Mediterranean Diet as a Shield against Male Infertility and Cancer Risk Induced by Environmental Pollutants: A Focus on Flavonoids
Abstract
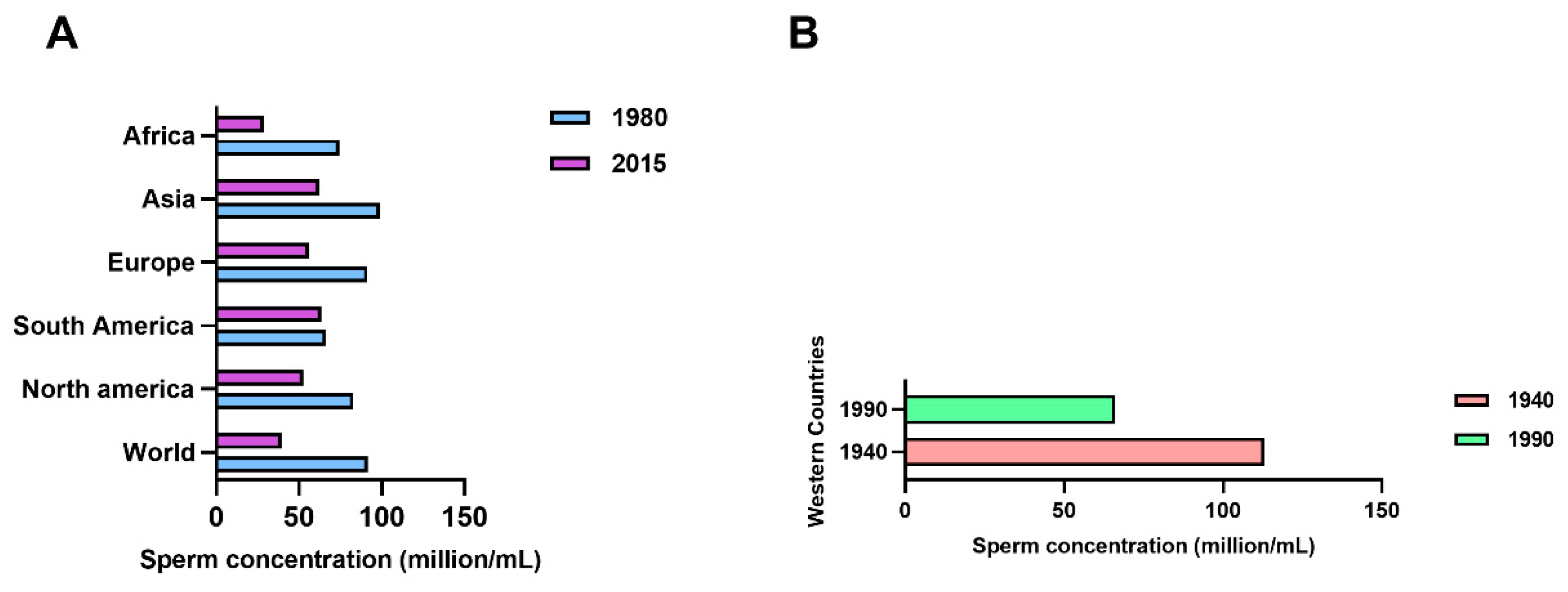
:1. Introduction
2. Mediterranean Diet
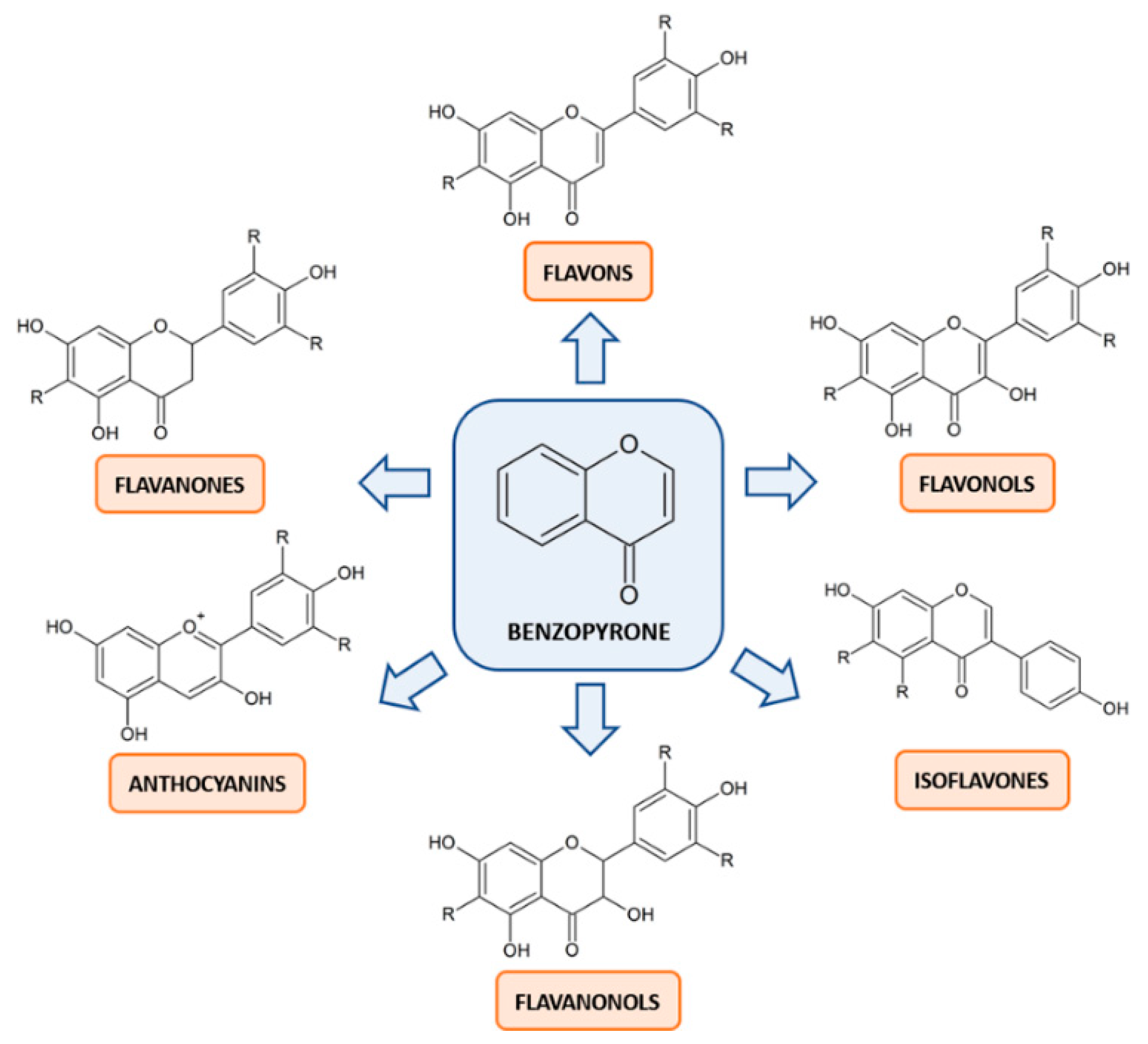
3. Flavonoids: Mechanisms of Action in Cancer
4. Flavonoids: Mechanisms of Action in Male Infertility
5. Heavy Metals
5.1. Cadmium (Cd)
5.2. Mercury (Hg)
5.3. Inorganic Arsenic (iAs)
6. Bisphenols
7. Polycyclic Aromatic Hydrocarbons
8. Dioxins
9. Phthalates
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morgan, H.L.; Watkins, A.J. Transgenerational Impact of Environmental Change. Adv. Exp. Med. Biol. 2019, 1200, 71–89. [Google Scholar] [CrossRef] [PubMed]
- Mortality, G.B.D.; Causes of Death, C. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar] [CrossRef]
- Ji, W.; Zhao, B. Estimating mortality derived from indoor exposure to particles of outdoor origin. PLoS ONE 2015, 10, e0124238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabir, E.R.; Rahman, M.S.; Rahman, I. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 2015, 40, 241–258. [Google Scholar] [CrossRef]
- Singh, R.; Kaur, B.; Kalina, I.; Popov, T.A.; Georgieva, T.; Garte, S.; Binkova, B.; Sram, R.J.; Taioli, E.; Farmer, P.B. Effects of environmental air pollution on endogenous oxidative DNA damage in humans. Mutat. Res. 2007, 620, 71–82. [Google Scholar] [CrossRef]
- Kruk, J.; Aboul-Enein, H.Y. Reactive Oxygen and Nitrogen Species in Carcinogenesis: Implications of Oxidative Stress on the Progression and Development of Several Cancer Types. Mini Rev. Med. Chem. 2017, 17, 904–919. [Google Scholar] [CrossRef]
- Hybertson, B.M.; Gao, B.; Bose, S.K.; McCord, J.M. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol. Asp. Med. 2011, 32, 234–246. [Google Scholar] [CrossRef]
- Williams, M.A.; Rangasamy, T.; Bauer, S.M.; Killedar, S.; Karp, M.; Kensler, T.W.; Yamamoto, M.; Breysse, P.; Biswal, S.; Georas, S.N. Disruption of the transcription factor Nrf2 promotes pro-oxidative dendritic cells that stimulate Th2-like immunoresponsiveness upon activation by ambient particulate matter. J. Immunol. 2008, 181, 4545–4559. [Google Scholar] [CrossRef] [Green Version]
- Montano, L. Reproductive biomarkers as early indicators for assessing environmental health risk. In Toxic Waste Management and Health Risk; Marfe, G.D.S., Ed.; Bentham Science Publisher: Sharjah, United Arab Emirates, 2020. [Google Scholar] [CrossRef]
- Montano, L.; Bergamo, P.; Andreassi, M.G.L. The role of human semen as an early and reliable tool of environmental impact assessment on human health. In Spermatozoa-Facts and Perspectives; Meccariello, R., Chianese, R., Eds.; InTechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Carlsen, E.; Giwercman, A.; Keiding, N.; Skakkebaek, N.E. Evidence for decreasing quality of semen during past 50 years. BMJ 1992, 305, 609–613. [Google Scholar] [CrossRef] [Green Version]
- Travison, T.G.; Araujo, A.B.; O’Donnell, A.B.; Kupelian, V.; McKinlay, J.B. A population-level decline in serum testosterone levels in American men. J. Clin. Endocrinol. Metab. 2007, 92, 196–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montano, L.; Donato, F.; Bianco, P.M.; Lettieri, G.; Guglielmino, A.; Motta, O.; Bonapace, I.M.; Piscopo, M. Semen quality as a potential susceptibility indicator to SARS-CoV-2 insults in polluted areas. Environ. Sci. Pollut. Res. Int. 2021, 28, 37031–37040. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Dutta, S.; Krajewska-Kulak, E. The Disappearing Sperms: Analysis of Reports Published Between 1980 and 2015. Am. J. Mens Health 2017, 11, 1279–1304. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Gong, T.T.; Jiang, Y.T.; Zhang, S.; Zhao, Y.H.; Wu, Q.J. Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories, 1990–2017: Results from a global burden of disease study, 2017. Aging (Albany NY) 2019, 11, 10952–10991. [Google Scholar] [CrossRef]
- Sharpe, R.M. The ‘oestrogen hypothesis’—Where do we stand now? Int. J. Androl. 2003, 26, 2–15. [Google Scholar] [CrossRef]
- Krausz, C. Male infertility: Pathogenesis and clinical diagnosis. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 271–285. [Google Scholar] [CrossRef]
- Lymperi, S.; Giwercman, A. Endocrine disruptors and testicular function. Metabolism 2018, 86, 79–90. [Google Scholar] [CrossRef]
- Ghazarian, A.A.; Kelly, S.P.; Altekruse, S.F.; Rosenberg, P.S.; McGlynn, K.A. Future of testicular germ cell tumor incidence in the United States: Forecast through 2026. Cancer 2017, 123, 2320–2328. [Google Scholar] [CrossRef] [Green Version]
- Scott, A.R.; Stoltzfus, K.C.; Tchelebi, L.T.; Trifiletti, D.M.; Lehrer, E.J.; Rao, P.; Bleyer, A.; Zaorsky, N.G. Trends in Cancer Incidence in US Adolescents and Young Adults, 1973–2015. JAMA Netw. Open 2020, 3, e2027738. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purdue, M.P.; Devesa, S.S.; Sigurdson, A.J.; McGlynn, K.A. International patterns and trends in testis cancer incidence. Int. J. Cancer 2005, 115, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Chia, V.M.; Quraishi, S.M.; Devesa, S.S.; Purdue, M.P.; Cook, M.B.; McGlynn, K.A. International trends in the incidence of testicular cancer, 1973-2002. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1151–1159. [Google Scholar] [CrossRef] [Green Version]
- Richiardi, L.; Bellocco, R.; Adami, H.O.; Torrang, A.; Barlow, L.; Hakulinen, T.; Rahu, M.; Stengrevics, A.; Storm, H.; Tretli, S.; et al. Testicular cancer incidence in eight northern European countries: Secular and recent trends. Cancer Epidemiol. Biomark. Prev. 2004, 13, 2157–2166. [Google Scholar]
- Beiki, O.; Granath, F.; Allebeck, P.; Akre, O.; Moradi, T. Subtype-specific risk of testicular tumors among immigrants and their descendants in Sweden, 1960 to 2007. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1053–1065. [Google Scholar] [CrossRef] [Green Version]
- Greene, M.H.; Kratz, C.P.; Mai, P.L.; Mueller, C.; Peters, J.A.; Bratslavsky, G.; Ling, A.; Choyke, P.M.; Premkumar, A.; Bracci, J.; et al. Familial testicular germ cell tumors in adults: 2010 summary of genetic risk factors and clinical phenotype. Endocr. Relat. Cancer 2010, 17, R109–R121. [Google Scholar] [CrossRef] [Green Version]
- Parkin, D.M.; Ferlay, J.; Curado, M.P.; Bray, F.; Edwards, B.; Shin, H.R.; Forman, D. Fifty years of cancer incidence: CI5 I-IX. Int. J. Cancer 2010, 127, 2918–2927. [Google Scholar] [CrossRef]
- Rosen, A.; Jayram, G.; Drazer, M.; Eggener, S.E. Global trends in testicular cancer incidence and mortality. Eur. Urol. 2011, 60, 374–379. [Google Scholar] [CrossRef]
- Turnbull, C.; Rahman, N. Genome-wide association studies provide new insights into the genetic basis of testicular germ-cell tumour. Int. J. Androl. 2011, 34, e86–e96. [Google Scholar] [CrossRef]
- Bray, F.; Richiardi, L.; Ekbom, A.; Pukkala, E.; Cuninkova, M.; Moller, H. Trends in testicular cancer incidence and mortality in 22 European countries: Continuing increases in incidence and declines in mortality. Int. J. Cancer 2006, 118, 3099–3111. [Google Scholar] [CrossRef]
- Lorenzetti, S.; Marcoccia, D.; Mantovani, A. Biomarkers of effect in endocrine disruption: How to link a functional assay to an adverse outcome pathway. Ann. Ist. Super. Sanita 2015, 51, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.V.; Yamada, H.Y.; Yao, Y.; Dai, W. Enhanced genomic instabilities caused by deregulated microtubule dynamics and chromosome segregation: A perspective from genetic studies in mice. Carcinogenesis 2009, 30, 1469–1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondal, G.; Ohashi, A.; Yang, L.; Rowley, M.; Couch, F.J. Tex14, a Plk1-regulated protein, is required for kinetochore-microtubule attachment and regulation of the spindle assembly checkpoint. Mol. Cell 2012, 45, 680–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, S.; Zhen, S.; Roumiantsev, S.; McDonald, L.T.; Yuan, G.C.; Orkin, S.H. Differential roles of Sall4 isoforms in embryonic stem cell pluripotency. Mol. Cell. Biol. 2010, 30, 5364–5380. [Google Scholar] [CrossRef] [Green Version]
