Role of Somatostatin Signalling in Neuroendocrine Tumours
Abstract
1. Introduction
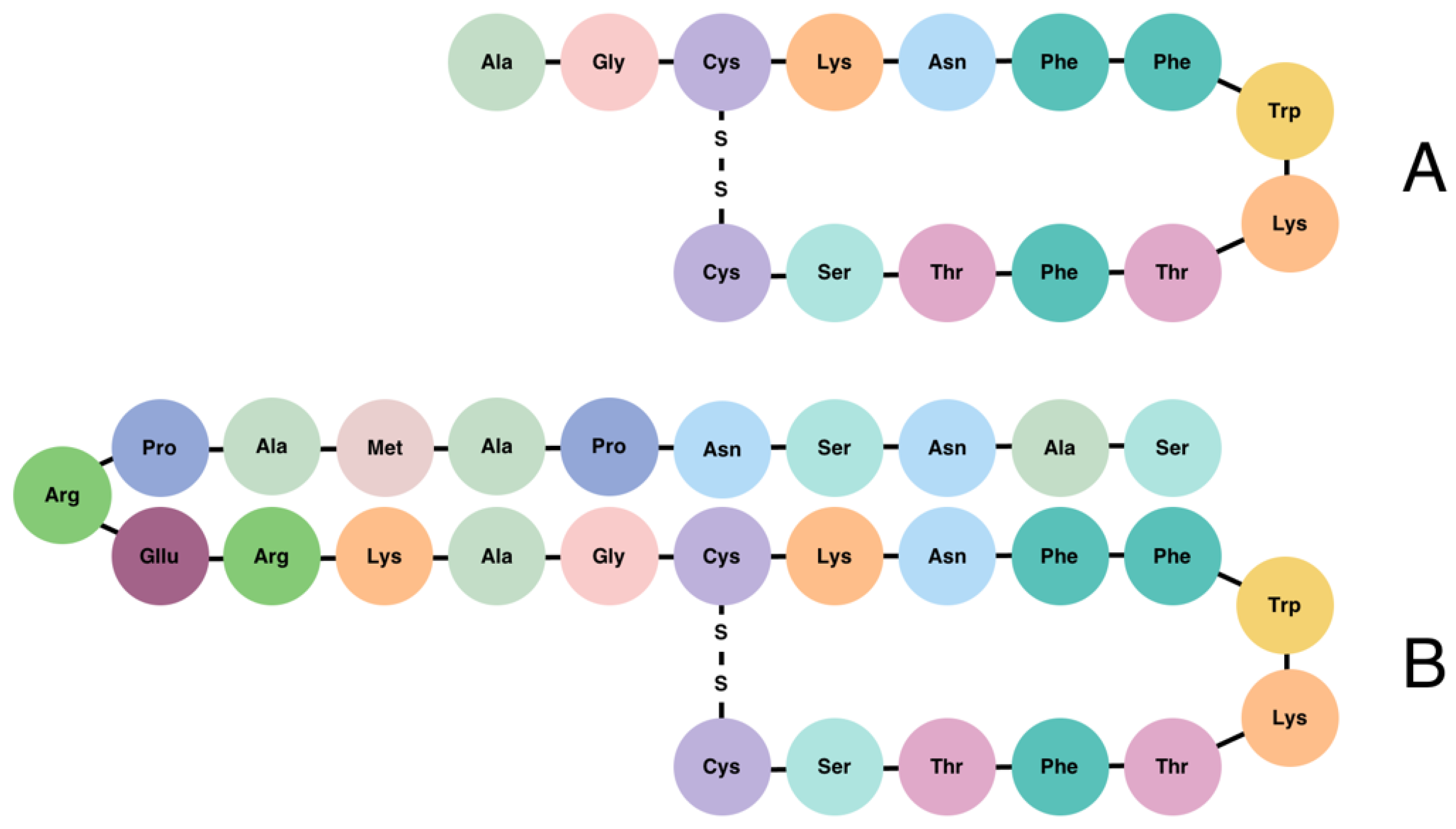
2. Somatostatin Signalling
3. Neuroendocrine Tumours
4. Somatostatin Signalling in NET Development and Prognostics
5. Somatostatin Therapy, NET Treatment, and Internal Regulation
6. Treatment with SSA Compared to Other Therapies, and the Continued Relevance of SSAs in the Treatment of NETs
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klöppel, G. Neuroendocrine Neoplasms: Dichotomy, Origin and Classifications. Visc. Med. 2017, 33, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Chen, L.-T.; Shan, Y.-S.; Chu, P.-Y.; Tsai, C.-R.; Tsai, H.-J. An Updated Analysis of the Epidemiologic Trends of Neuroendocrine Tumors in Taiwan. Sci. Rep. 2021, 11, 7881. [Google Scholar] [CrossRef] [PubMed]
- Modlin, I.; Öberg, K. A Century of Advances in Neuroendocrine Tumor Biology and Treatment, 1st ed.; Kidd, M., Öberg, K., Modlin, I., Eds.; Felsenstein C.C.C.P.: Hannover, Germany, 2007. [Google Scholar]
- Nishioka, H.; Inoshita, N. New WHO Classification of Pituitary Adenomas (4th Edition): Assessment of Pituitary Transcription Factors and the Prognostic Histological Factors. Brain Tumor Pathol. 2018, 35, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Ampofo, E.; Nalbach, L.; Menger, M.D.; Laschke, M.W. Regulatory Mechanisms of Somatostatin Expression. Int. J. Mol. Sci. 2020, 21, 4170. [Google Scholar] [CrossRef] [PubMed]
- Gatto, F.; Barbieri, F.; Arvigo, M.; Thellung, S.; Amarù, J.; Albertelli, M.; Ferone, D.; Florio, T. Biological and Biochemical Basis of the Differential Efficacy of First and Second Generation Somatostatin Receptor Ligands in Neuroendocrine Neoplasms. Int. J. Mol. Sci. 2019, 20, 3940. [Google Scholar] [CrossRef]
- Gomes-Porras, M.; Cárdenas-Salas, J.; Álvarez-Escolá, C. Somatostatin Analogs in Clinical Practice: A Review. Int. J. Mol. Sci. 2020, 21, 1682. [Google Scholar] [CrossRef]
- Stueven, A.K.; Kayser, A.; Wetz, C.; Amthauer, H.; Wree, A.; Tacke, F.; Wiedenmann, B.; Roderburg, C.; Jann, H. Somatostatin Analogues in the Treatment of Neuroendocrine Tumors: Past, Present and Future. Int. J. Mol. Sci. 2019, 20, 3049. [Google Scholar] [CrossRef]
- Rorsman, P.; Huising, M.O. The Somatostatin-Secreting Pancreatic δ-Cell in Health and Disease. Nat. Rev. Endocrinol. 2018, 14, 404–414. [Google Scholar] [CrossRef]
- Paragliola, R.M.; Prete, A.; Papi, G.; Torino, F.; Corsello, A.; Pontecorvi, A.; Corsello, S.M. Clinical Utility of Lanreotide Autogel® in Gastroenteropancreatic Neuroendocrine Tumors. Drug Des. Dev. Ther. 2016, 10, 3459–3470. [Google Scholar] [CrossRef]
- Kailey, B.; van de Bunt, M.; Cheley, S.; Johnson, P.R.; MacDonald, P.E.; Gloyn, A.L.; Rorsman, P.; Braun, M. SSTR2 Is the Functionally Dominant Somatostatin Receptor in Human Pancreatic-and-Cells. Am. J. Physiol. Endocrinol. Metab. 2012, 303, 1107–1116. [Google Scholar] [CrossRef]
- Brereton, M.F.; Vergari, E.; Zhang, Q.; Clark, A. Alpha-, Delta- and PP-Cells: Are They the Architectural Cornerstones of Islet Structure and Co-Ordination? J. Histochem. Cytochem. 2015, 63, 575–591. [Google Scholar] [CrossRef] [PubMed]
- Clark, S. Somatostatin 14. In xPharm: The Comprehensive Pharmacology Reference; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–3. [Google Scholar] [CrossRef]
- Clark, S. Somatostatin 28. In xPharm: The Comprehensive Pharmacology Reference; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–3. [Google Scholar] [CrossRef]
- Hadjidakis, D.J.; Raptis, S.A.; Souvatzoglou, A.; Karaiskos, C.; Diamantopoulos, E.J.; Moulopoulos, S.D. Differences between Somatostatin-28 and Somatostatin-14 with Respect to Their Biological Effects in Healthy Humans and Acromegalics. Clin. Physiol. Biochem. 1986, 4, 372–383. [Google Scholar] [PubMed]
- Neugebauer, V.; Mazzitelli, M.; Cragg, B.; Ji, G.; Navratilova, E.; Porreca, F. Amygdala, Neuropeptides, and Chronic Pain-Related Affective Behaviors. Neuropharmacology 2020, 170, 108052. [Google Scholar] [CrossRef] [PubMed]
- Cakir, M.; Dworakowska, D.; Grossman, A. Somatostatin Receptor Biology in Neuroendocrine and Pituitary Tumours: Part 1—Molecular Pathways. J. Cell. Mol. Med. 2010, 14, 2570–2584. [Google Scholar] [CrossRef]
