Fisetin Deters Cell Proliferation, Induces Apoptosis, Alleviates Oxidative Stress and Inflammation in Human Cancer Cells, HeLa
Abstract
:1. Introduction
2. Results
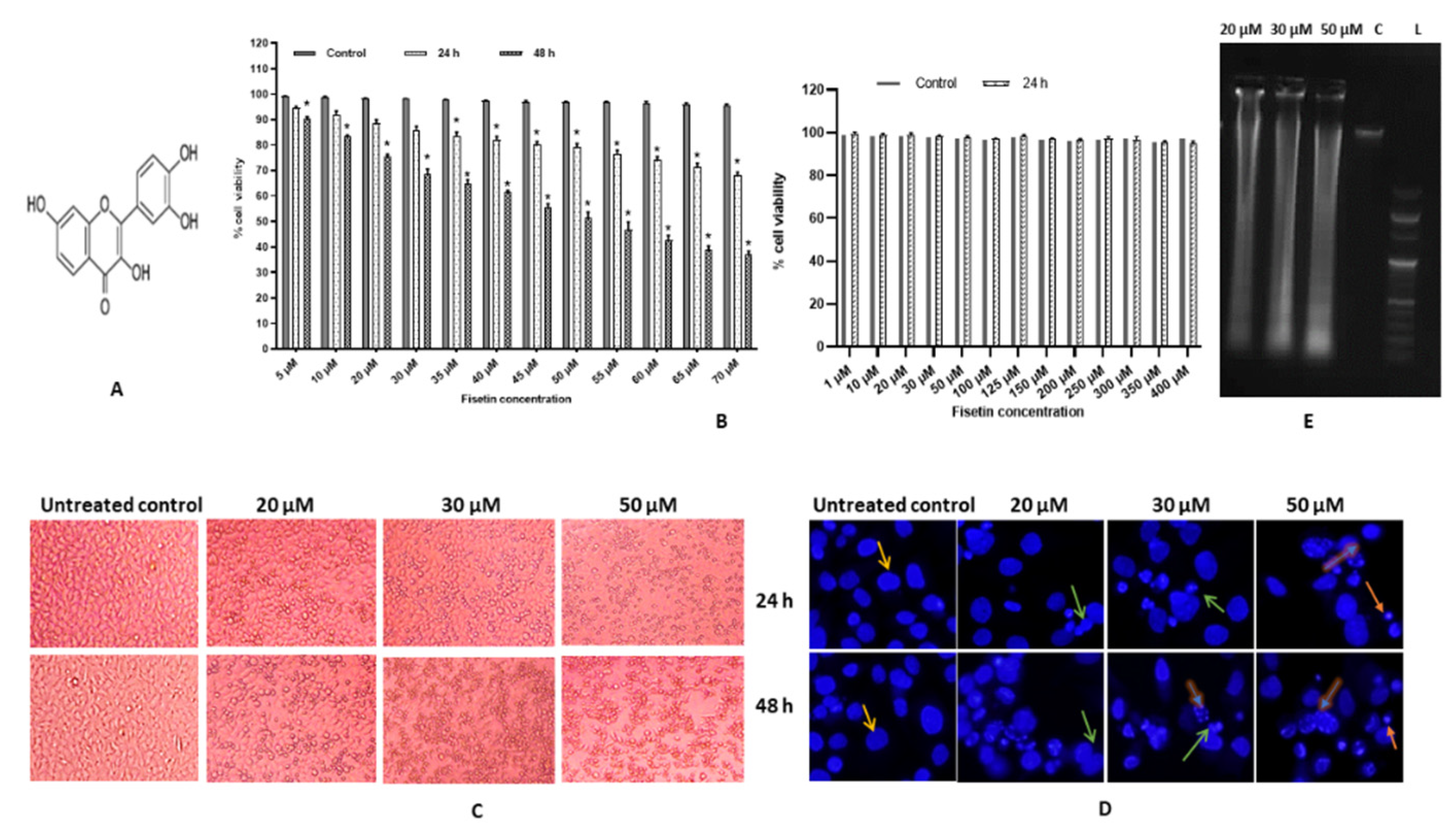
2.1. Fisetin Induces Morphological Changes and Inhibits Proliferation of HeLa Cells
2.2. Fisetin Changes Nuclear Morphology of HeLa Cells
2.3. Fisetin Leads DNA Fragmentation
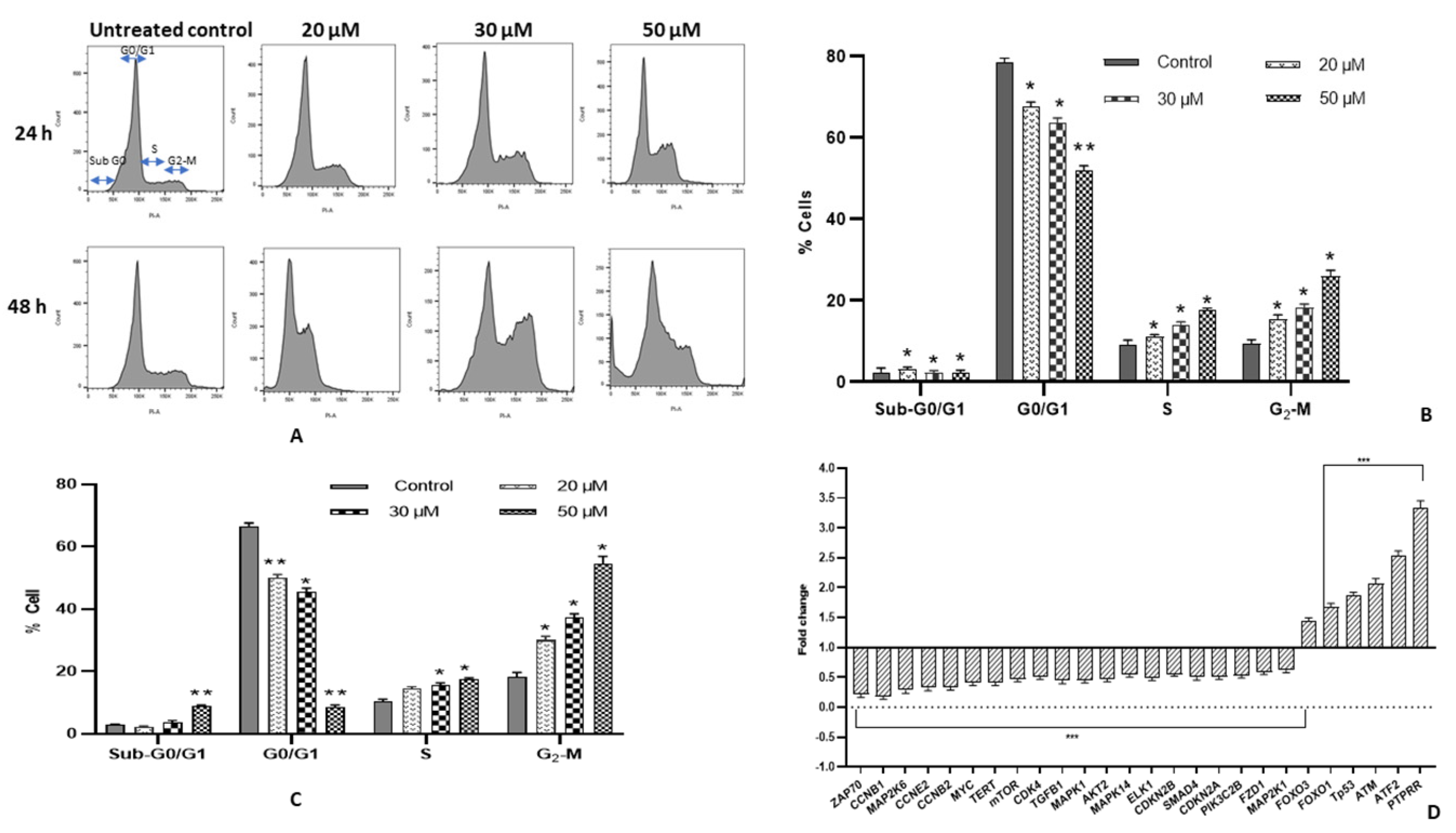
2.4. Fisetin Encourages G2/M Arrest and Modulates Cell Cycle Regulatory Genes
2.5. Fisetin Shows Early Apoptosis on HeLa Cells
2.6. Fisetin Decreases TMRE Fluorescent Intensity
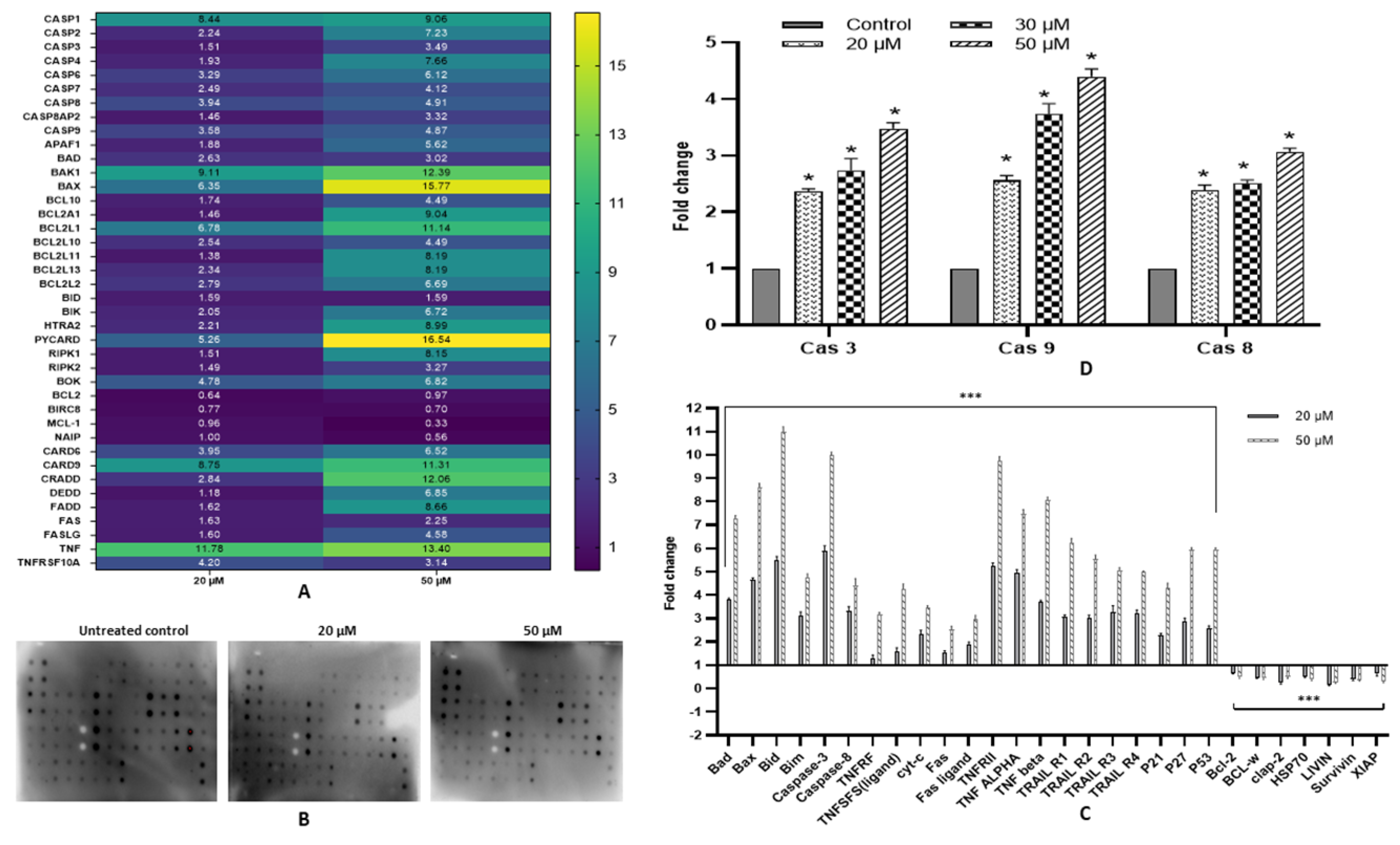
2.7. Fisetin Activates Extrinsic and Intrinsic Pathways
2.8. Fisetin Modulates Expression of Various Pro- and Anti-Apoptotic Proteins
2.9. Fisetin Elevates Caspase-3, Caspase-8 and Caspase-9 Activity
2.10. Fisetin Ameliorates Oxidation Stress in HeLa Cells by Upregulating GSH Activity
2.11. Fisetin Alleviates Inflammation in HeLa Cells
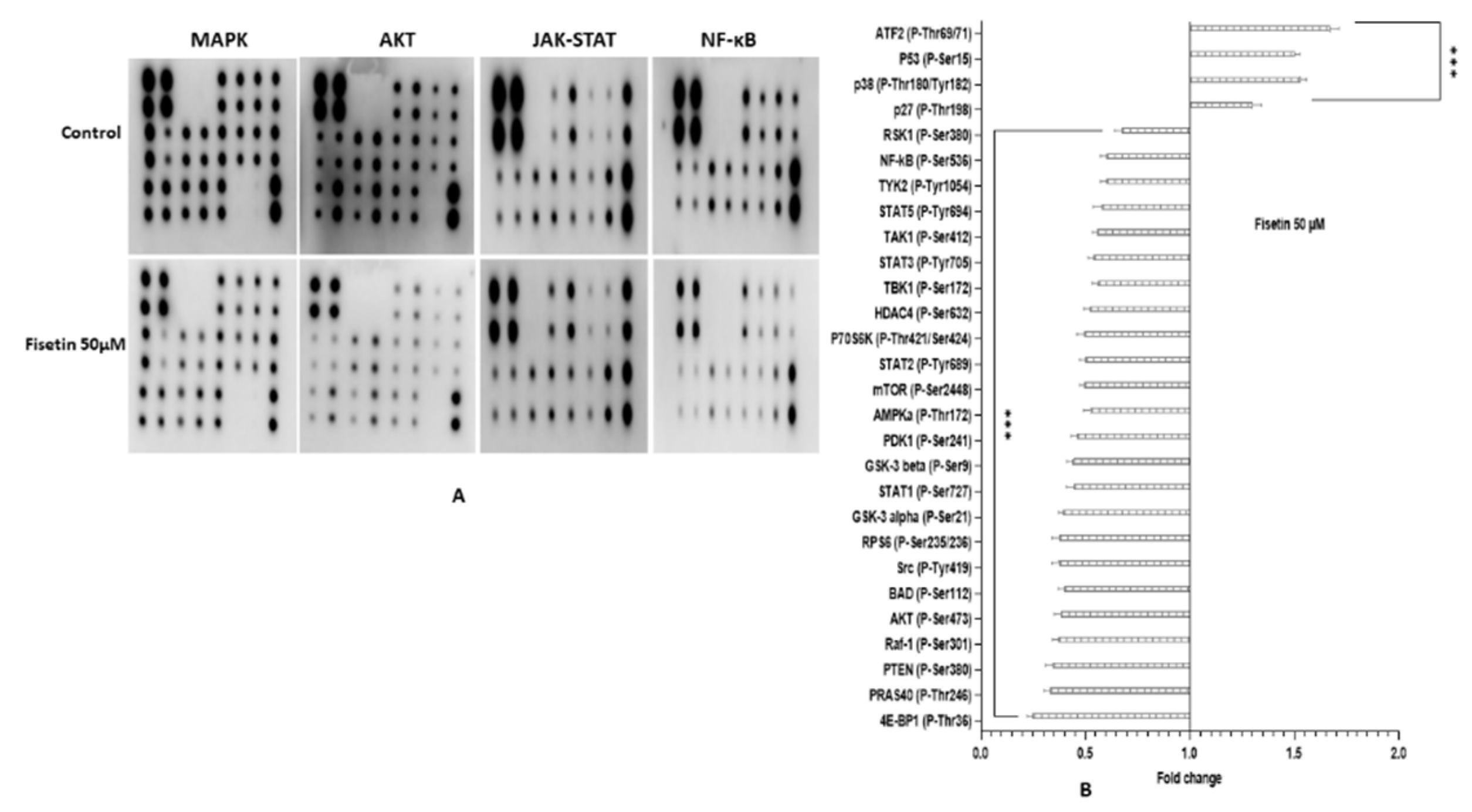
2.12. Fisetin Changes the Aberrant MAPK and PI3K/AKT/mTOR in HeLa Cells
3. Discussion
4. Materials and Methods
4.1. Cell lines and Reagents
4.2. Preparation of Drug Solutions
4.3. Cell Viability Assay
4.4. Nuclear Morphology by DAPI (4,6-diamidino-2-phenylindile) Staining
4.5. DNA Fragmentation Assay
4.6. Cell Cycle Analysis
4.7. Annexin V/Propidium Iodide Double Staining to Quantitate Apoptosis
4.8. TMRE Staining to Analyse Mitochondrial Membrane Potential
4.9. Gene Expression by TaqMan Apoptosis Array
4.10. Measurement of Apoptosis-Related Proteins Expression by Proteome Profiler Array
4.11. Phosphorylation Array
4.12. Caspases Multiplex Assay
4.13. Detection of GSH Activity in HeLa Cells
4.14. Inflammation Array
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TNF | Tumour necrotic factor |
| FASL | Fas ligand |
| TRAIL | Tumour necrosis factor-related apoptosis-inducing ligand |
| PARP | Poly [ADP ribose] polymerase 1 |
| PI | propidium iodide |
| PI3KCD | phosphotidyl-inositol-4,5-bisphosphate 3-kinase catalytic subunit delta |
| PTPRR | protein tyrosine phosphatase receptor type R |
| qPCR | quantitative real time polymerase chain reaction |
| TERT | telomerase reverse transcriptase |
| IL | Interleukin |
| MAPK | Mitogen activated protein kinase |
References
- Amararathna, M.; Johnston, M.R.; Rupasinghe, H.P. Plant polyphenols as chemopreventive agents for lung cancer. Int. J. Mol. Sci. 2016, 17, 1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patterson, S.L.; Colbert Maresso, K.; Hawk, E. Cancer chemoprevention: Successes and failures. Clin. Chem. 2013, 59, 94–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundarraj, K.; Raghunath, A.; Perumal, E. A review on the chemotherapeutic potential of fisetin: In vitro evidences. Biomed. Pharmacother. 2018, 97, 928–940. [Google Scholar] [CrossRef] [PubMed]
