Regulation of the Bud Dormancy Development and Release in Micropropagated Rhubarb ‘Malinowy’
Abstract
:1. Introduction
2. Results
2.1. Growth of In Vitro Propagated Plantlets in the Greenhouse and Dormancy Induction
2.2. Growth of In Vitro Propagated Plantlets in the Greenhouse and Dormancy Induction
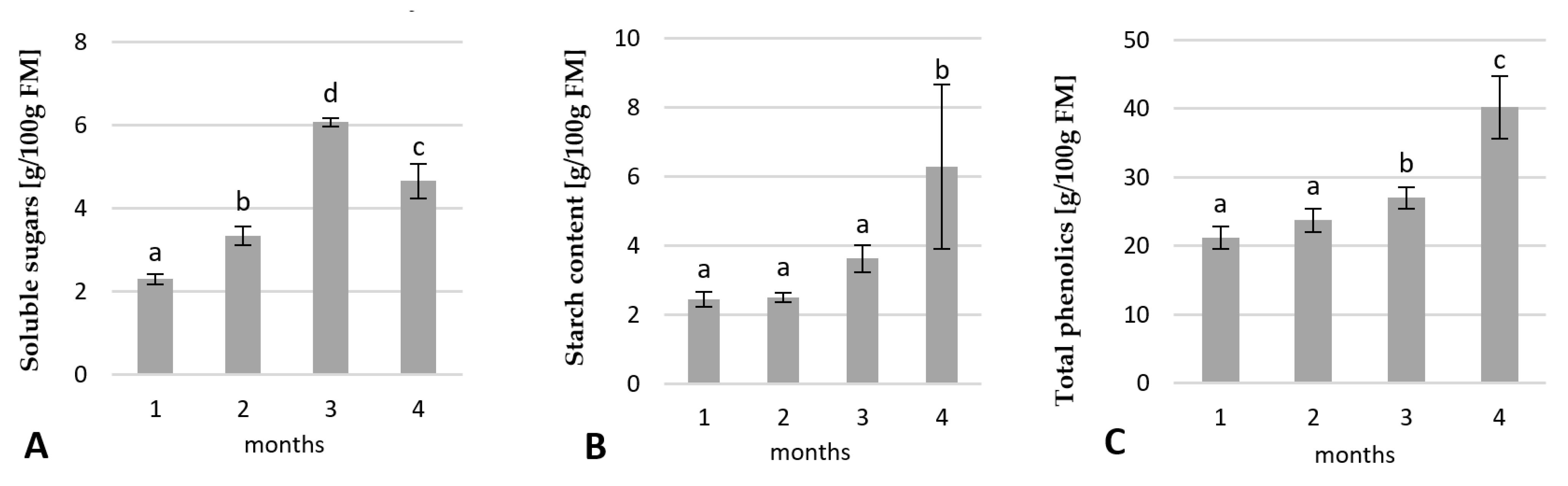
2.2.1. Changes in the Carbohydrates and Total Phenolic Contents
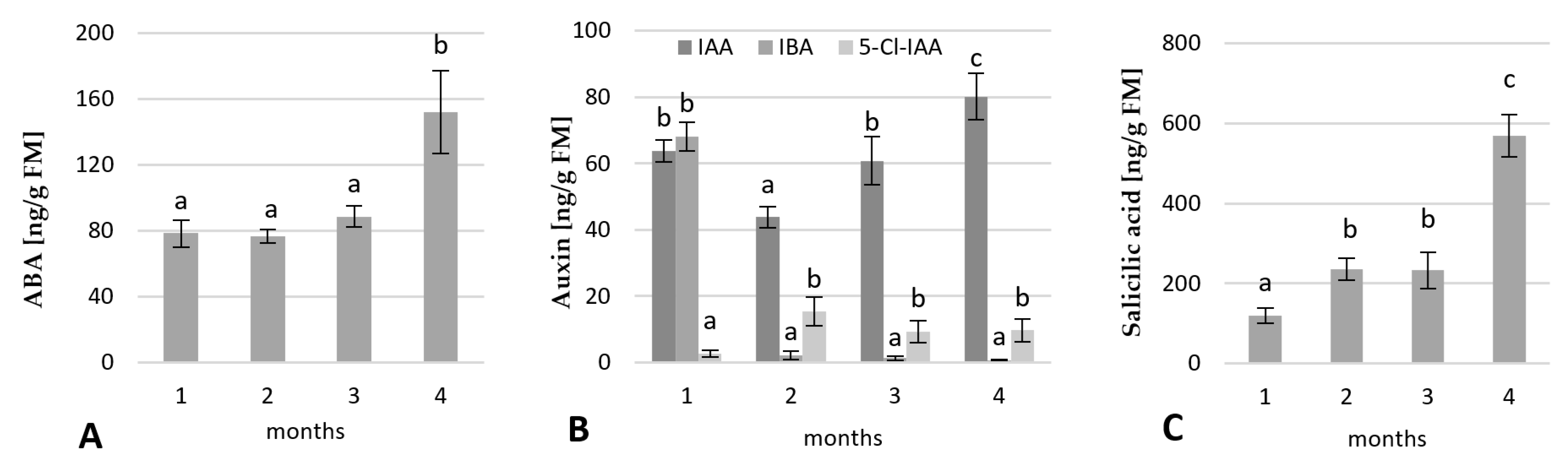
2.2.2. Change in the Phytohormone Contents
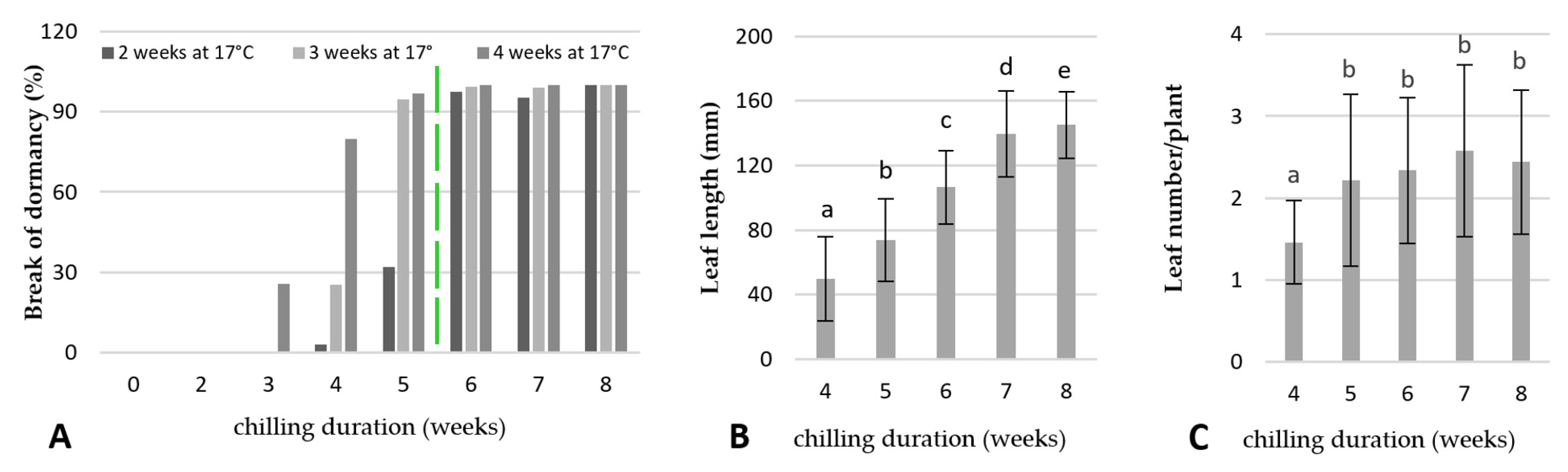
2.3. Break of Dormancy/Chilling Requirements for Shoot Induction and Growth
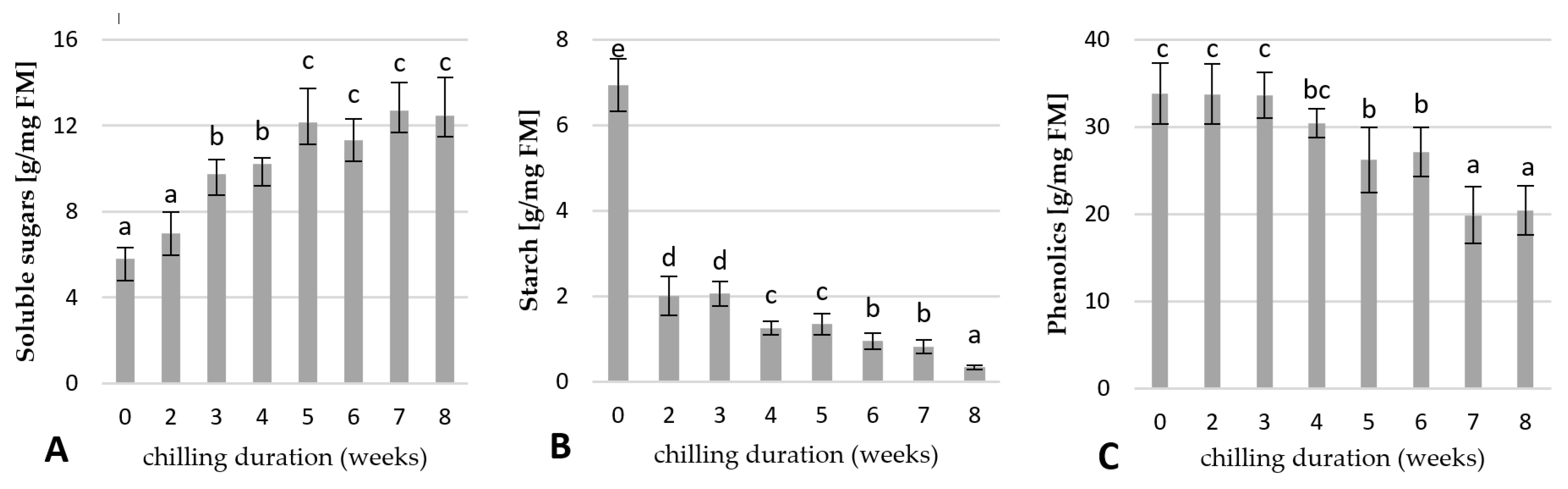
2.4. Physiological Responses of the Underground Buds during Cold Treatment
2.4.1. Changes in the Carbohydrates and Total Phenolic Contents
2.4.2. Change in the Endogenous Hormone Contents
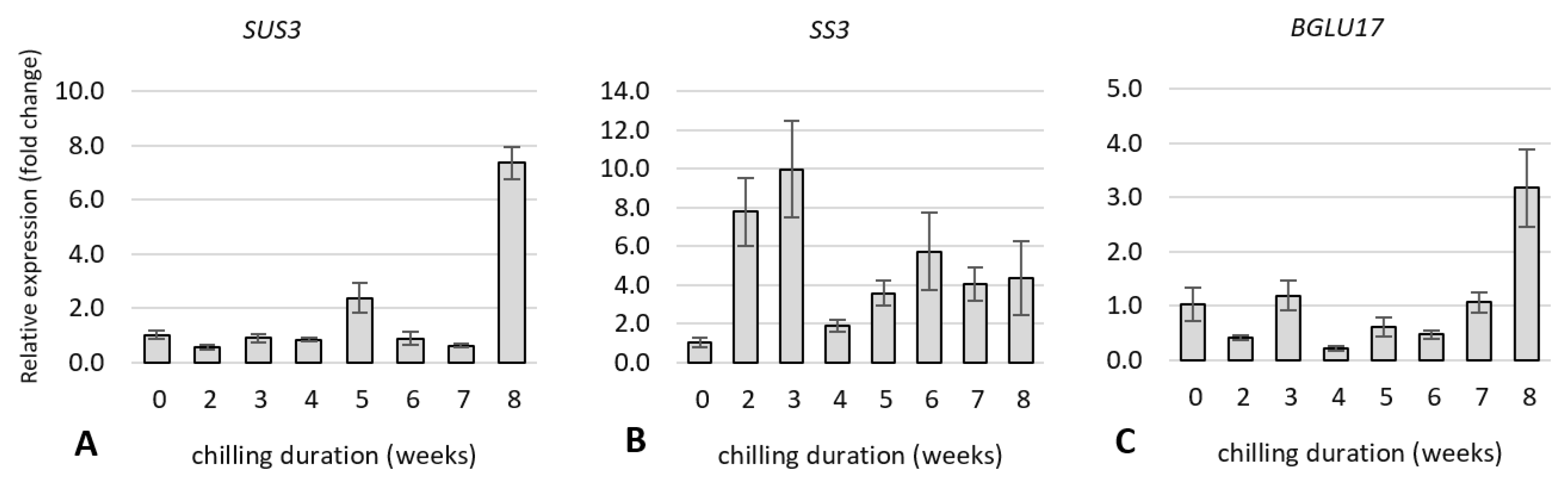
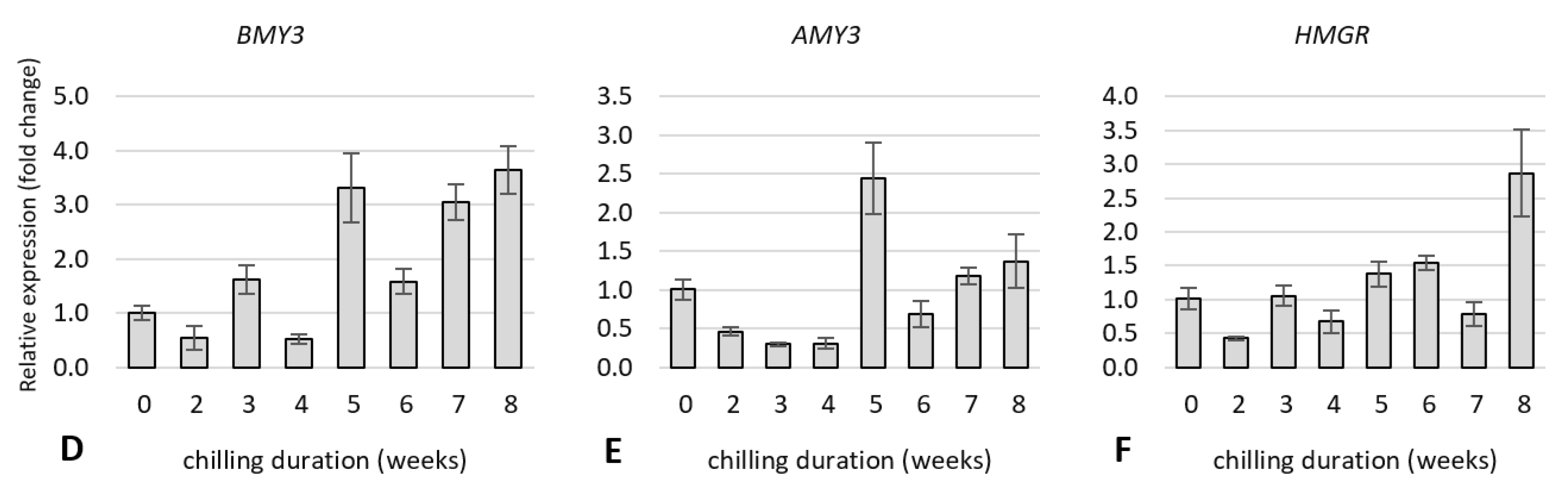
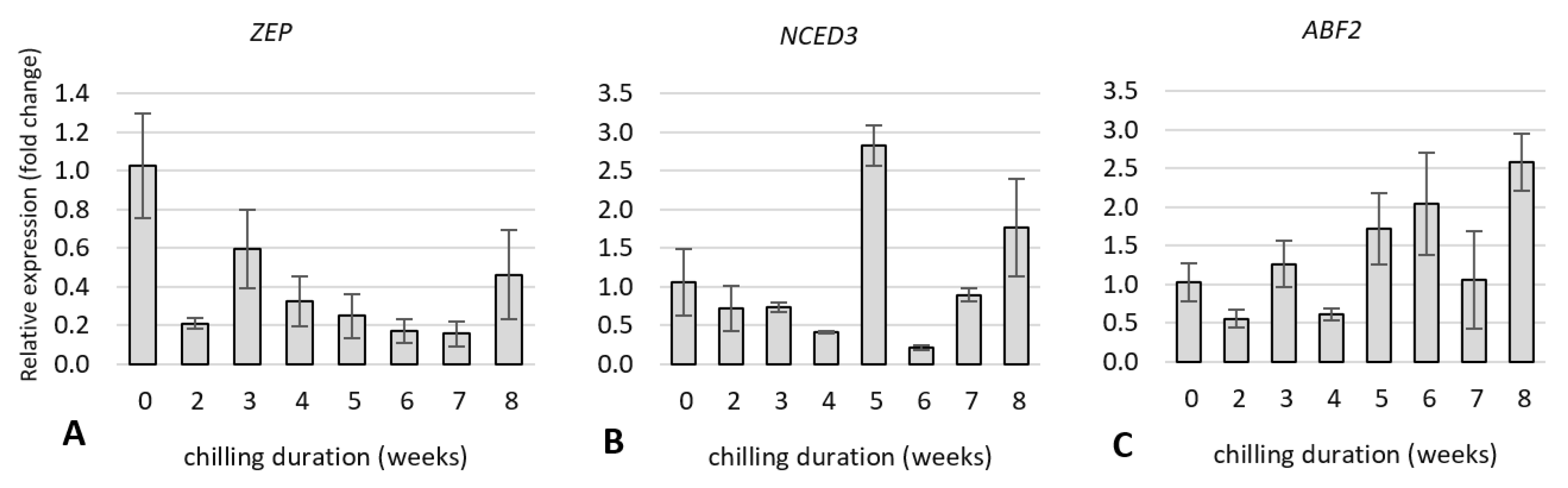
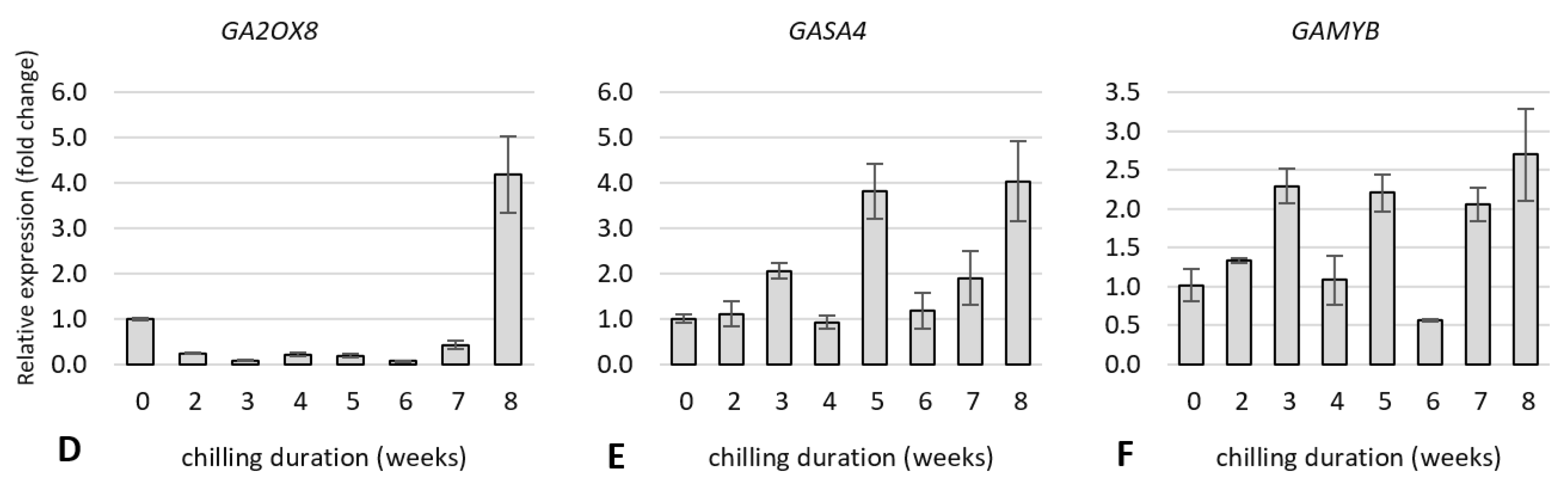
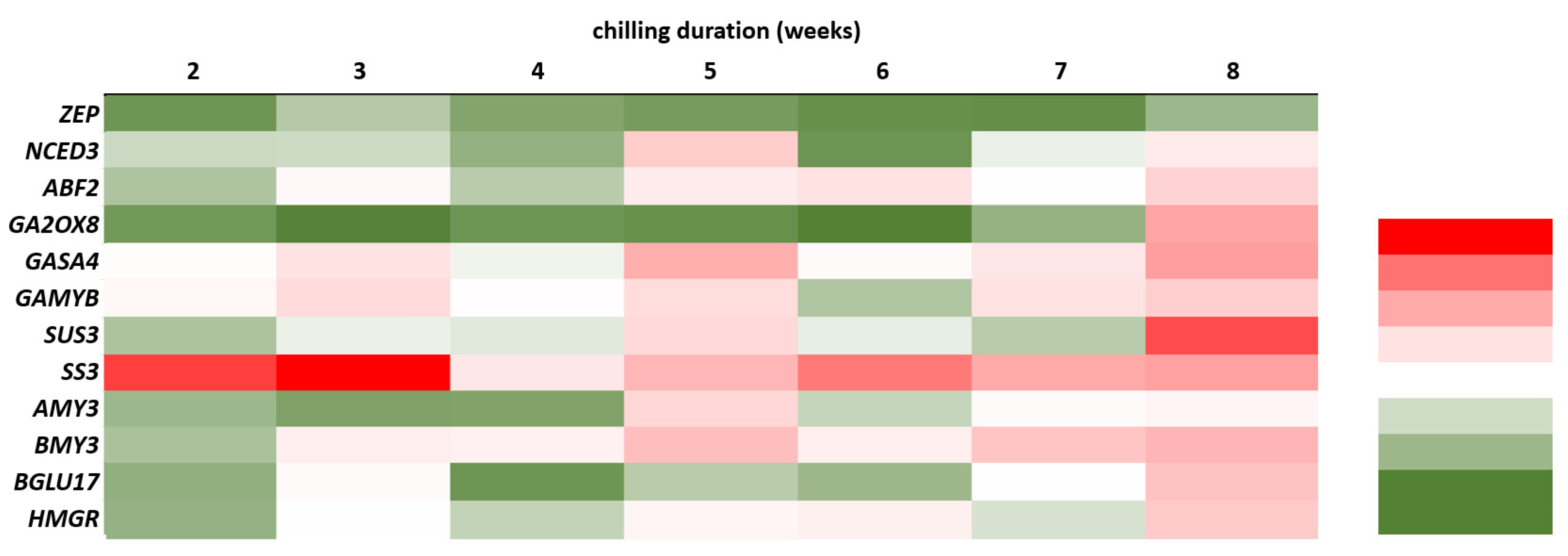
2.5. Expression Analysis of Dormancy-Related Genes in Plants during Cold Treatment
2.5.1. Expression of Genes Related to Carbohydrate and Flavonoid Metabolism
2.5.2. Expression of Genes Related to Hormone Metabolism and Hormone Signal Transduction Pathway
3. Discussion
3.1. Dormancy Induction
3.2. Dormancy Release
4. Materials and Methods
4.1. Plant Material
4.2. Growth in the Greenhouse/Dormancy Induction
4.3. Evaluation of Dormancy Breaking in Buds at Controlled Conditions
4.4. Measurements of Soluble Sugar Contents
4.5. Measurements of Starch Content
4.6. Measurements of Total Phenolic Contents
4.7. Quantification of Aux, ABA and SA
4.8. Molecular Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, J.V.; Chao, W.S.; Horvath, D.P. A current review on the regulation of dormancy in vegetative buds. Weed Sci. 2001, 49, 571–589. [Google Scholar] [CrossRef]
- Chao, W.S.; Foley, M.E.; Horvath, D.P.; Anderson, J.V. Signalling regulating dormancy in vegetative buds. Int. J. Plant Dev. Biol. 2007, 1, 49–56. [Google Scholar]
- Podwyszyńska, M. The mechanisms of in vitro storage organ formation in ornamental geophytes. Floricult. Ornam. Biotechnol 2012, 6, 9–23. [Google Scholar]
