A Review of Advanced Molecular Engineering Approaches to Enhance the Thermostability of Enzyme Breakers: From Prospective of Upstream Oil and Gas Industry
Abstract
1. Introduction
2. Enzyme Thermostability Issues in Upstream Oil and Gas Applications
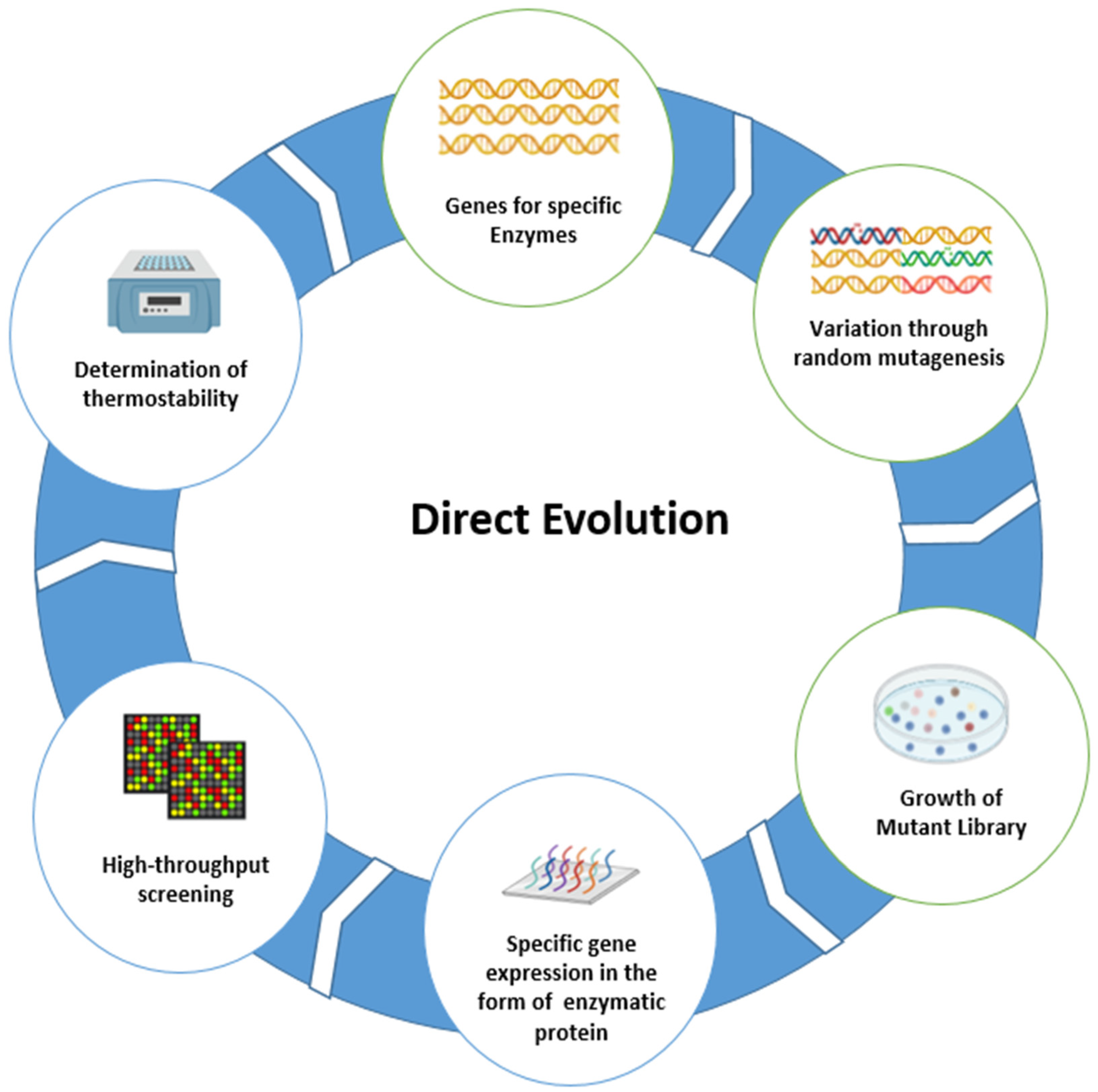
3. Direct Evolution
The Need for Direct Evolution to Increase the Thermostability of Enzyme Breakers
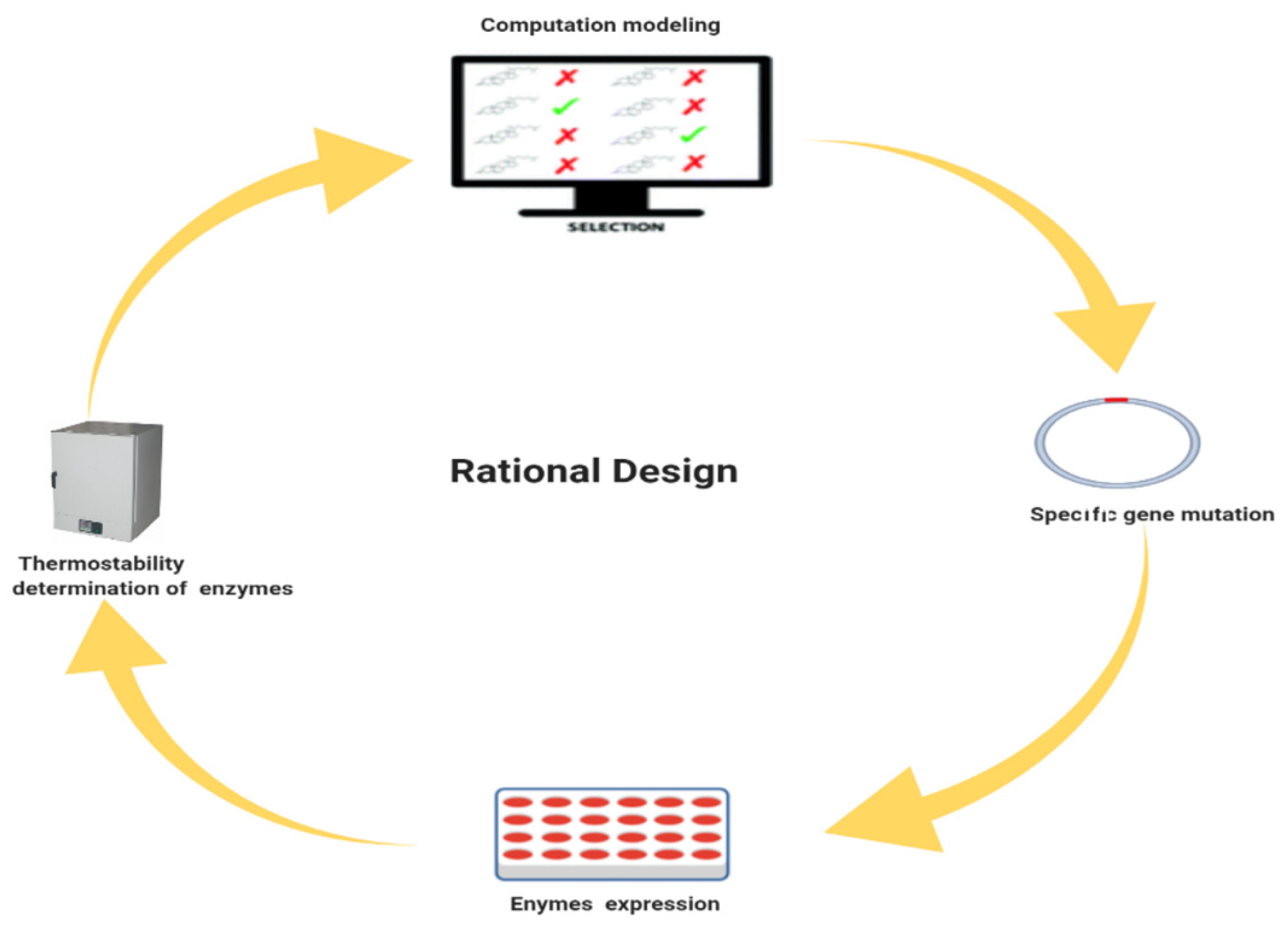
4. Rational Design
4.1. Rational Designed Algorithms for the Engineering of Thermostable Enzyme Breakers
4.1.1. FRESCO
4.1.2. PROSS
4.2. The Need for Rational Design to Increase the Thermostability of Enzyme Breakers
5. Semi-Rational Enzyme Engineering to Increase the Thermostability
6. Conclusions and Future Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Q.; Xing, H.; Liu, J.; Liu, X. A review on hydraulic fracturing of unconventional reservoir. Petroleum 2015, 1, 8–15. [Google Scholar] [CrossRef]
- Shiozawa, S. Designing Enhanced Geothermal and Hydraulic Fracturing Systems Based on Multiple Stages and Proppant. Doctoral Dissertation. 2015. Available online: https://repositories.lib.utexas.edu/handle/2152/34589 (accessed on 18 December 2021).