- Grassetti, D.; Giannandrea, F.; Paoli, D.; Masciandaro, P.; Figura, V.; Carlini, T.; Rizzo, F.; Lombardo, F.; Lenzi, A.; Gandini, L. Androgen receptor polymorphisms and testicular cancer risk. Andrology 2015, 3, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Tandstad, T.; Dahl, O.; Cohn-Cedermark, G.; Cavallin-Stahl, E.; Stierner, U.; Solberg, A.; Langberg, C.; Bremnes, R.M.; Laurell, A.; Wijkstrom, H.; et al. Risk-adapted treatment in clinical stage I nonseminomatous germ cell testicular cancer: The SWENOTECA management program. J. Clin. Oncol. 2009, 27, 2122–2128. [Google Scholar] [CrossRef]
- Magelssen, H.; Brydoy, M.; Fossa, S.D. The effects of cancer and cancer treatments on male reproductive function. Nat. Clin. Pract. Urol. 2006, 3, 312–322. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; McGlynn, K.A.; McCorkle, R.; Li, Y.; Erickson, R.L.; Ma, S.; Niebuhr, D.W.; Zhang, G.; Zhang, Y.; Bai, Y.; et al. Sexual functioning among testicular cancer survivors: A case-control study in the U.S. J. Psychosom. Res. 2012, 73, 68–73. [Google Scholar] [CrossRef] [Green Version]
- Global Burden of Disease Cancer; Fitzmaurice, C.; Akinyemiju, T.F.; Al Lami, F.H.; Alam, T.; Alizadeh-Navaei, R.; Allen, C.; Alsharif, U.; Alvis-Guzman, N.; Amini, E.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2018, 4, 1553–1568. [Google Scholar] [CrossRef]
- Cai, Q.; Chen, Y.; Zhang, D.; Pan, J.; Xie, Z.; Xu, C.; Li, S.; Zhang, X.; Gao, Y.; Hou, J.; et al. Estimates of over-time trends in incidence and mortality of testicular cancer from 1990 to 2030. Transl. Androl. Urol. 2020, 9, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Keys, A.; Menotti, A.; Karvonen, M.J.; Aravanis, C.; Blackburn, H.; Buzina, R.; Djordjevic, B.S.; Dontas, A.S.; Fidanza, F.; Keys, M.H.; et al. The diet and 15-year death rate in the seven countries study. Am. J. Epidemiol. 1986, 124, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Joint WHO/FAO Expert Consultation. Diet, nutrition and the prevention of chronic diseases. World Health Organ. Tech. Rep. Ser. 2003, 916, 1–149. [Google Scholar]
- American Heart Association Nutrition; Lichtenstein, A.H.; Appel, L.J.; Brands, M.; Carnethon, M.; Daniels, S.; Franch, H.A.; Franklin, B.; Kris-Etherton, P.; Harris, W.S.; et al. Diet and lifestyle recommendations revision 2006: A scientific statement from the American Heart Association Nutrition Committee. Circulation 2006, 114, 82–96. [Google Scholar] [CrossRef] [Green Version]
- de Lorenzo, A.; Andreoli, A.; Sorge, R.P.; Iacopino, L.; Montagna, S.; Promenzio, L.; Serrano, P. Modification of dietary habits (Mediterranean diet) and cancer mortality in a southern Italian village from 1960 to 1996. Ann. N. Y. Acad. Sci. 1999, 889, 224–229. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Simonetta, I.; Daidone, M.; Mogavero, A.; Ortello, A.; Pinto, A. Metabolic and Vascular Effect of the Mediterranean Diet. Int. J. Mol. Sci. 2019, 20, 4716. [Google Scholar] [CrossRef] [Green Version]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef] [Green Version]
- Kelly, F.J. Dietary antioxidants and environmental stress. Proc. Nutr. Soc. 2004, 63, 579–585. [Google Scholar] [CrossRef] [Green Version]
- Jamalan, M.; Ghaffari, M.A.; Hoseinzadeh, P.; Hashemitabar, M.; Zeinali, M. Human Sperm Quality and Metal Toxicants: Protective Effects of some Flavonoids on Male Reproductive Function. Int. J. Fertil. Steril. 2016, 10, 215–223. [Google Scholar] [CrossRef]
- Chung, R.T. Detoxification effects of phytonutrients against environmental toxicants and sharing of clinical experience on practical applications. Environ. Sci. Pollut. Res. Int. 2017, 24, 8946–8956. [Google Scholar] [CrossRef]
- Alegria-Torres, J.A.; Baccarelli, A.; Bollati, V. Epigenetics and lifestyle. Epigenomics 2011, 3, 267–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tedesco, I.; Russo, M.; Moccia, S.; Cervellera, C.; Spagnuolo, C.; Carbone, V.; Minasi, P.; Montano, L.; Verze, P.; Capece, M. Protective effect of curcumin towards cadmium and polycyclic aromatic hydrocarbons toxicities: The EcoNutraPrevention Project. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 540. [Google Scholar] [CrossRef]
- Baldi, F.; Mantovani, A. A new database for food safety: EDID (Endocrine disrupting chemicals—Diet Interaction Database). Ann. Ist. Super. Sanita 2008, 44, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Mosbah, R.; Yousef, M.I.; Maranghi, F.; Mantovani, A. Protective role of Nigella sativa oil against reproductive toxicity, hormonal alterations, and oxidative damage induced by chlorpyrifos in male rats. Toxicol. Ind. Health 2016, 32, 1266–1277. [Google Scholar] [CrossRef] [PubMed]
- Salas-Huetos, A.; Bullo, M.; Salas-Salvado, J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: A systematic review of observational studies. Hum. Reprod. Update 2017, 23, 371–389. [Google Scholar] [CrossRef]
- Ricci, E.; Al-Beitawi, S.; Cipriani, S.; Alteri, A.; Chiaffarino, F.; Candiani, M.; Gerli, S.; Vigano, P.; Parazzini, F. Dietary habits and semen parameters: A systematic narrative review. Andrology 2018, 6, 104–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alamo, A.; Condorelli, R.A.; Mongioi, L.M.; Cannarella, R.; Giacone, F.; Calabrese, V.; La Vignera, S.; Calogero, A.E. Environment and Male Fertility: Effects of Benzo-alpha-Pyrene and Resveratrol on Human Sperm Function In Vitro. J. Clin. Med. 2019, 8, 561. [Google Scholar] [CrossRef] [Green Version]
- Salas-Huetos, A.; Moraleda, R.; Giardina, S.; Anton, E.; Blanco, J.; Salas-Salvado, J.; Bullo, M. Effect of nut consumption on semen quality and functionality in healthy men consuming a Western-style diet: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 953–962. [Google Scholar] [CrossRef] [Green Version]
- Dominguez-Vias, G.; Segarra, A.B.; Martinez-Canamero, M.; Ramirez-Sanchez, M.; Prieto, I. Influence of a Virgin Olive Oil versus Butter Plus Cholesterol-Enriched Diet on Testicular Enzymatic Activities in Adult Male Rats. Int. J. Mol. Sci. 2017, 18, 1701. [Google Scholar] [CrossRef] [Green Version]
- Nassan, F.L.; Chavarro, J.E.; Tanrikut, C. Diet and men’s fertility: Does diet affect sperm quality? Fertil. Steril. 2018, 110, 570–577. [Google Scholar] [CrossRef] [Green Version]
- Montano, L.; Ceretti, E.; Donato, F.; Bergamo, P.; Zani, C.; Viola, G.C.V.; Notari, T.; Pappalardo, S.; Zani, D.; Ubaldi, S.; et al. Effects of a Lifestyle Change Intervention on Semen Quality in Healthy Young Men Living in Highly Polluted Areas in Italy: The FASt Randomized Controlled Trial. Eur. Urol. Focus 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Mitsunami, M.; Salas-Huetos, A.; Minguez-Alarcon, L.; Attaman, J.A.; Ford, J.B.; Kathrins, M.; Souter, I.; Chavarro, J.E.; Team, E.S. Men’s dietary patterns in relation to infertility treatment outcomes among couples undergoing in vitro fertilization. J. Assist. Reprod. Genet. 2021, 38, 2307–2318. [Google Scholar] [CrossRef] [PubMed]
- Mitsunami, M.; Salas-Huetos, A.; Minguez-Alarcon, L.; Attaman, J.A.; Ford, J.B.; Kathrins, M.; Souter, I.; Chavarro, J.E. A dietary score representing the overall relation of men’s diet with semen quality in relation to outcomes of infertility treatment with assisted reproduction. F&S Rep. 2021, 2, 396–404. [Google Scholar] [CrossRef]
