Electrospun Fiber-Coated Human Amniotic Membrane: A Potential Angioinductive Scaffold for Ischemic Tissue Repair
Abstract
:1. Introduction
2. Results
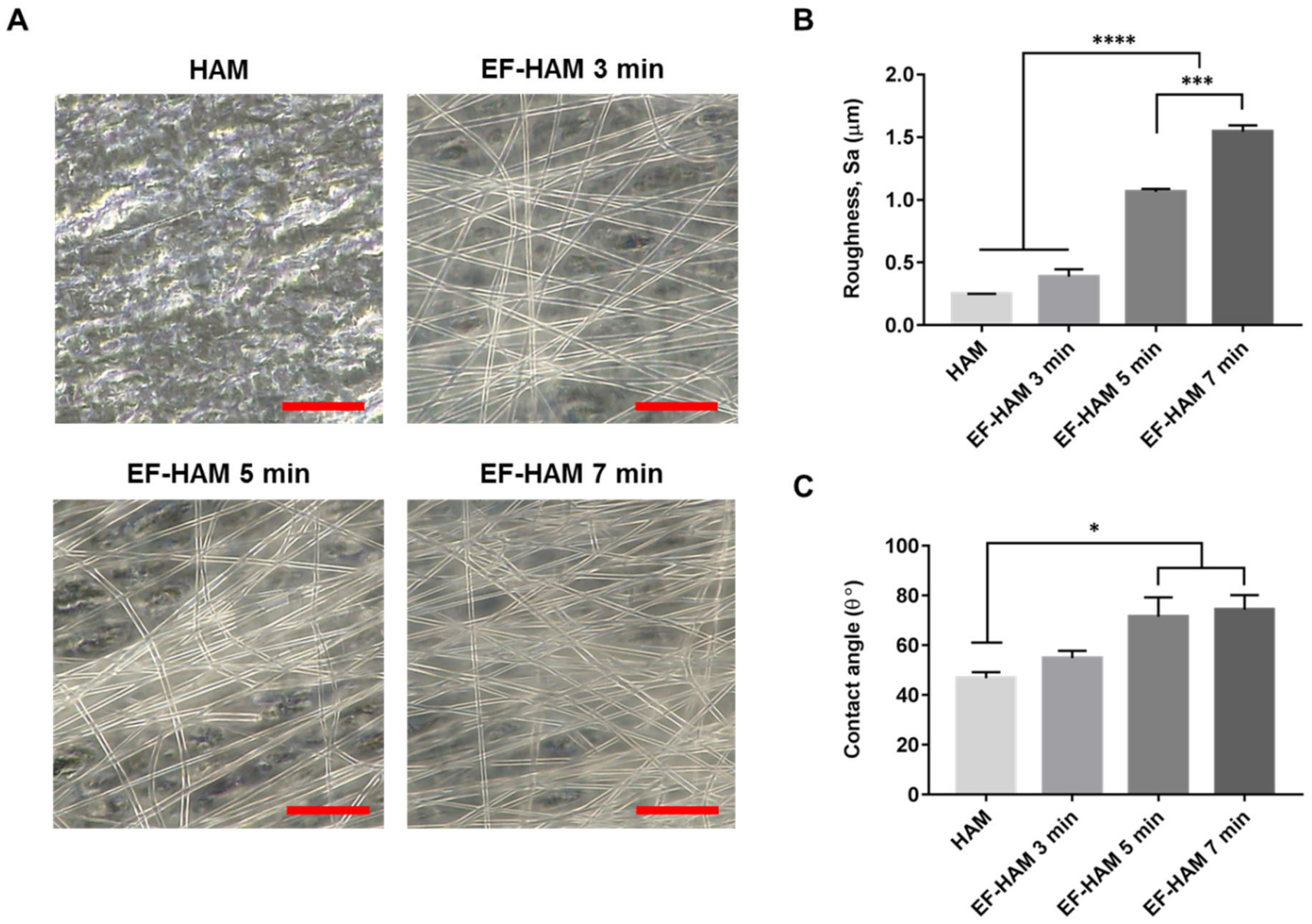
2.1. Physical Characteristics of EF-HAM Scaffolds
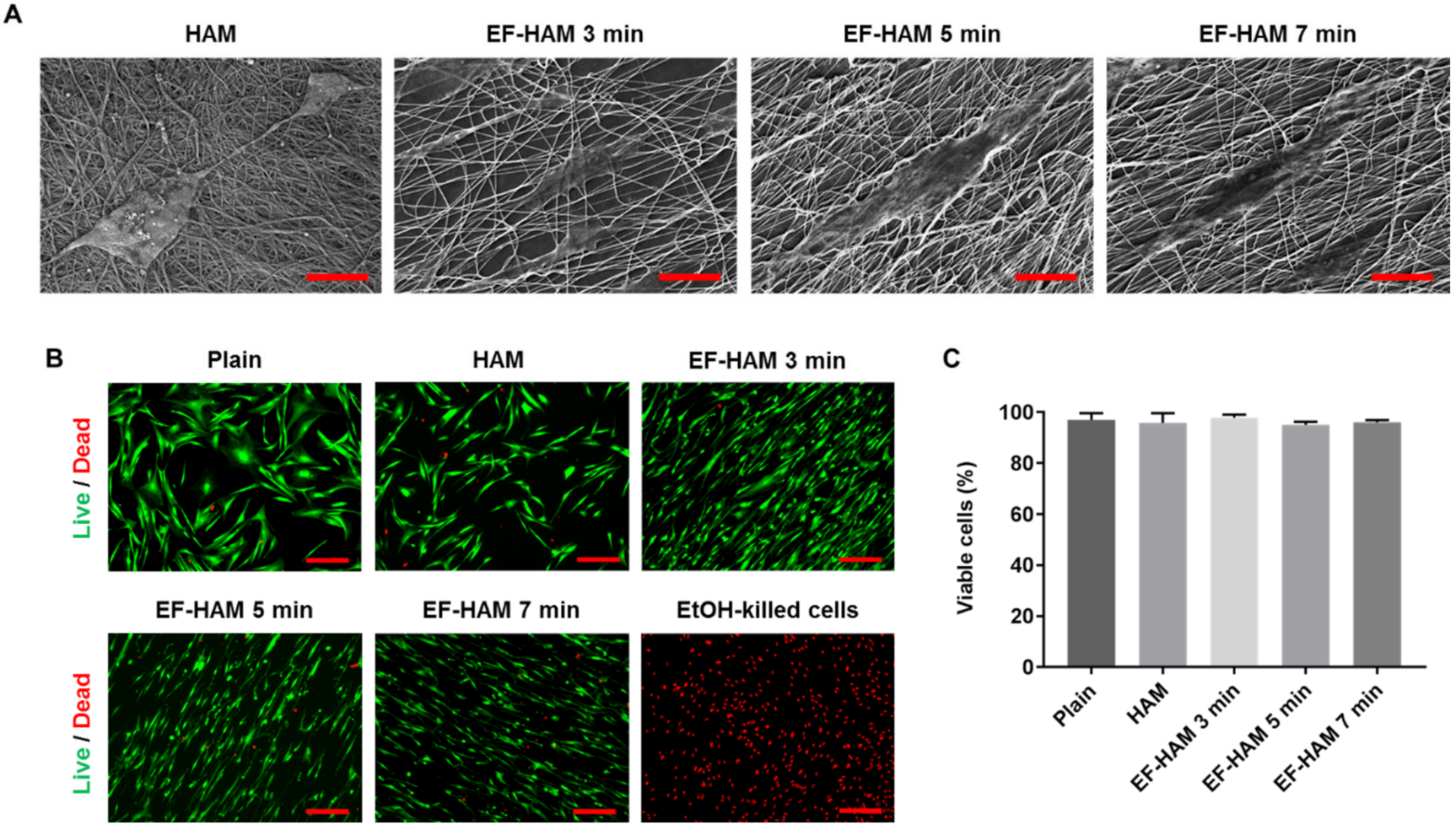
2.2. In Vitro Biocompatibility of EF-HAM Scaffolds
2.3. CM Derived from SkM-Seeded EF-HAM 7 Min Scaffolds Contained Higher Levels of Angiopoietin-1, IL-8, and VEGF-C
2.4. CM Derived from SkM-Seeded EF-HAM Scaffolds Enhanced Endothelial Cell Viability
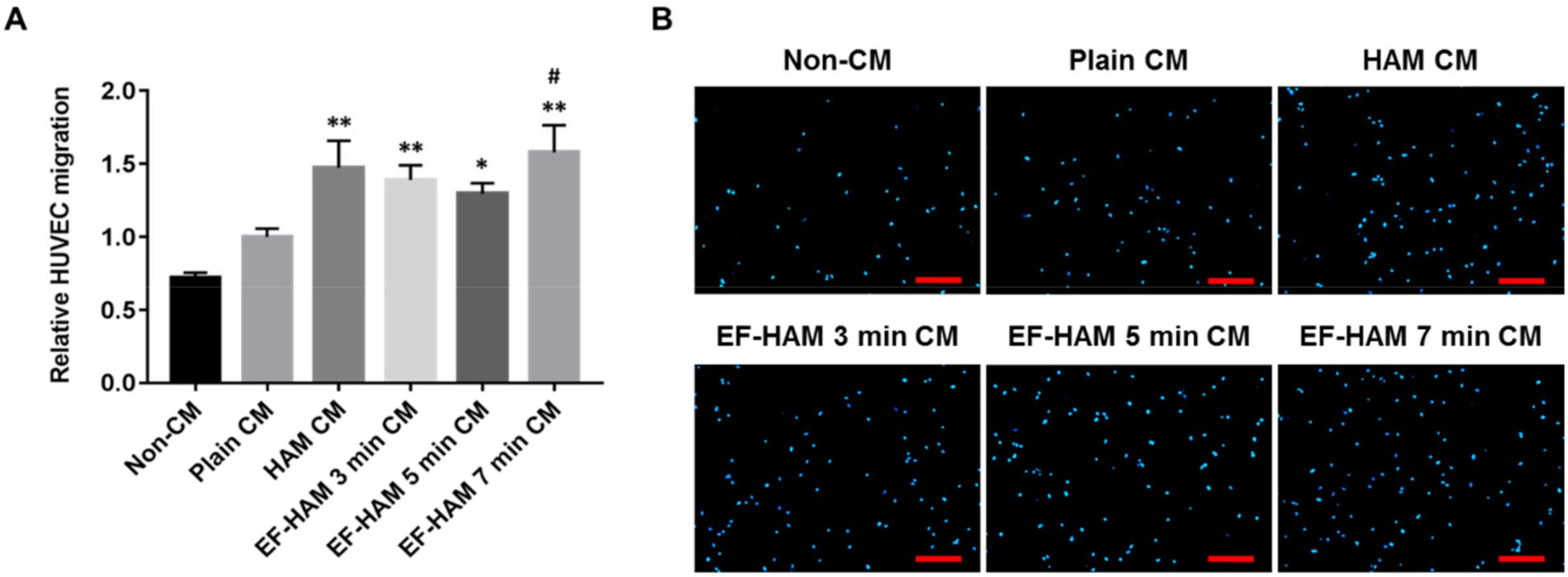
2.5. CM Derived from SkM-Seeded EF-HAM 7 Min Scaffolds Induced Higher Migration Capacity in HUVECs
2.6. EF-HAM 7 Min CM Promoted Longer Tube Formation in HUVECs
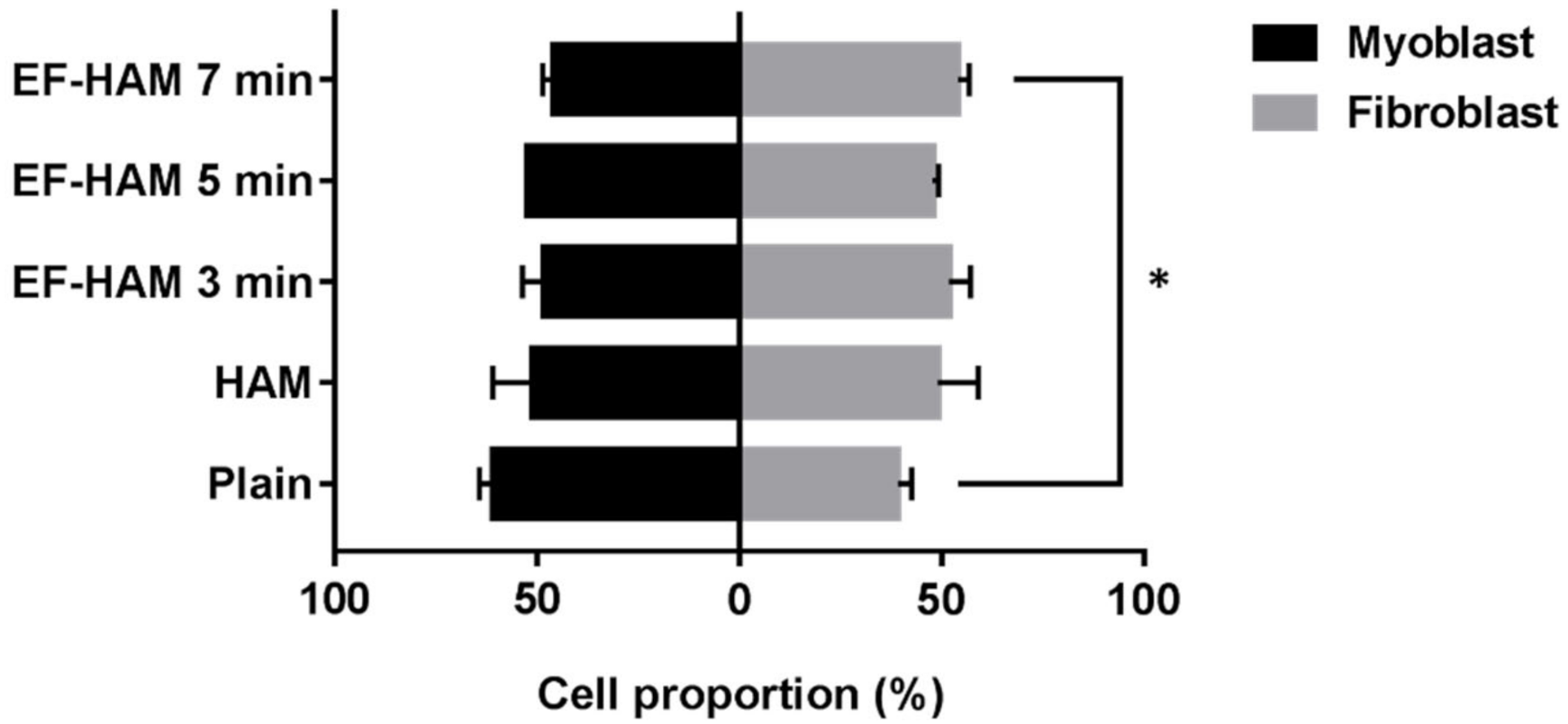
2.7. SkM-Seeded EF-HAM 7 Min Scaffolds Had a Higher Proportion of Skeletal Fibroblasts
3. Discussion
4. Materials and Methods
4.1. Skeletal Muscle Cells Isolation and Culture
4.2. HAM Processing and Decellularization
4.3. Fabrication of Composite EF-HAM Scaffolds
4.4. Surface Roughness Measurement
4.5. Scaffold Wettability
4.6. Scanning Electron Microscopy
4.7. Live and Dead Assay
4.8. Conditioned Media Collection and Angiogenic Paracrine Factors Quantification
4.9. HUVEC Viability, Migration and Tube Formation Assays
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| CM | Conditioned media |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DPBS | Dulbecco’s phosphate-buffered saline |
| ECM | Extracellular matrix |
| EF | Electrospun fiber |
| EF-HAM | Electrospun fiber-coated human amniotic membrane |
| EtOH | Ethanol |
| FBS | Fetal Bovine Serum |
| FCS | Fetal Calf Serum |
| FESEM | Field Emission Scanning Electron Microscope |
| HAM | Human amniotic membrane |
| HUVEC | Human umbilical vein endothelial cell |
| MFI | Median fluorescence intensity |
| MI | Myocardial infarction |
| PBS | Phosphate buffered saline |