- Costanzi, E.; Simioni, C.; Conti, I.; Laface, I.; Varano, G.; Brenna, C.; Neri, L.M. Two Neuroendocrine G Protein-coupled Receptor Molecules, Somatostatin and Melatonin: Physiology of Signal Transduction and Therapeutic Perspectives. J. Cell. Physiol. 2021, 236, 2505–2518. [Google Scholar] [CrossRef]
- Hofland, L.J.; Lamberts, S.W.J. The Pathophysiological Consequences of Somatostatin Receptor Internalization and Resistance. Endocr. Rev. 2003, 24, 28–47. [Google Scholar] [CrossRef]
- Klomp, M.J.; Dalm, S.U.; de Jong, M.; Feelders, R.A.; Hofland, J.; Hofland, L.J. Epigenetic Regulation of Somatostatin and Somatostatin Receptors in Neuroendocrine Tumors and Other Types of Cancer. Rev. Endocr. Metab. Disord. 2021, 22, 495–510. [Google Scholar] [CrossRef]
- Reisine, T.; Kong, H.; Raynor, K.; Yano, H.; Takeda, J.; Yasuda, K.; Bell, G.I. Splice Variant of the Somatostatin Receptor 2 Subtype, Somatostatin Receptor 2B, Couples to Adenylyl Cyclase. Mol. Pharmacol. 1993, 44, 1016–1020. [Google Scholar]
- Elliott, D.E.; Li, J.; Blum, A.M.; Metwali, A.; Patel, Y.C.; Weinstock, J. SSTR2A Is the Dominant Somatostatin Receptor Subtype Expressed by Inflammatory Cells, Is Widely Expressed and Directly Regulates T Cell IFN-Gamma Release. Eur. J. Immunol. 1999, 29, 2454–2463. [Google Scholar] [CrossRef]
- Kossut, M.; Łukomska, A.; Dobrzański, G.; Liguz-Lęcznar, M. Somatostatin receptors in the brain. Postepy Biochem. 2018, 64, 213–221. [Google Scholar] [CrossRef]
- Latorraca, N.R.; Venkatakrishnan, A.J.; Dror, R.O. GPCR Dynamics: Structures in Motion. Chem. Rev. 2017, 117, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.I.; Tan, H.; Zhao, Y.; Zhou, Y.; Cao, L.I.N. Expression and Selective Activation of Somatostatin Receptor Subtypes Induces Cell Cycle Arrest in Cancer Cells. Oncol. Lett. 2019, 17, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Günther, T.; Tulipano, G.; Dournaud, P.; Bousquet, C.; Csaba, Z.; Kreienkamp, H.J.; Lupp, A.; Korbonits, M.; Castaño, J.P.; Wester, H.J.; et al. International Union of Basic and Clinical Pharmacology. CV. Somatostatin Receptors: Structure, Function, Ligands, and New Nomenclature. Pharmacol. Rev. 2018, 70, 763–835. [Google Scholar] [CrossRef] [PubMed]
- Goo, T.; Akiba, Y.; Kaunitz, J.D. Mechanisms of Intragastric PH Sensing. Curr. Gastroenterol. Rep. 2010, 12, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Stengel, A.; Taché, Y. Central Somatostatin Signaling and Regulation of Food Intake. Ann. N. Y. Acad. Sci. 2019, 1455, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Kumar, U.; Singh, S. Role of Somatostatin in the Regulation of Central and Peripheral Factors of Satiety and Obesity. Int. J. Mol. Sci. 2020, 21, 2568. [Google Scholar] [CrossRef]
- ten Bokum, A.M.; Hofland, L.J.; van Hagen, P.M. Somatostatin and Somatostatin Receptors in the Immune System: A Review. Eur. Cytokine Netw. 2000, 11, 161–176. [Google Scholar]
- Chowers, Y.; Cahalon, L.; Lahav, M.; Schor, H.; Tal, R.; Bar-Meir, S.; Levite, M. Somatostatin Through Its Specific Receptor Inhibits Spontaneous and TNF-α- and Bacteria-Induced IL-8 and IL-1β Secretion from Intestinal Epithelial Cells. J. Immunol. 2000, 165, 2955–2961. [Google Scholar] [CrossRef]
- Weckbecker, G.; Lewis, I.; Albert, R.; Schmid, H.A.; Hoyer, D.; Bruns, C. Opportunities in Somatostatin Research: Biological, Chemical and Therapeutic Aspects. Nat. Rev. Drug Discov. 2003, 2, 999–1017. [Google Scholar] [CrossRef]
- Callison, J.C.; Walker, R.C.; Massion, P.P. Somatostatin Receptors in Lung Cancer: From Function to Molecular Imaging and Therapeutics. J. Lung Cancer 2011, 10, 69. [Google Scholar] [CrossRef][Green Version]
