotherapy, not only for esophageal cancer but also for other cancers, as well.
This study has two limitations. First, this mathematical model was able to simulate the frequency but not the shape and amplitude of Ca
2+ oscillations. The model used here was a modified version of one developed by Sneyd et al. [
6], with influence from Dupont et al. [
27]. The frequency modulation of signal transduction includes NF-κB, MAPK, etc. [
24], and our previous studies showed that the main downstream signaling pathway of Ca
2+ oscillations in ESCC cells is NF-κB [
14]. Therefore, we selected this relatively simple deterministic model to guide the experimental drug combination design. In the future, the model should be improved to include biochemical reactions, stochasticity, and to add spatial effects and other factors to better simulate both frequency and amplitude/shape of Ca
2+ oscillations. Second, this study was conducted only in cultured human ESCC cells without in vivo analysis. Future studies on the combined chemotherapy effects of afatinib and RP4010 are warranted to be conducted in ESCC animal models, such as orthotopic xenografted mice or NMBA-induced rats. It will also be interesting to use this mathematical simulation system to evaluate many FDA-approved TKIs other than afatinib and SOCE blockers. Regardless of the limitations, this study demonstrated that a simulation model of the intracellular Ca
2+ oscillations has the potential to be developed as an effective and rapid evaluation tool to be used to study whether the effects of the combination of drugs are synergistic, antagonistic or additive and to aid selection of the combinations of drugs producing the desired effects with the minimal dose. It could provide guidance for clinicians to determine optimal combination of TKIs and SOCE blockers.
4. Materials and Methods
4.1. Materials
RP4010 was obtained from Rhizen Pharmaceuticals, S.A (La Chaux-de-Fonds, Switzerland). The compound was dissolved in DMSO to make up a 10 µM stock solution. Afatinib was bought from Selleckchem company (Houston, TX, USA) and dissolved in DMSO to make a stock solution of 10 µM. Human ESCC (KYSE-30, KYSE-150, KYSE-70 and KYSE-790) and normal epithelial cell line (Het-1A) were used in this study [
13].
4.2. Cell Culture
ESCC and Het-1A cells were cultured at 37 °C in a 5% CO2 incubator and maintained in a 1:1 mixture of RPMI-1640 medium and Ham’s F12 Medium (Corning, NY, USA) supplemented with 5% fetal bovine serum (VWR, Radnor, PA, USA) and 1% penicillin/streptomycin (Corning, NY, USA).
4.3. Intracellular Ca2+ Oscillations Measurement
KYSE-150 cells were seeded in a black 96-well plate with a clear bottom. After attachment, cells were treated with RP4010 and afatinib, combined or separately, at different concentrations (details are shown in
Table 1), for 4 h. For RP4010, the concentration varied from 5 to 0.25 µM. The concentration of afatinib varied from 5 to 0.25 µM. For the combination of RP4010 and afatinib, the ratio of RP4010 to afatinib was 2:1. KYSE-150 cells were loaded with 5 µM Fluo-4 in 96-well imaging plates (BD Biosciences, Franklin Lakes, NJ, USA) at room temperature for 20 min. After washing, cells were kept in culture medium without phenol red. The intensity of fluorescent signals was recorded by using a Hamamatsu digital camera C11440 complemented with DMi8 inverted microscope (Leica, Wetzlar, Germany) with 20x objective (dry lens, NA 0.75). Time-lapse live-cell images were recorded every 5 s, for a total time period of 5 min, and the period between 2 peaks (2 Ca
2+ oscillations) was measured in order to calculate the corresponding periods.
4.4. Western Blot
Het-1A, KYSE-30, KYSE-150, KYSE-70 and KYSE-790 cell lines were cultured in a 6-well plate and harvest for Western blot. Cells were lysed with RIPA buffer (150 mM NaCl, 50 mM Tris-Cl, 1 mM EGTA, 1% Triton X-100, 0.1% SDS and 1% sodium deoxycholate, pH 8.0) and then supplemented with proteinase inhibitor cocktail (Sigma-Aldrich, Burlington, MA, USA). After that, protein concentrations were quantified by using a BCA kit (Thermo, Waltham, MA, USA). Primary antibodies used in this study included anti-EGFR (1:1000, Thermo, USA), anti-ERBB2 (1:1000, Thermo, Waltham, MA, USA), anti-ERBB3 (1:1000), anti-ERBB4 (1:1000) and anti-β-actin (1:1000, Proteintech, Rosemont, IL, USA). Secondary antibodies included HRP-labeled goat anti-rabbit IgG (1:5000) and anti-mouse IgG (1:5000, Cell Signaling Technology, Danvers, MA, USA). ECL substrate reagent (Cytiva Amersham, Marlborough, MA, USA) was used to visualize signals on ChemiDoc (BioRad, Hercules, CA, USA).
4.5. Cell Viability Measurement
Cell viability was measured by using MTT assay. KYSE-150 cells were seeded in a 96-well plate at the number of 104 in each well. After attachment, the cells were treated with RP4010 and afatinib, combined or separately, at different concentrations, for 24 h. For RP4010, the concentration varied from 10 to 0.625 µM. The concentration of afatinib varied from 5 to 0.3125 µM. For the combination of RP4010 and afatinib, the ratio of RP4010 to afatinib was 2:1. After 24 h, KYSE-150 cells were incubated with medium containing 10% of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT, 5 mg/mL), at 37 °C. After 4 h, formazan was dissolved in 150 µL DMSO. Absorbance was measured at 570 nm on SpectraMax i3 (Molecular Devices, San Jose, CA, USA). The survival curve was created by Graphpad Prism 5 (San Diego, CA, USA).