| PLGA | Poly lactic-co-glycolic acid |
| SkM | Skeletal muscle cells |
| TE | Trypsin-EDTA |
References
- Menasché, P. Skeletal myoblasts and cardiac repair. J. Mol. Cell. Cardiol. 2008, 45, 545–553. [Google Scholar] [CrossRef]
- Dib, N.; Dinsmore, J.; Lababidi, Z.; White, B.; Moravec, S.; Campbell, A.; Rosenbaum, A.; Seyedmadani, K.; Jaber, W.A.; Rizenhour, C.S.; et al. One-Year Follow-Up of Feasibility and Safety of the First U.S., Randomized, Controlled Study Using 3-Dimensional Guided Catheter-Based Delivery of Autologous Skeletal Myoblasts for Ischemic Cardiomyopathy (CAuSMIC Study). JACC Cardiovasc. Interv. 2009, 2, 9–16. [Google Scholar] [CrossRef]
- Povsic, T.J.; O’Connor, C.M.; Henry, T.; Taussig, A.; Kereiakes, D.J.; Fortuin, F.D.; Niederman, A.; Schatz, R.; Spencer, R.; Owens, D.; et al. A double-blind, randomized, controlled, multicenter study to assess the safety and cardiovascular effects of skeletal myoblast implantation by catheter delivery in patients with chronic heart failure after myocardial infarction. Am. Heart J. 2011, 162, 654–662.e1. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, L.; Cao, H.; Yang, J.; Wu, M.; Ma, Y.; Fan, H.; Zhan, Z.; Liu, Z. Myoblast transplantation improves cardiac function after myocardial infarction through attenuating inflammatory responses. Oncotarget 2017, 8, 68780–68794. [Google Scholar] [CrossRef] [Green Version]
- Hagège, A.A.; Marolleau, J.P.; Vilquin, J.T.; Alhéritière, A.; Peyrard, S.; Duboc, D.; Abergel, E.; Messas, E.; Mousseaux, E.; Schwartz, K.; et al. Skeletal myoblast transplantation in ischemic heart failure: Long-term follow-up of the first phase I cohort of patients. Circulation 2006, 114, I108–I113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, Y.; Asakura, Y.; Piras, B.A.; Hirai, H.; Tastad, C.T.; Verma, M.; Christ, A.J.; Zhang, J.; Yamazaki, T.; Yoshiyama, M.; et al. Increased angiogenesis and improved left ventricular function after transplantation of myoblasts lacking the MyoD gene into infarcted myocardium. PLoS ONE 2012, 7, e41736. [Google Scholar] [CrossRef]