- Wilson, A. An Innovative Approach to Gel Breakers for Hydraulic Fracturing. J. Pet. Technol. 2017, 69, 48–51. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Guo, J.; Lai, J.; Wang, D.; He, L.; Qin, Y. A study of relation between suspension behavior and microstructure and viscoelastic property of guar gum fracturing fluid. J. Pet. Sci. Eng. 2014, 124, 432–435. [Google Scholar] [CrossRef]
- Armistead, H.W. Composition for Fracturing Process. US Patent 3,396,107, 8 June 1968. [Google Scholar]
- Ihejirika, B.; Dosunmu, A.; Eme, C. Performance evaluation of guar gum as a carrier fluid for hydraulic fracturing. In Proceedings of the SPE Nigeria Annual International Conference and Exhibition, Lagos, Nigeria, 4–6 August 2015. [Google Scholar]
- Hasan, A.M.; Abdel-Raouf, M.E. Applications of guar gum and its derivatives in petroleum industry: A review. Egypt. J. Pet. 2018, 27, 1043–1050. [Google Scholar] [CrossRef]
- Song, J.; Fan, W.; Long, X.; Zhou, L.; Wang, C.; Li, G. Rheological behaviors of fluorinated hydrophobically associating cationic guar gum fracturing gel. J. Pet. Sci. Eng. 2016, 146, 999–1005. [Google Scholar] [CrossRef]
- Holtsclaw, J.; Funkhouser, G.P. A Crosslinkable Synthetic Polymer System for High-Temperature Hydraulic Fracturing Applications. In Proceedings of the SPE Tight Gas Completions Conference, San Antonio, TX, USA, 16–17 June 2009. [Google Scholar]
- Fuller, M.J. An innovative approach to gel breakers for hydraulic fracturing. In Proceedings of the SPE International Conference and Exhibition on Formation Damage Control, Lafayette, LA, USA, 24–26 February 2006. [Google Scholar]
- Meng, Y.; Zhao, F.; Jin, X.; Feng, Y.; Sun, G.; Lin, J.; Jia, B.; Li, P. Performance Evaluation of Enzyme Breaker for Fracturing Applications under Simulated Reservoir Conditions. Molecules 2021, 26, 3133. [Google Scholar] [CrossRef]
- Nasr-El-Din, H.A.; Al-Mohammed, A.M.; Al-Aamri, A.D.; Al-Fuwaires, O.A. A study of gel degradation, surface tension, and their impact on the productivity of hydraulically fractured gas wells. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dubai, United Arab Emirates, 4–6 December 2007. [Google Scholar]
- Sarwar, M.U.; Cawiezel, K.E.; Nasr-El-Din, H.A. Gel degradation studies of oxidative and enzyme breakers to optimize breaker type and concentration for effective break profiles at low and medium temperature ranges. In Proceedings of the SPE Hydraulic Fracturing Technology Conference, The Woodlands, TX, USA, 24–26 January 2011. [Google Scholar]
- Zhang, B.; Huston, A.; Whipple, L.; Urbina, H.; Barrett, K.; Wall, M.; Hutchins, R.; Mirakyan, A. A superior, high-performance enzyme for breaking borate crosslinked fracturing fluids under extreme well conditions. SPE Prod. Oper. 2013, 28, 210–216. [Google Scholar] [CrossRef]
- Leal, A.B.; Barroso, A.L.; Miranda, X.; Flores, L.; Medeiros, G.; Marcelino, C.; Moranezi, L.; Pereira, R.A.; Oliveira, F.S.; Cândido, H.B. Reservoir Drilling and Completion Best Practices: Well Productivity Assessment Applying Drill in Fluid, Chelant/Enzyme Breaker System and Stimulation Design on Onshore Well BHT Scenario in Brazil. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 6–9 May 2019. [Google Scholar]
- Chopade, P.; Fontenelle, L.; Reddy, B.; Coria, B. Novel stabilized enzyme breaker for fracturing applications. In Proceedings of the SPE Production and Operations Symposium, Oklahoma City, OK, USA, 1–5 March 2015. [Google Scholar]
