A Long Journey before Cycling: Regulation of Quiescence Exit in Adult Muscle Satellite Cells
Abstract
:1. Overview
2. Quiescence and MuSC Functions
3. MuSC Heterogeneity
4. Regulatory Mechanisms Underlying MuSC Quiescence Maintenance and Exit
4.1. Signaling Pathways
4.2. Post-Transcriptional Regulation
4.3. Epigenetic Regulation
4.4. Niche Signals
4.5. Metabolic Reprogramming during MuSC Quiescence Exit
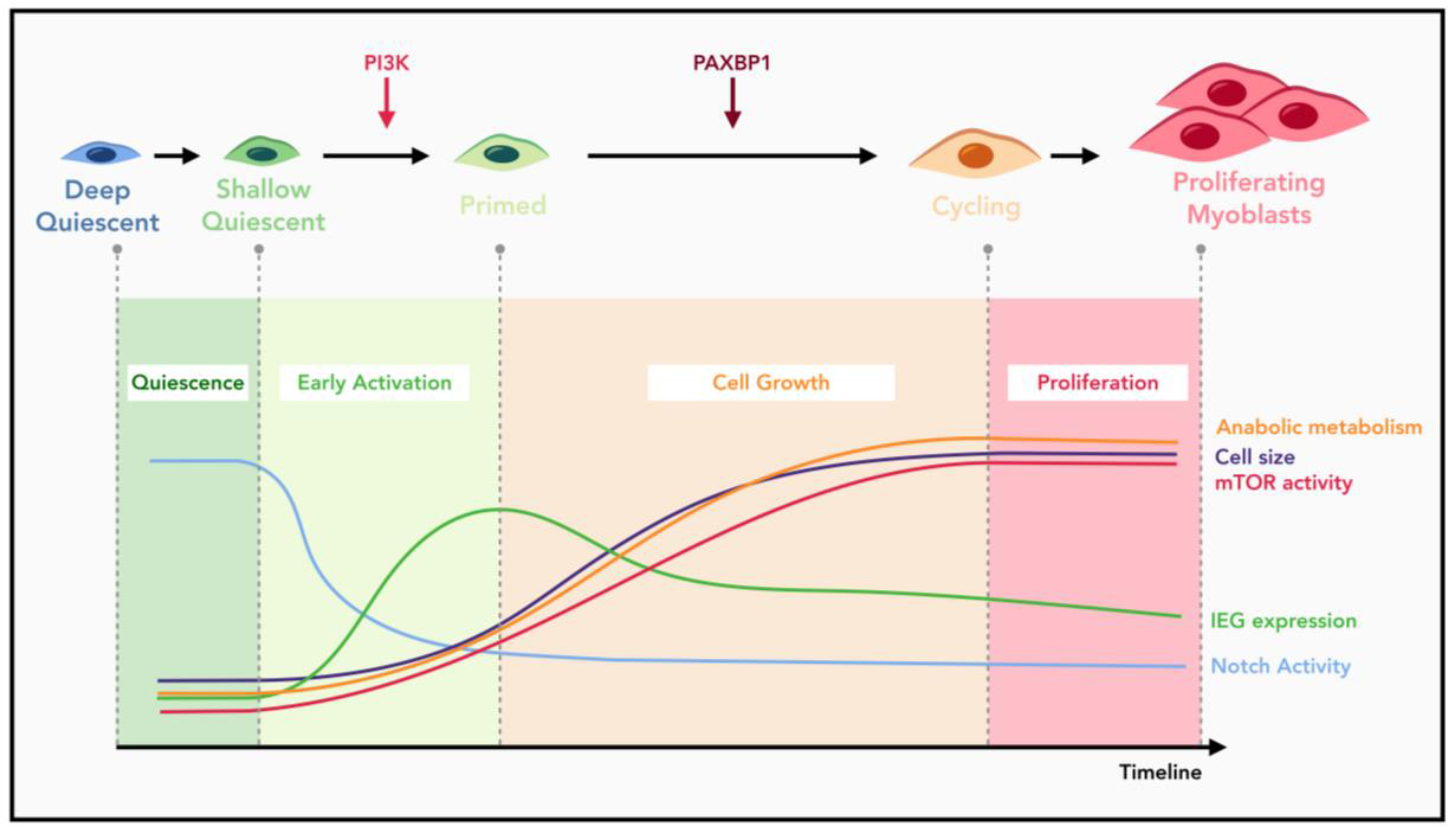
5. Multiple Checkpoints during MuSC Quiescence Exit
6. A Conserved Role of mTORC1 Signaling in Quiescence Exit
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evano, B.; Tajbakhsh, S. Skeletal Muscle Stem Cells in Comfort and Stress. Npj Regen. Med. 2018, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Relaix, F.; Bencze, M.; Borok, M.J.; Der Vartanian, A.; Gattazzo, F.; Mademtzoglou, D.; Perez-Diaz, S.; Prola, A.; Reyes-Fernandez, P.C.; Rotini, A.; et al. Perspectives on Skeletal Muscle Stem Cells. Nat. Commun. 2021, 12, 692. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Victor, P.; García-Prat, L.; Muñoz-Cánoves, P. Control of Satellite Cell Function in Muscle Regeneration and Its Disruption in Ageing. Nat. Rev. Mol. Cell Biol. 2021. [Google Scholar] [CrossRef] [PubMed]
- van Velthoven, C.T.J.; Rando, T.A. Stem Cell Quiescence: Dynamism, Restraint, and Cellular Idling. Cell Stem Cell 2019, 24, 213–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite Cells and the Muscle Stem Cell Niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef] [Green Version]
- Mauro, A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 1961, 9, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, M.; Relaix, F. PAX3 and PAX7 as Upstream Regulators of Myogenesis. Semin. Cell Dev. Biol. 2015, 44, 115–125. [Google Scholar] [CrossRef]
- Relaix, F.; Montarras, D.; Zaffran, S.; Gayraud-Morel, B.; Rocancourt, D.; Tajbakhsh, S.; Mansouri, A.; Cumano, A.; Buckingham, M. Pax3 and Pax7 Have Distinct and Overlapping Functions in Adult Muscle Progenitor Cells. J. Cell Biol. 2006, 172, 91–102. [Google Scholar] [CrossRef]
- Seale, P.; Sabourin, L.A.; Girgis-Gabardo, A.; Mansouri, A.; Gruss, P.; Rudnicki, M.A. Pax7 Is Required for the Specification of Myogenic Satellite Cells. Cell 2000, 102, 777–786. [Google Scholar] [CrossRef] [Green Version]
- Collins, C.A.; Olsen, I.; Zammit, P.S.; Heslop, L.; Petrie, A.; Partridge, T.A.; Morgan, J.E. Stem Cell Function, Self-Renewal, and Behavioral Heterogeneity of Cells from the Adult Muscle Satellite Cell Niche. Cell 2005, 122, 289–301. [Google Scholar] [CrossRef] [Green Version]
- Lepper, C.; Partridge, T.A.; Fan, C.-M. An Absolute Requirement for Pax7-Positive Satellite Cells in Acute Injury-Induced Skeletal Muscle Regeneration. Dev. Camb. Engl. 2011, 138, 3639–3646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, M.M.; Lawson, J.A.; Mathew, S.J.; Hutcheson, D.A.; Kardon, G. Satellite Cells, Connective Tissue Fibroblasts and Their Interactions Are Crucial for Muscle Regeneration. Dev. Camb. Engl. 2011, 138, 3625–3637. [Google Scholar] [CrossRef] [Green Version]
- Sacco, A.; Doyonnas, R.; Kraft, P.; Vitorovic, S.; Blau, H.M. Self-Renewal and Expansion of Single Transplanted Muscle Stem Cells. Nature 2008, 456, 502–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sambasivan, R.; Yao, R.; Kissenpfennig, A.; Van Wittenberghe, L.; Paldi, A.; Gayraud-Morel, B.; Guenou, H.; Malissen, B.; Tajbakhsh, S.; Galy, A. Pax7-Expressing Satellite Cells Are Indispensable for Adult Skeletal Muscle Regeneration. Dev. Camb. Engl. 2011, 138, 3647–3656. [Google Scholar] [CrossRef] [Green Version]
- Kuang, S.; Kuroda, K.; Le Grand, F.; Rudnicki, M.A. Asymmetric Self-Renewal and Commitment of Satellite Stem Cells in Muscle. Cell 2007, 129, 999–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocheteau, P.; Gayraud-Morel, B.; Siegl-Cachedenier, I.; Blasco, M.A.; Tajbakhsh, S. A Subpopulation of Adult Skeletal Muscle Stem Cells Retains All Template DNA Strands after Cell Division. Cell 2012, 148, 112–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guiraud, S.; Aartsma-Rus, A.; Vieira, N.M.; Davies, K.E.; van Ommen, G.-J.B.; Kunkel, L.M. The Pathogenesis and Therapy of Muscular Dystrophies. Annu. Rev. Genom. Hum. Genet. 2015, 16, 281–308. [Google Scholar] [CrossRef] [Green Version]
- Sousa-Victor, P.; Gutarra, S.; García-Prat, L.; Rodriguez-Ubreva, J.; Ortet, L.; Ruiz-Bonilla, V.; Jardí, M.; Ballestar, E.; González, S.; Serrano, A.L.; et al. Geriatric Muscle Stem Cells Switch Reversible Quiescence into Senescence. Nature 2014, 506, 316–321. [Google Scholar] [CrossRef]
- Duchenne Muscular Dystrophy and Dystrophin: Pathogenesis and Opportunities for Treatment. EMBO Rep. 2004, 5, 872–876. [CrossRef]
- Jejurikar, S.S.; Marcelo, C.L.; Kuzon, W.M. Skeletal Muscle Denervation Increases Satellite Cell Susceptibility to Apoptosis. Plast. Reconstr. Surg. 2002, 110, 160–168. [Google Scholar] [CrossRef]
- Wong, A.; Garcia, S.M.; Tamaki, S.; Striedinger, K.; Barruet, E.; Hansen, S.L.; Young, D.M.; Pomerantz, J.H. Satellite Cell Activation and Retention of Muscle Regenerative Potential after Long-Term Denervation. Stem Cells 2021, 39, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wei-LaPierre, L.; Klose, A.; Dirksen, R.T.; Chakkalakal, J.V. Inducible Depletion of Adult Skeletal Muscle Stem Cells Impairs the Regeneration of Neuromuscular Junctions. eLife 2015, 4, e09221. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Klose, A.; Forman, S.; Paris, N.D.; Wei-LaPierre, L.; Cortés-Lopéz, M.; Tan, A.; Flaherty, M.; Miura, P.; Dirksen, R.T.; et al. Loss of Adult Skeletal Muscle Stem Cells Drives Age-Related Neuromuscular Junction Degeneration. eLife 2017, 6, e26464. [Google Scholar] [CrossRef] [PubMed]