- Shudo, Y.; Miyagawa, S.; Ohkura, H.; Fukushima, S.; Saito, A.; Shiozaki, M.; Kawaguchi, N.; Matsuura, N.; Shimizu, T.; Okano, T.; et al. Addition of mesenchymal stem cells enhances the therapeutic effects of skeletal myoblast cell-sheet transplantation in a rat ischemic cardiomyopathy model. Tissue Eng. Part A 2014, 20, 728–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christman, K.L.; Fok, H.H.; Sievers, R.E.; Fang, Q.; Lee, R.J. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng. 2004, 10, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Formigli, L.; Perna, A.M.; Meacci, E.; Cinci, L.; Margheri, M.; Nistri, S.; Tani, A.; Silvertown, J.; Orlandini, G.; Porciani, C.; et al. Paracrine effects of transplanted myoblasts and relaxin on post-infarction heart remodelling. J. Cell. Mol. Med. 2007, 11, 1087–1100. [Google Scholar] [CrossRef] [Green Version]
- Niagara, M.I.; Haider, H.K.; Jiang, S.; Ashraf, M. Pharmacologically preconditioned skeletal myoblasts are resistant to oxidative stress and promote angiomyogenesis via release of paracrine factors in the infarcted heart. Circ. Res. 2007, 100, 545–555. [Google Scholar] [CrossRef] [Green Version]
- Menasché, P.; Alfieri, O.; Janssens, S.; McKenna, W.; Reichenspurner, H.; Trinquart, L.; Vilquin, J.T.; Marolleau, J.P.; Seymour, B.; Larghero, J.; et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: First randomized placebo-controlled study of myoblast transplantation. Circulation 2008, 117, 1189–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingason, A.B.; Goldstone, A.B.; Paulsen, M.J.; Thakore, A.D.; Truong, V.N.; Edwards, B.B.; Eskandari, A.; Bollig, T.; Steele, A.N.; Woo, Y.J. Angiogenesis precedes cardiomyocyte migration in regenerating mammalian hearts. J. Thorac. Cardiovasc. Surg. 2018, 155, 1118–1127.