- Barresi, V.; Alafaci, C.; Salpietro, F.; Tuccari, G. Sstr2A Immunohistochemical Expression in Human Meningiomas: Is There a Correlation with the Histological Grade, Proliferation or Microvessel Density? Oncol. Rep. 2008, 20, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Hankus, J.; Tomaszewska, R. Neuroendocrine Neoplasms and Somatostatin Receptor Subtypes Expression. Nucl. Med. Rev. 2016, 19, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Nakanishi, Y.; Kinukawa, N.; Ohni, S.; Obana, Y.; Nakazawa, A.; Nemoto, N. Expressions of Somatostatin Receptor Subtypes (SSTR-1, 2, 3, 4 and 5) in Neuroblastic Tumors; Special Reference to Clinicopathological Correlations with International Neuroblastoma Pathology Classification and Outcomes. Acta Histochem. Cytochem. 2014, 47, 219–229. [Google Scholar] [CrossRef][Green Version]
- de Vries, L.H.; Lodewijk, L.; Willems, S.M.; Dreijerink, K.M.A.; de Keizer, B.; van Diest, P.J.; Schepers, A.; Bonenkamp, H.J.; van Engen-van Grunsven, I.A.C.H.; Kruijff, S.; et al. SSTR2A Expression in Medullary Thyroid Carcinoma Is Correlated with Longer Survival. Endocrine 2018, 62, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Liu, Q.; Xu, H.; Wu, Z.; Zhou, L.; Gu, Z.; Gong, P.; Shen, J. Upregulated Expression of SSTR3 Is Involved in Neuronal Apoptosis After Intracerebral Hemorrhage in Adult Rats. Cell. Mol. Neurobiol. 2017, 37, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- van Opdenbosch, J.; Torfs, P.; de Winter, B.Y.; de Man, J.G.; Pelckmans, P.A.; van Marck, E.; Grundy, D.; van Nassauw, L.; Timmermans, J.P. Effect of Genetic SSTR4 Ablation on Inflammatory Peptide and Receptor Expression in the Non-Inflamed and Inflamed Murine Intestine. J. Cell. Mol. Med. 2009, 13, 3283–3295. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P.; Schluet, W.; Lippe, I.T.; Sametz, W. Involvement of Capsaicin-Sensitive Sensory Neurons in Gastrointestinal Function. Acta Physiol. Hung. 1987, 69, 403–411. [Google Scholar]
- Hu, Y.; Ye, Z.; Wang, F.; Qin, Y.; Xu, X.; Yu, X.; Ji, S. Role of Somatostatin Receptor in Pancreatic Neuroendocrine Tumor Development, Diagnosis, and Therapy. Front. Endocrinol. 2021, 12, 679000. [Google Scholar] [CrossRef]
- Trouillas, J.; Vasiljevic, A.; Lapoirie, M.; Chinezu, L.; Jouanneau, E.; Raverot, G. Pathological Markers of Somatotroph Pituitary Neuroendocrine Tumors Predicting the Response to Medical Treatment. Minerva Endocrinol. 2019, 44, 129–136. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Jin, K.; Fang, C.; Lin, Y.; Xue, L.; Feng, S.; Zhou, Z.; Shao, C.; Chen, M.; et al. Somatostatin Receptor Expression Indicates Improved Prognosis in Gastroenteropancreatic Neuroendocrine Neoplasm, and Octreotide Long-Acting Release Is Effective and Safe in Chinese Patients with Advanced Gastroenteropancreatic Neuroendocrine Tumors. Oncol. Lett. 2017, 13, 1165–1174. [Google Scholar] [CrossRef]
- Ioachimescu, A.G. Management of Neuroendocrine Tumors in the Twenty-First Century. Endocrinol. Metab. Clin. N. Am. 2018, 47, xiii–xiv. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Busam, K.J.; de Krijger, R.R.; Dietel, M.; El-Naggar, A.K.; et al. A Common Classification Framework for Neuroendocrine Neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) Expert Consensus Proposal. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef] [PubMed]
- Schimmack, S.; Svejda, B.; Lawrence, B.; Kidd, M.; Modlin, I.M. The Diversity and Commonalities of Gastroenteropancreatic Neuroendocrine Tumors. Langenbeck’s Arch. Surg. 2011, 396, 273–298. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; O’Neil, B.H. Summary of Emerging Personalized Medicine in Neuroendocrine Tumors: Are We on Track? J. Gastrointest. Oncol. 2016, 7, 804–818. [Google Scholar] [CrossRef][Green Version]
- Inzani, F.; Petrone, G.; Rindi, G. The New World Health Organization Classification for Pancreatic Neuroendocrine Neoplasia. Endocrinol. Metab. Clin. N. Am. 2018, 47, 463–470. [Google Scholar] [CrossRef]
- Popa, O.; Taban, S.; Pantea, S.; Plopeanu, A.; Barna, R.; Cornianu, M.; Pascu, A.-A.; Dema, A.L. The New WHO Classification of Gastrointestinal Neuroendocrine Tumors and Immunohistochemical Expression of Somatostatin Receptor 2 and 5. Exp. Ther. Med. 2021, 22, 10613. [Google Scholar] [CrossRef]
- Baghdadi, A.; Ghadimi, M.; Mirpour, S.; Hazhirkarzar, B.; Motaghi, M.; Pawlik, T.M.; Kamel, I.R. Imaging Neuroendocrine Tumors: Characterizing the Spectrum of Radiographic Findings. Surg. Oncol. 2021, 37, 1529. [Google Scholar] [CrossRef]
