Synthetic Collagen-like Polymer That Undergoes a Sol–Gel Transition Triggered by O–N Acyl Migration at Physiological pH
Abstract
1. Introduction
2. Results and Discussion
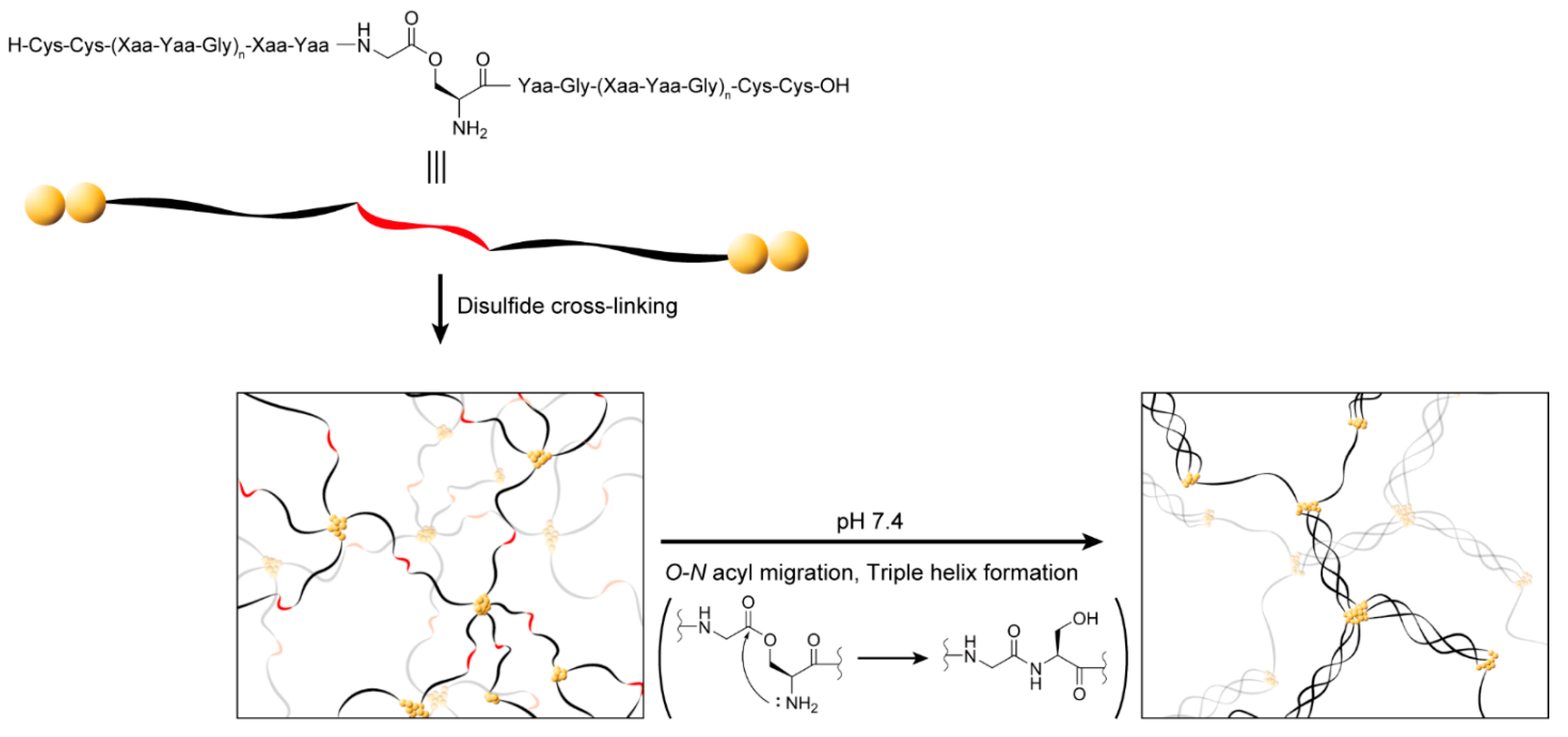
2.1. Design of a Collagen-like Peptide Polymer That Forms a Gel In Situ through a Sol–Gel Transition
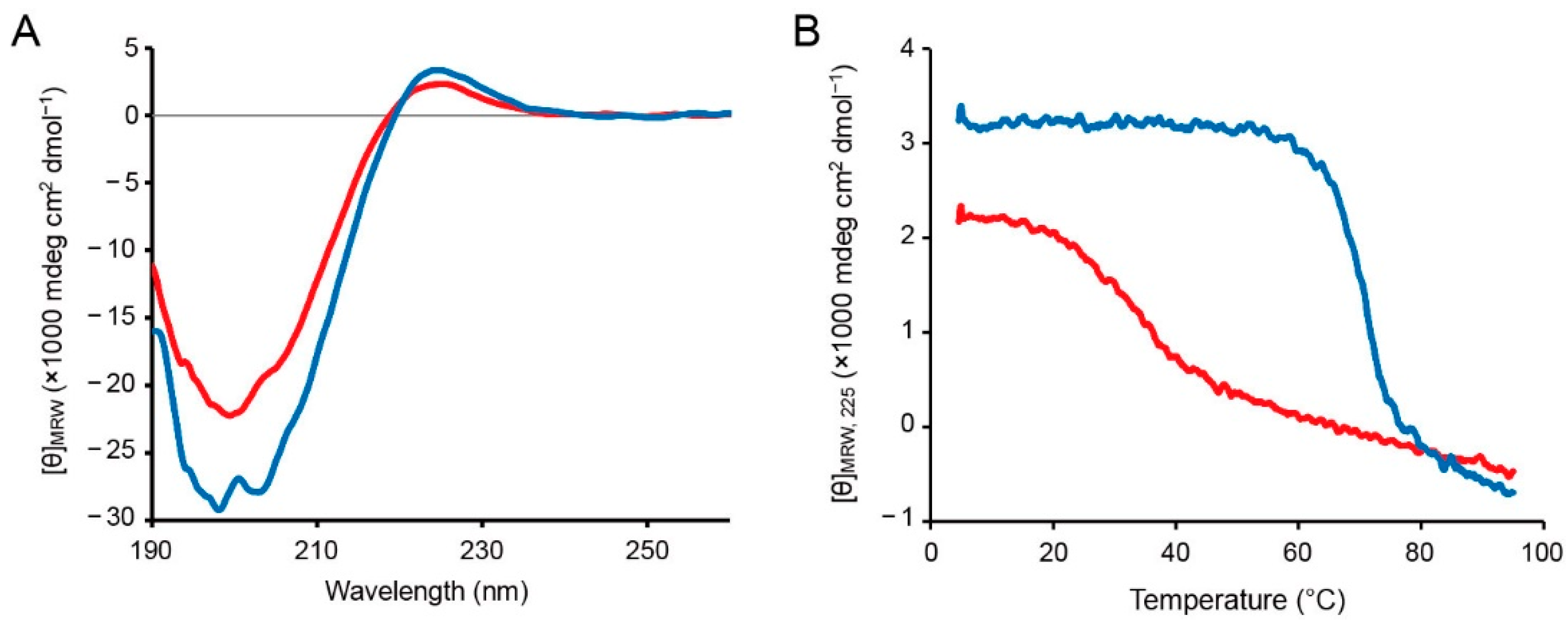
2.2. Synthesis and Structural Analysis of the Collagen-like Peptide Containing an O-Acyl Isopeptide Unit
2.3. Investigation of Gel Formation of the C2-Ester Polymer Induced by O–N Acyl Migration
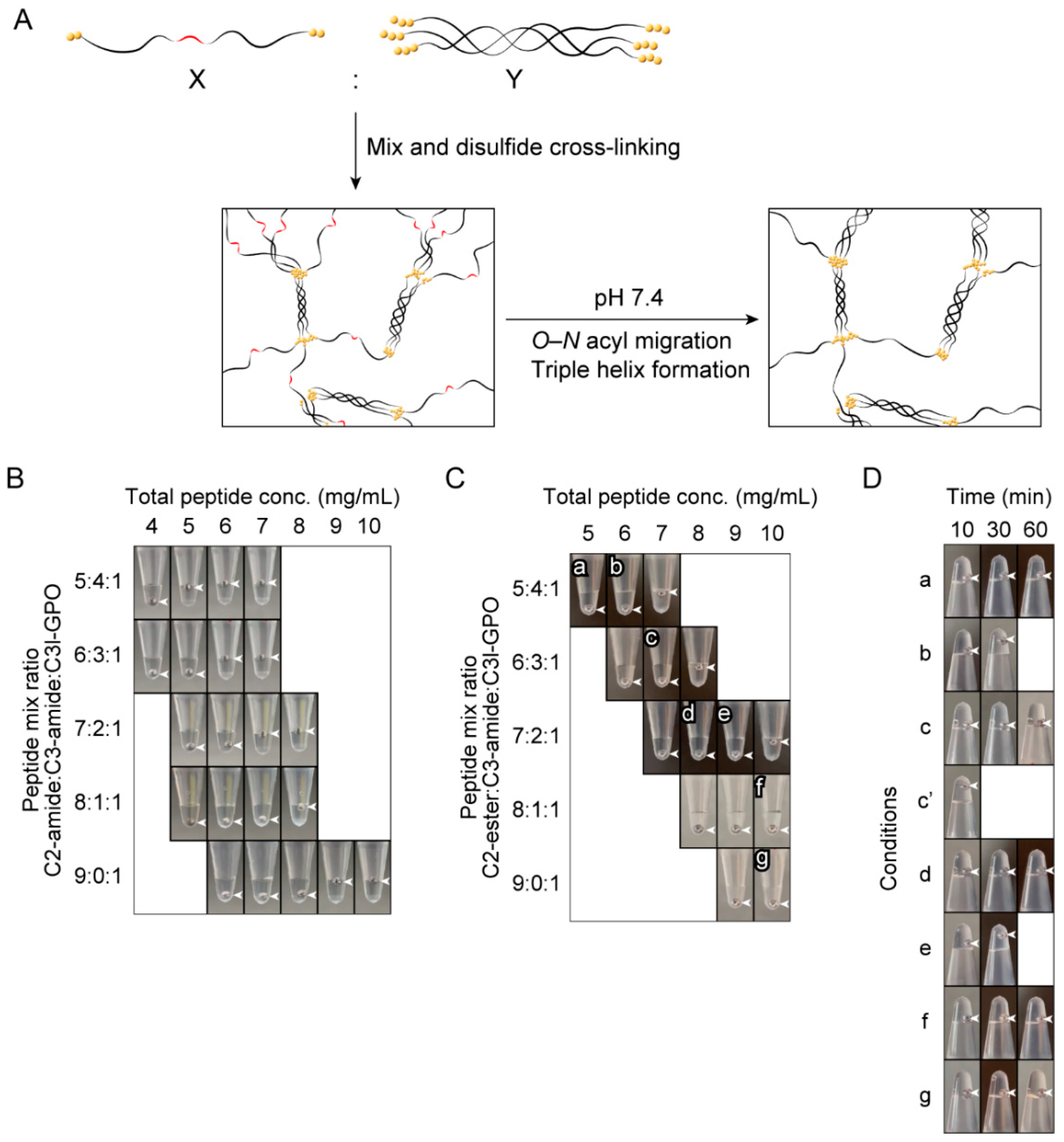
2.4. Investigation of the Gel-Forming Conditions of the Copolymers of C2-Ester and Triple-Helical Peptides
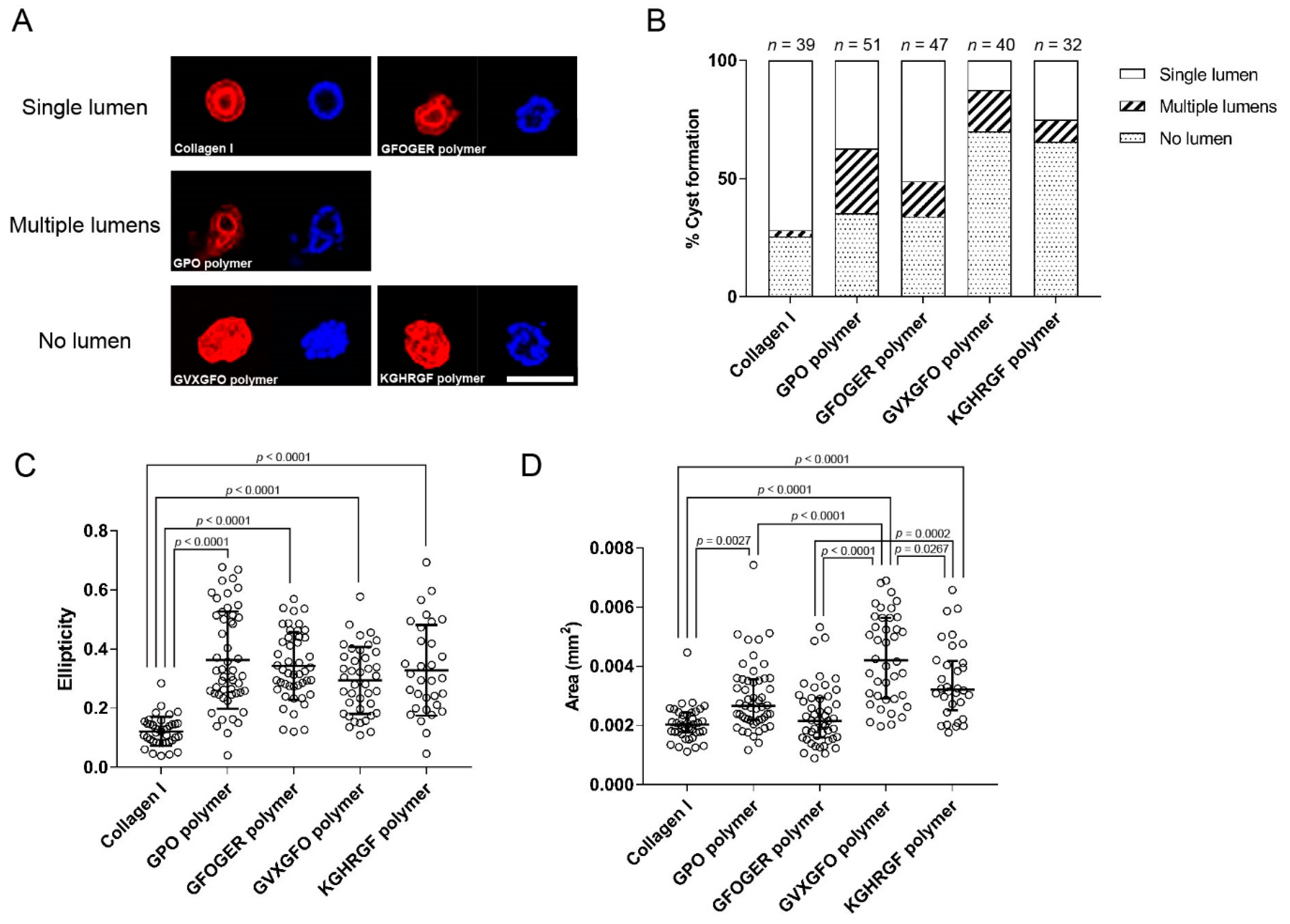
2.5. Application as a 3D Scaffold for Cell Culture
3. Materials and Methods
3.1. Cell Culture
3.2. Peptide Synthesis
3.3. CD Analysis
3.4. Preparation of the Peptide Polymers and Evaluation of Gel Formation
3.5. SEM
3.6. Three-Dimensional Cell Culture
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Beck, K.; Brodsky, B. Supercoiled protein motifs: The collagen triple-helix and the α-helical coiled coil. J. Struct. Biol. 1998, 122, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, K. Revisiting the Molecular Structure of Collagen. Connect. Tissue. Res. 2008, 49, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Persikov, A.V.; Ramshaw, J.A.; Kirkpatrick, A.; Brodsky, B. Amino acid propensities for the collagen triple-helix. Biochemistry 2000, 39, 14960–14967. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, D.A.; Tuckwell, D.S.; Eble, J.; Kühn, K.; Humphries, M.J. The integrin α1 A-domain is a ligand binding site for collagens and laminin. J. Biol. Chem. 1997, 272, 12311–12317. [Google Scholar] [CrossRef] [PubMed]