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, K.; Maeda, K.; Takefuji, M.; Kikuchi, R.; Morishita, Y.; Hirashima, M.; Murohara, T. Dynamics of angiogenesis in ischemic areas of the infarcted heart. Sci. Rep. 2017, 7, 7156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasmad, H.; Yusof, M.R.; Mohd Razi, Z.R.; Hj Idrus, R.B.; Chowdhury, S.R. Human amniotic membrane with aligned electrospun fiber as scaffold for aligned tissue regeneration. Tissue. Eng. Part C Methods 2018, 24, 368–378. [Google Scholar] [CrossRef]
- Gruss, J.S.; Jirsch, D.W. Human amniotic membrane: A versatile wound dressing. Can. Med. Assoc. J. 1978, 118, 1237–1246. [Google Scholar]
- Walkden, A. Amniotic membrane transplantation in ophthalmology: An updated perspective. Clin. Ophthalmol. 2020, 14, 2057–2072. [Google Scholar] [CrossRef]
- Liang, X.; Zhou, L.; Yan, J. Amniotic membrane for treating skin graft donor sites: A systematic review and meta-analysis. Burns 2020, 46, 621–629. [Google Scholar] [CrossRef]
- Malhotra, C.; Jain, A.K. Human amniotic membrane transplantation: Different modalities of its use in ophthalmology. World J. Transplant. 2014, 4, 111–121. [Google Scholar] [CrossRef]
- Adamowicz, J.; Pokrywczynska, M.; Tworkiewicz, J.; Kowalczyk, T.; Van Breda, S.V.; Tyloch, D.; Kloskowski, T.; Bodnar, M.; Skopinska-Wisniewska, J.; Marszałek, A.; et al. New Amniotic Membrane Based Biocomposite for Future Application in Reconstructive Urology. PLoS ONE 2016, 11, e0146012. [Google Scholar] [CrossRef] [Green Version]
- Arasteh, S.; Kazemnejad, S.; Khanjani, S.; Heidari-Vala, H.; Akhondi, M.M.; Mobini, S. Fabrication and characterization of nano-fibrous bilayer composite for skin regeneration application. Methods 2016, 99, 3–12. [Google Scholar] [CrossRef]