- Shim, D.; Ko, J.H.; Kim, W.C.; Wang, Q.; Keatthley, D.E.; Han, K.H. A molecular framework for seasonal growth-dormancy regulation in perennial plants. Hortic. Res. 2014, 1, 14059. [Google Scholar] [CrossRef] [Green Version]
- Lang, G.A.; Early, J.D.; Martin, G.C.; Darnell, R.L. Endo-, para-, and ecodormancy: Physiological terminology and classification for dormancy research. HortScience 1987, 22, 371–377. [Google Scholar]
- Zhuang, W.; Gao, Z.; Wen, L.; Huo, X.; Cai, B.; Zhang, Z. Metabolic changes upon flower bud break in Japanese apricot are enchanced by exogenous GA4. Hortic. Res. 2015, 2, 15046. [Google Scholar] [CrossRef] [Green Version]
- Michailidis, M.; Karagiannis, E.; Tanou, K.; Sarrou, E.; Adamakis, I.D.; Karamanoli, K.; Martens, S. Metabolic mechanisms underpinning vegetative bud dormancy release and shoot development in sweet cherry. Environ. Exp. Bot. 2018, 155, 1–11. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.; Shi, X.; Shao, L.; Xu, T.; Xia, Y.; Li, D.; Zhang, J. Chilling requirement validation and physiological and molecular responses of the bud endodormancy release in Paeonia lactiflora Pall. Int. J. Mol. Sci. 2021, 22, 8382. [Google Scholar] [CrossRef]
- Horvath, D.P.; Chao, W.S.; Suttle, J.C.; Thimmapuram, J.; Anderson, J.V. Transcriptome analysis identifies novel responses and potential regulatory genes involved in seasonal dormancy transitions of leafy spurge (Euphorbia esula L.). BMC Genom. 2008, 9, 536. [Google Scholar] [CrossRef] [Green Version]
- Xiang, H.; Zuo, J.; Guo, F.; Dong, D. What we already know about rhubarb: A comprehensive review. Chin. Med. 2020, 15, 88. [Google Scholar] [CrossRef]
- Bratsch, A.; Mainville, D. Specialty Crop Profile: Rhubarb; VTP: Blacksburg, VA, USA, 2005. [Google Scholar]
- Krafczyk, N.; Kötke, M.; Lehnert, N.; Glomb, M.A. Phenolic composition of rhubarb. Eur. Food Reseach Technol. 2008, 228, 187–196. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Dao, L.; Harden, L.; Pantoja, A.; Kuhl, J.C. Antioxidant activity, phenolic and anthocyanin contents of various rhubarb (Rheum spp.) varieties. Int. J. Food Sci. Technol. 2013, 48, 172–178. [Google Scholar] [CrossRef]
- Kalisz, S.; Oszmiański, J.; Kolniak-Ostek, J.; Grobelna, A.; Kieliszek, M.; Cendrowski, A. Effect of a variety of polyphenols compounds and antioxidant properties of rhubarb (Rheum rhabarbarum). Food Sci. Technol. 2020, 118, 108775. [Google Scholar] [CrossRef]