- Larouche, J.A.; Mohiuddin, M.; Choi, J.J.; Ulintz, P.J.; Fraczek, P.; Sabin, K.; Pitchiaya, S.; Kurpiers, S.J.; Castor-Macias, J.; Liu, W.; et al. Murine Muscle Stem Cell Response to Perturbations of the Neuromuscular Junction Are Attenuated with Aging. eLife 2021, 10, e66749. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.Y.; Huang, J.; Wang, H. Waking up Quiescent Neural Stem Cells: Molecular Mechanisms and Implications in Neurodevelopmental Disorders. PLoS Genet. 2020, 16, e1008653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzales, K.A.U.; Fuchs, E. Skin and Its Regenerative Powers: An Alliance between Stem Cells and Their Niche. Dev. Cell 2017, 43, 387–401. [Google Scholar] [CrossRef] [Green Version]
- Hsu, Y.-C.; Li, L.; Fuchs, E. Transit-Amplifying Cells Orchestrate Stem Cell Activity and Tissue Regeneration. Cell 2014, 157, 935–949. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Clevers, H. Coexistence of Quiescent and Active Adult Stem Cells in Mammals. Science 2010, 327, 542–545. [Google Scholar] [CrossRef] [Green Version]
- Urbán, N.; Blomfield, I.M.; Guillemot, F. Quiescence of Adult Mammalian Neural Stem Cells: A Highly Regulated Rest. Neuron 2019, 104, 834–848. [Google Scholar] [CrossRef]
- Wilson, A.; Laurenti, E.; Oser, G.; van der Wath, R.C.; Blanco-Bose, W.; Jaworski, M.; Offner, S.; Dunant, C.F.; Eshkind, L.; Bockamp, E.; et al. Hematopoietic Stem Cells Reversibly Switch from Dormancy to Self-Renewal during Homeostasis and Repair. Cell 2008, 135, 1118–1129. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Heller, E.; Beronja, S.; Oshimori, N.; Stokes, N.; Fuchs, E. An RNA Interference Screen Uncovers a New Molecule in Stem Cell Self-Renewal and Long-Term Regeneration. Nature 2012, 485, 104–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yilmaz, Ö.H.; Valdez, R.; Theisen, B.K.; Guo, W.; Ferguson, D.O.; Wu, H.; Morrison, S.J. Pten Dependence Distinguishes Haematopoietic Stem Cells from Leukaemia-Initiating Cells. Nature 2006, 441, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Yue, F.; Bi, P.; Wang, C.; Shan, T.; Nie, Y.; Ratliff, T.L.; Gavin, T.P.; Kuang, S. Pten Is Necessary for the Quiescence and Maintenance of Adult Muscle Stem Cells. Nat. Commun. 2017, 8, 14328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, T.H.; Quach, N.L.; Charville, G.W.; Liu, L.; Park, L.; Edalati, A.; Yoo, B.; Hoang, P.; Rando, T.A. Maintenance of Muscle Stem Cell Quiescence by MicroRNA-489. Nature 2012, 482, 524–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, T.H.; Rando, T.A. Molecular Regulation of Stem Cell Quiescence. Nat. Rev. Mol. Cell Biol. 2013, 14, 329–340. [Google Scholar] [CrossRef]
- Urbán, N.; Cheung, T.H. Stem Cell Quiescence: The Challenging Path to Activation. Development 2021, 148, dev165084. [Google Scholar] [CrossRef]
- Fukada, S.; Uezumi, A.; Ikemoto, M.; Masuda, S.; Segawa, M.; Tanimura, N.; Yamamoto, H.; Miyagoe-Suzuki, Y.; Takeda, S. Molecular Signature of Quiescent Satellite Cells in Adult Skeletal Muscle. Stem Cells 2007, 25, 2448–2459. [Google Scholar] [CrossRef]
- Machado, L.; de Lima, J.E.; Fabre, O.; Proux, C.; Legendre, R.; Szegedi, A.; Varet, H.; Ingerslev, L.R.; Barrès, R.; Relaix, F.; et al. In Situ Fixation Redefines Quiescence and Early Activation of Skeletal Muscle Stem Cells. Cell Rep. 2017, 21, 1982–1993. [Google Scholar] [CrossRef] [Green Version]
- Keyes, B.E.; Fuchs, E. Stem Cells: Aging and Transcriptional Fingerprints. J. Cell Biol. 2017, 217, 79–92. [Google Scholar] [CrossRef] [Green Version]
- Der Vartanian, A.; Quétin, M.; Michineau, S.; Auradé, F.; Hayashi, S.; Dubois, C.; Rocancourt, D.; Drayton-Libotte, B.; Szegedi, A.; Buckingham, M.; et al. PAX3 Confers Functional Heterogeneity in Skeletal Muscle Stem Cell Responses to Environmental Stress. Cell Stem Cell 2019, 24, 958–973.e9. [Google Scholar] [CrossRef]
- Scaramozza, A.; Park, D.; Kollu, S.; Beerman, I.; Sun, X.; Rossi, D.J.; Lin, C.P.; Scadden, D.T.; Crist, C.; Brack, A.S. Lineage Tracing Reveals a Subset of Reserve Muscle Stem Cells Capable of Clonal Expansion under Stress. Cell Stem Cell 2019, 24, 944–957.e5. [Google Scholar] [CrossRef] [PubMed]
- Walter, D.; Lier, A.; Geiselhart, A.; Thalheimer, F.B.; Huntscha, S.; Sobotta, M.C.; Moehrle, B.; Brocks, D.; Bayindir, I.; Kaschutnig, P.; et al. Exit from Dormancy Provokes DNA-Damage-Induced Attrition in Haematopoietic Stem Cells. Nature 2015, 520, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Latil, M.; Rocheteau, P.; Châtre, L.; Sanulli, S.; Mémet, S.; Ricchetti, M.; Tajbakhsh, S.; Chrétien, F. Skeletal Muscle Stem Cells Adopt a Dormant Cell State Post Mortem and Retain Regenerative Capacity. Nat. Commun. 2012, 3, 903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyson, N. The Regulation of E2F by PRB-Family Proteins. Genes Dev. 1998, 12, 2245–2262. [Google Scholar] [CrossRef] [Green Version]
- Hosoyama, T.; Nishijo, K.; Prajapati, S.I.; Li, G.; Keller, C. Rb1 Gene Inactivation Expands Satellite Cell and Postnatal Myoblast Pools. J. Biol. Chem. 2011, 286, 19556–19564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viatour, P.; Somervaille, T.C.; Venkatasubrahmanyam, S.; Kogan, S.; McLaughlin, M.E.; Weissman, I.L.; Butte, A.J.; Passegué, E.; Sage, J. Hematopoietic Stem Cell Quiescence Is Maintained by Compound Contributions of the Retinoblastoma Gene Family. Cell Stem Cell 2008, 3, 416–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakkalakal, J.V.; Christensen, J.; Xiang, W.; Tierney, M.T.; Boscolo, F.S.; Sacco, A.; Brack, A.S. Early Forming Label-Retaining Muscle Stem Cells Require P27kip1 for Maintenance of the Primitive State. Development 2014, 141, 1649–1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marescal, O.; Cheeseman, I.M. Cellular Mechanisms and Regulation of Quiescence. Dev. Cell 2020, 55, 259–271. [Google Scholar] [CrossRef]
- García-Prat, L.; Martínez-Vicente, M.; Perdiguero, E.; Ortet, L.; Rodríguez-Ubreva, J.; Rebollo, E.; Ruiz-Bonilla, V.; Gutarra, S.; Ballestar, E.; Serrano, A.L.; et al. Autophagy Maintains Stemness by Preventing Senescence. Nature 2016, 529, 37–42. [Google Scholar] [CrossRef]
- Evano, B.; Gill, D.; Hernando-Herraez, I.; Comai, G.; Stubbs, T.M.; Commere, P.-H.; Reik, W.; Tajbakhsh, S. Transcriptome and Epigenome Diversity and Plasticity of Muscle Stem Cells Following Transplantation. PLoS Genet. 2020, 16, e1009022. [Google Scholar] [CrossRef]