- Bose, C.C.; Alshatti, B.; Swartz, L.; Gupta, A.; Barati, R. Dual application of polyelectrolyte complex nanoparticles as enzyme breaker carriers and fluid loss additives for fracturing fluids. In Proceedings of the SPE/CSUR Unconventional Resources Conference—Canada, Calgary, AB, Canada, 30 September–2 October 2014. [Google Scholar]
- Brannon, H.D.; Tjon-Joe-Pin, R.M.; Carman, P.S.; Wood, W.D. Enzyme breaker technologies: A decade of improved well stimulation. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 5–8 October 2003. [Google Scholar]
- Al-Khaldi, M.H.; Ghosh, B.; Ghosh, D. A novel enzyme breaker for mudcake removal in high temperature horizontal and multi-lateral wells. In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition, Jakarta, Indonesia, 20–22 September 2011. [Google Scholar]
- Malone, M.R.; Nelson, S.G.; Jackson, R. Enzyme breaker technology increases production, Grayburg-Jackson field, southeast New Mexico: A case history. In Proceedings of the SPE Permian Basin Oil and Gas Recovery Conference, Midland, TX, USA, 21–23 March 2000. [Google Scholar]
- Asadi, M.; Penny, G.S. Large-scale comparative study of prepacked screen cleanup using acid and enzyme breaker. In Proceedings of the IADC/SPE Asia Pacific Drilling Technology, Kuala Lumpur, Malaysia, 11–13 September 2000. [Google Scholar]
- Himes, R.; Conway, M.; Schraufnagel, R. Enzyme breaker adsorption in sandstones and coal. In Proceedings of the SPE Formation Damage Control Symposium, Lafayette, LA, USA, 14–15 February 1996. [Google Scholar]
- Moore, W.; Beall, B.; Ali, S.A. Formation damage removal through the application of enzyme breaker technology. In Proceedings of the SPE Formation Damage Control Symposium, Lafayette, LA, USA, 14–15 February 1996. [Google Scholar]
- Gupta, D.; Pakulski, M.; Prasek, B.; Franklin, V. High-pH-tolerant enzyme breaker for oilfield applications. In Proceedings of the Permian Basin Oil and Gas Recovery Conference, Midland, TX, USA, 18–20 March 1992. [Google Scholar]
- Battistel, E.; Cobianco, S.; Bianchi, D.; Fornaroli, M.; Guglielmetti, G. Enzyme Breakers for Chemically Modified Starches. In Proceedings of the SPE European Formation Damage Conference, Sheveningen, The Netherlands, 25–27 May 2005. [Google Scholar]
- Coccia, M. A theory of classification and evolution of technologies within a Generalised Darwinism. Technol. Anal. Strateg. Manag. 2019, 31, 517–531. [Google Scholar] [CrossRef]
- Kaushal, J.; Mehandia, S.; Singh, G.; Raina, A.; Arya, S.K. Catalase enzyme: Application in bioremediation and food industry. Biocatal. Agric. Biotechnol. 2018, 16, 192–199. [Google Scholar] [CrossRef]
- Golchin, J.; Golchin, K.; Alidadian, N.; Ghaderi, S.; Eslamkhah, S.; Eslamkhah, M.; Akbarzadeh, A. Nanozyme applications in biology and medicine: An overview. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- 이재형; 정기준. Direct evolution of enzyme for enhanced production of biopolymer in Escherichia coli. In Proceedings of the 2017 KIChE Spring Meeting & International Symposium, Seoul, Korea, 26–28 April 2017. [Google Scholar]
- Li, Y.-x.; Yi, P.; Yan, Q.-j.; Qin, Z.; Liu, X.-q.; Jiang, Z.-q. Directed evolution of a β-mannanase from Rhizomucor miehei to improve catalytic activity in acidic and thermophilic conditions. Biotechnol. Biofuels 2017, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X. Studies on the Directed Evolution and Thermostability of β-Glucanase. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2006; pp. 64–94. [Google Scholar]