- Benatta, M.; Kettache, R.; Buchholz, N.; Trinchieri, A. The impact of nutrition and lifestyle on male fertility. Arch. Ital. Urol. Androl. 2020, 92. [Google Scholar] [CrossRef] [PubMed]
- Karayiannis, D.; Kontogianni, M.D.; Mendorou, C.; Douka, L.; Mastrominas, M.; Yiannakouris, N. Association between adherence to the Mediterranean diet and semen quality parameters in male partners of couples attempting fertility. Hum. Reprod. 2017, 32, 215–222. [Google Scholar] [CrossRef]
- Vigar, V.; Myers, S.; Oliver, C.; Arellano, J.; Robinson, S.; Leifert, C. A Systematic Review of Organic Versus Conventional Food Consumption: Is There a Measurable Benefit on Human Health? Nutrients 2019, 12, 7. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef]
- Moshammer, H.; Poteser, M.; Hutter, H.P. More pesticides-less children? Wien. Klin. Wochenschr. 2020, 132, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Baranski, M.; Srednicka-Tober, D.; Volakakis, N.; Seal, C.; Sanderson, R.; Stewart, G.B.; Benbrook, C.; Biavati, B.; Markellou, E.; Giotis, C.; et al. Higher antioxidant and lower cadmium concentrations and lower incidence of pesticide residues in organically grown crops: A systematic literature review and meta-analyses. Br. J. Nutr. 2014, 112, 794–811. [Google Scholar] [CrossRef] [Green Version]
- Ribes-Moya, A.M.; Adalid, A.M.; Raigon, M.D.; Hellin, P.; Fita, A.; Rodriguez-Burruezo, A. Variation in flavonoids in a collection of peppers (Capsicum sp.) under organic and conventional cultivation: Effect of the genotype, ripening stage, and growing system. J. Sci. Food Agric. 2020, 100, 2208–2223. [Google Scholar] [CrossRef]
- Hallmann, E.; Marszalek, K.; Lipowski, J.; Jasinska, U.; Kazimierczak, R.; Srednicka-Tober, D.; Rembialkowska, E. Polyphenols and carotenoids in pickled bell pepper from organic and conventional production. Food Chem. 2019, 278, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Srednicka-Tober, D.; Baranski, M.; Seal, C.J.; Sanderson, R.; Benbrook, C.; Steinshamn, H.; Gromadzka-Ostrowska, J.; Rembialkowska, E.; Skwarlo-Sonta, K.; Eyre, M.; et al. Higher PUFA and n-3 PUFA, conjugated linoleic acid, alpha-tocopherol and iron, but lower iodine and selenium concentrations in organic milk: A systematic literature review and meta- and redundancy analyses. Br. J. Nutr. 2016, 115, 1043–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurtado-Barroso, S.; Tresserra-Rimbau, A.; Vallverdu-Queralt, A.; Lamuela-Raventos, R.M. Organic food and the impact on human health. Crit. Rev. Food Sci. Nutr. 2019, 59, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, B.; Du, Y.; Snetselaar, L.G.; Sun, Q.; Hu, F.B.; Bao, W. Inverse Association between Organic Food Purchase and Diabetes Mellitus in US Adults. Nutrients 2018, 10, 1877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, J.; Liang, X.; Cao, P.; Wang, X.; Gao, P.; Ma, N.; Li, N.; Xu, H. A Preliminary Investigation of Naturally Occurring Aluminum in Grains, Vegetables, and Fruits from Some Areas of China and Dietary Intake Assessment. J. Food Sci. 2019, 84, 701–710. [Google Scholar] [CrossRef]
- Glibowski, P. Organic food and health. Rocz. Panstw. Zakl. Hig. 2020, 71, 131–136. [Google Scholar] [CrossRef]
- Crinnion, W.J. Organic foods contain higher levels of certain nutrients, lower levels of pesticides, and may provide health benefits for the consumer. Altern. Med. Rev. 2010, 15, 4–12. [Google Scholar]
- Chiu, Y.H.; Williams, P.L.; Gillman, M.W.; Gaskins, A.J.; Minguez-Alarcon, L.; Souter, I.; Toth, T.L.; Ford, J.B.; Hauser, R.; Chavarro, J.E.; et al. Association Between Pesticide Residue Intake From Consumption of Fruits and Vegetables and Pregnancy Outcomes Among Women Undergoing Infertility Treatment With Assisted Reproductive Technology. JAMA Intern. Med. 2018, 178, 17–26. [Google Scholar] [CrossRef]
- Baudry, J.; Assmann, K.E.; Touvier, M.; Alles, B.; Seconda, L.; Latino-Martel, P.; Ezzedine, K.; Galan, P.; Hercberg, S.; Lairon, D.; et al. Association of Frequency of Organic Food Consumption With Cancer Risk: Findings From the NutriNet-Sante Prospective Cohort Study. JAMA Intern. Med. 2018, 178, 1597–1606. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Superfruits: Phytochemicals, antioxidant efficacies, and health effects—A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1580–1604. [Google Scholar] [CrossRef]
- Vanduchova, A.; Anzenbacher, P.; Anzenbacherova, E. Isothiocyanate from Broccoli, Sulforaphane, and Its Properties. J. Med. Food 2019, 22, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Paolini, M.; Perocco, P.; Canistro, D.; Valgimigli, L.; Pedulli, G.F.; Iori, R.; Croce, C.D.; Cantelli-Forti, G.; Legator, M.S.; Abdel-Rahman, S.Z. Induction of cytochrome P450, generation of oxidative stress and in vitro cell-transforming and DNA-damaging activities by glucoraphanin, the bioprecursor of the chemopreventive agent sulforaphane found in broccoli. Carcinogenesis 2004, 25, 61–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, S.; Chen, C.; Satoh, J.; Yim, S.; Gonzalez, F.J. Dietary phytochemicals regulate whole-body CYP1A1 expression through an arylhydrocarbon receptor nuclear translocator-dependent system in gut. J. Clin. Investig. 2007, 117, 1940–1950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdull Razis, A.F.; Noor, N.M. Cruciferous vegetables: Dietary phytochemicals for cancer prevention. Asian Pac. J. Cancer Prev. 2013, 14, 1565–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zona, A.; Iavarone, I.; Buzzoni, C.; Conti, S.; Santoro, M.; Fazzo, L.; Pasetto, R.; Pirastu, R.; Bruno, C.; Ancona, C.; et al. SENTIERI: Epidemiological Study of Residents in National Priority Contaminated Sites. Fifth Report. Epidemiol. Prev. 2019, 43, 1–208. [Google Scholar] [CrossRef] [PubMed]
- Maresca, V.; Fusaro, L.; Sorbo, S.; Siciliano, A.; Loppi, S.; Paoli, L.; Monaci, F.; Karam, E.A.; Piscopo, M.; Guida, M.; et al. Functional and structural biomarkers to monitor heavy metal pollution of one of the most contaminated freshwater sites in Southern Europe. Ecotoxicol. Environ. Saf. 2018, 163, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Basile, A.; Loppi, S.; Piscopo, M.; Paoli, L.; Vannini, A.; Monaci, F.; Sorbo, S.; Lentini, M.; Esposito, S. The biological response chain to pollution: A case study from the “Italian Triangle of Death” assessed with the liverwort Lunularia cruciata. Environ. Sci. Pollut. Res. Int. 2017, 24, 26185–26193. [Google Scholar] [CrossRef]
- Bergamo, P.; Volpe, M.G.; Lorenzetti, S.; Mantovani, A.; Notari, T.; Cocca, E.; Cerullo, S.; Di Stasio, M.; Cerino, P.; Montano, L. Human semen as an early, sensitive biomarker of highly polluted living environment in healthy men: A pilot biomonitoring study on trace elements in blood and semen and their relationship with sperm quality and RedOx status. Reprod. Toxicol. 2016, 66, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, S.; Gentile, M.; Esposito, G.; Gentile, T.; Ferrara, I.; Crescenzo, C.; Palmieri, M.; Cuomo, F.; De Filippo, S.; Lettieri, G.; et al. Could Kallikrein-Related Serine Peptidase 3 Be an Early Biomarker of Environmental Exposure in Young Women? Int. J. Environ. Res. Public Health 2021, 18, 8833. [Google Scholar] [CrossRef]
- Montano, L.; Porciello, G.; Crispo, A.; Lorenzetti, S.; Salvatore, R.; Ubaldi, S.; Caputo, M. The role of the Mediterranean diet on sperm morphology in healthy men living in polluted area (EcoFoodFertility project). Reprod. Toxicol. 2017, 72, 45. [Google Scholar] [CrossRef]