- Chakkalakal, J.V.; Jones, K.M.; Basson, M.A.; Brack, A.S. The Aged Niche Disrupts Muscle Stem Cell Quiescence. Nature 2012, 490, 355–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinin, V.; Gayraud-Morel, B.; Gomès, D.; Tajbakhsh, S. Asymmetric Division and Cosegregation of Template DNA Strands in Adult Muscle Satellite Cells. Nat. Cell Biol. 2006, 8, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Webster, M.T.; Manor, U.; Lippincott-Schwartz, J.; Fan, C.-M. Intravital Imaging Reveals Ghost Fibers as Architectural Units Guiding Myogenic Progenitors during Regeneration. Cell Stem Cell 2016, 18, 243–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Prat, L.; Perdiguero, E.; Alonso-Martín, S.; Dell’Orso, S.; Ravichandran, S.; Brooks, S.R.; Juan, A.H.; Campanario, S.; Jiang, K.; Hong, X.; et al. FoxO Maintains a Genuine Muscle Stem-Cell Quiescent State until Geriatric Age. Nat. Cell Biol. 2020, 22, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- de Morree, A.; Klein, J.D.D.; Gan, Q.; Farup, J.; Urtasun, A.; Kanugovi, A.; Bilen, B.; van Velthoven, C.T.J.; Quarta, M.; Rando, T.A. Alternative Polyadenylation of Pax3 Controls Muscle Stem Cell Fate and Muscle Function. Science 2019, 366, 734–738. [Google Scholar] [CrossRef]
- Porpiglia, E.; Samusik, N.; Ho, A.T.V.; Cosgrove, B.D.; Mai, T.; Davis, K.L.; Jager, A.; Nolan, G.P.; Bendall, S.C.; Fantl, W.J.; et al. High-Resolution Myogenic Lineage Mapping by Single-Cell Mass Cytometry. Nat. Cell Biol. 2017, 19, 558–567. [Google Scholar] [CrossRef] [Green Version]
- De Micheli, A.J.; Laurilliard, E.J.; Heinke, C.L.; Ravichandran, H.; Fraczek, P.; Soueid-Baumgarten, S.; De Vlaminck, I.; Elemento, O.; Cosgrove, B.D. Single-Cell Analysis of the Muscle Stem Cell Hierarchy Identifies Heterotypic Communication Signals Involved in Skeletal Muscle Regeneration. Cell Rep. 2020, 30, 3583–3595.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barruet, E.; Garcia, S.M.; Striedinger, K.; Wu, J.; Lee, S.; Byrnes, L.; Wong, A.; Xuefeng, S.; Tamaki, S.; Brack, A.S.; et al. Functionally Heterogeneous Human Satellite Cells Identified by Single Cell RNA Sequencing. eLife 2020, 9, e51576. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.S.; Doles, J.D. Single Cell Transcriptome Analysis of Muscle Satellite Cells Reveals Widespread Transcriptional Heterogeneity. Gene 2017, 636, 54–63. [Google Scholar] [CrossRef]
- Dell’Orso, S.; Juan, A.H.; Ko, K.-D.; Naz, F.; Perovanovic, J.; Gutierrez-Cruz, G.; Feng, X.; Sartorelli, V. Single Cell Analysis of Adult Mouse Skeletal Muscle Stem Cells in Homeostatic and Regenerative Conditions. Development 2019, 146. [Google Scholar] [CrossRef] [Green Version]
- Giordani, L.; He, G.J.; Negroni, E.; Sakai, H.; Law, J.Y.C.; Siu, M.M.; Wan, R.; Corneau, A.; Tajbakhsh, S.; Cheung, T.H.; et al. High-Dimensional Single-Cell Cartography Reveals Novel Skeletal Muscle-Resident Cell Populations. Mol. Cell 2019, 74, 609–621.e6. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.T.; King, K.Y.; Brett, J.O.; Cromie, M.J.; Charville, G.W.; Maguire, K.K.; Brunson, C.; Mastey, N.; Liu, L.; Tsai, C.-R.; et al. MTORC1 Controls the Adaptive Transition of Quiescent Stem Cells from G0 to GAlert. Nature 2014, 510, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.T.; Schroeder, M.D.; Ma, C.; Rando, T.A. HGFA Is an Injury-Regulated Systemic Factor That Induces the Transition of Stem Cells into GAlert. Cell Rep. 2017, 19, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Laurenti, E.; Frelin, C.; Xie, S.; Ferrari, R.; Dunant, C.F.; Zandi, S.; Neumann, A.; Plumb, I.; Doulatov, S.; Chen, J.; et al. CDK6 Levels Regulate Quiescence Exit in Human Hematopoietic Stem Cells. Cell Stem Cell 2015, 16, 302–313. [Google Scholar] [CrossRef] [Green Version]
- Llorens-Bobadilla, E.; Zhao, S.; Baser, A.; Saiz-Castro, G.; Zwadlo, K.; Martin-Villalba, A. Single-Cell Transcriptomics Reveals a Population of Dormant Neural Stem Cells That Become Activated upon Brain Injury. Cell Stem Cell 2015, 17, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Mourikis, P.; Tajbakhsh, S. Distinct Contextual Roles for Notch Signalling in Skeletal Muscle Stem Cells. BMC Dev. Biol. 2014, 14, 2. [Google Scholar] [CrossRef] [Green Version]
- Bjornson, C.R.R.; Cheung, T.H.; Liu, L.; Tripathi, P.V.; Steeper, K.M.; Rando, T.A. Notch Signaling Is Necessary to Maintain Quiescence in Adult Muscle Stem Cells. Stem Cells 2012, 30, 232–242. [Google Scholar] [CrossRef] [Green Version]
- Mourikis, P.; Sambasivan, R.; Castel, D.; Rocheteau, P.; Bizzarro, V.; Tajbakhsh, S. A Critical Requirement for Notch Signaling in Maintenance of the Quiescent Skeletal Muscle Stem Cell State. Stem Cells 2012, 30, 243–252. [Google Scholar] [CrossRef]
- Fukada, S.; Yamaguchi, M.; Kokubo, H.; Ogawa, R.; Uezumi, A.; Yoneda, T.; Matev, M.M.; Motohashi, N.; Ito, T.; Zolkiewska, A.; et al. Hesr1 and Hesr3 Are Essential to Generate Undifferentiated Quiescent Satellite Cells and to Maintain Satellite Cell Numbers. Dev. Camb. Engl. 2011, 138, 4609–4619. [Google Scholar] [CrossRef] [Green Version]
- Wen, Y.; Bi, P.; Liu, W.; Asakura, A.; Keller, C.; Kuang, S. Constitutive Notch Activation Upregulates Pax7 and Promotes the Self-Renewal of Skeletal Muscle Satellite Cells. Mol. Cell. Biol. 2012, 32, 2300–2311. [Google Scholar] [CrossRef] [Green Version]
- Buas, M.F.; Kabak, S.; Kadesch, T. The Notch Effector Hey1 Associates with Myogenic Target Genes to Repress Myogenesis. J. Biol. Chem. 2010, 285, 1249–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bröhl, D.; Vasyutina, E.; Czajkowski, M.T.; Griger, J.; Rassek, C.; Rahn, H.-P.; Purfürst, B.; Wende, H.; Birchmeier, C. Colonization of the Satellite Cell Niche by Skeletal Muscle Progenitor Cells Depends on Notch Signals. Dev. Cell 2012, 23, 469–481. [Google Scholar] [CrossRef] [Green Version]