- Lall, R.K.; Adhami, V.M.; Mukhtar, H. Dietary flavonoid fisetin for cancer prevention and treatment. Mol. Nutr. Food Res. 2016, 60, 1396–1405. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.G.; Murillo, G.; Naithani, R.; Peng, X. Cancer chemoprevention by natural products: How far have we come? Pharm. Res. 2010, 27, 950–961. [Google Scholar] [CrossRef]
- Raj, L.; Ide, T.; Gurkar, A.U.; Foley, M.; Schenone, M.; Li, X.; Tolliday, N.J.; Golub, T.R.; Carr, S.A.; Shamji, A.F.; et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature 2011, 475, 231–234. [Google Scholar] [CrossRef]
- Sudhakar, A. History of cancer, ancient and modern treatment methods. J. Cancer Sci. Ther. 2009, 1, 1. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Rauch, J.; Kolch, W. Targeting MAPK signaling in cancer: Mechanisms of drug resistance and sensitivity. Int. J. Mol. Sci. 2020, 21, 1102. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Bode, A.M.; Dong, Z.; Lee, M.-H. AKT as a therapeutic target for cancer. Cancer Res. 2019, 79, 1019–1031. [Google Scholar] [CrossRef] [Green Version]
- Porta, C.; Paglino, C.; Mosca, A. Targeting PI3K/Akt/mTOR signaling in cancer. Front. Oncol. 2014, 4, 64. [Google Scholar] [CrossRef] [Green Version]
- Panji, M.; Behmard, V.; Zare, Z.; Malekpour, M.; Nejadbiglari, H.; Yavari, S.; Dizaj, T.N.; Safaeian, A.; Maleki, N.; Abbasi, M.; et al. Suppressing effects of Green tea extract and Epigallocatechin-3-gallate [EGCG] on TGF-β-induced Epithelial-to-mesenchymal transition via ROS/Smad signaling in human cervical cancer cells. Gene 2021, 794, 145774. [Google Scholar] [CrossRef] [PubMed]
- Scheid, M.P.; Schubert, K.M.; Duronio, V. Regulation of Bad phosphorylation and association with Bcl-xL by the MAPK/Erk kinase. J. Biol. Chem. 1999, 274, 31108–31113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kedhari Sundaram, M.; Raina, R.; Afroze, N.; Bajbouj, K.; Hamad, M.; Haque, S.; Hussain, A. Quercetin modulates signaling pathways and induces apoptosis in cervical cancer cells. Biosci Rep. 2019, 39, BSR20190720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samarghandian, S.; Azimi Nezhad, M.; Mohammadi, G. Role of caspases, Bax and Bcl-2 in chrysin-induced apoptosis in the A549 human lung adenocarcinoma epithelial cells. Anti-Cancer Agents Med. Chem. 2014, 14, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ma, X.; Li, Q.; Yang, Y.; Xu, X.; Sun, J.; Yu, M.; Cao, K.; Yang, L.; Yang, G.; et al. Anti-cancer effects of fisetin on mammary carcinoma cells via regulation of the PI3K/Akt/mTOR pathway: In vitro and in vivo studies. Int. J. Mol. Med. 2018, 42, 811–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, C.