- Aitken, R.J. Oxidative stress and the etiology of male infertility. J. Assist. Reprod. Genet. 2016, 33, 1691–1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aitken, R.J.; Gibb, Z.; Baker, M.A.; Drevet, J.; Gharagozloo, P. Causes and consequences of oxidative stress in spermatozoa. Reprod. Fertil. Dev. 2016, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Montano, L.; Donato, F.; Bianco, P.M.; Lettieri, G.; Guglielmino, A.; Motta, O.; Bonapace, I.M.; Piscopo, M. Air Pollution and COVID-19: A Possible Dangerous Synergy for Male Fertility. Int. J. Environ. Res. Public Health 2021, 18, 6846. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, L.; Maugeri, A.; Cirmi, S.; Lombardo, G.E.; Russo, C.; Gangemi, S.; Calapai, G.; Navarra, M. Citrus fruits and their flavonoids in inflammatory bowel disease: An overview. Nat. Prod. Res. 2020, 34, 122–136. [Google Scholar] [CrossRef]
- Cirmi, S.; Navarra, M.; Woodside, J.V.; Cantwell, M.M. Citrus fruits intake and oral cancer risk: A systematic review and meta-analysis. Pharmacol. Res. 2018, 133, 187–194. [Google Scholar] [CrossRef] [Green Version]
- Cirmi, S.; Bisignano, C.; Mandalari, G.; Navarra, M. Anti-infective potential of Citrus bergamia Risso et Poiteau (bergamot) derivatives: A systematic review. Phytother. Res. 2016, 30, 1404–1411. [Google Scholar] [CrossRef]
- Marino, A.; Paterniti, I.; Cordaro, M.; Morabito, R.; Campolo, M.; Navarra, M.; Esposito, E.; Cuzzocrea, S. Role of natural antioxidants and potential use of bergamot in treating rheumatoid arthritis. PharmaNutrition 2015, 3, 53–59. [Google Scholar] [CrossRef]
- Mannucci, C.; Casciaro, M.; Sorbara, E.E.; Calapai, F.; Di Salvo, E.; Pioggia, G.; Navarra, M.; Calapai, G.; Gangemi, S. Nutraceuticals against Oxidative Stress in Autoimmune Disorders. Antioxidants 2021, 10, 261. [Google Scholar] [CrossRef]
- Maugeri, A.; Cirmi, S.; Minciullo, P.L.; Gangemi, S.; Calapai, G.; Mollace, V.; Navarra, M. Citrus fruits and inflammaging: A systematic review. Phytochem. Rev. 2019, 18, 1025–1049. [Google Scholar] [CrossRef]
- Citraro, R.; Navarra, M.; Leo, A.; Donato Di Paola, E.; Santangelo, E.; Lippiello, P.; Aiello, R.; Russo, E.; De Sarro, G. The Anticonvulsant Activity of a Flavonoid-Rich Extract from Orange Juice Involves both NMDA and GABA-Benzodiazepine Receptor Complexes. Molecules 2016, 21, 1261. [Google Scholar] [CrossRef] [Green Version]
- Mannucci, C.; Navarra, M.; Calapai, F.; Squeri, R.; Gangemi, S.; Calapai, G. Clinical Pharmacology of Citrus bergamia: A Systematic Review. Phytother. Res. 2017, 31, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, C.; Calapai, F.; Cardia, L.; Inferrera, G.; D’Arena, G.; Di Pietro, M.; Navarra, M.; Gangemi, S.; Ventura Spagnolo, E.; Calapai, G. Clinical Pharmacology of Citrus aurantium and Citrus sinensis for the Treatment of Anxiety. Evid.-Based Complement. Alternat. Med. 2018, 2018, 3624094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Das, S.; Patra, S.K.; Efferth, T.; Jena, M.; Bhutia, S.K. Dietary polyphenols in chemoprevention and synergistic effect in cancer: Clinical evidences and molecular mechanisms of action. Phytomedicine 2021, 90, 153554. [Google Scholar] [CrossRef] [PubMed]
- Cirmi, S.; Maugeri, A.; Ferlazzo, N.; Gangemi, S.; Calapai, G.; Schumacher, U.; Navarra, M. Anticancer Potential of Citrus Juices and Their Extracts: A Systematic Review of Both Preclinical and Clinical Studies. Front. Pharmacol. 2017, 8, 420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adorisio, S.; Argentieri, M.; Avato, P.; Caderni, G.; Chioccioli, S.; Cirmi, S.; Delfino, D.; Greco, G.; Hrelia, P.; Iriti, M. The molecular basis of the anticancer properties of quercetin. Pharmadvances 2021, 3, 496–522. [Google Scholar] [CrossRef]
- Navarra, M.; Ursino, M.R.; Ferlazzo, N.; Russo, M.; Schumacher, U.; Valentiner, U. Effect of Citrus bergamia juice on human neuroblastoma cells in vitro and in metastatic xenograft models. Fitoterapia 2014, 95, 83–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarra, M.; Femia, A.P.; Romagnoli, A.; Tortora, K.; Luceri, C.; Cirmi, S.; Ferlazzo, N.; Caderni, G. A flavonoid-rich extract from bergamot juice prevents carcinogenesis in a genetic model of colorectal cancer, the Pirc rat (F344/NTac-Apc(am1137)). Eur. J. Nutr. 2020, 59, 885–894. [Google Scholar] [CrossRef]
- Clementino, M.; Shi, X.; Zhang, Z. Prevention of Polyphenols against Carcinogenesis Induced by Environmental Carcinogens. J. Environ. Pathol. Toxicol. Oncol. 2017, 36, 87–98. [Google Scholar] [CrossRef]
- Petriello, M.C.; Newsome, B.; Hennig, B. Influence of nutrition in PCB-induced vascular inflammation. Environ. Sci. Pollut. Res. Int. 2014, 21, 6410–6418. [Google Scholar] [CrossRef] [Green Version]
- Ferlazzo, N.; Visalli, G.; Cirmi, S.; Lombardo, G.E.; Lagana, P.; Di Pietro, A.; Navarra, M. Natural iron chelators: Protective role in A549 cells of flavonoids-rich extracts of Citrus juices in Fe(3+)-induced oxidative stress. Environ. Toxicol. Pharmacol. 2016, 43, 248–256. [Google Scholar] [CrossRef]
- Link, A.; Balaguer, F.; Goel, A. Cancer chemoprevention by dietary polyphenols: Promising role for epigenetics. Biochem. Pharmacol. 2010, 80, 1771–1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Symonowicz, M.; Kolanek, M. Flavonoids and Their Properties to Form Chelate Complexes; Lodz University of Technology Repository: Łódź, Poland, 2012. [Google Scholar]
- Ferlazzo, N.; Cirmi, S.; Calapai, G.; Ventura-Spagnolo, E.; Gangemi, S.; Navarra, M. Anti-Inflammatory Activity of Citrus bergamia Derivatives: Where Do We Stand? Molecules 2016, 21, 1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gugliandolo, E.; Fusco, R.; D’Amico, R.; Peditto, M.; Oteri, G.; Di Paola, R.; Cuzzocrea, S.; Navarra, M. Treatment With a Flavonoid-Rich Fraction of Bergamot Juice Improved Lipopolysaccharide-Induced Periodontitis in Rats. Front. Pharmacol. 2018, 9, 1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fusco, R.; Cirmi, S.; Gugliandolo, E.; Di Paola, R.; Cuzzocrea, S.; Navarra, M. A flavonoid-rich extract of orange juice reduced oxidative stress in an experimental model of inflammatory bowel disease. J. Funct. Foods 2017, 30, 168–178. [Google Scholar] [CrossRef]
- Maugeri, A.; Ferlazzo, N.; De Luca, L.; Gitto, R.; Navarra, M. The link between the AMPK/SIRT1 axis and a flavonoid-rich extract of Citrus bergamia juice: A cell-free, in silico, and in vitro study. Phytother. Res. 2019, 33, 1805–1814. [Google Scholar] [CrossRef]
- Curro, M.; Risitano, R.; Ferlazzo, N.; Cirmi, S.; Gangemi, C.; Caccamo, D.; Ientile, R.; Navarra, M. Citrus bergamia Juice Extract Attenuates beta-Amyloid-Induced Pro-Inflammatory Activation of THP-1 Cells Through MAPK and AP-1 Pathways. Sci. Rep. 2016, 6, 20809. [Google Scholar] [CrossRef] [Green Version]
- Ferlazzo, N.; Cirmi, S.; Maugeri, A.; Russo, C.; Lombardo, G.E.; Gangemi, S.; Calapai, G.; Mollace, V.; Navarra, M. Neuroprotective Effect of Bergamot Juice in 6-OHDA-Induced SH-SY5Y Cell Death, an In Vitro Model of Parkinson’s Disease. Pharmaceutics 2020, 12, 326. [Google Scholar] [CrossRef] [Green Version]
- Cirmi, S.; Maugeri, A.; Lombardo, G.E.; Russo, C.; Musumeci, L.; Gangemi, S.; Calapai, G.; Barreca, D.; Navarra, M. A Flavonoid-Rich Extract of Mandarin Juice Counteracts 6-OHDA-Induced Oxidative Stress in SH-SY5Y Cells and Modulates Parkinson-Related Genes. Antioxidants 2021, 10, 539. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [Green Version]
- Giacoppo, S.; Galuppo, M.; Lombardo, G.E.; Ulaszewska, M.M.; Mattivi, F.; Bramanti, P.; Mazzon, E.; Navarra, M. Neuroprotective effects of a polyphenolic white grape juice extract in a mouse model of experimental autoimmune encephalomyelitis. Fitoterapia 2015, 103, 171–186. [Google Scholar] [CrossRef]