- Gaudenzi, G.; Carra, S.; Dicitore, A.; Cantone, M.C.; Persani, L.; Vitale, G. Fishing for Neuroendocrine Tumors. Endocr.-Relat. Cancer 2020, 27, R163–R176. [Google Scholar] [CrossRef]
- Lopes, M.B.S. The 2017 World Health Organization Classification of Tumors of the Pituitary Gland: A Summary. Acta Neuropathol. 2017, 134, 521–535. [Google Scholar] [CrossRef]
- Ma, Z.Y.; Gong, Y.F.; Zhuang, H.K.; Zhou, Z.X.; Huang, S.Z.; Zou, Y.P.; Huang, B.W.; Sun, Z.H.; Zhang, C.Z.; Tang, Y.Q.; et al. Pancreatic Neuroendocrine Tumors: A Review of Serum Biomarkers, Staging, and Management. World J. Gastroenterol. 2020, 26, 2305–2322. [Google Scholar] [CrossRef]
- Modlin, I.M.; Oberg, K.; Chung, D.C.; Jensen, R.T.; de Herder, W.W.; Thakker, R.; Caplin, M.; Fave, G.D.; Kaltsas, G.A.; Krenning, E.P.; et al. Gastroenteropancreatic Neuroendocrine Tumours. Lancet Oncol. 2008, 9, 61–72. [Google Scholar] [CrossRef]
- Öberg, K. The Genesis of the Neuroendocrine Tumors Concept: From Oberndorfer to 2018. Endocrinol. Metab. Clin. N. Am. 2018, 47, 711–731. [Google Scholar] [CrossRef] [PubMed]
- Cukier, M.; Vergara, R.; Mendez-Rios, J.D.; Castillo, O.; Barrera, I.; Tello, E.; el Achtar, O.; Loo, Y.; Tapia, H.; Perez, G.; et al. Neuroendocrine Tumors in Panama: A Nationwide Database Analysis. Mol. Clin. Oncol. 2021, 15, 2319. [Google Scholar] [CrossRef] [PubMed]
- Kanakis, G.; Kaltsas, G. Biochemical Markers for Gastroenteropancreatic Neuroendocrine Tumours (GEP-NETs). Best Pract. Res. Clin. Gastroenterol. 2012, 26, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Massironi, S.; Fraquelli, M.; Paggi, S.; Sangiovanni, A.; Conte, D.; Sciola, V.; Ciafardini, C.; Colombo, M.; Peracchi, M. Chromogranin A Levels in Chronic Liver Disease and Hepatocellular Carcinoma. Dig. Liver Dis. 2009, 41, 31–35. [Google Scholar] [CrossRef]
- Marotta, V.; Zatelli, M.C.; Sciammarella, C.; Ambrosio, M.R.; Bondanelli, M.; Colao, A.; Faggiano, A. Chromogranin A as Circulating Marker for Diagnosis and Management of Neuroendocrine Neoplasms: More Flaws than Fame. Endocr.-Relat. Cancer 2018, 25, R11–R29. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Y.; Li, Z.; Cheng, C.; Yang, T.; Wang, C.; Liu, L.; Liu, S. Diagnostic Value of Circulating Chromogranin A for Neuroendocrine Tumors: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0124884. [Google Scholar] [CrossRef]
- Taupenot, L.; Harper, K.L.; O’Connor, D.T. The Chromogranin–Secretogranin Family. N. Engl. J. Med. 2003, 348, 1134–1149. [Google Scholar] [CrossRef]
- Kalligeros, M.; Diamantopoulos, L.; Toumpanakis, C. Biomarkers in Small Intestine NETs and Carcinoid Heart Disease: A Comprehensive Review. Biology 2021, 10, 950. [Google Scholar] [CrossRef]
- Tellez, M.R.; Mamikunian, G.; O’Dorisio, T.M.; Vinik, A.I.; Woltering, E.A. A Single Fasting Plasma 5-HIAA Value Correlates With 24-Hour Urinary 5-HIAA Values and Other Biomarkers in Midgut Neuroendocrine Tumors (NETs). Pancreas 2013, 42, 405–410. [Google Scholar] [CrossRef]
- De Mestier, L.; Savagner, F.; Brixi, H.; do Cao, C.; Dominguez-Tinajero, S.; Roquin, G.; Goichot, B.; Hentic, O.; Dubreuil, O.; Hautefeuille, V.; et al. Plasmatic and Urinary 5-Hydroxyindolacetic Acid Measurements in Patients with Midgut Neuroendocrine Tumors: A GTE Study. J. Clin. Endocrinol. Metab. 2021, 106, e1673–e1682. [Google Scholar] [CrossRef] [PubMed]
- van der Lely, A.J.; de Herder, W.W. Carcinoid Syndrome: Diagnosis and Medical Management. Arq. Bras. Endocrinol. Metabol. 2005, 49, 850–860. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bocchini, M.; Nicolini, F.; Severi, S.; Bongiovanni, A.; Ibrahim, T.; Simonetti, G.; Grassi, I.; Mazza, M. Biomarkers for Pancreatic Neuroendocrine Neoplasms (PanNENs) Management—An Updated Review. Front. Oncol. 2020, 10, 831. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Martínez, A.D.; Hofland, L.J.; Gálvez Moreno, M.A.; Castaño, J.P.; de Herder, W.W.; Feelders, R.A. Neuroendocrine Neoplasms: Current and Potential Diagnostic, Predictive and Prognostic Markers. Endocr.-Relat. Cancer 2019, 26, R157–R179. [Google Scholar] [CrossRef] [PubMed]