- Schuster-Gossler, K.; Cordes, R.; Gossler, A. Premature Myogenic Differentiation and Depletion of Progenitor Cells Cause Severe Muscle Hypotrophy in Delta1 Mutants. Proc. Natl. Acad. Sci. USA 2007, 104, 537–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasyutina, E.; Lenhard, D.C.; Wende, H.; Erdmann, B.; Epstein, J.A.; Birchmeier, C. RBP-J (Rbpsuh) Is Essential to Maintain Muscle Progenitor Cells and to Generate Satellite Cells. Proc. Natl. Acad. Sci. USA 2007, 104, 4443–4448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baghdadi, M.B.; Firmino, J.; Soni, K.; Evano, B.; Di Girolamo, D.; Mourikis, P.; Castel, D.; Tajbakhsh, S. Notch-Induced MiR-708 Antagonizes Satellite Cell Migration and Maintains Quiescence. Cell Stem Cell 2018. [Google Scholar] [CrossRef]
- Baghdadi, M.B.; Castel, D.; Machado, L.; Fukada, S.; Birk, D.E.; Relaix, F.; Tajbakhsh, S.; Mourikis, P. Reciprocal Signalling by Notch–Collagen V–CALCR Retains Muscle Stem Cells in Their Niche. Nature 2018, 557, 714–718. [Google Scholar] [CrossRef]
- Low, S.; Barnes, J.L.; Zammit, P.S.; Beauchamp, J.R. Delta-Like 4 Activates Notch 3 to Regulate Self-Renewal in Skeletal Muscle Stem Cells. Stem Cells 2018, 36, 458–466. [Google Scholar] [CrossRef] [Green Version]
- Verma, M.; Asakura, Y.; Murakonda, B.S.R.; Pengo, T.; Latroche, C.; Chazaud, B.; McLoon, L.K.; Asakura, A. Muscle Satellite Cell Cross-Talk with a Vascular Niche Maintains Quiescence via VEGF and Notch Signaling. Cell Stem Cell 2018, 23, 530–543.e9. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Zhu, H.; Situ, C.; Han, L.; Yu, Y.; Cheung, T.H.; Liu, K.; Wu, Z. P110α of PI3K Is Necessary and Sufficient for Quiescence Exit in Adult Muscle Satellite Cells. EMBO J. 2018, e98239. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. MTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.Y.; Sabatini, D.M. MTOR at the Nexus of Nutrition, Growth, Ageing and Disease. Nat. Rev. Mol. Cell Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Lin, Y.; Ortiz-Vega, S.; Yonezawa, K.; Avruch, J. Rheb Binds and Regulates the MTOR Kinase. Curr. Biol. 2005, 15, 702–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoki, K.; Li, Y.; Zhu, T.; Wu, J.; Guan, K.-L. TSC2 Is Phosphorylated and Inhibited by Akt and Suppresses MTOR Signalling. Nat. Cell Biol. 2002, 4, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Inoki, K.; Li, Y.; Xu, T.; Guan, K.-L. Rheb GTPase Is a Direct Target of TSC2 GAP Activity and Regulates MTOR Signaling. Genes Dev. 2003, 17, 1829–1834. [Google Scholar] [CrossRef] [Green Version]
- Inoki, K.; Zhu, T.; Guan, K.-L. TSC2 Mediates Cellular Energy Response to Control Cell Growth and Survival. Cell 2003, 115, 577–590. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Chen, Z.; Erdjument-Bromage, H.; Tempst, P.; Pandolfi, P.P. Phosphorylation and Functional Inactivation of TSC2 by Erk: Implications for Tuberous Sclerosisand Cancer Pathogenesis. Cell 2005, 121, 179–193. [Google Scholar] [CrossRef] [Green Version]
- Menon, S.; Dibble, C.C.; Talbott, G.; Hoxhaj, G.; Valvezan, A.J.; Takahashi, H.; Cantley, L.C.; Manning, B.D. Spatial Control of the TSC Complex Integrates Insulin and Nutrient Regulation of MTORC1 at the Lysosome. Cell 2014, 156, 771–785. [Google Scholar] [CrossRef] [Green Version]
- Potter, C.J.; Pedraza, L.G.; Xu, T. Akt Regulates Growth by Directly Phosphorylating Tsc2. Nat. Cell Biol. 2002, 4, 658–665. [Google Scholar] [CrossRef]
- Roux, P.P.; Ballif, B.A.; Anjum, R.; Gygi, S.P.; Blenis, J. Tumor-Promoting Phorbol Esters and Activated Ras Inactivate the Tuberous Sclerosis Tumor Suppressor Complex via P90 Ribosomal S6 Kinase. Proc. Natl. Acad. Sci. USA 2004, 101, 13489–13494. [Google Scholar] [CrossRef] [Green Version]
- Hara, K.; Yonezawa, K.; Weng, Q.P.; Kozlowski, M.T.; Belham, C.; Avruch, J. Amino Acid Sufficiency and MTOR Regulate P70 S6 Kinase and EIF-4E BP1 through a Common Effector Mechanism. J. Biol. Chem. 1998, 273, 14484–14494. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.; Goraksha-Hicks, P.; Li, L.; Neufeld, T.P.; Guan, K.-L. Regulation of TORC1 by Rag GTPases in Nutrient Response. Nat. Cell Biol. 2008, 10, 935–945. [Google Scholar] [CrossRef] [Green Version]
- Rogala, K.B.; Gu, X.; Kedir, J.F.; Abu-Remaileh, M.; Bianchi, L.F.; Bottino, A.M.S.; Dueholm, R.; Niehaus, A.; Overwijn, D.; Fils, A.-C.P.; et al. Structural Basis for the Docking of MTORC1 on the Lysosomal Surface. Science 2019, 366, 468–475. [Google Scholar] [CrossRef]
- Sancak, Y.; Peterson, T.R.; Shaul, Y.D.; Lindquist, R.A.; Thoreen, C.C.; Bar-Peled, L.; Sabatini, D.M. The Rag GTPases Bind Raptor and Mediate Amino Acid Signaling to MTORC1. Science 2008, 320, 1496–1501. [Google Scholar] [CrossRef] [Green Version]
- Sancak, Y.; Bar-Peled, L.; Zoncu, R.; Markhard, A.L.; Nada, S.; Sabatini, D.M. Ragulator-Rag Complex Targets MTORC1 to the Lysosomal Surface and Is Necessary for Its Activation by Amino Acids. Cell 2010, 141, 290–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rion, N.; Castets, P.; Lin, S.; Enderle, L.; Reinhard, J.R.; Eickhorst, C.; Rüegg, M.A. MTOR Controls Embryonic and Adult Myogenesis via MTORC1. Development 2019, 146(7), dev172460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopinath, S.D.; Webb, A.E.; Brunet, A.; Rando, T.A. FOXO3 Promotes Quiescence in Adult Muscle Stem Cells during the Process of Self-Renewal. Stem Cell Rep. 2014, 2, 414–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, H.S.; Kang, K.H.; Joe, C.O.; Kim, J.W. Pten Coordinates Retinal Neurogenesis by Regulating Notch Signalling. EMBO J. 2012, 31, 817–828. [Google Scholar] [CrossRef] [Green Version]
- Serra, H.; Chivite, I.; Angulo-Urarte, A.; Soler, A.; Sutherland, J.D.; Arruabarrena-Aristorena, A.; Ragab, A.; Lim, R.; Malumbres, M.; Fruttiger, M.; et al. PTEN Mediates Notch-Dependent Stalk Cell Arrest in Angiogenesis. Nat. Commun. 2015, 6, 7935. [Google Scholar] [CrossRef] [Green Version]