- Cersosimo, F.; Lonardi, S.; Bernardini, G.; Telfer, B.; Mandelli, G.E.; Santucci, A.; Vermi, W.; Giurisato, E. Tumor-Associated Macrophages in Osteosarcoma: From Mechanisms to Therapy. Int. J. Mol. Sci. 2020, 21, 5207. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Zhang, T.; Zhu, X.; Yang, C.; Wang, Y.; Zhou, N.; Ju, B.; Zhou, T.; Deng, G.; Qiu, C. Hyperoside Induces Breast Cancer Cells Apoptosis via ROS-Mediated NF-kappaB Signaling Pathway. Int. J. Mol. Sci. 2019, 21, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celano, M.; Maggisano, V.; De Rose, R.F.; Bulotta, S.; Maiuolo, J.; Navarra, M.; Russo, D. Flavonoid Fraction of Citrus reticulata Juice Reduces Proliferation and Migration of Anaplastic Thyroid Carcinoma Cells. Nutr. Cancer 2015, 67, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Seba, V.; Silva, G.; Santos, M.B.D.; Baek, S.J.; Franca, S.C.; Fachin, A.L.; Regasini, L.O.; Marins, M. Chalcone Derivatives 4′-Amino-1-Naphthyl-Chalcone (D14) and 4′-Amino-4-Methyl-1-Naphthyl-Chalcone (D15) Suppress Migration and Invasion of Osteosarcoma Cells Mediated by p53 Regulating EMT-Related Genes. Int. J. Mol. Sci. 2018, 19, 2838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Z.Y.; Liang, I.C.; Li, H.J.; Wu, C.C.; Lo, H.M.; Chang, D.C.; Hung, C.F. Chrysin Inhibits High Glucose-Induced Migration on Chorioretinal Endothelial Cells via VEGF and VEGFR Down-Regulation. Int. J. Mol. Sci. 2020, 21, 5541. [Google Scholar] [CrossRef]
- Vemuri, S.K.; Banala, R.R.; Subbaiah, G.; Srivastava, S.K.; Reddy, A.G.; Malarvili, T. Anti-cancer potential of a mix of natural extracts of turmeric, ginger and garlic: A cell-based study. Egypt. J. Basic Appl. Sci. 2017, 4, 332–344. [Google Scholar] [CrossRef]
- Vincent, T.L.; Gatenby, R.A. An evolutionary model for initiation, promotion, and progression in carcinogenesis. Int. J. Oncol. 2008, 32, 729–737. [Google Scholar]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Busselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef] [Green Version]
- Masuelli, L.; Benvenuto, M.; Di Stefano, E.; Mattera, R.; Fantini, M.; De Feudis, G.; De Smaele, E.; Tresoldi, I.; Giganti, M.G.; Modesti, A.; et al. Curcumin blocks autophagy and activates apoptosis of malignant mesothelioma cell lines and increases the survival of mice intraperitoneally transplanted with a malignant mesothelioma cell line. Oncotarget 2017, 8, 34405–34422. [Google Scholar] [CrossRef] [Green Version]
- Prieto-Dominguez, N.; Garcia-Mediavilla, M.V.; Sanchez-Campos, S.; Mauriz, J.L.; Gonzalez-Gallego, J. Autophagy as a Molecular Target of Flavonoids Underlying their Protective Effects in Human Disease. Curr. Med. Chem. 2018, 25, 814–838. [Google Scholar] [CrossRef]
- Chae, H.S.; Xu, R.; Won, J.Y.; Chin, Y.W.; Yim, H. Molecular Targets of Genistein and Its Related Flavonoids to Exert Anticancer Effects. Int. J. Mol. Sci. 2019, 20, 2420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cernelev, O.; Mantovani, A. Natural substances in supplements and nutraceuticals as Endocrine Disruptors. In Challenges in Endocrine Disruptor Toxicology and Risk Assessment; Mantovani, A., Fucic, A., Eds.; Royal Society of Chemistry: London, UK, 2020; pp. 356–376. [Google Scholar]
- Ye, R.J.; Yang, J.M.; Hai, D.M.; Liu, N.; Ma, L.; Lan, X.B.; Niu, J.G.; Zheng, P.; Yu, J.Q. Interplay between male reproductive system dysfunction and the therapeutic effect of flavonoids. Fitoterapia 2020, 147, 104756. [Google Scholar] [CrossRef] [PubMed]
- Moretti, E.; Mazzi, L.; Terzuoli, G.; Bonechi, C.; Iacoponi, F.; Martini, S.; Rossi, C.; Collodel, G. Effect of quercetin, rutin, naringenin and epicatechin on lipid peroxidation induced in human sperm. Reprod. Toxicol. 2012, 34, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Osawe, S.O.; Farombi, E.O. Querce.etin and rutin ameliorates sulphasalazine-induced spermiotoxicity, alterations in reproductive hormones and steroidogenic enzyme imbalance in rats. Andrologia 2018, 50, e12981. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liu, W.; Li, Z.; Xiao, W. Effect of epigallocatechin-3-gallate (EGCG) on embryos inseminated with oxidative stress-induced DNA damage sperm. Syst. Biol. Reprod. Med. 2020, 66, 244–254. [Google Scholar] [CrossRef]
- Duracka, M.; Lukac, N.; Kacaniova, M.; Kantor, A.; Hleba, L.; Ondruska, L.; Tvrda, E. Antibiotics Versus Natural Biomolecules: The Case of In Vitro Induced Bacteriospermia by Enterococcus Faecalis in Rabbit Semen. Molecules 2019, 24, 4329. [Google Scholar] [CrossRef] [Green Version]
- Pei, Y.; Yang, L.; Wu, L.; He, H.; Geng, G.; Xu, D.; Chen, H.; Li, Q. Combined effect of apigenin and ferulic acid on frozen-thawed boar sperm quality. Anim. Sci. J. 2018, 89, 956–965. [Google Scholar] [CrossRef]
- Xu, D.; Wu, L.; Yang, L.; Liu, D.; Chen, H.; Geng, G.; Li, Q. Rutin protects boar sperm from cryodamage via enhancing the antioxidative defense. Anim. Sci. J. 2020, 91, e13328. [Google Scholar] [CrossRef]
- Prabu, S.M.; Shagirtha, K.; Renugadevi, J. Amelioration of cadmium-induced oxidative stress, impairment in lipids and plasma lipoproteins by the combined treatment with quercetin and alpha-tocopherol in rats. J. Food Sci. 2010, 75, T132–T140. [Google Scholar] [CrossRef]
- Farombi, E.O.; Adedara, I.A.; Akinrinde, S.A.; Ojo, O.O.; Eboh, A.S. Protective effects of kolaviron and quercetin on cadmium-induced testicular damage and endocrine pathology in rats. Andrologia 2012, 44, 273–284. [Google Scholar] [CrossRef]
- Couture, R.; Mora, N.; Al Bittar, S.; Najih, M.; Touaibia, M.; Martin, L.J. Luteolin modulates gene expression related to steroidogenesis, apoptosis, and stress response in rat LC540 tumor Leydig cells. Cell Biol. Toxicol. 2020, 36, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Allam, J.P.; Duan, Y.G.; Haidl, G. Influence of reactive oxygen species on human sperm functions and fertilizing capacity including therapeutical approaches. Arch. Gynecol. Obstet. 2013, 288, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Appiah, M.O.; Li, W.; Zhao, J.; Liu, H.; Dong, Y.; Xiang, J.; Wang, J.; Lu, W. Quercetin supplemented casein-based extender improves the post-thaw quality of rooster semen. Cryobiology 2020, 94, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Ghaniei, A.; Eslami, M.; Zadeh Hashem, E.; Rezapour, R.; Talebi, A. Quercetin attenuates H2O2-induced toxicity of rooster semen during liquid storage at 4 degrees C. J. Anim. Physiol. Anim. Nutr. 2019, 103, 713–722. [Google Scholar] [CrossRef]
- Sun, J.; Wang, H.; Liu, B.; Shi, W.; Shi, J.; Zhang, Z.; Xing, J. Rutin attenuates H2O2-induced oxidation damage and apoptosis in Leydig cells by activating PI3K/Akt signal pathways. Biomed. Pharmacother. 2017, 88, 500–506. [Google Scholar] [CrossRef]
- Samie, A.; Sedaghat, R.; Baluchnejadmojarad, T.; Roghani, M. Hesperetin, a citrus flavonoid, attenuates testicular damage in diabetic rats via inhibition of oxidative stress, inflammation, and apoptosis. Life Sci. 2018, 210, 132–139. [Google Scholar] [CrossRef]
- Ma, B.; Zhang, J.; Zhu, Z.; Zhao, A.; Zhou, Y.; Ying, H.; Zhang, Q. Luteolin Ameliorates Testis Injury and Blood-Testis Barrier Disruption through the Nrf2 Signaling Pathway and by Upregulating Cx43. Mol. Nutr. Food Res. 2019, 63, e1800843. [Google Scholar] [CrossRef]
- Chen, M.; Hao, J.; Yang, Q.; Li, G. Effects of icariin on reproductive functions in male rats. Molecules 2014, 19, 9502–9514. [Google Scholar] [CrossRef] [Green Version]
- Bharti, S.; Misro, M.M.; Rai, U. Quercetin supplementation restores testicular function and augments germ cell survival in the estrogenized rats. Mol. Cell. Endocrinol. 2014, 383, 10–20. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, Y.J.; Seo, Y.R. An Overview of Carcinogenic Heavy Metal: Molecular Toxicity Mechanism and Prevention. J. Cancer Prev. 2015, 20, 232–240. [Google Scholar] [CrossRef]