- Daskalakis, K.; Norlén, O.; Hellman, P.; Stålberg, P. Applying the Use of Novel Biomarkers for Neuroendocrine Tumors in the Clinic: Where Are We Now? Int. J. Endocr. Oncol. 2019, 6, IJE14. [Google Scholar] [CrossRef]
- Zatelli, M.C.; Grossrubatscher, E.M.; Guadagno, E.; Sciammarella, C.; Faggiano, A.; Colao, A. Circulating Tumor Cells and MiRNAs as Prognostic Markers in Neuroendocrine Neoplasms. Endocr.-Relat. Cancer 2017, 24, R223–R237. [Google Scholar] [CrossRef]
- Lewis, M.A. Hereditary Syndromes in Neuroendocrine Tumors. Curr. Treat. Options Oncol. 2020, 21, 50. [Google Scholar] [CrossRef]
- Cives, M.; Pelle, E.; Quaresmini, D.; Rizzo, F.M.; Tucci, M.; Silvestris, F. The Tumor Microenvironment in Neuroendocrine Tumors: Biology and Therapeutic Implications. Neuroendocrinology 2019, 109, 83–99. [Google Scholar] [CrossRef]
- Vitali, E.; Piccini, S.; Trivellin, G.; Smiroldo, V.; Lavezzi, E.; Zerbi, A.; Pepe, G.; Lania, A.G. The Impact of SST2 Trafficking and Signaling in the Treatment of Pancreatic Neuroendocrine Tumors. Mol. Cell. Endocrinol. 2021, 527, 111226. [Google Scholar] [CrossRef]
- Theodoropoulou, M.; Stalla, G.K. Somatostatin Receptors: From Signaling to Clinical Practice. Front. Neuroendocrinol. 2013, 34, 228–252. [Google Scholar] [CrossRef]
- Lee, H.; Suh, M.; Choi, H.; Ha, S.; Paeng, J.C.; Cheon, G.J.; Kang, K.W.; Lee, D.S. A Pan-Cancer Analysis of the Clinical and Genetic Portraits of Somatostatin Receptor Expressing Tumor as a Potential Target of Peptide Receptor Imaging and Therapy. EJNMMI Res. 2020, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- García De La Torre, N.; Wass, J.A.H.; Turner, H.E. Antiangiogenic Effects of Somatostatin Analogues. Clin. Oncol. 2002, 57, 425–441. [Google Scholar] [CrossRef] [PubMed]
- Rai, U.; Thrimawithana, T.R.; Valery, C.; Young, S.A. Therapeutic Uses of Somatostatin and Its Analogues: Current View and Potential Applications. Pharmacol. Ther. 2015, 152, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, M.; Luque, R.M.; Durn-Prado, M.; Baragli, A.; Grande, C.; Volante, M.; Gahete, M.D.; Deltetto, F.; Camanni, M.; Ghigo, E.; et al. Somatostatin and Somatostatin Analogues Reduce PDGF-Induced Endometrial Cell Proliferation and Motility. Hum. Reprod. 2012, 27, 2117–2129. [Google Scholar] [CrossRef] [PubMed]
- Pola, S.; Cattaneo, M.G.; Vicentini, L.M. Anti-Migratory and Anti-Invasive Effect of Somatostatin in Human Neuroblastoma Cells: Involvement of Rac and Map Kinase Activity. J. Biol. Chem. 2003, 278, 40601–40606. [Google Scholar] [CrossRef] [PubMed]
- Brunner, P.; Jörg, A.C.; Glatz, K.; Bubendorf, L.; Radojewski, P.; Umlauft, M.; Marincek, N.; Spanjol, P.M.; Krause, T.; Dumont, R.A.; et al. The Prognostic and Predictive Value of Sstr2-Immunohistochemistry and Sstr2-Targeted Imaging in Neuroendocrine Tumors. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 468–475. [Google Scholar] [CrossRef]
- Casar-Borota, O.; Heck, A.; Schulz, S.; Nesland, J.M.; Ramm-Pettersen, J.; Lekva, T.; Alafuzoff, I.; Bollerslev, J. Expression of SSTR2a, but Not of SSTRs 1, 3, or 5 in Somatotroph Adenomas Assessed by Monoclonal Antibodies Was Reduced by Octreotide and Correlated with the Acute and Long-Term Effects of Octreotide. J. Clin. Endocrinol. Metab. 2013, 98, 2145. [Google Scholar] [CrossRef]
- Corleto, V.D.; Falconi, M.; Panzuto, F.; Milione, M.; de Luca, O.; Perri, P.; Cannizzaro, R.; Bordi, C.; Pederzoli, P.; Scarpa, A.; et al. Somatostatin Receptor Subtypes 2 and 5 Are Associated with Better Survival in Well-Differentiated Endocrine Carcinomas. Neuroendocrinology 2009, 89, 223–230. [Google Scholar] [CrossRef]
- Diakatou, E.; Kaltsas, G.; Tzivras, M.; Kanakis, G.; Papaliodi, E.; Kontogeorgos, G. Somatostatin and Dopamine Receptor Profile of Gastroenteropancreatic Neuroendocrine Tumors: An Immunohistochemical Study. Endocr. Pathol. 2011, 22, 24–30. [Google Scholar] [CrossRef]
- Diakatou, E.; Alexandraki, K.I.; Tsolakis, A.V.; Kontogeorgos, G.; Chatzellis, E.; Leonti, A.; Kaltsas, G.A. Somatostatin and Dopamine Receptor Expression in Neuroendocrine Neoplasms: Correlation of Immunohistochemical Findings with Somatostatin Receptor Scintigraphy Visual Scores. Clin. Endocrinol. 2015, 83, 420–428. [Google Scholar] [CrossRef]