- Kozak, D.; Sałata, A. Effect of cytokinins on in vitro multiplication of rhubarb (Rheum rhaponticum L.) ‘Karpov Lipskiego’ shoots and ex vitro acclimatization and growth. Acta Sci. Pol. Hortorum Cultus 2011, 10, 75–87. [Google Scholar]
- Clapa, D.; Borsai, O.; Hârta, M.; Bonta, V.; Szabo, K.; Coman, V.; Bobis, O. Micropropagation, genetic fidelity and phenolic compound of Rheum rhabarbarum L. Plants 2020, 9, 656. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J. Virus Identification and Development of Long-Term Management Strategies for the Rhubarb Industry; Final Report Hal Project VG05053; Department of Agriculture, Forestry and Fisheries: Pretoria, South Africa, 2011; p. 93.
- Wojtania, A.; Mieszczakowska-Frąc, M. In Vitro Propagation Method for Production of Phenolic-Rich Planting Material of Culinary Rhubarb ‘Malinowy’. Plants 2021, 10, 1768. [Google Scholar] [CrossRef]
- Schrader, W.L. Rhubarb Production in California; UCANR Publications: San Diego, CA, USA, 2000. [Google Scholar]
- Palonem, P. Vegetative growth, cold acclimation, and dormancy as affected by temperature and photoperiod in six reed raspberry (Rubus idaeus L.) cultivars. Eur. J. Hort. Sci. 2006, 71, 1–6. [Google Scholar]
- Anderson, J.V.; Gesch, R.W.; Jia, Y.; Chao, W.S.; Horvath, D.P. Seasonal shifts in dormancy status, carbohydrate metabolism, and related gene expression in crown buds of leafy spurge. Plant Cell Environ. 2005, 28, 1567–1578. [Google Scholar] [CrossRef]
- Yuxi, Z.; Dan, Y.; Chunyng, L.; Shupeng, G. Dynamic of carbohydrate metabolism and the related genes highlights PPP pathway activation chilling induced bud dormancy release in tree peony (Paeonia suffruticosa). Sci. Hortic. 2018, 242, 36–42. [Google Scholar] [CrossRef]
- Baldermann, S.; Homann, T.; Neugart, S.; Chmielewski, F.M.; Götz, K.P.; Gödeke, K.P.; Huschek, G.; Morlock, G.E.; Rawal, H.M. Selected plant metabolites involved in oxidation-reduction process during bud dormancy and ontogenetic development in sweet cherry buds (Prunus avium L.). Molecules 2018, 23, 1197. [Google Scholar] [CrossRef] [Green Version]
- Horvath, D.P. Role of mature leaves in inhibition of root bud growth in Euphorbia esula L. Weed Sci. 1999, 47, 544–550. [Google Scholar] [CrossRef]