- Lettieri, G.; Notariale, R.; Carusone, N.; Giarra, A.; Trifuoggi, M.; Manna, C.; Piscopo, M. New Insights into Alterations in PL Proteins Affecting Their Binding to DNA after Exposure of Mytilus galloprovincialis to Mercury-A Possible Risk to Sperm Chromatin Structure? Int. J. Mol. Sci. 2021, 22, 5893. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, G.; Notariale, R.; Ambrosino, A.; Di Bonito, A.; Giarra, A.; Trifuoggi, M.; Manna, C.; Piscopo, M. Spermatozoa Transcriptional Response and Alterations in PL Proteins Properties after Exposure of Mytilus galloprovincialis to Mercury. Int. J. Mol. Sci. 2021, 22, 1618. [Google Scholar] [CrossRef] [PubMed]
- Piscopo, M.; Trifuoggi, M.; Notariale, R.; Labar, S.; Troisi, J.; Giarra, A.; Rabbito, D.; Puoti, R.; de Benedictis, D.; Brundo, M.V.; et al. Protamine-like proteins’ analysis as an emerging biotechnique for cadmium impact assessment on male mollusk Mytilus galloprovincialis (Lamarck 1819). Acta Biochim. Pol. 2018, 65, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Piscopo, M.; Notariale, R.; Rabbito, D.; Ausio, J.; Olanrewaju, O.S.; Guerriero, G. Mytilus galloprovincialis (Lamarck, 1819) spermatozoa: hsp70 expression and protamine-like protein property studies. Environ. Sci. Pollut. Res. Int. 2018, 25, 12957–12966. [Google Scholar] [CrossRef]
- De Guglielmo, V.; Puoti, R.; Notariale, R.; Maresca, V.; Ausio, J.; Troisi, J.; Verrillo, M.; Basile, A.; Febbraio, F.; Piscopo, M. Alterations in the properties of sperm protamine-like II protein after exposure of Mytilus galloprovincialis (Lamarck 1819) to sub-toxic doses of cadmium. Ecotoxicol. Environ. Saf. 2019, 169, 600–606. [Google Scholar] [CrossRef]
- Lettieri, G.; Mollo, V.; Ambrosino, A.; Caccavale, F.; Troisi, J.; Febbraio, F.; Piscopo, M. Molecular effects of copper on the reproductive system of mytilus galloprovincialis. Mol. Reprod. Dev. 2019, 86, 1357–1368. [Google Scholar] [CrossRef]
- Lettieri, G.; D’Agostino, G.; Mele, E.; Cardito, C.; Esposito, R.; Cimmino, A.; Giarra, A.; Trifuoggi, M.; Raimondo, S.; Notari, T.; et al. Discovery of the Involvement in DNA Oxidative Damage of Human Sperm Nuclear Basic Proteins of Healthy Young Men Living in Polluted Areas. Int. J. Mol. Sci. 2020, 21, 4198. [Google Scholar] [CrossRef]
- Lettieri, G.; Marra, F.; Moriello, C.; Prisco, M.; Notari, T.; Trifuoggi, M.; Giarra, A.; Bosco, L.; Montano, L.; Piscopo, M. Molecular Alterations in Spermatozoa of a Family Case Living in the Land of Fires. A First Look at Possible Transgenerational Effects of Pollutants. Int. J. Mol. Sci. 2020, 21, 6710. [Google Scholar] [CrossRef]
- Yang, O.; Kim, H.L.; Weon, J.I.; Seo, Y.R. Endocrine-disrupting Chemicals: Review of Toxicological Mechanisms Using Molecular Pathway Analysis. J. Cancer Prev. 2015, 20, 12–24. [Google Scholar] [CrossRef]
- Togawa, K.; Le Cornet, C.; Feychting, M.; Tynes, T.; Pukkala, E.; Hansen, J.; Olsson, A.; Oksbjerg Dalton, S.; Nordby, K.C.; Uuksulainen, S.; et al. Parental Occupational Exposure to Heavy Metals and Welding Fumes and Risk of Testicular Germ Cell Tumors in Offspring: A Registry-Based Case-Control Study. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1426–1434. [Google Scholar] [CrossRef] [Green Version]
- Morales-Suarez-Varela, M.M.; Toft, G.V.; Jensen, M.S.; Ramlau-Hansen, C.; Kaerlev, L.; Thulstrup, A.M.; Llopis-Gonzalez, A.; Olsen, J.; Bonde, J.P. Parental occupational exposure to endocrine disrupting chemicals and male genital malformations: A study in the Danish National Birth Cohort study. Environ. Health 2011, 10, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giordano, F.; Abballe, A.; De Felip, E.; di Domenico, A.; Ferro, F.; Grammatico, P.; Ingelido, A.M.; Marra, V.; Marrocco, G.; Vallasciani, S.; et al. Maternal exposures to endocrine disrupting chemicals and hypospadias in offspring. Birth Defects Res. A Clin. Mol. Teratol. 2010, 88, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Jahan, S.; Khan, M.; Ahmed, S.; Ullah, H. Comparative analysis of antioxidants against cadmium induced reproductive toxicity in adult male rats. Syst. Biol. Reprod. Med. 2014, 60, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Iwasaki, M.; Sawada, N.; Takachi, R.; Kasuga, Y.; Yokoyama, S.; Onuma, H.; Nishimura, H.; Kusama, R.; Yokoyama, K.; et al. Dietary cadmium intake and breast cancer risk in Japanese women: A case-control study. Int. J. Hyg. Environ. Health 2014, 217, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Henson, M.C.; Chedrese, P.J. Endocrine disruption by cadmium, a common environmental toxicant with paradoxical effects on reproduction. Exp. Biol. Med. (Maywood) 2004, 229, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Monsefi, M.; Alaee, S.; Moradshahi, A.; Rohani, L. Cadmium-induced infertility in male mice. Environ. Toxicol. 2010, 25, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, X.; Sun, J.; Zhu, C.; Li, X.; Tian, L.; Liu, L.; Bai, W. Cytoprotective effects of dietary flavonoids against cadmium-induced toxicity. Ann. N. Y. Acad. Sci. 2017, 1398, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Arafa, M.H.; Mohammad, N.S.; Atteia, H.H. Fenugreek seed powder mitigates cadmium-induced testicular damage and hepatotoxicity in male rats. Exp. Toxicol. Pathol. 2014, 66, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziem, S.H.; El-Nekeety, A.A.; Barakat, I.A.; Mohamed, M.I.; Abdel-Wahhab, M.A. Aquilegia vulgaris extract protects against the oxidative stress and the mutagenic effects of cadmium in Balb/c mice. Exp. Toxicol. Pathol. 2011, 63, 337–344. [Google Scholar] [CrossRef]
- Cirmi, S.; Maugeri, A.; Micali, A.; Marini, H.R.; Puzzolo, D.; Santoro, G.; Freni, J.; Squadrito, F.; Irrera, N.; Pallio, G.; et al. Cadmium-Induced Kidney Injury in Mice Is Counteracted by a Flavonoid-Rich Extract of Bergamot Juice, Alone or in Association with Curcumin and Resveratrol, via the Enhancement of Different Defense Mechanisms. Biomedicines 2021, 9, 1797. [Google Scholar] [CrossRef]
- Ferlazzo, N.; Micali, A.; Marini, H.R.; Freni, J.; Santoro, G.; Puzzolo, D.; Squadrito, F.; Pallio, G.; Navarra, M.; Cirmi, S.; et al. A Flavonoid-Rich Extract from Bergamot Juice, Alone or in Association with Curcumin and Resveratrol, Shows Protective Effects in a Murine Model of Cadmium-Induced Testicular Injury. Pharmaceuticals 2021, 14, 386. [Google Scholar] [CrossRef] [PubMed]
- Alshatwi, A.A.; Hasan, T.N.; Alqahtani, A.M.; Syed, N.A.; Shafi, G.; Al-Assaf, A.H.; Al-Khalifa, A.S. Delineating the anti-cytotoxic and anti-genotoxic potentials of catechin hydrate against cadmium toxicity in human peripheral blood lymphocytes. Environ. Toxicol. Pharmacol. 2014, 38, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Trombetta, D.; Smeriglio, A.; Mandalari, G.; Romeo, O.; Felice, M.R.; Gattuso, G.; Nabavi, S.M. Food flavonols: Nutraceuticals with complex health benefits and functionalities. Trends Food Sci. Technol. 2021, 117, 194–204. [Google Scholar] [CrossRef]
- El-Diasty, H.H.; El-Sayyad, H.; Refaat, S.; El-Ghaweet, H.A. Efficacy of Quercetin-Sensitized Cisplatin against N-Nitroso-NMethylurea Induced Testicular Carcinogenesis in Wistar Rats. Asian Pac. J. Cancer Prev. 2021, 22, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Psotova, J.; Lasovsky, J.; Vicar, J. Metal-chelating properties, electrochemical behavior, scavenging and cytoprotective activities of six natural phenolics. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech. Repub. 2003, 147, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Kilic, C.S.; Sytar, O.; et al. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef]