- Fougner, S.L.; Borota, O.C.; Berg, J.P.; Hald, J.K.; Ramm-Pettersen, J.; Bollerslev, J. The Clinical Response to Somatostatin Analogues in Acromegaly Correlates to the Somatostatin Receptor Subtype 2a Protein Expression of the Adenoma. Clin. Endocrinol. 2008, 68, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Franck, S.E.; Gatto, F.; van der Lely, A.J.; Janssen, J.A.M.J.L.; Dallenga, A.H.G.; Nagtegaal, A.P.; Hofland, L.J.; Neggers, S.J.C.M.M. Somatostatin Receptor Expression in GH-Secreting Pituitary Adenomas Treated with Long-Acting Somatostatin Analogues in Combination with Pegvisomant. Neuroendocrinology 2017, 105, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Kaemmerer, D.; Träger, T.; Hoffmeister, M.; Sipos, B.; Hommann, M.; Sänger, J.; Schulz, S.; Lupp, A. Inverse Expression of Somatostatin and CXCR4 Chemokine Receptors in Gastroenteropancreatic Neuroendocrine Neoplasms of Different Malignancy. Oncotarget 2015, 6, 27566–27579. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; de Reuver, P.R.; Gill, P.; Andrici, J.; D’Urso, L.; Mittal, A.; Pavlakis, N.; Clarke, S.; Samra, J.S.; Gill, A.J. Somatostatin Receptor SSTR-2a Expression Is a Stronger Predictor for Survival than Ki-67 in Pancreatic Neuroendocrine Tumors. Medicine 2015, 94, 1281. [Google Scholar] [CrossRef]
- Papotti, M.; Bongiovanni, M.; Volante, M.; Allìa, E.; Landolfi, S.; Helboe, L.; Schindler, M.; Cole, S.; Bussolati, G. Expression of Somatostatin Receptor Types 1-5 in 81 Cases of Gastrointestinal and Pancreatic Endocrine Tumors: A Correlative Immunohistochemical and Reverse-Transcriptase Polymerase Chain Reaction Analysis. Virchows Arch. 2002, 440, 461–475. [Google Scholar] [CrossRef]
- Remes, S.M.; Leijon, H.L.; Vesterinen, T.J.; Arola, J.T.; Haglund, C.H. Immunohistochemical Expression of Somatostatin Receptor Subtypes in a Panel of Neuroendocrine Neoplasias. J. Histochem. Cytochem. 2019, 67, 735–743. [Google Scholar] [CrossRef]
- Körner, M.; Eltschinger, V.; Waser, B.; Schonbrunn, A.; Reubi, J.C. Value of Immunohistochemistry for Somatostatin Receptor Subtype Sst2A in Cancer Tissues. Am. J. Surg. Pathol. 2005, 29, 1642–1651. [Google Scholar] [CrossRef]
- Zamora, V.; Cabanne, A.; Salanova, R.; Bestani, C.; Domenichini, E.; Marmissolle, F.; Giacomi, N.; O’Connor, J.; Méndez, G.; Roca, E. Immunohistochemical Expression of Somatostatin Receptors in Digestive Endocrine Tumours. Dig. Liver Dis. 2010, 42, 220–225. [Google Scholar] [CrossRef]
- Nasir, A.; Stridsberg, M.; Strosberg, J.; Su, P.-H.; Livingston, S.; Malik, H.A.; Kelley, S.T.; Centeno, B.A.; Coppola, D.; Malafa, M.E.; et al. Somatostatin Receptor Profiling in Hepatic Metastases from Small Intestinal and Pancreatic Neuroendocrine Neoplasms: Immunohistochemical Approach with Potential Clinical Utility. Cancer Control 2006, 13, 52–60. [Google Scholar] [CrossRef]
- Nielsen, K.; Binderup, T.; Langer, S.W.; Kjaer, A.; Knigge, P.; Grøndahl, V.; Melchior, L.; Federspiel, B.; Knigge, U. P53, Somatostatin Receptor 2a and Chromogranin A Immunostaining as Prognostic Markers in High Grade Gastroenteropancreatic Neuroendocrine Neoplasms. BMC Cancer 2020, 20, 27. [Google Scholar] [CrossRef]
- Okuwaki, K.; Kida, M.; Mikami, T.; Yamauchi, H.; Imaizumi, H.; Miyazawa, S.; Iwai, T.; Takezawa, M.; Saegusa, M.; Watanabe, M.; et al. Clinicopathologic Characteristics of Pancreatic Neuroendocrine Tumors and Relation of Somatostatin Receptor Type 2A to Outcomes. Cancer 2013, 119, 4094–4102. [Google Scholar] [CrossRef] [PubMed]
- Righi, L.; Volante, M.; Tavaglione, V.; Billè, A.; Daniele, L.; Angusti, T.; Inzani, F.; Pelosi, G.; Rindi, G.; Papotti, M. Somatostatin Receptor Tissue Distribution in Lung Neuroendocrine Tumours: A Clinicopathologic and Immunohistochemical Study of 218 “clinically Aggressive” Cases. Ann. Oncol. 2009, 21, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Srirajaskanthan, R.; Watkins, J.; Marelli, L.; Khan, K.; Caplin, M.E. Expression of Somatostatin and Dopamine 2 Receptors in Neuroendocrine Tumours and the Potential Role for New Biotherapies. Neuroendocrinology 2009, 89, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.G.; Scott, A.T.; Li, G.; Sherman, S.K.; Ear, P.H.; Howe, J.R. Metastatic Pancreatic Neuroendocrine Tumors Have Decreased Somatostatin Expression and Increased Akt Signaling. Surgery 2021, 169, 155–161. [Google Scholar] [CrossRef]