- Emsley, J.; Knight, C.G.; Farndale, R.W.; Barnes, M.J.; Liddington, R.C. Structural basis of collagen recognition by integrin α2β1. Cell 2000, 101, 47–56. [Google Scholar] [CrossRef]
- Konitsiotis, A.D.; Raynal, N.; Bihan, D.; Hohenester, E.; Farndale, R.W.; Leitinger, B. Characterization of high affinity binding motifs for the discoidin domain receptor DDR2 in collagen. J. Biol. Chem. 2008, 283, 6861–6868. [Google Scholar] [CrossRef]
- Lisman, T.; Raynal, N.; Groeneveld, D.; Maddox, B.; Peachey, A.R.; Huizinga, E.G.; de Groot, P.G.; Farndale, R.W. A single high-affinity binding site for von Willebrand factor in collagen III, identified using synthetic triple-helical peptides. Blood 2006, 108, 3753–3756. [Google Scholar] [CrossRef] [PubMed]
- Giudici, C.; Raynal, N.; Wiedemann, H.; Cabral, W.A.; Marini, J.C.; Timpl, R.; Bächinger, H.P.; Farndale, R.W.; Sasaki, T.; Tenni, R. Mapping of SPARC/BM-40/osteonectin-binding sites on fibrillar collagens. J. Biol. Chem. 2008, 283, 19551–19560. [Google Scholar] [CrossRef]
- Hohenester, E.; Sasaki, T.; Giudici, C.; Farndale, R.W.; Bächinger, H.P. Structural basis of sequence-specific collagen recognition by SPARC. Proc. Natl. Acad. Sci. USA 2008, 105, 18273–18277. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, S.M.; Guy, C.A.; Fields, G.B.; San Antonio, J.D. Defining the domains of type I collagen involved in heparin- binding and endothelial tube formation. Proc. Natl. Acad. Sci USA 1998, 95, 7275–7280. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, A.; Okano-Kosugi, H.; Yamazaki, C.M.; Koide, T. Pigment epithelium-derived factor (PEDF) shares binding sites in collagen with heparin/heparan sulfate proteoglycans. J. Biol. Chem. 2011, 286, 26364–26374. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, K.; Yoshida, T.; Maruno, T.; Oki, H.; Ohkubo, T.; Koide, T.; Kobayashi, T. Spatiotemporal regulation of PEDF signaling by type I collagen remodeling. Proc. Natl. Acad. Sci. USA 2020, 117, 11450–11458. [Google Scholar] [CrossRef] [PubMed]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Luo, T.; Kiick, K.L. Collagen-like peptides and peptide-polymer conjugates in the design of assembled materials. Eur. Polym. J. 2013, 49, 2998–3009. [Google Scholar] [CrossRef]
- Valot, L.; Maumus, M.; Brunel, L.; Martinez, J.; Amblard, M.; Noël, D.; Mehdi, A.; Subra, G. A Collagen-Mimetic Organic-Inorganic Hydrogel for Cartilage Engineering. Gels 2021, 7, 73. [Google Scholar] [CrossRef]
- Kotch, F.W.; Raines, R.T. Self-assembly of synthetic collagen triple helices. Proc. Natl. Acad. Sci. USA 2006, 103, 3028–3033. [Google Scholar] [CrossRef]
- Koide, T.; Homma, D.L.; Asada, S.; Kitagawa, K. Self-complementary peptides for the formation of collagen-like triple helical supramolecules. Bioorg. Med. Chem. Lett. 2005, 15, 5230–5233. [Google Scholar] [CrossRef]
- Yamazaki, C.M.; Asada, S.; Kitagawa, K.; Koide, T. Artificial collagen gels via self-assembly of de novo designed peptides. Biopolymers 2008, 90, 816–823. [Google Scholar] [CrossRef]