- Ramesh, B.; Chandrasekaran, J.; Jeevankumar, S.; Jacob, G.; Cherian, K.M. Hybrid Amniotic Membrane Dressing with Green Silver Nanoparticles as Bioengineered Skin for Wounds and Burns: A Pilot Studies. J. Biotechnol. Biomater. 2017, 7, 272. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Samadikuchaksaraei, A.; Seifalian, A.M.; Urbanska, A.M.; Ghanbarian, H.; Hardy, J.G.; Omrani, M.D.; Mozafari, M.; Reis, R.L.; Kundu, S.C. Silk fibroin/amniotic membrane 3D bi-layered artificial skin. Biomed. Mater. 2017, 13, 035003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Zhou, Z.; Lin, H.; Wu, J.; Ginn, B.; Choi, J.S.; Jiang, X.; Chung, L.; Elisseeff, J.H.; Yiu, S.; et al. Synthetic Nanofiber-Reinforced Amniotic Membrane via Interfacial Bonding. ACS Appl. Mater. Interfaces 2018, 10, 14559–14569. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Long, D.; Hsu, C.-C.; Liu, H.; Chen, L.; Slavin, B.; Lin, H.; Li, X.; Tang, J.; Yiu, S.; et al. Nanofiber-reinforced decellularized amniotic membrane improves limbal stem cell transplantation in a rabbit model of corneal epithelial defect. Acta Biomater. 2019, 97, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tian, S.; Bai, J.; Yu, K.; Liu, L.; Liu, G.; Dong, R.; Tian, D. Regulation of ERK1/2 and SMAD2/3 Pathways by Using Multi-Layered Electrospun PCL–Amnion Nanofibrous Membranes for the Prevention of Post-Surgical Tendon Adhesion. Int. J. Nanomed. 2020, 15, 927–942. [Google Scholar] [CrossRef] [Green Version]
- Gholipourmalekabadi, M.; Seifalian, A.; Urbanska, A.M.; Omrani, M.D.; Hardy, J.G.; Madjd, Z.; Hashemi, S.M.; Ghanbarian, H.; Milan, P.B.; Mozafari, M.; et al. 3D Protein-Based Bilayer Artificial Skin for the Guided Scarless Healing of Third-Degree Burn Wounds in Vivo. Biomacromolecules 2018, 19, 2409–2422. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-W.; Won, J.-Y.; Lee, H.-Y.; Lee, H.-J.; Youn, S.-W.; Lee, J.-Y.; Cho, C.-H.; Cho, H.-J.; Oh, S.; Chae, I.-H.; et al. Angiopoietin-1 Protects Heart against Ischemia/Reperfusion Injury through VE-Cadherin Dephosphorylation and Myocardiac Integrin-β1/ERK/Caspase-9 Phosphorylation Cascade. Mol. Med. 2011, 17, 1095–1106. [Google Scholar] [CrossRef]