- Whelan, J.T.; Forbes, S.L.; Bertrand, F.E. CBF-1 (RBP-J Kappa) Binds to the PTEN Promoter and Regulates PTEN Gene Expression. Cell Cycle 2007, 6, 80–84. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-Q.; Liang, Y.-K.; Wu, Y.; Chen, M.; Chen, W.-L.; Li, R.-H.; Zeng, Y.-Z.; Huang, W.-H.; Wu, J.-D.; Zeng, D.; et al. Notch3 Inhibits Cell Proliferation and Tumorigenesis and Predicts Better Prognosis in Breast Cancer through Transactivating PTEN. Cell Death Dis. 2021, 12, 502. [Google Scholar] [CrossRef] [PubMed]
- von Maltzahn, J.; Chang, N.C.; Bentzinger, C.F.; Rudnicki, M.A. Wnt Signaling in Myogenesis. Trends Cell Biol. 2012, 22, 602–609. [Google Scholar] [CrossRef] [Green Version]
- Pawlikowski, B.; Vogler, T.O.; Gadek, K.; Olwin, B.B. Regulation of Skeletal Muscle Stem Cells by Fibroblast Growth Factors. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2017, 246, 359–367. [Google Scholar] [CrossRef] [Green Version]
- Brack, A.S.; Conboy, I.M.; Conboy, M.J.; Shen, J.; Rando, T.A. A Temporal Switch from Notch to Wnt Signaling in Muscle Stem Cells Is Necessary for Normal Adult Myogenesis. Cell Stem Cell 2008, 2, 50–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, M.M.; Keefe, A.C.; Lawson, J.A.; Flygare, S.D.; Yandell, M.; Kardon, G. Transiently Active Wnt/β-Catenin Signaling Is Not Required but Must Be Silenced for Stem Cell Function during Muscle Regeneration. Stem Cell Rep. 2014, 3, 475–488. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Wang, H.; Hu, P. Stem Cell Activation in Skeletal Muscle Regeneration. Cell. Mol. Life Sci. 2015, 72, 1663–1677. [Google Scholar] [CrossRef] [Green Version]
- Parisi, A.; Lacour, F.; Giordani, L.; Colnot, S.; Maire, P.; Le Grand, F. APC Is Required for Muscle Stem Cell Proliferation and Skeletal Muscle Tissue Repair. J. Cell Biol. 2015, 210, 717–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eliazer, S.; Muncie, J.M.; Christensen, J.; Sun, X.; D’Urso, R.S.; Weaver, V.M.; Brack, A.S. Wnt4 from the Niche Controls the Mechano-Properties and Quiescent State of Muscle Stem Cells. Cell Stem Cell 2019, 25, 654–665.e4. [Google Scholar] [CrossRef]
- Bentzinger, C.F.; Wang, Y.X.; von Maltzahn, J.; Soleimani, V.D.; Yin, H.; Rudnicki, M.A. Fibronectin Regulates Wnt7a Signaling and Satellite Cell Expansion. Cell Stem Cell 2013, 12, 75–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otto, A.; Schmidt, C.; Luke, G.; Allen, S.; Valasek, P.; Muntoni, F.; Lawrence-Watt, D.; Patel, K. Canonical Wnt Signalling Induces Satellite-Cell Proliferation during Adult Skeletal Muscle Regeneration. J. Cell Sci. 2008, 121, 2939–2950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernet, J.D.; Doles, J.D.; Hall, J.K.; Kelly Tanaka, K.; Carter, T.A.; Olwin, B.B. P38 MAPK Signaling Underlies a Cell-Autonomous Loss of Stem Cell Self-Renewal in Skeletal Muscle of Aged Mice. Nat. Med. 2014, 20, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Shea, K.L.; Xiang, W.; LaPorta, V.S.; Licht, J.D.; Keller, C.; Basson, M.A.; Brack, A.S. Sprouty1 Regulates Reversible Quiescence of a Self-Renewing Adult Muscle Stem Cell Pool during Regeneration. Cell Stem Cell 2010, 6, 117–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, M.; Watanabe, Y.; Ohtani, T.; Uezumi, A.; Mikami, N.; Nakamura, M.; Sato, T.; Ikawa, M.; Hoshino, M.; Tsuchida, K.; et al. Calcitonin Receptor Signaling Inhibits Muscle Stem Cells from Escaping the Quiescent State and the Niche. Cell Rep. 2015, 13, 302–314. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Fan, C.-M. A CREB-MPP7-AMOT Regulatory Axis Controls Muscle Stem Cell Expansion and Self-Renewal Competence. Cell Rep. 2017, 21, 1253–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Rozo, M.; Yue, S.; Zheng, X.; Tan, F.J.; Lepper, C.; Fan, C.-M. Muscle Stem Cell Renewal Suppressed by GAS1 Can Be Reversed by GDNF in Mice. Nat. Metab. 2019, 1, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Chenette, D.M.; Cadwallader, A.B.; Antwine, T.L.; Larkin, L.C.; Wang, J.; Olwin, B.B.; Schneider, R.J. Targeted MRNA Decay by RNA Binding Protein AUF1 Regulates Adult Muscle Stem Cell Fate, Promoting Skeletal Muscle Integrity. Cell Rep. 2016, 16, 1379–1390. [Google Scholar] [CrossRef] [Green Version]
- Hausburg, M.A.; Doles, J.D.; Clement, S.L.; Cadwallader, A.B.; Hall, M.N.; Blackshear, P.J.; Lykke-Andersen, J.; Olwin, B.B. Post-Transcriptional Regulation of Satellite Cell Quiescence by TTP-Mediated MRNA Decay. eLife 2015, 4, e03390. [Google Scholar] [CrossRef]
- de Morrée, A.; van Velthoven, C.T.J.; Gan, Q.; Salvi, J.S.; Klein, J.D.D.; Akimenko, I.; Quarta, M.; Biressi, S.; Rando, T.A. Staufen1 Inhibits MyoD Translation to Actively Maintain Muscle Stem Cell Quiescence. Proc. Natl. Acad. Sci. USA 2017, 114, E8996–E9005. [Google Scholar] [CrossRef] [Green Version]
- Crist, C.G.; Montarras, D.; Buckingham, M. Muscle Satellite Cells Are Primed for Myogenesis but Maintain Quiescence with Sequestration of Myf5 MRNA Targeted by MicroRNA-31 in MRNP Granules. Cell Stem Cell 2012, 11, 118–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, L.; Wan, R.; Luan, S.; Zeng, W.; Cheung, T.H. Dek Modulates Global Intron Retention during Muscle Stem Cells Quiescence Exit. Dev. Cell 2020, 53, 661–676.e6. [Google Scholar] [CrossRef]
- Zismanov, V.; Chichkov, V.; Colangelo, V.; Jamet, S.; Wang, S.; Syme, A.; Koromilas, A.E.; Crist, C. Phosphorylation of EIF2α Is a Translational Control Mechanism Regulating Muscle Stem Cell Quiescence and Self-Renewal. Cell Stem Cell 2016, 18, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Gayraud-Morel, B.; Le Bouteiller, M.; Commere, P.-H.; Cohen-Tannoudji, M.; Tajbakhsh, S. Notchless Defines a Stage-Specific Requirement for Ribosome Biogenesis during Lineage Progression in Adult Skeletal Myogenesis. Development 2018, 145, dev162636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitajima, Y.; Suzuki, N.; Nunomiya, A.; Osana, S.; Yoshioka, K.; Tashiro, Y.; Takahashi, R.; Ono, Y.; Aoki, M.; Nagatomi, R. The Ubiquitin-Proteasome System Is Indispensable for the Maintenance of Muscle Stem Cells. Stem Cell Rep. 2018, 11, 1523–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Cheung, T.H.; Charville, G.W.; Hurgo, B.M.C.; Leavitt, T.; Shih, J.; Brunet, A.; Rando, T.A. Chromatin Modifications as Determinants of Muscle Stem Cell Quiescence and Chronological Aging. Cell Rep. 2013, 4, 189–204. [Google Scholar] [CrossRef] [Green Version]