- Horvath, D.; Anderson, J.V.; Chao, W.S.; Foley, M.E. Knowing when to grow: Signals regulating bud dormancy. Trends Plant Sci. 2003, 8, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.S.; Serpe, M.D.; Anderson, J.V.; Gesch, R.W.; Horvath, D.P. Sugars, hormones, and environment affects the dormancy status in underground adventitious buds of leafy spurge (Euphorbia esula). Weed Sci. 2006, 54, 59–68. [Google Scholar] [CrossRef]
- Tarancón, C.; González-Grandío, E.; Oliveros, J.C.; Nicolasand, M.; Cubas, P. Conserved carbon starvation response underlies bud dormancy in woody and herbaceous species. Front. Plant Sci. 2017, 8, 788. [Google Scholar] [CrossRef] [Green Version]
- Larisch, C.; Dittrich, M.; Wildhagen, H.; Lautner, S.; Fromm, J.; Polle, A.; Hedrich, R.; Rennenberg, H.; Müller, T.; Ache, T. Poplar wood rays are involved in seasonal remodeling of tree physiology. Plant Physiol. 2012, 160, 1515–1529. [Google Scholar] [CrossRef] [Green Version]
- Zheng, C.; Halaly, T.; Acheampong, A.K.; Takebayashi, Y.; Jikumaru, Y.; Kamiya, Y.; Or, E. Abscisic acid (ABA) regulates grape bud dormancy, and dormancy release stimuli may act through modification of ABA metabolism. J. Exp. Bot. 2015, 66, 1527–1542. [Google Scholar] [CrossRef]
- Wang, D.; Gao, Z.; Du, P.; Xiao, W.; Tan, Q.; Chen, X.; Li, L.; Gao, D. Expression of ABA metabolism-related genes suggests similarities and differences between seed dormancy and bud dormancy of peach (Prunus persica). Front. Plant Sci. 2016, 6, 1248. [Google Scholar] [CrossRef]
- Tuan, P.A.; Bai, S.; Saito, T.; Ito, A.; Moriguchi, T. Dormancy-Associated MADS-Box (DAM) and the abscisic acid pathway regulate pear endodormancy through a feedback mechanism. Plant Cell Physiol. 2017, 58, 1378–1390. [Google Scholar] [CrossRef]
- Rubio, S.; Noriega, X.; Pérez, F.J. ABA promotes starch synthesis and storage metabolism in dormant grapevine buds. J. Plant Physiol. 2019, 234, 1–8. [Google Scholar] [CrossRef]
- Sorce, C.; Lombardi, L.; Giorgetti, N.; Parisi, B.; Ranalli, P.; Lorenzi, R. Indoleacetic acid concetration and metabolism changes during bud development in tubers of two potato (Solanum tuberosum) cultivars. J. Plant Physiol. 2009, 166, 1023–1033. [Google Scholar] [CrossRef]