- Yamazaki, C.M.; Kadoya, Y.; Hozumi, K.; Okano-Kosugi, H.; Asada, S.; Kitagawa, K.; Nomizu, M.; Koide, T. A collagen-mimetic triple helical supramolecule that evokes integrin-dependent cell responses. Biomaterials 2010, 31, 1925–1934. [Google Scholar] [CrossRef]
- Rele, S.; Song, Y.; Apkarian, R.P.; Qu, Z.; Conticello, V.P.; Chaikof, E.L. D-periodic collagen-mimetic microfibers. J. Am. Chem. Soc. 2007, 129, 14780–14787. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, L.E.R.; Fallas, J.A.; Bakota, E.L.; Kang, M.K.; Hartgerink, J.D. Multi-hierarchical self-assembly of a collagen mimetic peptide from triple helix to nanofibre and hydrogel. Nat. Chem. 2011, 3, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Ichise, S.F.; Takeuchi, S.; Aoki, S.; Kuroda, K.C.; Nose, H.; Masuda, R.; Koide, T. Development of a collagen-like peptide polymer via end-to-end disulfide cross-linking and its application as a biomaterial. Acta Biomater. 2019, 94, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.P.; Wu, C.R.; Liu, W.; Zhang, J.W. Disulfide bond formation in peptides by dimethyl sulfoxide. Scope and applications. J. Am. Chem. Soc. 1991, 113, 6657–6662. [Google Scholar] [CrossRef]
- Sohma, Y.; Hayashi, Y.; Skwarczynski, M.; Hamada, Y.; Sasaki, M.; Kimura, T.; Kiso, Y. O-N intramolecular acyl migration reaction in the development of prodrugs and the synthesis of difficult sequence-containing bioactive peptides. Biopolymers 2004, 76, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, A.; Sohma, Y.; Hirayama, Y.; Mukai, H.; Kimura, T.; Hayashi, Y.; Matsuzaki, K.; Kiso, Y. “Click Peptide”: pH-Triggered in Situ Production and Aggregation of Monomer Aβ1–42. Chembiochem 2009, 10, 710–715. [Google Scholar] [CrossRef]