- Xie, Q.; Sun, Z.; Chen, M.; Zhong, Q.; Yang, T.; Yi, J. IL-8 up-regulates proliferative angiogenesis in ischemic myocardium in rabbits through phosphorylation of Akt/GSK-3β(ser9) dependent pathways. Int. J. Clin. Exp. Med. 2015, 8, 12498–12508. [Google Scholar]
- Zhao, T.; Zhao, W.; Meng, W.; Liu, C.; Chen, Y.; Gerling, I.C.; Weber, K.T.; Bhattacharya, S.K.; Kumar, R.; Sun, Y. VEGF-C/VEGFR-3 pathway promotes myocyte hypertrophy and survival in the infarcted myocardium. Am. J. Transl. Res. 2015, 7, 697–709. [Google Scholar]
- Koblizek, T.I.; Weiss, C.; Yancopoulos, G.D.; Deutsch, U.; Risau, W. Angiopoietin-1 induces sprouting angiogenesis in vitro. Curr. Biol. 1998, 8, 529–532. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.; Kim, H.G.; So, J.-N.; Kim, J.H.; Kwak, H.J.; Koh, G.Y. Angiopoietin-1 Regulates Endothelial Cell Survival Through the Phosphatidylinositol 3′-Kinase/Akt Signal Transduction Pathway. Circ. Res. 2000, 86, 24–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, A.; Dubey, S.; Varney, M.L.; Dave, B.J.; Singh, R.K. IL-8 Directly Enhanced Endothelial Cell Survival, Proliferation, and Matrix Metalloproteinases Production and Regulated Angiogenesis. J. Immunol. 2003, 170, 3369–3376. [Google Scholar] [CrossRef]
- Cao, Y.; Linden, P.; Farnebo, J.; Cao, R.; Eriksson, A.; Kumar, V.; Qi, J.-H.; Claesson-Welsh, L.; Alitalo, K. Vascular endothelial growth factor C induces angiogenesis in vivo. Proc. Natl. Acad. Sci. USA 1998, 95, 14389–14394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishimoto, K.; Liu, S.; Tsuji, T.; Olson, A.K.; Hu, G.