- Zhu, M.; Zhang, L.; Yang, F.; Cha, Y.; Li, S.; Zhuo, M.; Huang, S.; Li, J. A Recombinant β-Mannanase from Thermoanaerobacterium aotearoense SCUT27: Biochemical characterization and its thermostability improvement. J. Agric. Food Chem. 2019, 68, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Yoav, S.; Stern, J.; Salama-Alber, O.; Frolow, F.; Anbar, M.; Karpol, A.; Hadar, Y.; Morag, E.; Bayer, E.A. Directed evolution of Clostridium thermocellum β-Glucosidase a towards enhanced thermostability. Int. J. Mol. Sci. 2019, 20, 4701. [Google Scholar] [CrossRef] [PubMed]
- Fogang, L.T.; Kamal, M.S.; Sultan, A.S. Viscosity-Reducing Agents (Breakers) for Viscoelastic Surfactant Gels for Well Stimulation. Energy Fuels 2020, 34, 15686–15700. [Google Scholar] [CrossRef]
- Nishimoto, M. Large scale production of lacto-N-biose I, a building block of type I human milk oligosaccharides, using sugar phosphorylases. Biosci. Biotechnol. Biochem. 2020, 84, 17–24. [Google Scholar] [CrossRef]
- Saini, R.; Saini, H.S.; Dahiya, A. Amylases: Characteristics and industrial applications. J. Pharmacogn. Phytochem. 2017, 6, 1865–1871. [Google Scholar]
- Kim, Y.-W.; Choi, J.-H.; Kim, J.-W.; Park, C.; Kim, J.-W.; Cha, H.; Lee, S.-B.; Oh, B.-H.; Moon, T.-W.; Park, K.-H. Directed evolution of Thermus maltogenic amylase toward enhanced thermal resistance. Appl. Environ. Microbiol. 2003, 69, 4866–4874. [Google Scholar] [CrossRef][Green Version]
- Stephens, D.E.; Rumbold, K.; Permaul, K.; Prior, B.A.; Singh, S. Directed evolution of the thermostable xylanase from Thermomyces lanuginosus. J. Biotechnol. 2007, 127, 348–354. [Google Scholar] [CrossRef]
- Song, H.-Y.; El Sheikha, A.F.; Hu, D.-M. The positive impacts of microbial phytase on its nutritional applications. Trends Food Sci. Technol. 2019, 86, 553–562. [Google Scholar] [CrossRef]
- Tang, Z.; Jin, W.; Sun, R.; Liao, Y.; Zhen, T.; Chen, H.; Wu, Q.; Gou, L.; Li, C. Improved thermostability and enzyme activity of a recombinant phyA mutant phytase from Aspergillus niger N25 by directed evolution and site-directed mutagenesis. Enzym. Microb. Technol. 2018, 108, 74–81. [Google Scholar] [CrossRef]
- Reichmann, H. Dopamine agonist therapy in advanced Parkinson’s disease. J. Mov. Disord. 2009, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Zeng, W.; Du, G.; Chen, J.; Zhou, J. Site-directed mutagenesis to improve the thermostability of tyrosine phenol-lyase. J. Biotechnol. 2020, 310, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Thyer, R.; d’Oelsnitz, S.; Blevins, M.S.; Klein, D.R.; Brodbelt, J.S.; Ellington, A.D. Directed evolution of an improved aminoacyl-tRNA synthetase for incorporation of L-3, 4-dihydroxyphenylalanine (L-DOPA). Angew. Chem. 2021, 60, 14811–14816. [Google Scholar] [CrossRef] [PubMed]
- Kitaoka, M.; Tian, J.; Nishimoto, M. Novel putative galactose operon involving lacto-N-biose phosphorylase in Bifidobacterium longum. Appl. Environ. Microbiol. 2005, 71, 3158–3162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hess, H. Toward rational design of high-efficiency enzyme cascades. ACS Catal. 2017, 7, 6018–6027. [Google Scholar] [CrossRef]
- Steiner, K.; Schwab, H. Recent advances in rational approaches for enzyme engineering. Comput. Struct. Biotechnol. J. 2012, 2, e201209010. [Google Scholar] [CrossRef]
- Yang, Q.; Tang, B.; Li, S. Improving the thermostability of α-amylase from Rhizopus oryzae by rational design. Chin. J. Biotechnol. 2018, 34, 1117–1127. [Google Scholar]
- Korkegian, A.; Black, M.E.; Baker, D.; Stoddard, B.L. Computational thermostabilization of an enzyme. Science 2005, 308, 857–860. [Google Scholar] [CrossRef][Green Version]