- Wang, H.; Kakizawa, T.; Taniguchi, A.; Mizuguchi, T.; Kimura, T.; Kiso, Y. Synthesis of amyloid β peptide 1-42 (E22Δ) click peptide: pH-triggered in situ production of its native form. Bioorg. Med. Chem. 2009, 17, 4881–4887. [Google Scholar] [CrossRef]
- Kanai, S.; Machida, K.; Masuda, R.; Koide, T. Peptide precursors that acquire denatured collagen-hybridizing ability by O-to-N acyl migration at physiological pH. Org. Biomol. Chem. 2020, 18, 2823–2827. [Google Scholar] [CrossRef]
- Long, C.G.; Braswell, E.; Zhu, D.; Apigo, J.; Baum, J.; Brodsky, B. Characterization of collagen-like peptides containing interruptions in the repeating Gly-X-Y sequence. Biochemistry 1993, 32, 11688–11695. [Google Scholar] [CrossRef]
- Kar, K.; Wang, Y.; Brodsky, B. Sequence dependence of kinetics and morphology of collagen model peptide self-assembly into higher order structures. Protein Sci. 2008, 17, 1086–1095. [Google Scholar] [CrossRef]
- Wang, A.Z.; Ojakian, G.K.; Nelson, W.J. Steps in the morphogenesis of a polarized epithelium. I. Uncoupling the roles of cell-cell and cell-substratum contact in establishing plasma membrane polarity in multicellular epithelial (MDCK) cysts. J. Cell Sci. 1990, 95, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Z.; Ojakian, G.K.; Nelson, W.J. Steps in the morphogenesis of a polarized epithelium. II. Disassembly and assembly of plasma membrane domains during reversal of epithelial cell polarity in multicellular epithelial (MDCK) cysts. J. Cell Sci. 1990, 95, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Bihan, D.; Chang, F.; Huang, P.H.; Farndale, R.W.; Leitinger, B. Discoidin Domain Receptors Promote α1β1- and α2β1-Integrin Mediated Cell Adhesion to Collagen by Enhancing Integrin Activation. PLoS ONE 2012, 7, e52209. [Google Scholar] [CrossRef] [PubMed]