- Li, C.; Junttila, O.; Ernstsen, A.; Heino, P.; Palva, E.T. Photoperiodic control of growth, cold acclimation and dormancy development in silver birch (Betula pendula) ecotypes. Physiol. Plant. 2003, 117, 206–212. [Google Scholar] [CrossRef]
- Nagar, P.K. Changes in abscisic acid, phenols and indole acetic acid in bulbs of tuberose (Polianthes tuberosa L.) during dormancy andsprouting. Sci. Hortic. 1995, 63, 77–82. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Zhao, Y.; Feng, Z.; Li, Q.; Yang, H.Q.; Luan, S.; Li, J.; He, Z.H. Auxin controls seed dormancy. Proc. Natl. Acad. Sci. USA 2013, 110, 15485–154490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Zhang, J.; Liu, Y.; Zhao, J.; Fu, J.; Ren, X.; Wang, G.; Wang, J. Exogenous auxin regulates multi-metabolic network and embryo development, controlling seed secondary dormancy and germination in Nicotiana tabacum L. BMC Plant Biol. 2016, 16, 41. [Google Scholar] [CrossRef] [Green Version]
- Sharma, E.; Sharma, R.; Borah, P.; Jain, M.; Khurana, J.P. Emerging roles of auxin in abiotic stress responses. In Elucidation of Abiotic Stress Signaling in Plants; Pandey, G.K., Ed.; Springer: New York, NY, USA, 2015; pp. 299–328. [Google Scholar]
- Franklin, G.; Dias, A.C.P. Chlorogenic acid participates in the regulation of shoot, root and root hair development in Hypericum perforatum. Plant Physiol. Biochem. 2011, 49, 835–842. [Google Scholar] [CrossRef]
- Bach, A.; Kapczyńska, A.; Dziurka, K.; Dziurka, M. The importance of applied light quality on the process of shoot organogenesis and production of phenolics and carbohydrates in Lachenalia sp. cultures in vitro. S. Afr. J. Bot. 2018, 114, 14–19. [Google Scholar] [CrossRef]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Zhang, Z.L.; Hanzlik, S.; Cook, E.; Shen, Q.J. Salicylic acid inhibits gibberellin-induced alpha-amylase expression and seed germination via a pathway involving an abscisic-acid-inducible WRKY gene. Plant Mol. Biol. 2007, 64, 293–303. [Google Scholar] [CrossRef]
- Bankeblia, N. Low temperature and breaking of dormancy effects on respiration rate, sugars, phenolics and peroxidase activity changes in inner buds of onion Allium cepa L. Acta Agric. Scand. Sect. B Soil Plant Sci. 2003, 53, 16–20. [Google Scholar] [CrossRef]
- Podwyszynska, M. Improvement of bulb formation in micropropagated tulips by treatment with NAA and paclobutrazol or ancymidol. Acta Hortic. 2004, 725, 679–684. [Google Scholar] [CrossRef]