- Juan, A.H.; Derfoul, A.; Feng, X.; Ryall, J.G.; Dell’Orso, S.; Pasut, A.; Zare, H.; Simone, J.M.; Rudnicki, M.A.; Sartorelli, V. Polycomb EZH2 Controls Self-Renewal and Safeguards the Transcriptional Identity of Skeletal Muscle Stem Cells. Genes Dev. 2011, 25, 789–794. [Google Scholar] [CrossRef] [Green Version]
- Woodhouse, S.; Pugazhendhi, D.; Brien, P.; Pell, J.M. Ezh2 Maintains a Key Phase of Muscle Satellite Cell Expansion but Does Not Regulate Terminal Differentiation. J. Cell Sci. 2013, 126, 565–579. [Google Scholar] [CrossRef] [Green Version]
- Faralli, H.; Wang, C.; Nakka, K.; Benyoucef, A.; Sebastian, S.; Zhuang, L.; Chu, A.; Palii, C.G.; Liu, C.; Camellato, B.; et al. UTX Demethylase Activity Is Required for Satellite Cell–Mediated Muscle Regeneration. Available online: https://www.jci.org/articles/view/83239/pdf (accessed on 4 January 2022).
- Boonsanay, V.; Zhang, T.; Georgieva, A.; Kostin, S.; Qi, H.; Yuan, X.; Zhou, Y.; Braun, T. Regulation of Skeletal Muscle Stem Cell Quiescence by Suv4-20h1-Dependent Facultative Heterochromatin Formation. Cell Stem Cell 2016, 18, 229–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryall, J.G.; Dell’Orso, S.; Derfoul, A.; Juan, A.; Zare, H.; Feng, X.; Clermont, D.; Koulnis, M.; Gutierrez-Cruz, G.; Fulco, M.; et al. The NAD+-Dependent SIRT1 Deacetylase Translates a Metabolic Switch into Regulatory Epigenetics in Skeletal Muscle Stem Cells. Cell Stem Cell 2015, 16, 171–183. [Google Scholar] [CrossRef] [Green Version]
- Ly, C.H.; Lynch, G.S.; Ryall, J.G. A Metabolic Roadmap for Somatic Stem Cell Fate. Cell Metab. 2020, 31, 1052–1067. [Google Scholar] [CrossRef]
- Ryall, J.G.; Cliff, T.; Dalton, S.; Sartorelli, V. Metabolic Reprogramming of Stem Cell Epigenetics. Cell Stem Cell 2015, 17, 651–662. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Günther, S.; Looso, M.; Künne, C.; Krüger, M.; Kim, J.; Zhou, Y.; Braun, T. Prmt5 Is a Regulator of Muscle Stem Cell Expansion in Adult Mice. Nat. Commun. 2015, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Naito, M.; Mori, M.; Inagawa, M.; Miyata, K.; Hashimoto, N.; Tanaka, S.; Asahara, H. Dnmt3a Regulates Proliferation of Muscle Satellite Cells via P57Kip2. PLoS Genet 2016, 12, e1006167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteves de Lima, J.; Bou Akar, R.; Machado, L.; Li, Y.; Drayton-Libotte, B.; Dilworth, F.J.; Relaix, F. HIRA Stabilizes Skeletal Muscle Lineage Identity. Nat. Commun. 2021, 12, 3450. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, E.; Blau, H.M. Tissue Stem Cells: Architects of Their Niches. Cell Stem Cell 2020, 27, 532–556. [Google Scholar] [CrossRef] [PubMed]
- Sampath, S.C.; Sampath, S.C.; Ho, A.T.V.; Corbel, S.Y.; Millstone, J.D.; Lamb, J.; Walker, J.; Kinzel, B.; Schmedt, C.; Blau, H.M. Induction of Muscle Stem Cell Quiescence by the Secreted Niche Factor Oncostatin M. Nat. Commun. 2018, 9, 1531. [Google Scholar] [CrossRef]
- Urciuolo, A.; Quarta, M.; Morbidoni, V.; Gattazzo, F.; Molon, S.; Grumati, P.; Montemurro, F.; Tedesco, F.S.; Blaauw, B.; Cossu, G.; et al. Collagen VI Regulates Satellite Cell Self-Renewal and Muscle Regeneration. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [Green Version]
- Goel, A.J.; Rieder, M.-K.; Arnold, H.-H.; Radice, G.L.; Krauss, R.S. Niche Cadherins Control the Quiescence-to-Activation Transition in Muscle Stem Cells. Cell Rep. 2017, 21, 2236–2250. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Shih, C.-H.; Wosczyna, M.N.; Mueller, A.A.; Cho, J.; Aggarwal, A.; Rando, T.A.; Feldman, B.J. Macrophage-Released ADAMTS1 Promotes Muscle Stem Cell Activation. Nat. Commun. 2017, 8, 669. [Google Scholar] [CrossRef]
- Lukjanenko, L.; Karaz, S.; Stuelsatz, P.; Gurriaran-Rodriguez, U.; Michaud, J.; Dammone, G.; Sizzano, F.; Mashinchian, O.; Ancel, S.; Migliavacca, E.; et al. Aging Disrupts Muscle Stem Cell Function by Impairing Matricellular WISP1 Secretion from Fibro-Adipogenic Progenitors. Cell Stem Cell 2019, 24, 433–446.e7. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Zhou, J.; Li, Y.; Zhao, Y.; Yuan, J.; Cao, Y.; Wang, L.; Zhang, Z.; Zhang, B.; Wang, C.C.; et al. YY1 Regulates Skeletal Muscle Regeneration through Controlling Metabolic Reprogramming of Satellite Cells. EMBO J. 2019, 38. [Google Scholar] [CrossRef]
- Pala, F.; Di Girolamo, D.; Mella, S.; Yennek, S.; Chatre, L.; Ricchetti, M.; Tajbakhsh, S. Distinct Metabolic States Govern Skeletal Muscle Stem Cell Fates during Prenatal and Postnatal Myogenesis. J. Cell Sci. 2018, 131, jcs212977. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Ryu, D.; Wu, Y.; Gariani, K.; Wang, X.; Luan, P.; DAmico, D.; Ropelle, E.R.; Lutolf, M.P.; Aebersold, R.; et al. NAD+ Repletion Improves Mitochondrial and Stem Cell Function and Enhances Life Span in Mice. Science 2016, 352, 1436–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, A.H.; Rando, T.A. Induction of Autophagy Supports the Bioenergetic Demands of Quiescent Muscle Stem Cell Activation. EMBO J. 2014, 33, 2782–2797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Han, L.; Weng, M.; Zhu, H.; Heng, Y.; Wang, G.; Shen, Z.; Chen, X.; Fu, X.; Zhang, M.; et al. Paxbp1 Controls a Key Checkpoint for Cell Growth and Survival during Early Activation of Quiescent Muscle Satellite Cells. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Lunt, S.Y.; Vander Heiden, M.G. Aerobic Glycolysis: Meeting the Metabolic Requirements of Cell Proliferation. Annu. Rev. Cell Dev. Biol. 2011, 27, 441–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yucel, N.; Wang, Y.X.; Mai, T.; Porpiglia, E.; Lund, P.J.; Markov, G.; Garcia, B.A.; Bendall, S.C.; Angelo, M.; Blau, H.M. Glucose Metabolism Drives Histone Acetylation Landscape Transitions That Dictate Muscle Stem Cell Function. Cell Rep. 2019, 27, 3939–3955.e6. [Google Scholar] [CrossRef] [Green Version]