- Myllymäki, S.M.; Teräväinen, T.P.; Manninen, A. Two distinct integrin-mediated mechanisms contribute to apical lumen formation in epithelial cells. PLoS ONE 2011, 6, e19453. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Datta, A.; Leroy, P.; O’Brien, L.E.; Mak, G.; Jou, T.; Matlin, K.S.; Mostov, K.E.; Zegers, M.M.P. β1-integrin orients epithelial polarity via Rac1 and laminin. Mol. Biol. Cell 2005, 16, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.; Enemchukwu, N.O.; Khaja, S.D.; Murthy, N.; Mantalaris, A.; García, A.J. Bioadhesive hydrogel microenvironments to modulate epithelial morphogenesis. Biomaterials 2008, 29, 2637–2645. [Google Scholar] [CrossRef][Green Version]
- Enemchukwu, N.O.; Cruz-Acuña, R.; Bongiorno, T.; Johnson, C.T.; García, J.R.; Sulchek, T.; García, A.J. Synthetic matrices reveal contributions of ECM biophysical and biochemical properties to epithelial morphogenesis. J. Cell. Biol. 2016, 212, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Yoshiya, T.; Kawashima, H.; Sohma, Y.; Kimura, T.; Kiso, Y. O-acyl isopeptide method: Efficient synthesis of isopeptide segment and application to racemization-free segment condensation. Org. Biomol. Chem. 2009, 7, 2894–2904. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]

| Peptide | Sequence |
|---|---|
| C2-ester | H-Cys-Cys-(Host)-Pro-Hyp-Z-Hyp-Gly-(Host)-Cys-Cys-OH |