- Kim, H.S.; Le, Q.A.T.; Kim, Y.H. Development of thermostable lipase B from Candida antarctica (CalB) through in silico design employing B-factor and RosettaDesign. Enzym. Microb. Technol. 2010, 47, 1–5. [Google Scholar] [CrossRef]
- Shirke, A.N.; Basore, D.; Butterfoss, G.L.; Bonneau, R.; Bystroff, C.; Gross, R.A. Toward rational thermostabilization of Aspergillus oryzae cutinase: Insights into catalytic and structural stability. Proteins Struct. Funct. Bioinform. 2016, 84, 60–72. [Google Scholar] [CrossRef]
- Lutz, S. Beyond directed evolution—Semi-rational protein engineering and design. Curr. Opin. Biotechnol. 2010, 21, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Pongsupasa, V.; Anuwan, P.; Maenpuen, S.; Wongnate, T. Rational-Design Engineering to Improve Enzyme Thermostability. In Enzyme Engineering; Springer: New York, NY, USA, 2022; pp. 159–178. [Google Scholar]
- Yang, H.; Liu, L.; Li, J.; Chen, J.; Du, G. Rational design to improve protein thermostability: Recent advances and prospects. ChemBioEng Rev. 2015, 2, 87–94. [Google Scholar] [CrossRef]
- Yang, M.; Yang, S.-X.; Liu, Z.-M.; Li, N.-N.; Li, L.; Mou, H.-J. Rational design of alginate lyase from Microbulbifer sp. Q7 to improve thermal stability. Mar. Drugs 2019, 17, 378. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, L.; Liang, Z.; Zhang, Y. Protein-imprinted materials: Rational design, application and challenges. Anal. Bioanal. Chem. 2012, 403, 2173–2183. [Google Scholar] [CrossRef]
- Sutherland, T.D.; Huson, M.G.; Rapson, T.D. Rational design of new materials using recombinant structural proteins: Current state and future challenges. J. Struct. Biol. 2018, 201, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Wijma, H.J.; Floor, R.J.; Jekel, P.A.; Baker, D.; Marrink, S.J.; Janssen, D.B. Computationally designed libraries for rapid enzyme stabilization. Protein Eng. Des. Sel. 2014, 27, 49–58. [Google Scholar] [CrossRef]
- Gu, J.; Bai, F.; Li, H.; Wang, X. A generic force field for protein coarse-grained molecular dynamics simulation. Int. J. Mol. Sci. 2012, 13, 14451–14469. [Google Scholar] [CrossRef]
- Goldenzweig, A.; Goldsmith, M.; Hill, S.E.; Gertman, O.; Laurino, P.; Ashani, Y.; Dym, O.; Unger, T.; Albeck, S.; Prilusky, J.; et al. Automated structure-and sequence-based design of proteins for high bacterial expression and stability. Mol. Cell 2016, 63, 337–346. [Google Scholar] [CrossRef]
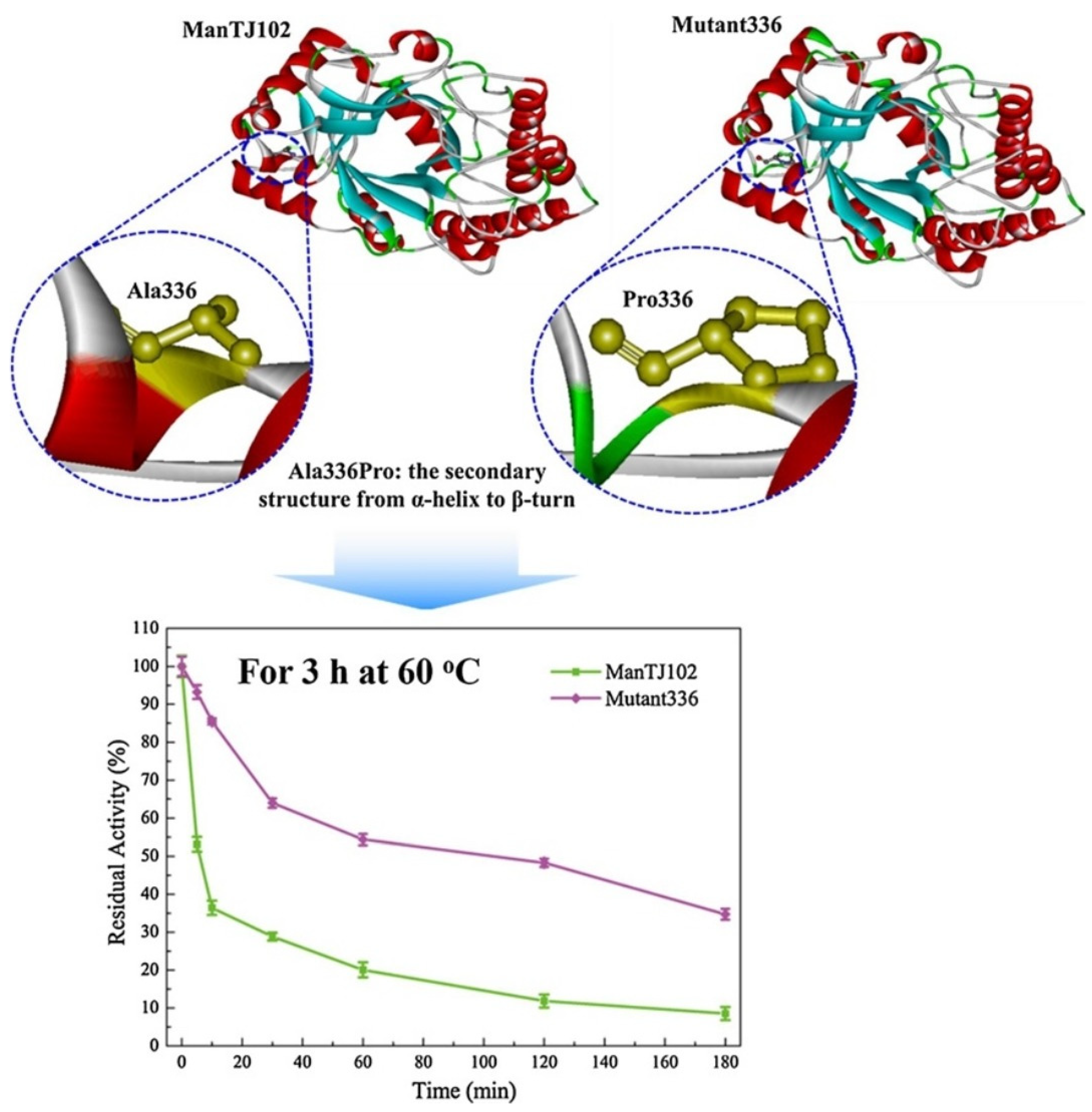
- Wang, X.-C.; You, S.-P.; Zhang, J.-X.; Dai, Y.-M.; Zhang, C.-Y.; Qi, W.; Dou, T.-Y.; Su, R.-X.; He, Z.-M. Rational design of a thermophilic β-mannanase fromBacillus subtilis TJ-102 to improve its thermostability. Enzym. Microb. Technol. 2018, 118, 50–56. [Google Scholar] [CrossRef]
- Heinzelman, P.; Snow, C.D.; Wu, I.; Nguyen, C.; Villalobos, A.; Govindarajan, S.; Minshull, J.; Arnold, F.H. A family of thermostable fungal cellulases created by structure-guided recombination. Proc. Natl. Acad. Sci. USA 2009, 106, 5610–5615. [Google Scholar] [CrossRef]
- Xu, P.; Ni, Z.-F.; Zong, M.-H.; Ou, X.-Y.; Yang, J.-G.; Lou, W.-Y. Improving the thermostability and activity of Paenibacillus pasadenensis chitinase through semi-rational design. Int. J. Biol. Macromol. 2020, 150, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Spadiut, O.; Leitner, C.; Salaheddin, C.; Varga, B.; Vertessy, B.G.; Tan, T.C.; Divne, C.; Haltrich, D. Improving thermostability and catalytic activity of pyranose 2-oxidase from Trametes multicolor by rational and semi-rational design. FEBS J. 2009, 276, 776–792. [Google Scholar] [CrossRef] [PubMed]