-S.; Shen, K.-H.; Huang, J.-S.; Ko, S.-C.; Shih, Y.-W. Antimetastatic potential of fisetin involves inactivation of the PI3K/Akt and JNK signaling pathways with downregulation of MMP-2/9 expressions in prostate cancer PC-3 cells. Mol. Cell Biochem. 2010, 333, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Pal, H.C.; Sharma, S.; Elmets, C.A.; Athar, M.; Afaq, F. Fisetin inhibits growth, induces G 2/M arrest and apoptosis of human epidermoid carcinoma A 431 cells: Role of mitochondrial membrane potential disruption and consequent caspases activation. Exp. Dermatol. 2013, 22, 470–475. [Google Scholar] [CrossRef] [Green Version]
- Kang, K.A.; Piao, M.J.; Hyun, J.W. Fisetin induces apoptosis in human nonsmall lung cancer cells via a mitochondria-mediated pathway. Vitr. Cell Dev. Biol. 2015, 51, 300–309. [Google Scholar] [CrossRef]
- Smith, M.L.; Murphy, K.; Doucette, C.D.; Greenshields, A.L.; Hoskin, D.W. The dietary flavonoid fisetin causes cell cycle arrest, caspase-dependent apoptosis, and enhanced cytotoxicity of chemotherapeutic drugs in triple-negative breast cancer cells. J. Cell Biochem. 2016, 117, 1913–1925. [Google Scholar] [CrossRef]
- Li, J.; Cheng, Y.; Qu, W.; Sun, Y.; Wang, Z.; Wang, H.; Tian, B. Fisetin, a Dietary Flavonoid, Induces Cell Cycle Arrest and Apoptosis through Activation of p53 and Inhibition of NF-Kappa B Pathways in Bladder Cancer Cells. Basic Clin. Pharmacol. Toxicol. 2011, 108, 84–93. [Google Scholar] [CrossRef]
- Yang, P.-M.; Tseng, H.-H.; Peng, C.-W.; Chen, W.-S.; Chiu, S.-J. Dietary flavonoid fisetin targets caspase-3-deficient human breast cancer MCF-7 cells by induction of caspase-7-associated apoptosis and inhibition of autophagy. Int. J. Oncol. 2012, 40, 469–478. [Google Scholar]
- Szliszka, E.; Helewski, K.J.; Mizgala, E.; Krol, W. The dietary flavonol fisetin enhances the apoptosis-inducing potential of TRAIL in prostate cancer cells. Int. J. Oncol. 2011, 39, 771–779. [Google Scholar]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.-M.; An, J. Cytokines, inflammation and pain. Int. Anesthesiol. Clin. 2007, 45, 27. [Google Scholar] [CrossRef] [Green Version]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ying, T.H.; Yang, S.F.; Tsai, S.J.; Hsieh, S.C.; Huang, Y.C.; Bau, D.T.; Hsieh, Y.H. Fisetin induces apoptosis in human cervical cancer HeLa cells through ERK1/2-mediated activation of caspase-8-/caspase-3-dependent pathway. Arch. Toxicol. 