| C2-amide | H-Cys-Cys-(Host)-Pro-Hyp-Gly-Ser-Hyp-Gly-(Host)-Cys-Cys-OH |
| C3-amide | H-Cys-Cys-Cys-(Host)-Pro-Hyp-Gly-Pro-Arg-Gly-(Host)-Cys-Cys-Cys-OH |
| C3l-GPO | H-Cys-Cys-Cys-(Host)-Pro-Pro-Gly-Pro-Pro-Gly-Pro-Arg-Gly-Pro-Pro-Gly-(Host)-Cys-Cys-Cys-OH |
| C3l-GFOGER | H-Cys-Cys-Cys-(Host)-Pro-Pro-Gly-Phe-Hyp-Gly-Glu-Arg-Gly-Pro-Pro-Gly-(Host)-Cys-Cys-Cys-OH |
| C3l-GVXGFO | H-Cys-Cys-Cys-(Host)-Pro-Arg-Gly-Gln-Hyp-Gly-Val-Nle-Gly-Phe-Hyp-Gly-(Host)-Cys-Cys-Cys-OH |
| C3l-KGHRGF | H-Cys-Cys-Cys-(Host)-Pro-Lys-Gly-His-Arg-Gly-Phe-Ser-Gly-Leu-Hyp-Gly-(Host)-Cys-Cys-Cys-OH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ichise, S.F.; Koide, T. Synthetic Collagen-like Polymer That Undergoes a Sol–Gel Transition Triggered by O–N Acyl Migration at Physiological pH. Int. J. Mol. Sci. 2022, 23, 1584. https://doi.org/10.3390/ijms23031584
Ichise SF, Koide T. Synthetic Collagen-like Polymer That Undergoes a Sol–Gel Transition Triggered by O–N Acyl Migration at Physiological pH. International Journal of Molecular Sciences. 2022; 23(3):1584. https://doi.org/10.3390/ijms23031584
Chicago/Turabian StyleIchise, Shinichiro F., and Takaki Koide. 2022. "Synthetic Collagen-like Polymer That Undergoes a Sol–Gel Transition Triggered by O–N Acyl Migration at Physiological pH" International Journal of Molecular Sciences 23, no. 3: 1584. https://doi.org/10.3390/ijms23031584
APA StyleIchise, S. F., & Koide, T. (2022). Synthetic Collagen-like Polymer That Undergoes a Sol–Gel Transition Triggered by O–N Acyl Migration at Physiological pH. International Journal of Molecular Sciences, 23(3), 1584. https://doi.org/10.3390/ijms23031584