- Su, W.-B.; Zhu, C.-Y.; Zhou, H.-P.; Gao, J.; Zhang, Y.-W. A single site mutation significantly improves the thermostability and activity of heparinase I from Bacteroides eggerthii. Biocatal. Biotransform. 2021, 39, 1–7. [Google Scholar] [CrossRef]


| Application | Stabilizer/Enzyme | Notes | Ref |
|---|---|---|---|
| Filter cake removal | Chelant/enzyme | The system was stable and effective to break hydroxypropyl starch and xanthan gum | [15] |
| Fracturing fluid | Lignosulfonates/enzyme (mannanase) | Efficient breaking of guar-based fluid at high pH (10.5) and high temperature (160 °F) | [16] |
| Fracturing fluid | Polyelectrolyte complex nanoparticles (enzyme carrier) | Fracture conductivity enhanced compared to the old technique due to the efficient removal of HPG polymer | [17] |
| Well stimulation | Enzymes as polymer breaker | Enzymes showed better polymer clean up compared to conventional breakers (sodium persulfate) and better well productivity | [18] |
| Mud cake removal | a-amylase system (old) and structurally reinforced a-Helix system (new) | The old enzyme caused damage at 212 °F and precipitated. The new system effectively hydrolyzed the biopolymer at high temperatures. | [19] |
| Fracturing fluid | Enzyme breaker | Efficient cleaning of polymer and long-term sustained production from the well. Low temperature, 100 °F | [20] |
| Gravel pack clean up | Enzyme breaker | The polymer was cleaned and the plugging of the gravel was reduced | [21] |
| Polymer and breaker adsorption | Enzyme | Care should be taken when using real rocks saturated with oil compared to cleaned rocks. Oil may affect enzyme activity. | [22] |
| Drilling fluid damage | Enzyme | Starch was broken using polymer linkage-specific enzymes. This process reduced the formation damage due to polymer in the drilling fluid. | [23] |
| Fracturing fluid | High-pH tolerant enzymes (modified hemicellulose) | The enzyme remains active at up to pH values of 11.5. Crosslinked guar was broken using this enzyme at a low temperature (120 °F). | [24] |
| Crosslinked fluids | Enzymes versus oxidative breakers | Enzyme breakers yielded better and homogenous breaking compared to oxidative breakers | [13] |
| Filter cake removal | linkage specific enzymes | The modified enzyme showed better polymer breaking compared to a mixture of generic hydrolytic enzymes. Amylases, cellulases, and glucosidases enzymes were very efficient in breaking the modified crosslinked starch in the filter cake during drilling operations. | [25] |
| Enzymes | Substrate | Applications | Findings | Ref |
|---|---|---|---|---|
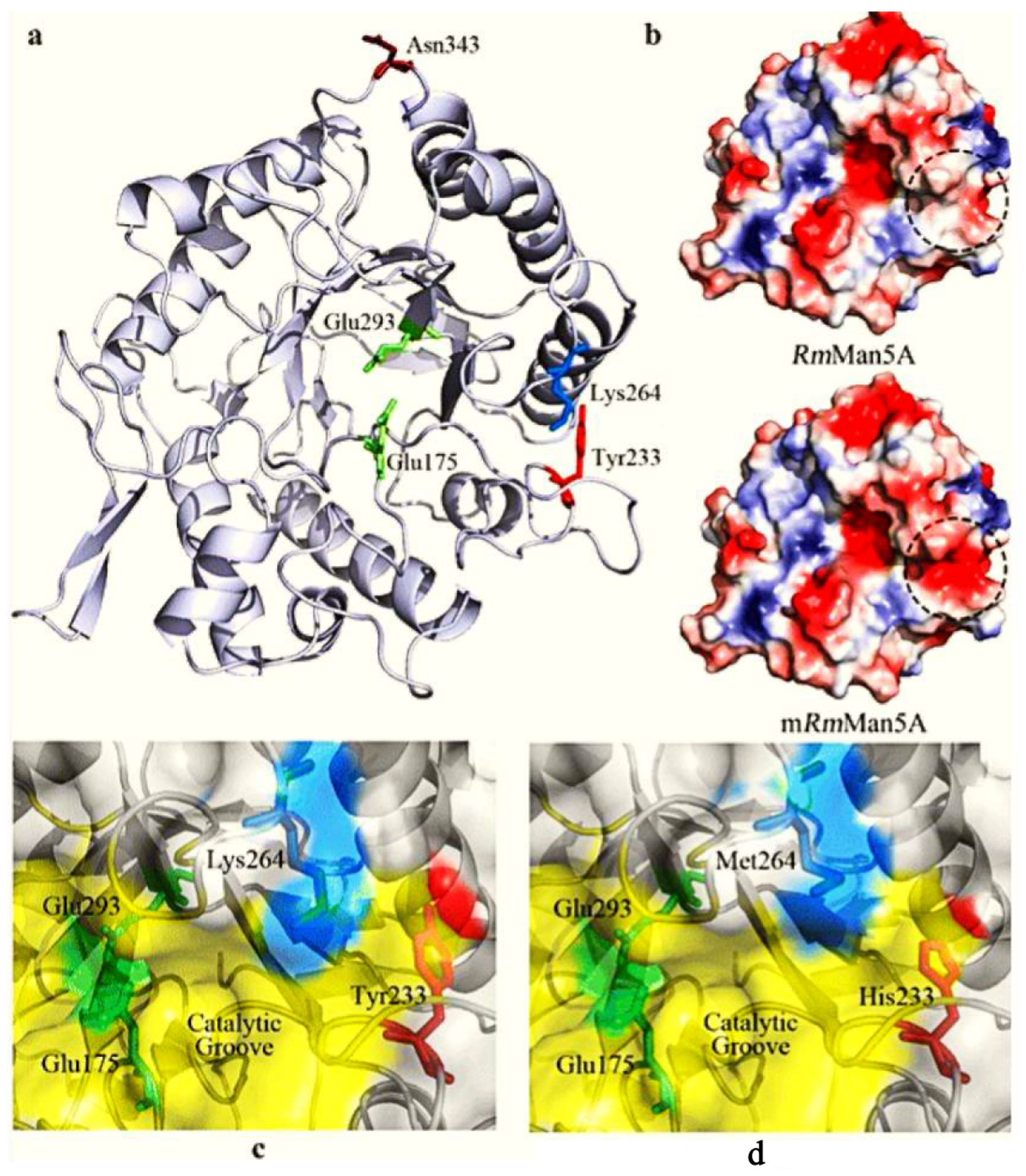
| Mannanase enzyme | β-1,4-glycosidic bonds | Upstream oil, Feed and medicine industry | Through direct evolution three mutations (Tyr23His, Lys264Met and Asn343Ser) were identified that, has increased their catalytic and thermostability. Through iterative mutagenesis, out of 240,000 clones isolated one mutant (ManM3-3) outperformed under high temperature. | [30,31] |
| Cellulase | β-1,4-glycosidic bonds, Cellulose | Enzyme breaker or Gel breaker in oil Industry, feed, textile Industry | With direct evolution two mutants, EGsl and EGs2 were generated with enhanced thermostability at 149 °F and 153 °F respectively, which exhibit 37 °F and 41 °F higher working temperature than wild type (112 °F). | [33] |