- Tang, N.; Jia, R.; Yin, J.; Wang, Y.; Tang, D. Effects of cold treatments on seedling emergence and growth of Lilium davidii var. unicolor bulblets. HortScience 2021, 56, 1119–1124. [Google Scholar] [CrossRef]
- Country Folks Grower. Growing Rhubarb in Warmer Climates. Available online: https://cfgrower.com/growing-rhubarb-in-warmer-climates (accessed on 28 December 2021).
- Evans, M.R.; Anderson, N.O.; Wilkins, H.F. Temperature and GA3 effects on emergence and flowering of potted Paeonia lactiflora. HortScience 1990, 25, 923–924. [Google Scholar] [CrossRef]
- Xu, R.Y.; Niimi, Y.; Han, D.S. Changes in endogenous abscisic acid and soluble sugars levels during dormancy-release in bulbs of Lilium rubellum. Sci. Hortic. 2006, 111, 68–72. [Google Scholar] [CrossRef]
- Gibson, S.I. Sugar and phytohormone response pathways: Navigating a signalling network. J. Exp. Bot. 2004, 55, 253–264. [Google Scholar] [CrossRef]
- Ciereszko, I. Regulatory roles of sugars in plant growth and development. Acta Soc. Bot. Pol. 2018, 87, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Saniewski, M.; Kawa-Miszczak, L.; Węgrzynowicz-Lesiak, E.; Okubo, H. Role of ABA, gibberellins and auxin in dormancy and dormancy release of tulip bulbs. In Dormancy in Plants: From Whole Plant Behaviour to Cellular Control; Viémont, J.D., Crabbé, J., Eds.; CABI Publishing: Wallingford, UK, 2000. [Google Scholar]
- Yuxi, Z.; Yanchao, Y.; Zejun, L.; Tao, Z.; Feng, L.; Chunying, L.; Shupeng, G. GA3 is superior to GA4 in promoting bud endodormancy release in tree peony (Paeonia suffruticosa) and their potential working mechanism. BMC Plant Biol. 2021, 21, 323. [Google Scholar] [CrossRef]
- Pan, W.; Liang, J.; Sui, J.; Li, J.; Liu, C.; Xin, Y.; Zhand, Y.; Wang, S.; Zhao, Y.; Zhang, J.; et al. ABA and bud dormancy in perennials: Current knowledge and future perspective. Genes 2021, 12, 1635. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhuo, X.; Zhao, K.; Zheng, T.; Han, Y.; Yuan, C.; Zhang, Q. Transcriptome profiles reveal the crucial roles of hormone and sugar in the bud dormancy of Prunus mume. Sci. Rep. 2018, 8, 5090. [Google Scholar] [CrossRef] [Green Version]