2012, 86, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.Y.; Chen, M.C.; Tseng, Y.S.; Chen, M.C.; Zhou, Z.; Yang, J.J.; Lin, Y.M.; Viswanadha, V.P.; Wang, G.; Huang, C.Y. Fisetin activates Hippo pathway and JNK/ERK/AP-1 signaling to inhibit proliferation and induce apoptosis of human osteosarcoma cells via ZAK overexpression. Environ. Toxicol. 2019, 34, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Zou, J.; Fang, Y.; Meng, Y.; Xiao, C.; Fu, J.; Liu, S.; Bai, P.; Yao, Y. Fisetin and polymeric micelles encapsulating fisetin exhibit potent cytotoxic effects towards ovarian cancer cells. BMC Complement. Altern. Med. 2018, 18, 91. [Google Scholar] [CrossRef] [Green Version]
- Pak, F.; Oztopcu-Vatan, P. Fisetin effects on cell proliferation and apoptosis in glioma cells. Z. Nat. C 2019, 74, 295–302. [Google Scholar] [CrossRef]
- Raina, R.; Afroze, N.; Sundaram, M.K.; Haque, S.; Bajbouj, K.; Hamad, M.; Hussain, A. Chrysin inhibits propagation of HeLa cells by attenuating cell survival and inducing apoptotic pathways. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2206–2220. [Google Scholar]
- Kashyap, D.; Garg, V.K.; Tuli, H.S.; Yerer, M.B.; Sak, K.; Sharma, A.K.; Kumar, M.; Aggarwal, V.; Sandhu, S.S. Fisetin and quercetin: Promising flavonoids with chemopreventive potential. Biomolecules 2019, 9, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, Y.; Wang, R.; Shao, N.; Zhi, F.; Yang, Y. Luteolin suppresses tumor proliferation through inducing apoptosis and autophagy via MaPK activation in glioma. Onco Targets Ther. 2019, 12, 2383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youns, M.; Hegazy, W.A.H. The natural flavonoid fisetin inhibits cellular proliferation of hepatic, colorectal, and pancreatic cancer cells through modulation of multiple signaling pathways. PLoS ONE 2017, 12, e0169335. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Jin, K.; Lan, H. Luteolin inhibits cell cycle progression and induces apoptosis of breast cancer cells through downregulation of human telomerase reverse transcriptase. Oncol. Lett. 2019, 17, 3842–3850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Gui, L.S.; Zhao, X.L.; Zhu, L.L.; Li, Q.W. FOXO1 is a tumor suppressor in cervical cancer. Genet. Mol. Res. 2015, 14, 6605–6616. [Google Scholar] [CrossRef]
- Zhang, J.; Ng, S.; Wang, J.; Zhou, J.; Tan, S.-H.; Yang, N.; Lin, Q.; Xia, D.; Shen, H.-M. Histone deacetylase inhibitors induce autophagy through FOXO1-dependent pathways. Autophagy 2015, 11, 629–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Ehren, J.L.; Maher, P. Concurrent regulation of the transcription factors Nrf2 and ATF4 mediates the enhancement of glutathione levels by the flavonoid fisetin. Biochem. Pharmacol. 2013, 85, 1816–1826. [Google Scholar] [CrossRef]