- Venegas-Moreno, E.; Vazquez-Borrego, M.C.; Dios, E.; Gros-Herguido, N.; Flores-Martinez, A.; Rivero-Cortés, E.; Madrazo-Atutxa, A.; Japón, M.A.; Luque, R.M.; Castaño, J.P.; et al. Association between Dopamine and Somatostatin Receptor Expression and Pharmacological Response to Somatostatin Analogues in Acromegaly. J. Cell. Mol. Med. 2018, 22, 1640–1649. [Google Scholar] [CrossRef]
- Fuentes-Fayos, A.C.; García-Martínez, A.; Herrera-Martínez, A.D.; Jiménez-Vacas, J.M.; Vázquez-Borrego, M.C.; Castaño, J.P.; Picó, A.; Gahete, M.D.; Luque, R.M. Molecular Determinants of the Response to Medical Treatment of Growth Hormone Secreting Pituitary Neuroendocrine Tumors. Minerva Endocrinol. 2019, 44, 109–128. [Google Scholar] [CrossRef]
- Colao, A.; Auriemma, R.S.; Pivonello, R. The Effects of Somatostatin Analogue Therapy on Pituitary Tumor Volume in Patients with Acromegaly. Pituitary 2016, 19, 210–221. [Google Scholar] [CrossRef]
- Yonezawa, N.; Nishida, E.; Sakai, H.; Koyasu, S.; Matsuzaki, F.; Iida, K.; Yahara, I. Purification and Characterization of the 90-KDa Heat-Shock Protein from Mammalian Tissues. Eur. J. Biochem. 1988, 177, 1–7. [Google Scholar] [CrossRef]
- Curt, A.M.; Popa Ilie, I.R.; Cainap, C.; Balacescu, O.; Ghervan, C. MicroRNAs and Treatment with Somatostatin Analogs in Gastro- Entero-Pancreatic Neuroendocrine Neoplasms: Challenges in Personalized Medicine. J. Gastrointest. Liver Dis. 2020, 29, 647–659. [Google Scholar] [CrossRef]
- Cantone, M.C.; Dicitore, A.; Vitale, G. Somatostatin-Dopamine Chimeric Molecules in Neuroendocrine Neoplasms. J. Clin. Med. 2021, 10, 501. [Google Scholar] [CrossRef]
- Ferone, D.; Saveanu, A.; Culler, M.D.; Arvigo, M.; Rebora, A.; Gatto, F.; Minuto, F.; Jaquet, P. Novel Chimeric Somatostatin Analogs: Facts and Perspectives. Eur. J. Endocrinol. 2007, 156, S23–S28. [Google Scholar] [CrossRef] [PubMed]
- Jaquet, P.; Gunz, G.; Saveanu, A.; Dufour, H.; Taylor, J.; Dong, J.; Kim, S.; Moreau, J.-P.; Enjalbert, A.; Culler, M.D. Efficacy of Chimeric Molecules Directed towards Multiple Somatostatin and Dopamine Receptors on Inhibition of GH and Prolactin Secretion from GH-Secreting Pituitary Adenomas Classified as Partially Responsive to Somatostatin Analog Therapy. Eur. J. Endocrinol. 2005, 153, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.-G.; Kim, S.; Taylor, J.; Dong, J.; Moreau, J.-P.; Culler, M.D.; Melmed, S. Suppression of Rat and Human Growth Hormone and Prolactin Secretion by a Novel Somatostatin/Dopaminergic Chimeric Ligand. J. Clin. Endocrinol. Metab. 2003, 88, 5414–5421. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Herrera-Martínez, A.D.; van den Dungen, R.; Dogan-Oruc, F.; van Koetsveld, P.M.; Culler, M.D.; de Herder, W.W.; Luque, R.M.; Feelders, R.A.; Hofland, L.J. Effects of Novel Somatostatin-Dopamine Chimeric Drugs in 2D and 3D Cell Culture Models of Neuroendocrine Tumors. Endocr.-Relat. Cancer 2019, 26, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Borrego, M.C.; López, F.; Gálvez-Moreno, M.A.; Fuentes-Fayos, A.C.; Venegas-Moreno, E.; Herrera-Martínez, A.D.; Blanco-Acevedo, C.; Solivera, J.; Landsman, T.; Gahete, M.D.; et al. A New Generation Somatostatin-Dopamine Analogue Exerts Potent Antitumoral Actions on Pituitary Neuroendocrine Tumor Cells. Neuroendocrinology 2020, 110, 70–82. [Google Scholar] [CrossRef]
- Dicitore, A.; Cantone, M.C.; Gaudenzi, G.; Saronni, D.; Carra, S.; Borghi, M.O.; Albertelli, M.; Ferone, D.; Hofland, L.J.; Persani, L.; et al. Efficacy of a Novel Second-Generation Somatostatin-Dopamine Chimera (TBR-065) in Human Medullary Thyroid Cancer: A Preclinical Study. Neuroendocrinology 2021, 111, 937–950. [Google Scholar] [CrossRef]
- de Boon, W.M.I.; van Esdonk, M.J.; Stuurman, F.E.; Biermasz, N.R.; Pons, L.; Paty, I.; Burggraaf, J. A Novel Somatostatin-Dopamine Chimera (BIM23B065) Reduced GH Secretion in a First-in-Human Clinical Trial. J. Clin. Endocrinol. Metab. 2019, 104, 883–891. [Google Scholar] [CrossRef]