- Ola-Mudathir, K.F.; Suru, S.M.; Fafunso, M.A.; Obioha, U.E.; Faremi, T.Y. Protective roles of onion and garlic extracts on cadmium-induced changes in sperm characteristics and testicular oxidative damage in rats. Food Chem. Toxicol. 2008, 46, 3604–3611. [Google Scholar] [CrossRef]
- Bu, T.; Mi, Y.; Zeng, W.; Zhang, C. Protective effect of quercetin on cadmium-induced oxidative toxicity on germ cells in male mice. Anat. Rec. (Hoboken) 2011, 294, 520–526. [Google Scholar] [CrossRef]
- Jia, Y.; Lin, J.; Mi, Y.; Zhang, C. Quercetin attenuates cadmium-induced oxidative damage and apoptosis in granulosa cells from chicken ovarian follicles. Reprod. Toxicol. 2011, 31, 477–485. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, H.; Wang, K.; Yang, Z.; Liu, Z. Protective effect of quercetin on rat testes against cadmium toxicity by alleviating oxidative stress and autophagy. Environ. Sci. Pollut. Res. Int. 2020, 27, 25278–25286. [Google Scholar] [CrossRef]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef]
- Henriques, M.C.; Loureiro, S.; Fardilha, M.; Herdeiro, M.T. Exposure to mercury and human reproductive health: A systematic review. Reprod. Toxicol. 2019, 85, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Tortora, F.; Notariale, R.; Maresca, V.; Good, K.V.; Sorbo, S.; Basile, A.; Piscopo, M.; Manna, C. Phenol-Rich Feijoa sellowiana (Pineapple Guava) Extracts Protect Human Red Blood Cells from Mercury-Induced Cellular Toxicity. Antioxidants 2019, 8, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piscopo, M.; Tenore, G.C.; Notariale, R.; Maresca, V.; Maisto, M.; de Ruberto, F.; Heydari, M.; Sorbo, S.; Basile, A. Antimicrobial and antioxidant activity of proteins from Feijoa sellowiana Berg. fruit before and after in vitro gastrointestinal digestion. Nat. Prod. Res. 2020, 34, 2607–2611. [Google Scholar] [CrossRef] [PubMed]
- Manzolli, E.S.; Serpeloni, J.M.; Grotto, D.; Bastos, J.K.; Antunes, L.M.; Barbosa Junior, F.; Barcelos, G.R. Protective effects of the flavonoid chrysin against methylmercury-induced genotoxicity and alterations of antioxidant status, in vivo. Oxid. Med. Cell Longev. 2015, 2015, 602360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Notariale, R.; Infantino, R.; Palazzo, E.; Manna, C. Erythrocytes as a Model for Heavy Metal-Related Vascular Dysfunction: The Protective Effect of Dietary Components. Int. J. Mol. Sci. 2021, 22, 6604. [Google Scholar] [CrossRef] [PubMed]
- Barbhuiya, S.N.; Barhoi, D.; Giri, S. Impact of Arsenic on Reproductive Health. In Environmental Health; Otsuki, T., Ed.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Zadorozhnaja, T.D.; Little, R.E.; Miller, R.K.; Mendel, N.A.; Taylor, R.J.; Presley, B.J.; Gladen, B.C. Concentrations of arsenic, cadmium, copper, lead, mercury, and zinc in human placentas from two cities in Ukraine. J. Toxicol. Environ. Health A 2000, 61, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Li, X.F.; Cullen, W.R.; Weinfeld, M.; Le, X.C. Arsenic binding to proteins. Chem. Rev. 2013, 113, 7769–7792. [Google Scholar] [CrossRef] [PubMed]
- Pullella, K.; Kotsopoulos, J. Arsenic Exposure and Breast Cancer Risk: A Re-Evaluation of the Literature. Nutrients 2020, 12, 3305. [Google Scholar] [CrossRef] [PubMed]
- Quiller, G.; Merida-Ortega, A.; Rothenberg, S.J.; Cebrian, M.E.; Gandolfi, A.J.; Franco-Marina, F.; Lopez-Carrillo, L. Dietary flavonoids improve urinary arsenic elimination among Mexican women. Nutr. Res. 2018, 55, 65–71. [Google Scholar] [CrossRef]
- Authority, E.F.S. Bisphenol A: EFSA Draft Opinion Proposes Lowering the Tolerable Daily Intake. Available online: https://www.efsa.europa.eu/en/news/bisphenol-efsa-draft-opinion-proposes-lowering-tolerable-daily-intake (accessed on 15 December 2021).
- De Toni, L.; Sabovic, I.; Cosci, I.; Ghezzi, M.; Foresta, C.; Garolla, A. Testicular Cancer: Genes, Environment, Hormones. Front. Endocrinol. (Lausanne) 2019, 10, 408. [Google Scholar] [CrossRef] [PubMed]
- Matuszczak, E.; Komarowska, M.D.; Debek, W.; Hermanowicz, A. The Impact of Bisphenol A on Fertility, Reproductive System, and Development: A Review of the Literature. Int. J. Endocrinol. 2019, 2019, 4068717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maffini, M.V.; Rubin, B.S.; Sonnenschein, C.; Soto, A.M. Endocrine disruptors and reproductive health: The case of bisphenol-A. Mol. Cell. Endocrinol. 2006, 254–255, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Markey, C.M.; Luque, E.H.; Munoz De Toro, M.; Sonnenschein, C.; Soto, A.M. In utero exposure to bisphenol A alters the development and tissue organization of the mouse mammary gland. Biol. Reprod. 2001, 65, 1215–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulayeva, N.N.; Watson, C.S. Xenoestrogen-induced ERK-1 and ERK-2 activation via multiple membrane-initiated signaling pathways. Environ. Health Perspect. 2004, 112, 1481–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noguchi, S.; Nakatsuka, M.; Asagiri, K.; Habara, T.; Takata, M.; Konishi, H.; Kudo, T. Bisphenol A stimulates NO synthesis through a non-genomic estrogen receptor-mediated mechanism in mouse endothelial cells. Toxicol. Lett. 2002, 135, 95–101. [Google Scholar] [CrossRef]
- Wei, Y.; Han, C.; Li, S.; Cui, Y.; Bao, Y.; Shi, W. Cuscuta chinensis flavonoids down-regulate the DNA methylation of the H19/Igf2 imprinted control region and estrogen receptor alpha promoter of the testis in bisphenol A exposed mouse offspring. Food Funct. 2020, 11, 787–798. [Google Scholar] [CrossRef]
- Han, D.H.; Denison, M.S.; Tachibana, H.; Yamada, K. Relationship between estrogen receptor-binding and estrogenic activities of environmental estrogens and suppression by flavonoids. Biosci. Biotechnol. Biochem. 2002, 66, 1479–1487. [Google Scholar] [CrossRef] [Green Version]
- Meli, R.; Monnolo, A.; Annunziata, C.; Pirozzi, C.; Ferrante, M.C. Oxidative Stress and BPA Toxicity: An Antioxidant Approach for Male and Female Reproductive Dysfunction. Antioxidants 2020, 9, 405. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, A.; Thaker, R. Air pollutants and impairments of male reproductive health-an overview. Rev. Environ. Health 2021, 36, 565–575. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC Monogr. Eval. Carcinog. Risks Hum. 2010, 92, 1–853. [Google Scholar]
- Vogel, C.F.A.; Van Winkle, L.S.; Esser, C.; Haarmann-Stemmann, T. The aryl hydrocarbon receptor as a target of environmental stressors—Implications for pollution mediated stress and inflammatory responses. Redox Biol. 2020, 34, 101530. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Vazquez, C.; Quintana, F.J. Regulation of the Immune Response by the Aryl Hydrocarbon Receptor. Immunity 2018, 48, 19–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, W.; Wong, E.W.; Mruk, D.D.; Cheng, C.Y. TGF-beta3 and TNFalpha perturb blood-testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: A new concept of BTB regulation during spermatogenesis. Dev. Biol. 2009, 327, 48–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roychoudhury, S.; Chakraborty, S.; Choudhury, A.P.; Das, A.; Jha, N.K.; Slama, P.; Nath, M.; Massanyi, P.; Ruokolainen, J.; Kesari, K.K. Environmental Factors-Induced Oxidative Stress: Hormonal and Molecular Pathway Disruptions in Hypogonadism and Erectile Dysfunction. Antioxidants 2021, 10, 837. [Google Scholar] [CrossRef] [PubMed]