- Naidu, M.S.K.; Suryakar, A.N.; Swami, S.C.; Katkam, R.V.; Kumbar, K.M. Oxidative stress and antioxidant status in cervical cancer patients. Indian J. Clin. Biochem. 2007, 22, 140–144. [Google Scholar] [CrossRef] [Green Version]
- Tavsan, Z.; Kayali, H.A. Flavonoids showed anticancer effects on the ovarian cancer cells: Involvement of reactive oxygen species, apoptosis, cell cycle and invasion. Biomed. Pharmacother. 2019, 116, 109004. [Google Scholar] [CrossRef] [PubMed]
- Zahra, K.; Patel, S.; Dey, T.; Pandey, U.; Mishra, S.P. A study of oxidative stress in cervical cancer-an institutional study. Biochem. Biophys. Rep. 2021, 25, 100881. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodgers, E.H.; Grant, M.H. The effect of the flavonoids, quercetin, myricetin and epicatechin on the growth and enzyme activities of MCF7 human breast cancer cells. Chem. Biol. Interact. 1998, 116, 213–228. [Google Scholar] [CrossRef]
- Moskaug, J.Ø.; Carlsen, H.; Myhrstad, M.C.W.; Blomhoff, R. Polyphenols and glutathione synthesis regulation. Am. J. Clin. Nutr. 2005, 81, 277S–283S. [Google Scholar] [CrossRef]
- Giovannini, C.; Filesi, C.; D’Archivio, M.; Scazzocchio, B.; Santangelo, C.; Masella, R. Polyphenols and endogenous antioxidant defences: Effects on glutathione and glutathione related enzymes. Ann. Dell’istituto Super. Sanita 2006, 42, 336–347. [Google Scholar]
- Mukundan, H.; Bahadur, A.K.; Kumar, A.; Sardana, S.; Naik, S.L.D.; Ray, A.; Sharma, B.K. Glutathione Level and Its Relation to Radiation Therapy in Patients with Cancer of Uterine Cervix; NISCAIR-CSIR: Delhi, India, 1999. [Google Scholar]
- Althunibat, O.Y.; Al Hroob, A.M.; Abukhalil, M.H.; Germoush, M.O.; Bin-Jumah, M.; Mahmoud, A.M. Fisetin ameliorates oxidative stress, inflammation and apoptosis in diabetic cardiomyopathy. Life Sci. 2019, 221, 83–92. [Google Scholar] [CrossRef]
- Briukhovetska, D.; Dörr, J.; Endres, S.; Libby, P.; Dinarello, C.A.; Kobold, S. Interleukins in cancer: From biology to therapy. Nat. Rev. Cancer 2021, 21, 481–499. [Google Scholar] [CrossRef]
- Chen, G.Y.; Shaw, M.H.; Redondo, G.; Núñez, G. Innate immune receptor nod1 protects the intestine from inflammation-induced tumorigenesis. Cancer Res. 2008, 68, 10060–10067. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.; Lin, Y.; Ye, X.; Feng, C.; Lu, Y.; Yang, G.; Dong, C. Expression of IL-1α and IL-6 is associated with progression and prognosis of human cervical cancer. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 4475. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.-L.; Maeda, S.; Hsu, L.