- Seo, M.; Hanada, A.; Kuwahara, A.; Endo, A.; Okamoto, M.; Yamauchi, Y.; North, H.; Marion-Poll, A.; Sun, T.; Koshiba, T.; et al. Regulation of hormone metabolism in Arabidopsis seeds: Phytochrome regulation of abscisic acid metabolism and abscisic acid regulation of gibberellin metabolism. Plant J. 2006, 48, 354–366. [Google Scholar] [CrossRef]
- Ariizumi, T.; Hauvermale, A.L.; Nelson, S.K.; Hanada, A.; Yamaguchi, S.; Steber, C.M. Lifting DELLA repression of Arabidopsis seed germination by nonproteolytic gibberellin signaling. Plant Physiol. 2013, 162, 2125–2139. [Google Scholar] [CrossRef] [Green Version]
- Korasick, D.A.; Enders, T.A.; Strader, L.C. Auxin biosynthesis and storage forms. J. Exp. Bot. 2013, 64, 2541–2555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frick, E.M.; Strader, L.C. Roles for IBA-derived auxin in plant development. J. Exp. Bot. 2018, 69, 169–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagar, P.K.; Sood, S. Changes in endogenous auxins during winter dormancy in tea (Camellia sinensis L.) O. Kuntze. Acta Physiol. Plant. 2006, 28, 165–169. [Google Scholar] [CrossRef]
- Said, Q.; Ez-Zohra, I.F.; Mohamed, F.; Tayeb, K. Changes in levels of hydrogen peroxide and phenolic compounds in grapevine latent buds during the annual cycle. Int. J. Sci. Res. Public 2014, 4, 1–5. [Google Scholar]
- Karimi, R.; Ershadi, A. Role of exogenous abscisic acid in adapting of ‘Sultana’ grapevine to low temperature stress. Acta Physiol. Plant. 2015, 37, 151. [Google Scholar] [CrossRef]
- Pakkish, Z.; Rahemi, M.; Baghizadeh, A. Seasonal changes of peroxidase, polyphenol oxidase enzyme activity and phenol content during and after rest in pistachio (Pistacia vera L.) flower buds. World Appl. Sci. J. 2009, 6, 1193–1199. [Google Scholar]
- Gheshlaghi, E.A.; Rabiei, V.; Ghasemi, M.; Fattahi, J.; Razavi, F. Phenolic metabolism and antioxidant activity during endodormancy of Kiwifruit buds. Adv. Hortic. Sci. 2017, 31, 225–337. [Google Scholar]
- Benkeblia, N.; Selselet-Attou, G. Effects of low temperature on chandes in oligosaccharides, phenolics and peroxidase in inner bud of onion Allium cepa L. during break of dormancy. Acta Agric. Scand. Sect. B Soil Plant Sci. 1999, 49, 98–102. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Singleton, V.S.; Rossi, J.J.A. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagent. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Dobrev, P.I.; Kaminek, M. Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction. J. Chromatogr. A 2002, 950, 21–29. [Google Scholar] [CrossRef]