- L’honoré, A.; Commère, P.-H.; Negroni, E.; Pallafacchina, G.; Friguet, B.; Drouin, J.; Buckingham, M.; Montarras, D. The Role of Pitx2 and Pitx3 in Muscle Stem Cells Gives New Insights into P38α MAP Kinase and Redox Regulation of Muscle Regeneration. eLife 2018, 7, e32991. [Google Scholar] [CrossRef]
- Le Roux, I.; Konge, J.; Le Cam, L.; Flamant, P.; Tajbakhsh, S. Numb Is Required to Prevent P53-Dependent Senescence Following Skeletal Muscle Injury. Nat. Commun. 2015, 6, 8528. [Google Scholar] [CrossRef] [Green Version]
- Coller, H.A. The Paradox of Metabolism in Quiescent Stem Cells. FEBS Lett. 2019, 593, 2817–2839. [Google Scholar] [CrossRef]
- Aimee, F.; John, S.; Abby, K.; David, J.; Matilde, M.; Melina, G.; Daniel, B.; White Andrew, C.; Jessica, Z.; Nick, G.; et al. Lactate Dehydrogenase Activity Drives Hair Follicle Stem Cell Activation. Nat. Cell Biol. 2017, 19, 1017–1026. [Google Scholar] [CrossRef]
- Beckervordersandforth, R.; Ebert, B.; Schäffner, I.; Moss, J.; Fiebig, C.; Shin, J.; Moore, D.L.; Ghosh, L.; Trinchero, M.F.; Stockburger, C.; et al. Role of Mitochondrial Metabolism in the Control of Early Lineage Progression and Aging Phenotypes in Adult Hippocampal Neurogenesis. Neuron 2017, 93, 560–573.e6. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.; Berg, D.A.; Zhu, Y.; Shin, J.Y.; Song, J.; Bonaguidi, M.A.; Enikolopov, G.; Nauen, D.W.; Christian, K.M.; Ming, G.; et al. Single-Cell RNA-Seq with Waterfall Reveals Molecular Cascades Underlying Adult Neurogenesis. Cell Stem Cell 2015, 17, 360–372. [Google Scholar] [CrossRef] [Green Version]
- Simsek, T.; Kocabas, F.; Zheng, J.; DeBerardinis, R.J.; Mahmoud, A.I.; Olson, E.N.; Schneider, J.W.; Zhang, C.C.; Sadek, H.A. The Distinct Metabolic Profile of Hematopoietic Stem Cells Reflects Their Location in a Hypoxic Niche. Cell Stem Cell 2010, 7, 380–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takubo, K.; Nagamatsu, G.; Kobayashi, C.I.; Nakamura-Ishizu, A.; Kobayashi, H.; Ikeda, E.; Goda, N.; Rahimi, Y.; Johnson, R.S.; Soga, T.; et al. Regulation of Glycolysis by Pdk Functions as a Metabolic Checkpoint for Cell Cycle Quiescence in Hematopoietic Stem Cells. Cell Stem Cell 2013, 12, 49–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Dillon, C.P.; Shi, L.Z.; Milasta, S.; Carter, R.; Finkelstein, D.; McCormick, L.L.; Fitzgerald, P.; Chi, H.; Munger, J.; et al. The Transcription Factor Myc Controls Metabolic Reprogramming upon T Lymphocyte Activation. Immunity 2011, 35, 871–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, K.; Shrestha, S.; Zeng, H.; Karmaus, P.W.F.; Neale, G.; Vogel, P.; Guertin, D.A.; Lamb, R.F.; Chi, H. T Cell Exit from Quiescence and Differentiation into Th2 Cells Depend on Raptor-MTORC1-Mediated Metabolic Reprogramming. Immunity 2013, 39, 1043–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, R.; Arif, T.; Kalmykova, S.; Kasianov, A.; Lin, M.; Menon, V.; Qiu, J.; Bernitz, J.M.; Moore, K.; Lin, F.; et al. Restraining Lysosomal Activity Preserves Hematopoietic Stem Cell Quiescence and Potency. Cell Stem Cell 2020, 26, 359–376.e7. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Carracedo, A.; Weiss, D.; Arai, F.; Ala, U.; Avigan, D.E.; Schafer, Z.T.; Evans, R.M.; Suda, T.; Lee, C.-H.; et al. A PML–PPAR-δ Pathway for Fatty Acid Oxidation Regulates Hematopoietic Stem Cell Maintenance. Nat. Med. 2012, 18, 1350–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ron-Harel, N.; Santos, D.; Ghergurovich, J.M.; Sage, P.T.; Reddy, A.; Lovitch, S.B.; Dephoure, N.; Satterstrom, F.K.; Sheffer, M.; Spinelli, J.B.; et al. Mitochondrial Biogenesis and Proteome Remodeling Promote One-Carbon Metabolism for T Cell Activation. Cell Metab. 2016, 24, 104–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, H.; Yang, K.; Li, Y.; Shaw, T.I.; Wang, Y.; Blanco, D.B.; Wang, X.; Cho, J.-H.; Wang, H.; Rankin, S.; et al. Integrative Proteomics and Phosphoproteomics Profiling Reveals Dynamic Signaling Networks and Bioenergetics Pathways Underlying T Cell Activation. Immunity 2017, 46, 488–503. [Google Scholar] [CrossRef] [Green Version]
- van Velthoven, C.T.J.; de Morree, A.; Egner, I.M.; Brett, J.O.; Rando, T.A. Transcriptional Profiling of Quiescent Muscle Stem Cells In Vivo. Cell Rep. 2017, 21, 1994–2004. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Liu, Y.; Liu, R.; Ikenoue, T.; Guan, K.-L.; Liu, Y.; Zheng, P. TSC–MTOR Maintains Quiescence and Function of Hematopoietic Stem Cells by Repressing Mitochondrial Biogenesis and Reactive Oxygen Species. J. Exp. Med. 2008, 205, 2397–2408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diao, Y.; Guo, X.; Li, Y.; Sun, K.; Lu, L.; Jiang, L.; Fu, X.; Zhu, H.; Sun, H.; Wang, H.; et al. Pax3/7BP Is a Pax7- and Pax3-Binding Protein That Regulates the Proliferation of Muscle Precursor Cells by an Epigenetic Mechanism. Cell Stem Cell 2012, 11, 231–241. [Google Scholar] [CrossRef] [Green Version]
- Meng, D.; Frank, A.R.; Jewell, J.L. MTOR Signaling in Stem and Progenitor Cells. Development 2018, 145, dev152595. [Google Scholar] [CrossRef] [Green Version]
- Valcourt, J.R.; Lemons, J.M.S.; Haley, E.M.; Kojima, M.; Demuren, O.O.; Coller, H.A. Staying Alive: Metabolic Adaptations to Quiescence. Cell Cycle 2012, 11, 1680–1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, P.; He, X.C.; Paulson, A.; Li, Z.; Tao, F.; Perry, J.M.; Guo, F.; Zhao, M.; Zhi, L.; Venkatraman, A.; et al. The Dlk1-Gtl2 Locus Preserves LT-HSC Function by Inhibiting the PI3K-MTOR Pathway to Restrict Mitochondrial Metabolism. Cell Stem Cell 2016, 18, 214–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Grindley, J.C.; Yin, T.; Jayasinghe, S.; He, X.C.; Ross, J.T.; Haug, J.S.; Rupp, D.; Porter-Westpfahl, K.S.; Wiedemann, L.M.; et al. PTEN Maintains Haematopoietic Stem Cells and Acts in Lineage Choice and Leukaemia Prevention. Nature 2006, 441, 518–522. [Google Scholar] [CrossRef]