4.6. Mathematical Modeling
The mathematical model used was a modified version of one developed by Sneyd et al., with influence from Dupont et al. [
6,
27]. The governing dynamical equations for the Ca
2+ signaling pathway are as follows:
where
c denotes the Ca
2+ concentration in the cytoplasm, [Ca
2+ ]
cyto;
Ce is the Ca
2+ concentration in the ER, [Ca
2+ ]
ER;
p is the concentration of IP
3, [IP
3]; and
h is the rate at which Ca
2+ can activate IP
3Rs.
The fluxes in the above equations were given as follows:
where the
J terms represent the Ca
2+ fluxes across the cell and ER membranes.
JIP3R modeled the flow of Ca
2+ from the ER through the IP3R channel and depended upon both [Ca
2+]
cyto and [Ca
2+]
ER. As
c decreased, the difference between
c and
Ce increased, which resulted in an increase for
JIP3R. The parameter
kf was a scaling factor used to control the IP3R density.
PO represented the open probability of IP
3R and depended on the activation and inactivation rates of IP
3R, which were both affected by the binding of Ca
2+ and IP
3. The SERCA pump moved Ca
2+ from the cytoplasm into the ER and changed depending on
c and
Ce. As
Ce decreased with respect to
c, the entire term increased. In order to model Ca
2+ flow into the cell,
Jin combined α
0, which accounted for leaks into the cytoplasm through unspecified channels, and
, which represented SOCE-mediated Ca
2+ influx. These channels open and close in response to [Ca
2+]
ER,
Ce. As
Ce increased, the denominator in
increased, causing the entire term to decrease, implying that the cell does not need to continue to fill up ER Ca
2+ stores. The opposite applied if
Ce decreased, meaning that the cell needs to increase ER Ca
2+ stores. A Hill equation was used to model
Jpm, the flow of Ca
2+ through the plasma membrane pump, with
Kpm being the concentration of Ca
2+, where half of the binding sites at the pump were occupied, and
Vpm is the maximum capacity of the plasma pump. The parameter
γ denoted the ratio of the volume of the cytoplasm to the volume of the ER, and the parameter
δ was dimensionless, as it was a scale factor relating the fluxes through the plasma membrane and ER membrane. To model IP3 concentration,
βp represents the speed it takes p to decay to the steady state
ps and
ν = βp ps.The model was further expanded upon with the introduction of the functions
α and
β:
The function α(c,p) modeled the rate at which [Ca2+ ] inactivating IP3Rs and β(c,p,t) modeled the rate at which [Ca2+ ] activates IP3Rs.
4.7. Addition of TKI and SOCE Blocker in the Computational Model
A new feature in this computational model was the incorporation of the effects of one drug or of the combination of two different drugs acting on different Ca
2+ resources. TKI (afatinib) and SOCE blocker (RP4010) were incorporated into the model through
D1 and
D2, which represent the overall drug effect on each of the channels:
As a SOCE channel inhibitor, RP4010 decreased [Ca
2+]
cyto and was expected to reduce the frequency of the oscillations. As a TKI, afatinib inhibits EGFR tyrosine kinase activity and thus affects the steady state of IP3. It was expected that it negatively affected the ER Ca
2+ release through the IP3R channel and, again, reducing [Ca
2+]
cyto. In order to describe the relationship between the effectiveness of the drug to the dose of drug given to the cell, we used the following equations:
where the functions
fi were described through the Michaelis–Menten kinetics:
where
S1 and
S2 represent the doses of afatinib and RP4010, respectively.
All parameters used in the mathematical model were summarized in
Table 3.
4.8. Validation of the Mathematical Model
We fitted the parameters of the mathematical model and validated it by using experimental data for different concentrations of drugs individually or in combination with a ratio of RP4010:afatinib = 2:1. The observed experimental data showed that [Ca2+] oscillates in ESCC cells had a period of 32.8 s. We used this value to adjust the model parameters kf, α1 and τmax, accordingly, to fit. In non-tumorous esophageal epithelial cells, which contain much less SOCE and ER Ca2+ release, we divided the values of k f by 5 and α1 by 3 to reflect such differences. The working assumption was that increasing concentrations of TKI or SOCE blockers would gradually decrease the level of intracellular Ca2+ oscillations in tumor cells until to the level in Het-1A cells. Finally, to find the coefficients ci and ki in the Michaelis–Menten equations, we matched the experimental data regarding drug doses and their corresponding periods.
To carry out the numerical simulations, the MATLAB’s ode23s built-in function, based on the explicit Runge–Kutta (2,3) pair of Bogacki and Shampine [
28], was used to solve the systems of differential equations. The MATLAB’s lsqcurvefit built-in function was implemented by using the Levenberg–Marquardt algorithm [
29] that solves a minimization problem involving a least-squares data-fitting term for fitting the model to the experimental data presented in
Table 1.
4.9. Statistical Analysis
In this study, at least 50 cells in each concentration group were included to calculate the period (peak–peak duration) of Ca
2+ oscillations. Cancer cells present different patterns of Ca
2+ oscillations during cell-cycle progression, and we focused on the G(1)/S phase, a determining phase for cell proliferation that contains majority cells with Ca
2+ oscillations. To simplify the model, we trimmed the dataset by removing any data outside 1.5 times the interquartile range. After trimming, the mean values of the period calculated from the rest of the dataset were used for the mathematical simulation, as presented in
Table 1 and
Table 2.
The combination index (CI) was calculated from the CI equation algorithms by using CompuSyn software (CompuSyn, Inc., Paramus, NJ, USA). Dose–effect Curve and combination index (CI) versus fraction affected (fa) plots were analyzed and exported from CompuSyn [
30].