- Stefancic, M.; Stampar, F.; Veberic, R.; Osterc, G. The levels of IAA, IAAsp and some phenolics in cherry rootstock ‘GiSelA 5′ leafy cuttings pretreated with IAA and IBA. Sci. Hortic. 2007, 112, 399–405. [Google Scholar] [CrossRef]
- Chang, S.; Puryear, J.; Cairney, J. A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Rep. 1993, 11, 113–116. [Google Scholar] [CrossRef]
- Mala, D.; Awasthi, S.; Sharma, N.K.; Swarnkar, M.K.; Shankar, R.; Kumar, S. Comparative transcriptome analysis of Rheum australe, an endangered medicinal herb, growing in its natural habitat and those grown in controlled growth chambers. Sci. Rep. 2021, 11, 3702. [Google Scholar] [CrossRef]

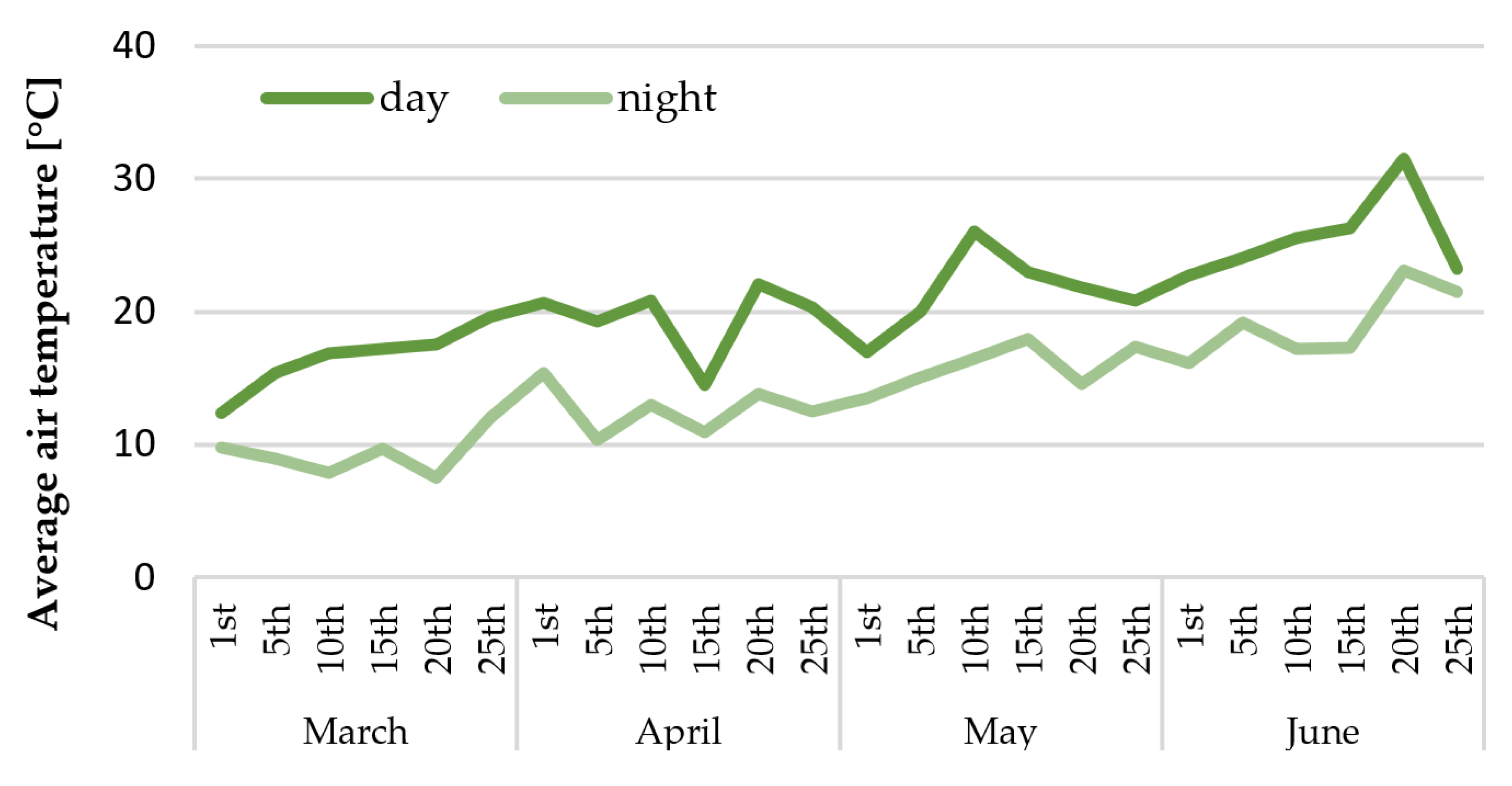
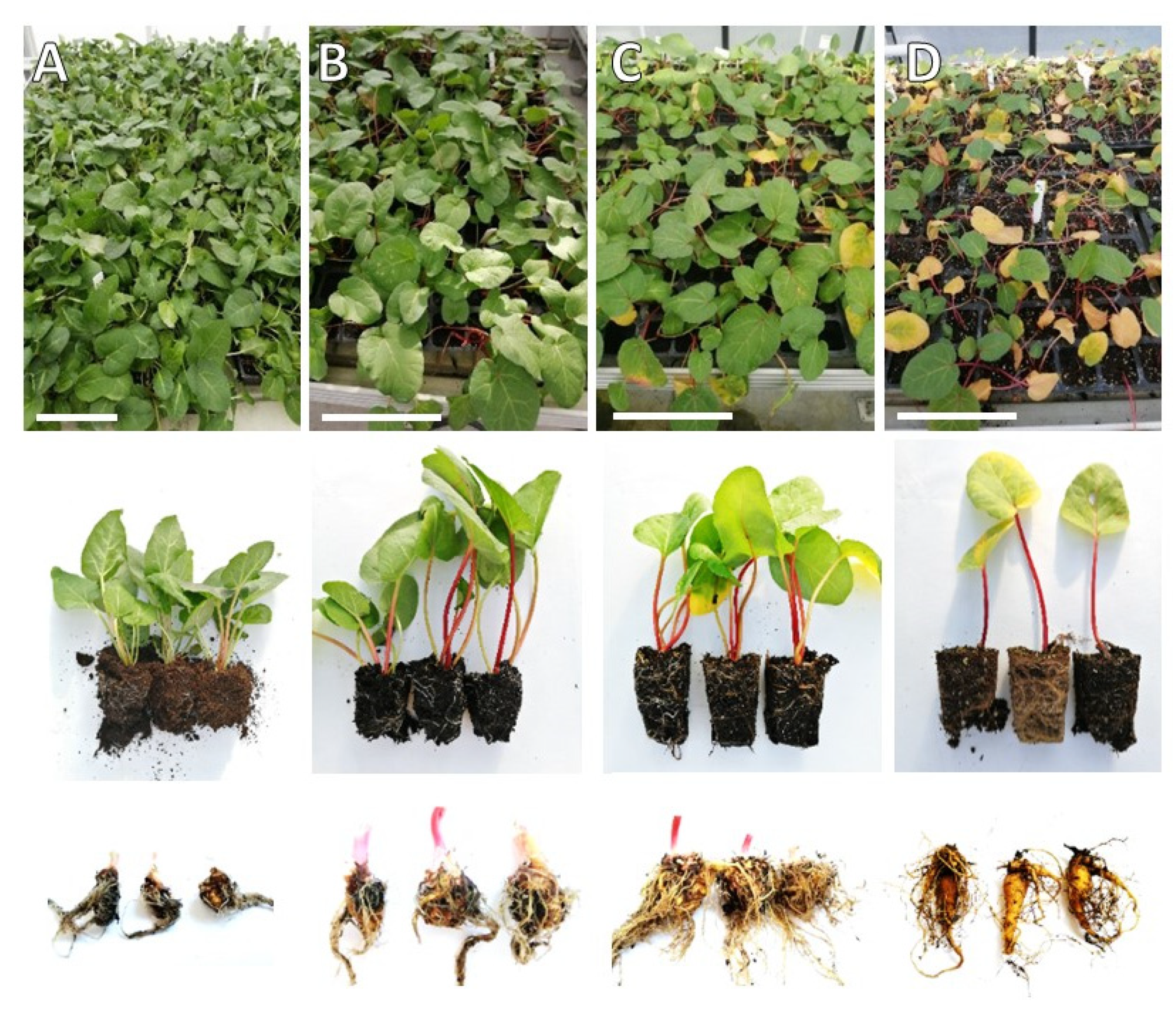

| Growth Duration | Length of Leaf Petioles (cm) | Leaf Area (cm2) | Yellow Leaves (%) | Rhizome Mass (g) |
|---|---|---|---|---|
| 1 month | 7.27 a | 28.74 a | - | 0.883 a |
| 2 months | 10.25 b | 74.75 b | - | 2.200 b |
| 3 months | 9.63 b | 74.21 b | 29.6 | 4.325 c |
| 4 months | - | - | 79.7 | 6.574 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojtania, A.; Markiewicz, M.; Waligórski, P. Regulation of the Bud Dormancy Development and Release in Micropropagated Rhubarb ‘Malinowy’. Int. J. Mol. Sci. 2022, 23, 1480. https://doi.org/10.3390/ijms23031480
Wojtania A, Markiewicz M, Waligórski P. Regulation of the Bud Dormancy Development and Release in Micropropagated Rhubarb ‘Malinowy’. International Journal of Molecular Sciences. 2022; 23(3):1480. https://doi.org/10.3390/ijms23031480
Chicago/Turabian StyleWojtania, Agnieszka, Monika Markiewicz, and Piotr Waligórski. 2022. "Regulation of the Bud Dormancy Development and Release in Micropropagated Rhubarb ‘Malinowy’" International Journal of Molecular Sciences 23, no. 3: 1480. https://doi.org/10.3390/ijms23031480
APA StyleWojtania, A., Markiewicz, M., & Waligórski, P. (2022). Regulation of the Bud Dormancy Development and Release in Micropropagated Rhubarb ‘Malinowy’. International Journal of Molecular Sciences, 23(3), 1480. https://doi.org/10.3390/ijms23031480