- Poljsak, B.; Fink, R. The protective role of antioxidants in the defence against ROS/RNS-mediated environmental pollution. Oxid. Med. Cell. Longev. 2014, 2014, 671539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matzkin, M.E.; Calandra, R.S.; Rossi, S.P.; Bartke, A.; Frungieri, M.B. Hallmarks of Testicular Aging: The Challenge of Anti-Inflammatory and Antioxidant Therapies Using Natural and/or Pharmacological Compounds to Improve the Physiopathological Status of the Aged Male Gonad. Cells 2021, 10, 3114. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, K.; Aggarwal, B.B.; Singh, R.B.; Buttar, H.S.; Wilson, D.; De Meester, F. Food Antioxidants and Their Anti-Inflammatory Properties: A Potential Role in Cardiovascular Diseases and Cancer Prevention. Diseases 2016, 4, 28. [Google Scholar] [CrossRef]
- Mani, V.M.; Soundari, A.; Balasubramanian, B.; Park, S.; Issara, U.; Preethi, K.; Liu, W.C. Evaluation of Dimer of Epicatechin from an Endophytic Fungus Curvularia australiensis FC2AP on Acute Toxicity Levels, Anti-Inflammatory and Anti-Cervical Cancer Activity in Animal Models. Molecules 2021, 26, 654. [Google Scholar] [CrossRef]
- Kim, M.; Jee, S.C.; Kim, K.S.; Kim, H.S.; Yu, K.N.; Sung, J.S. Quercetin and Isorhamnetin Attenuate Benzo[a]pyrene-Induced Toxicity by Modulating Detoxification Enzymes through the AhR and NRF2 Signaling Pathways. Antioxidants 2021, 10, 787. [Google Scholar] [CrossRef]
- Singletary, K.W.; Jung, K.J.; Giusti, M. Anthocyanin-rich grape extract blocks breast cell DNA damage. J. Med. Food 2007, 10, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Androutsopoulos, V.P.; Tsatsakis, A.M. Benzo[a]pyrene sensitizes MCF7 breast cancer cells to induction of G1 arrest by the natural flavonoid eupatorin-5-methyl ether, via activation of cell signaling proteins and CYP1-mediated metabolism. Toxicol. Lett. 2014, 230, 304–313. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; et al. Risk for animal and human health related to the presence of dioxins and dioxin-like PCBs in feed and food. EFSA J. 2018, 16, e05333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Ye, Y.; Huang, F.; Chen, H.; Wu, H.; Huang, J.; Hu, J.; Xia, D.; Wu, Y. Association between dioxin and cancer incidence and mortality: A meta-analysis. Sci. Rep. 2016, 6, 38012. [Google Scholar] [CrossRef]
- Mocarelli, P.; Gerthoux, P.M.; Patterson, D.G., Jr.; Milani, S.; Limonta, G.; Bertona, M.; Signorini, S.; Tramacere, P.; Colombo, L.; Crespi, C.; et al. Dioxin exposure, from infancy through puberty, produces endocrine disruption and affects human semen quality. Environ. Health Perspect. 2008, 116, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Kolluri, S.K.; Jin, U.H.; Safe, S. Role of the aryl hydrocarbon receptor in carcinogenesis and potential as an anti-cancer drug target. Arch. Toxicol. 2017, 91, 2497–2513. [Google Scholar] [CrossRef] [PubMed]
- Gu, A.; Ji, G.; Long, Y.; Zhou, Y.; Shi, X.; Song, L.; Wang, X. Assessment of an association between an aryl hydrocarbon receptor gene (AHR) polymorphism and risk of male infertility. Toxicol. Sci. 2011, 122, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Goya-Jorge, E.; Jorge Rodriguez, M.E.; Veitia, M.S.; Giner, R.M. Plant Occurring Flavonoids as Modulators of the Aryl Hydrocarbon Receptor. Molecules 2021, 26, 2315. [Google Scholar] [CrossRef]
- Romagnolo, D.F.; Donovan, M.G.; Papoutsis, A.J.; Doetschman, T.C.; Selmin, O.I. Genistein Prevents BRCA1 CpG Methylation and Proliferation in Human Breast Cancer Cells with Activated Aromatic Hydrocarbon Receptor. Curr. Dev. Nutr. 2017, 1, e000562. [Google Scholar] [CrossRef] [Green Version]
- Donovan, M.G.; Selmin, O.I.; Doetschman, T.C.; Romagnolo, D.F. Epigenetic Activation of BRCA1 by Genistein In Vivo and Triple Negative Breast Cancer Cells Linked to Antagonism toward Aryl Hydrocarbon Receptor. Nutrients 2019, 11, 2559. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.Q.; Chiu-Leung, L.C.; Lin, S.M.; Leung, L.K. The citrus flavonone hesperetin attenuates the nuclear translocation of aryl hydrocarbon receptor. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2018, 210, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Kitakaze, T.; Makiyama, A.; Nakai, R.; Kimura, Y.; Ashida, H. Kaempferol modulates TCDD- and t-BHQ-induced drug-metabolizing enzymes and luteolin enhances this effect. Food Funct. 2020, 11, 3668–3680. [Google Scholar] [CrossRef] [PubMed]
- Jin, U.H.; Park, H.; Li, X.; Davidson, L.A.; Allred, C.; Patil, B.; Jayaprakasha, G.; Orr, A.A.; Mao, L.; Chapkin, R.S.; et al. Structure-Dependent Modulation of Aryl Hydrocarbon Receptor-Mediated Activities by Flavonoids. Toxicol. Sci. 2018, 164, 205–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP); Aids, P.; Silano, V.; Barat Baviera, J.M.; Bolognesi, C.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; et al. Update of the risk assessment of di-butylphthalate (DBP), butyl-benzyl-phthalate (BBP), bis(2-ethylhexyl)phthalate (DEHP), di-isononylphthalate (DINP) and di-isodecylphthalate (DIDP) for use in food contact materials. EFSA J. 2019, 17, e05838. [Google Scholar] [CrossRef] [Green Version]
- Hlisnikova, H.; Petrovicova, I.; Kolena, B.; Sidlovska, M.; Sirotkin, A. Effects and Mechanisms of Phthalates’ Action on Reproductive Processes and Reproductive Health: A Literature Review. Int. J. Environ. Res. Public Health 2020, 17, 6811. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Ma, B.F.; Liu, B.; Ding, P.; Wei, J.H.; Cheng, P.; Li, S.Y.; Chen, D.X.; Sun, Z.J.; Li, Z. The Involvement of the Chemokine RANTES in Regulating Luminal Acidification in Rat Epididymis. Front. Immunol. 2020, 11, 583274. [Google Scholar] [CrossRef] [PubMed]
- Rebourcet, D.; Odet, F.; Verot, A.; Combe, E.; Meugnier, E.; Pesenti, S.; Leduque, P.; Dechaud, H.; Magre, S.; Le Magueresse-Battistoni, B. The effects of an in utero exposure to 2,3,7,8-tetrachloro-dibenzo-p-dioxin on male reproductive function: Identification of Ccl5 as a potential marker. Int. J. Androl. 2010, 33, 413–424. [Google Scholar] [CrossRef] [Green Version]
- Hsu, Y.L.; Hsieh, C.J.; Tsai, E.M.; Hung, J.Y.; Chang, W.A.; Hou, M.F.; Kuo, P.L. Didymin reverses phthalate ester-associated breast cancer aggravation in the breast cancer tumor microenvironment. Oncol. Lett. 2016, 11, 1035–1042. [Google Scholar] [CrossRef] [Green Version]
- Merida-Ortega, A.; Hernandez-Alcaraz, C.; Hernandez-Ramirez, R.U.; Garcia-Martinez, A.; Trejo-Valdivia, B.; Salinas-Rodriguez, A.; Svensson, K.; Cebrian, M.E.; Franco-Marina, F.; Lopez-Carrillo, L. Phthalate exposure, flavonoid consumption and breast cancer risk among Mexican women. Environ. Int. 2016, 96, 167–172. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montano, L.; Maugeri, A.; Volpe, M.G.; Micali, S.; Mirone, V.; Mantovani, A.; Navarra, M.; Piscopo, M. Mediterranean Diet as a Shield against Male Infertility and Cancer Risk Induced by Environmental Pollutants: A Focus on Flavonoids. Int. J. Mol. Sci. 2022, 23, 1568. https://doi.org/10.3390/ijms23031568
Montano L, Maugeri A, Volpe MG, Micali S, Mirone V, Mantovani A, Navarra M, Piscopo M. Mediterranean Diet as a Shield against Male Infertility and Cancer Risk Induced by Environmental Pollutants: A Focus on Flavonoids. International Journal of Molecular Sciences. 2022; 23(3):1568. https://doi.org/10.3390/ijms23031568
Chicago/Turabian StyleMontano, Luigi, Alessandro Maugeri, Maria Grazia Volpe, Salvatore Micali, Vincenzo Mirone, Alberto Mantovani, Michele Navarra, and Marina Piscopo. 2022. "Mediterranean Diet as a Shield against Male Infertility and Cancer Risk Induced by Environmental Pollutants: A Focus on Flavonoids" International Journal of Molecular Sciences 23, no. 3: 1568. https://doi.org/10.3390/ijms23031568
APA StyleMontano, L., Maugeri, A., Volpe, M. G., Micali, S., Mirone, V., Mantovani, A., Navarra, M., & Piscopo, M. (2022). Mediterranean Diet as a Shield against Male Infertility and Cancer Risk Induced by Environmental Pollutants: A Focus on Flavonoids. International Journal of Molecular Sciences, 23(3), 1568. https://doi.org/10.3390/ijms23031568