| Galacto-N-biose/lacto-N-biose I phosphorylase (GLNBP) | Lactose- N-biose I | Milk Industry, Food industry | That study increased the thermostability of GLNBP enzyme by 68 °F by creating the C236Y and D576V mutants. | [44] |
| Amylase | Carbohydrates | Food, textile and paper/pulp, Baking Industry | In this study total 7 single gene mutations were accumulated in thermostable mutant after four rounds of direct evolution. | [37] |
| Endo-B-1, 4-xylanase(XYnA) | Xylan | Biofuel production and pharmaceutical industry | Their study increased the thermostability of XynA enzyme, the four mutants’ exhibit higher thermal stability in the first generation produced through random mutagenesis. The 2B7-10 mutant produced in second generation out- performed than the wild type. | [38] |
| Phytase | Phytate | Feed industry | Three mutants were generated in first generation through direct evolution which has 84% higher activity than wild type. The subsequent second generation has increased the thermal stability. | [40] |
| Tyrosine phenol-lyase | Tyrosine | Pharmaceutical industry | The 25 mutants were generated through direct evolution, among these only two shows the higher thermostability than other. | [42] |
| Enzymes | Substrate | Applications | Findings | Ref |
|---|---|---|---|---|
| β-mannanase | The upstream oil industry, feed, and medicine | The mutant (mutant336 (A336 P) with enhanced thermostability generated through rational design. | [47] | |
| Amylase | Maltose | The upstream oil industry, feed, and medicine | The three generated mutations (G128L/K269L/G393P) through rational design have increased from 122 °F to 150 °F. | [48] |
| Cytosine deaminase | Cytosine | Anticancer | The three thermostable mutations were identified through rational design with Rosetta software that has increased the melting temperature (Tm) of enzyme up to 50 °F. | [49] |
| Lipase B | Triglycerides | Pharmaceutical and food industry | The rational design with computational modeling software Rosetta, that increases the Tm up to 45 °F. | [50] |
| Cutinase | Degrade the cuticle polymer | Pharmaceutical industry | The rational design has significantly improved the thermostability by 10-fold higher than the wild type. | [51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naeem, M.; Khalil, A.B.; Tariq, Z.; Mahmoud, M. A Review of Advanced Molecular Engineering Approaches to Enhance the Thermostability of Enzyme Breakers: From Prospective of Upstream Oil and Gas Industry. Int. J. Mol. Sci. 2022, 23, 1597. https://doi.org/10.3390/ijms23031597
Naeem M, Khalil AB, Tariq Z, Mahmoud M. A Review of Advanced Molecular Engineering Approaches to Enhance the Thermostability of Enzyme Breakers: From Prospective of Upstream Oil and Gas Industry. International Journal of Molecular Sciences. 2022; 23(3):1597. https://doi.org/10.3390/ijms23031597
Chicago/Turabian StyleNaeem, Muhammad, Amjad Bajes Khalil, Zeeshan Tariq, and Mohamed Mahmoud. 2022. "A Review of Advanced Molecular Engineering Approaches to Enhance the Thermostability of Enzyme Breakers: From Prospective of Upstream Oil and Gas Industry" International Journal of Molecular Sciences 23, no. 3: 1597. https://doi.org/10.3390/ijms23031597
APA StyleNaeem, M., Khalil, A. B., Tariq, Z., & Mahmoud, M. (2022). A Review of Advanced Molecular Engineering Approaches to Enhance the Thermostability of Enzyme Breakers: From Prospective of Upstream Oil and Gas Industry. International Journal of Molecular Sciences, 23(3), 1597. https://doi.org/10.3390/ijms23031597