-C.; Yagita, H.; Karin, M. Inhibition of NF-κB in cancer cells converts inflammation-induced tumor growth mediated by TNFα to TRAIL-mediated tumor regression. Cancer Cell 2004, 6, 297–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, A.J.; Putoczki, T. JAK-STAT Signalling Pathway in Cancer. Cancers 2020, 12, 1971. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Hoya, A.; Soto-Cruz, I. Role of the JAK/STAT pathway in cervical cancer: Its relationship with HPV E6/E7 oncoproteins. Cells 2020, 9, 2297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-J.; Jia, S.-S. Fisetin inhibits laryngeal carcinoma through regulation of AKT/NF-κB/mTOR and ERK1/2 signaling pathways. Biomed. Pharmacother. 2016, 83, 1164–1174. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gong, X.; Jiang, R.; Lin, D.; Zhou, T.; Zhang, A.; Li, H.; Zhang, X.; Wan, J.; Kuang, G.; et al. Fisetin inhibited growth and metastasis of triple-negative breast cancer by reversing epithelial-to-mesenchymal transition via PTEN/Akt/GSK3β signal pathway. Front. Pharmacol. 2018, 9, 772. [Google Scholar] [CrossRef]
- Fang, X.; Yu, S.; Eder, A.; Mao, M.; Bast, R.C.; Boyd, D.; Mills, G.B. Regulation of BAD phosphorylation at serine 112 by the Ras-mitogen-activated protein kinase pathway. Oncogene 1999, 18, 6635–6640. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, J.; Ohmichi, M.; Kurachi, H.; Kanda, Y.; Hisamoto, K.; Nishio, Y.; Adachi, K.; Tasaka, K.; Kanzaki, T.; Murata, Y. Inhibition of BAD phosphorylation either at serine 112 via extracellular signal-regulated protein kinase cascade or at serine 136 via Akt cascade sensitizes human ovarian cancer cells to cisplatin. Cancer Res. 2000, 60, 5988–5994. [Google Scholar]
- Yang, J.; Nie, J.; Ma, X.; Wei, Y.; Peng, Y.; Wei, X. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019, 18, 26. [Google Scholar] [CrossRef] [Green Version]
- Nakahata, S.; Ichikawa, T.; Maneesaay, P.; Saito, Y.; Nagai, K.; Tamura, T.; Manachai, N.; Yamakawa, N.; Hamasaki, M.; Kitabayashi, I.; et al. Loss of NDRG2 expression activates PI3K-AKT signalling via PTEN phosphorylation in ATLL and other cancers. Nat. Commun. 2014, 5, 3393. [Google Scholar] [CrossRef] [Green Version]
- Germann, U.A.; Furey, B.F.; Markland, W.; Hoover, R.R.; Aronov, A.M.; Roix, J.J.; Hale, M.; Boucher, D.M.; Sorrell, D.A.; Martinez-Botella, G.; et al. Targeting the MAPK signaling pathway in cancer: Promising preclinical activity with the novel selective ERK1/2 inhibitor BVD-523 [ulixertinib]. Mol. Cancer Ther. 2017, 16, 2351–2363. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, M.-H.; Tsai, J.-P.; Yang, S.-F.; Chiou, H.-L.; Lin, C.-L.; Hsieh, Y.-H.; Chang, H.