-F. Endogenous angiogenin in endothelial cells is a general requirement for cell proliferation and angiogenesis. Oncogene 2004, 24, 445–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadir, N.D.; Yang, Z.; Hassan, A.; Denslin, V.; Lee, E.H. Electrospun fibers enhanced the paracrine signaling of mesenchymal stem cells for cartilage regeneration. Stem Cell Res. Ther. 2021, 12, 100. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Gao, P.-L.; Wang, K.; Wang, J.-Y.; Zhong, Y.; Luo, Y. Fibrous scaffolds potentiate the paracrine function of mesenchymal stem cells: A new dimension in cell-material interaction. Biomaterials 2017, 141, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Thummarati, P.; Kino-Oka, M. Effect of Co-culturing Fibroblasts in Human Skeletal Muscle Cell Sheet on Angiogenic Cytokine Balance and Angiogenesis. Front. Bioeng. Biotechnol. 2020, 8, 578140. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Ismail, A.B.; Chee, S.C.; Bin Laupa, M.S.; Jaffri, F.B.; Saberi, S.E.M.; Idrus, R.B.H. One-Step Purification of Human Skeletal Muscle Myoblasts and Subsequent Expansion Using Laminin-Coated Surface. Tissue Eng. Part C Methods 2015, 21, 1135–1142. [Google Scholar] [CrossRef]
- Afandi, M.A.M.; Maarof, M.; Chowdhury, S.R.; Idrus, R.B.H. Synergistic Effect of Laminin and Epidermal Growth Factor on Biological and Morphological Properties of Co-Cultured Myoblasts and Fibroblasts. Tissue Eng. Regen. Med. 2020, 17, 835–845. [Google Scholar] [CrossRef]
- Zahari, N.K.; Idrus, R.B.H.; Chowdhury, S.R. Laminin-Coated Poly(Methyl Methacrylate) (PMMA) Nanofiber Scaffold Facilitates the Enrichment of Skeletal Muscle Myoblast Population. Int. J. Mol. Sci. 2017, 18, 2242. [Google Scholar] [CrossRef] [Green Version]
- Gobinathan, S.; Zainol, S.S.; Azizi, S.F.; Iman, N.M.; Muniandy, R.; Hasmad, H.N.; Bin Yusof, M.R.; Husain, S.; Abd Aziz, H.; Lokanathan, Y. Decellularization and genipin crosslinking of amniotic membrane suitable for tissue engineering applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 2051–2067. [Google Scholar] [CrossRef] [PubMed]