- Paliouras, G.N.; Hamilton, L.K.; Aumont, A.; Joppé, S.E.; Barnabé-Heider, F.; Fernandes, K.J.L. Mammalian Target of Rapamycin Signaling Is a Key Regulator of the Transit-Amplifying Progenitor Pool in the Adult and Aging Forebrain. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 15012–15026. [Google Scholar] [CrossRef] [Green Version]
- Deng, Z.; Lei, X.; Zhang, X.; Zhang, H.; Liu, S.; Chen, Q.; Hu, H.; Wang, X.; Ning, L.; Cao, Y.; et al. MTOR Signaling Promotes Stem Cell Activation via Counterbalancing BMP-Mediated Suppression during Hair Regeneration. J. Mol. Cell Biol. 2015, 7, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Chapman, N.M.; Zeng, H.; Nguyen, T.-L.M.; Wang, Y.; Vogel, P.; Dhungana, Y.; Liu, X.; Neale, G.; Locasale, J.W.; Chi, H. MTOR Coordinates Transcriptional Programs and Mitochondrial Metabolism of Activated Treg Subsets to Protect Tissue Homeostasis. Nat. Commun. 2018, 9, 2095. [Google Scholar] [CrossRef]
- Yang, K.; Neale, G.; Green, D.R.; He, W.; Chi, H. The Tumor Suppressor Tsc1 Enforces Quiescence of Naive T Cells to Promote Immune Homeostasis and Function. Nat. Immunol. 2011, 12, 888–897. [Google Scholar] [CrossRef]
- Fu, X.; Xiao, J.; Wei, Y.; Li, S.; Liu, Y.; Yin, J.; Sun, K.; Sun, H.; Wang, H.; Zhang, Z.; et al. Combination of Inflammation-Related Cytokines Promotes Long-Term Muscle Stem Cell Expansion. Cell Res. 2015, 25, 655–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Brink, S.C.; Sage, F.; Vértesy, Á.; Spanjaard, B.; Peterson-Maduro, J.; Baron, C.S.; Robin, C.; van Oudenaarden, A. Single-Cell Sequencing Reveals Dissociation-Induced Gene Expression in Tissue Subpopulations. Nat. Methods 2017, 14, 935–936. [Google Scholar] [CrossRef] [PubMed]
- Machado, L.; Relaix, F.; Mourikis, P. Stress Relief: Emerging Methods to Mitigate Dissociation-Induced Artefacts. Trends Cell Biol. 2021, 31, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Machado, L.; Geara, P.; Camps, J.; Dos Santos, M.; Teixeira-Clerc, F.; Van Herck, J.; Varet, H.; Legendre, R.; Pawlotsky, J.-M.; Sampaolesi, M.; et al. Tissue Damage Induces a Conserved Stress Response That Initiates Quiescent Muscle Stem Cell Activation. Cell Stem Cell 2021, 28, 1125–1135.e7. [Google Scholar] [CrossRef] [PubMed]
- Rodriques, S.G.; Stickels, R.R.; Goeva, A.; Martin, C.A.; Murray, E.; Vanderburg, C.R.; Welch, J.; Chen, L.M.; Chen, F.; Macosko, E.Z. Slide-Seq: A Scalable Technology for Measuring Genome-Wide Expression at High Spatial Resolution. Science 2019, 363, 1463–1467. [Google Scholar] [CrossRef]
- Srivatsan, S.R.; Regier, M.C.; Barkan, E.; Franks, J.M.; Packer, J.S.; Grosjean, P.; Duran, M.; Saxton, S.; Ladd, J.J.; Spielmann, M.; et al. Embryo-Scale, Single-Cell Spatial Transcriptomics. Science 2021, 373, 111–117. [Google Scholar] [CrossRef]
- Shah, S.; Lubeck, E.; Zhou, W.; Cai, L. In Situ Transcription Profiling of Single Cells Reveals Spatial Organization of Cells in the Mouse Hippocampus. Neuron 2016, 92, 342–357. [Google Scholar] [CrossRef] [Green Version]
- Takei, Y.; Yun, J.; Zheng, S.; Ollikainen, N.; Pierson, N.; White, J.; Shah, S.; Thomassie, J.; Suo, S.; Eng, C.-H.L.; et al. Integrated Spatial Genomics Reveals Global Architecture of Single Nuclei. Nature 2021, 590, 344–350. [Google Scholar] [CrossRef]
- Katzenelenbogen, Y.; Sheban, F.; Yalin, A.; Yofe, I.; Svetlichnyy, D.; Jaitin, D.A.; Bornstein, C.; Moshe, A.; Keren-Shaul, H.; Cohen, M.; et al. Coupled ScRNA-Seq and Intracellular Protein Activity Reveal an Immunosuppressive Role of TREM2 in Cancer. Cell 2020, 182, 872–885.e19. [Google Scholar] [CrossRef]
- Stoeckius, M.; Hafemeister, C.; Stephenson, W.; Houck-Loomis, B.; Chattopadhyay, P.K.; Swerdlow, H.; Satija, R.; Smibert, P. Simultaneous Epitope and Transcriptome Measurement in Single Cells. Nat. Methods 2017, 14, 865–868. [Google Scholar] [CrossRef] [Green Version]

| Niche Factors | Sources | Functions | References |
|---|---|---|---|
| OSM | Muscle fibers | Induce MuSC quiescence | Sampath et al., 2018 [135] |
| WNT4 | Muscle fibers | Maintain MuSC tension and quiescence | Eliazer et al., 2019 [107] |
| HGFA | Serum | Process pro-HGF and promote MuSC entry into Galert state | Rodgers et al., 2017 [63] |
| COLV | MuSC | Interact with Calcitonin receptor and maintain MuSC quiescence | Baghdadi et al., 2018 [75,76] |
| Fibronectin | MuSC | Stimulate WNT7A signaling and MuSC expansion | Bentzinger et al., 2013 [108] |
| FGF2 | Muscle fibers | Expression of FGF2 in aged muscle fibers breaks MuSC quiescence | Chakkalakal et al., 2012 [51] |
| COLVI | Fibroblasts | Required for MuSC self-renewal after injury | Urciuolo et al., 2013 [136] |
| DLL4 | Newly formed myotubes/endothelial cells | Activate Notch signaling and promote MuSC quiescence | Low et al., 2018 [77]; Verma et al., 2018 [78] |
| NCAD/MCAD | Muscle fibers | Forms adhesive junctions between MuSC and myofibers and maintain MuSC quiescence | Goel et al., 2017 [137] |
| ADAMTS1 | Macrophages | Reduce Notch signaling and induce MuSC activation | Du et al., 2017 [138] |
| WISP1 | FAP | WISP1 stimulates asymmetric MuSC commitment | Lukjanenko et al., 2018 [139] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Han, L.; Wu, Z. A Long Journey before Cycling: Regulation of Quiescence Exit in Adult Muscle Satellite Cells. Int. J. Mol. Sci. 2022, 23, 1748. https://doi.org/10.3390/ijms23031748
Zhou S, Han L, Wu Z. A Long Journey before Cycling: Regulation of Quiescence Exit in Adult Muscle Satellite Cells. International Journal of Molecular Sciences. 2022; 23(3):1748. https://doi.org/10.3390/ijms23031748
Chicago/Turabian StyleZhou, Shaopu, Lifang Han, and Zhenguo Wu. 2022. "A Long Journey before Cycling: Regulation of Quiescence Exit in Adult Muscle Satellite Cells" International Journal of Molecular Sciences 23, no. 3: 1748. https://doi.org/10.3390/ijms23031748
APA StyleZhou, S., Han, L., & Wu, Z. (2022). A Long Journey before Cycling: Regulation of Quiescence Exit in Adult Muscle Satellite Cells. International Journal of Molecular Sciences, 23(3), 1748. https://doi.org/10.3390/ijms23031748