-R. Fisetin suppresses the proliferation and metastasis of renal cell carcinoma through upregulation of MEK/ERK-targeting CTSS and ADAM9. Cells 2019, 8, 948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, E.J.; Ahn, W.S. Kaempferol induced the apoptosis via cell cycle arrest in human breast cancer MDA-MB-453 cells. Nutr. Res. Pract. 2008, 2, 322–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, P.; Roy, A.S.; Chaudhury, S.; Jana, S.K.; Chaudhury, K.; Dasgupta, S. Preparation of albumin based nanoparticles for delivery of fisetin and evaluation of its cytotoxic activity. Int. J. Biol. Macromol. 2016, 86, 408–417. [Google Scholar] [CrossRef]
- Feng, C.; Yuan, X.; Chu, K.; Zhang, H.; Ji, W.; Rui, M. Preparation and optimization of poly [lactic acid] nanoparticles loaded with fisetin to improve anti-cancer therapy. Int. J. Biol. Macromol. 2019, 125, 700–710. [Google Scholar] [CrossRef]
- Kadari, A.; Gudem, S.; Kulhari, H.; Bhandi, M.M.; Borkar, R.M.; Kolapalli, V.R.M.; Sistla, R. Enhanced oral bioavailability and anticancer efficacy of fisetin by encapsulating as inclusion complex with HPβCD in polymeric nanoparticles. Drug Deliv. 2017, 24, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Mehta, P.; Pawar, A.; Mahadik, K.; Bothiraja, C. Emerging novel drug delivery strategies for bioactive flavonol fisetin in biomedicine. Biomed. Pharmacother. 2018, 106, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Pawar, A.; Singh, S.; Rajalakshmi, S.; Shaikh, K.; Bothiraja, C. Development of fisetin-loaded folate functionalized pluronic micelles for breast cancer targeting. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 1), 347–361. [Google Scholar] [CrossRef] [Green Version]
- Raina, R.; Pramodh, S.; Rais, N.; Haque, S.; Shafarin, J.; Bajbouj, K.; Hamad, M.; Hussain, A. Luteolin inhibits proliferation, triggers apoptosis and modulates Akt/mTOR and MAP kinase pathways in HeLa cells. Oncol. Lett. 2021, 21, 1. [Google Scholar] [CrossRef]


| Hall Mark | Molecular Target | Transcript Expression | Protein Expression | ||
|---|---|---|---|---|---|
| Upregulation | Downregulation | Upregulation | Downregulation | ||
| Apoptosis | Caspases | CASP9, CASP7, CASP3, CASP6, CASP4, CASP8AP2, CASP2, CASP1 and CASP8 | Caspase-3 and Caspase-8 | ||
| Pro-apoptotic gene | APAF-1, BCL10, BCL2A1, BCL2L1, BCL2L13, BCL2L2, BAD, BAK1 and BAX, BOK, HTRA2, PYCARD, RIPK1, RIPK2, BID, PYCARD, RIPK2 and RIPK1. | Bad, Bax, Bid, Bim, P21, p53, p27, (ligand), cyt-c and HSP27. | |||
| Death receptors | FAS, FASL, CARD6, CARD9, CRADD, DEDD, FADD, TNF, TNFRSFS10A and TNFRSFS10B | Fas, Fas ligand, TNFRII, TNFα, TNF β, TNFRF, TNFSFS, TRAIL R1 to TRAIL R4. | |||
| Anti-apoptotic gene | BCL2, MCL1, BIRC5, and NAIP | Bcl-2, BCL-w, clap-2, HSP70, LIVIN, Survivin and XIAP. | |||