- Maarof, M.; Lokanathan, Y.; Ruszymah, H.I.; Bin Saim, A.; Chowdhury, S.R. Proteomic Analysis of Human Dermal Fibroblast Conditioned Medium (DFCM). J. Protein Chem. 2018, 37, 589–607. [Google Scholar] [CrossRef] [PubMed]



| Conditioned Medium | Culture Condition |
|---|---|
| Non-CM | Negative control; SkM-free, serum-free culture medium |
| Plain CM | Derived from SkM cultured on the plain tissue culture surface |
| HAM CM | Derived from SkM-seeded HAM scaffolds |
| EF-HAM 3 min CM | Derived from SkM-seeded EF-HAM 3 min scaffolds |
| EF-HAM 5 min CM | Derived from SkM-seeded EF-HAM 5 min scaffolds |
| EF-HAM 7 min CM | Derived from SkM-seeded EF-HAM 7 min scaffolds |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasmad, H.N.; Bt Hj Idrus, R.; Sulaiman, N.; Lokanathan, Y. Electrospun Fiber-Coated Human Amniotic Membrane: A Potential Angioinductive Scaffold for Ischemic Tissue Repair. Int. J. Mol. Sci. 2022, 23, 1743. https://doi.org/10.3390/ijms23031743
Hasmad HN, Bt Hj Idrus R, Sulaiman N, Lokanathan Y. Electrospun Fiber-Coated Human Amniotic Membrane: A Potential Angioinductive Scaffold for Ischemic Tissue Repair. International Journal of Molecular Sciences. 2022; 23(3):1743. https://doi.org/10.3390/ijms23031743
Chicago/Turabian StyleHasmad, Hanis Nazihah, Ruszymah Bt Hj Idrus, Nadiah Sulaiman, and Yogeswaran Lokanathan. 2022. "Electrospun Fiber-Coated Human Amniotic Membrane: A Potential Angioinductive Scaffold for Ischemic Tissue Repair" International Journal of Molecular Sciences 23, no. 3: 1743. https://doi.org/10.3390/ijms23031743
APA StyleHasmad, H. N., Bt Hj Idrus, R., Sulaiman, N., & Lokanathan, Y. (2022). Electrospun Fiber-Coated Human Amniotic Membrane: A Potential Angioinductive Scaffold for Ischemic Tissue Repair. International Journal of Molecular Sciences, 23(3), 1743. https://doi.org/10.3390/ijms23031743