| Sustained cell proliferation | Cell cycle regulation | CCNB1, CCNB2, CCNE2, CDKN2A and CDK4. | |||
| Anti-proliferation and TSGs (Tumour suppressor genes) | PTPRR, FOXO 1, FOXO 3. ATM, ATF2 and TP53 | TERT | |||
| Inflammation and anti-oxidation | IL-2 and MYC, | IL-10 and IL-13 | IL-1α, IL-1β, IL-4, IL-7, IL-11 IL-16, IL-12p70, MIG, MCP-1, MCP-2, MIP-1β, MIP-1γ, MCF, I-309 and EOTAXIN | ||
| Hall Mark | Molecular Target | Transcript Expression | Protein Expression | ||
|---|---|---|---|---|---|
| Upregulation | Downregulation | Upregulation | Downregulation | ||
| Pathways:Anti-proliferation, anti-inflammation and apoptosis-inducing pathway | MAPK | MAPK1, MAK14, MAP2K1, MAP2K6, ELK 1 | p38 (P-Thr180/Tyr182) and P53 (P-Ser15) | RSK1 (P-Ser380) and Raf-1 (P-Ser301) | |
| AKT/MTPR/PI3K | AKT2, MTOR, PIK3C2B, PIK3CB. | p27 (P-Thr198) | GSK3a (p-ser21), GSK3b (p-ser9), MTOR (p-ser2448), PRAS 40 (p-Ther246), BAD (p-ser112), PTEN (p-ser380), AKT (p-ser473), AMPKa (P-Thr172), RPS6 (P-Ser235/236), and 4E-BP1 (P-Thr36). | ||
| JAK-STAT and NF-kB | Src (P-Tyr419), STAT1 (P-Ser727), STAT2 (P-Tyr689), STAT3 (P-Tyr705), STAT5 (P-Tyr694), TYK2 (P-Tyr1054), HDAC4 (P-Ser632), NF-kB (P-Ser536), TAK1 (P-Ser412) and TBK1 (P-Ser172). | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afroze, N.; Pramodh, S.; Shafarin, J.; Bajbouj, K.; Hamad, M.; Sundaram, M.K.; Haque, S.; Hussain, A. Fisetin Deters Cell Proliferation, Induces Apoptosis, Alleviates Oxidative Stress and Inflammation in Human Cancer Cells, HeLa. Int. J. Mol. Sci. 2022, 23, 1707. https://doi.org/10.3390/ijms23031707
Afroze N, Pramodh S, Shafarin J, Bajbouj K, Hamad M, Sundaram MK, Haque S, Hussain A. Fisetin Deters Cell Proliferation, Induces Apoptosis, Alleviates Oxidative Stress and Inflammation in Human Cancer Cells, HeLa. International Journal of Molecular Sciences. 2022; 23(3):1707. https://doi.org/10.3390/ijms23031707
Chicago/Turabian StyleAfroze, Nazia, Sreepoorna Pramodh, Jasmin Shafarin, Khuloud Bajbouj, Mawieh Hamad, Madhumitha Kedhari Sundaram, Shafiul Haque, and Arif Hussain. 2022. "Fisetin Deters Cell Proliferation, Induces Apoptosis, Alleviates Oxidative Stress and Inflammation in Human Cancer Cells, HeLa" International Journal of Molecular Sciences 23, no. 3: 1707. https://doi.org/10.3390/ijms23031707
APA StyleAfroze, N., Pramodh, S., Shafarin, J., Bajbouj, K., Hamad, M., Sundaram, M. K., Haque, S., & Hussain, A. (2022). Fisetin Deters Cell Proliferation, Induces Apoptosis, Alleviates Oxidative Stress and Inflammation in Human Cancer Cells, HeLa. International Journal of Molecular Sciences, 23(3), 1707. https://doi.org/10.3390/ijms23031707