- Schmitz, R.L.; Weissbach, J.; Kleilein, J.; Bell, J.; Hüttelmaier, S.; Viol, F.; Clauditz, T.; Grabowski, P.; Laumen, H.; Rosendahl, J.; et al. Targeting Hdacs in Pancreatic Neuroendocrine Tumor Models. Cells 2021, 10, 1408. [Google Scholar] [CrossRef]
- Cives, M.; Simone, V.; Rizzo, F.M.; Silvestris, F. NETs: Organ-Related Epigenetic Derangements and Potential Clinical Applications. Oncotarget 2016, 7, 57414–57429. [Google Scholar] [CrossRef]
- Sun, L.; Qian, Q.; Sun, G.; Mackey, L.V.; Fuselier, J.A.; Coy, D.H.; Yu, C.-Y. Valproic Acid Induces NET Cell Growth Arrest and Enhances Tumor Suppression of the Receptor-Targeted Peptide–Drug Conjugate via Activating Somatostatin Receptor Type II. J. Drug Target. 2016, 24, 169–177. [Google Scholar] [CrossRef]
- Arvidsson, Y.; Johanson, V.; Pfragner, R.; Wängberg, B.; Nilsson, O. Cytotoxic Effects of Valproic Acid on Neuroendocrine Tumour Cells. Neuroendocrinology 2016, 103, 578–591. [Google Scholar] [CrossRef]
- Baradari, V.; Huether, A.; Höpfner, M.; Schuppan, D.; Scherübl, H. Antiproliferative and Proapoptotic Effects of Histone Deacetylase Inhibitors on Gastrointestinal Neuroendocrine Tumor Cells. Endocr.-Relat. Cancer 2006, 13, 1237–1250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-Y.; Rumilla, K.M.; Jin, L.; Nakamura, N.; Stilling, G.A.; Ruebel, K.H.; Hobday, T.J.; Erlichman, C.; Erickson, L.A.; Lloyd, R.V. Association of DNA Methylation and Epigenetic Inactivation of RASSF1A and Beta-Catenin with Metastasis in Small Bowel Carcinoid Tumors. Endocrine 2006, 30, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Vinita, M.A.; Madhuchhanda, R.; Kristen, A.S.; Muthusamy, K.; Herbert, C. Azacytidine Induces Cell Cycle Arrest and Suppression of Neuroendocrine Markers in Carcinoids. Int. J. Clin. Exp. Med. 2010, 3, 95–102. [Google Scholar]
- Mohammed, T.A.; Holen, K.D.; Jaskula-Sztul, R.; Mulkerin, D.; Lubner, S.J.; Schelman, W.R.; Eickhoff, J.; Chen, H.; LoConte, N.K. A Pilot Phase II Study of Valproic Acid for Treatment of Low-Grade Neuroendocrine Carcinoma. Oncologist 2011, 16, 835–843. [Google Scholar] [CrossRef]
- Shah, M.H.; Binkley, P.; Chan, K.; Xiao, J.; Arbogast, D.; Collamore, M.; Farra, Y.; Young, D.; Grever, M. Cardiotoxicity of Histone Deacetylase Inhibitor Depsipeptide in Patients with Metastatic Neuroendocrine Tumors. Clin. Cancer Res. 2006, 12, 3997–4003. [Google Scholar] [CrossRef]
- Jin, N.; Lubner, S.J.; Mulkerin, D.L.; Rajguru, S.; Carmichael, L.; Chen, H.; Holen, K.D.; LoConte, N.K. A Phase II Trial of a Histone Deacetylase Inhibitor Panobinostat in Patients with Low-Grade Neuroendocrine Tumors. Oncologist 2016, 21, 785. [Google Scholar] [CrossRef]
- Zanini, S.; Renzi, S.; Giovinazzo, F.; Bermano, G. MTOR Pathway in Gastroenteropancreatic Neuroendocrine Tumor (GEP-NETs). Front. Endocrinol. 2020, 11, 562505. [Google Scholar] [CrossRef]
- Wolin, E.M. The Expanding Role of Somatostatin Analogs in the Management of Neuroendocrine Tumors. Gastrointest. Cancer Res. 2012, 5, 161–168. [Google Scholar]
- von der Ohe, M.R.; Camilleri, M.; Thomforde, G.M.; Klee, G.G. Differential Regional Effects of Octreotide on Human Gastrointestinal Motor Function. Gut 1995, 36, 743–748. [Google Scholar] [CrossRef]
- Kulaksiz, H. Identification of Somatostatin Receptor Subtypes 1, 2A, 3, and 5 in Neuroendocrine Tumours with Subtype Specific Antibodies. Gut 2002, 50, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Shi, X.; Ma, H.; Wang, H.; Li, B.; Song, B.; Guo, S.; Jin, G. The Effect of Using Long-Acting Octreotide as Adjuvant Therapy for Patients with Grade 2 Pancreatic Neuroendocrine Tumors after Radical Resection. J. Pancreatol. 2020, 3, 167–172. [Google Scholar] [CrossRef]
- Janson, E.T.; Knigge, U.; Dam, G.; Federspiel, B.; Grønbaek, H.; Stålberg, P.; Langer, S.W.; Kjaer, A.; Arola, J.; Schalin-Jäntti, C.; et al. Nordic Guidelines 2021 for Diagnosis and Treatment of Gastroenteropancreatic Neuroendocrine Neoplasms. Acta Oncol. 2021, 60, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.E.; Invernizzi, P.; Mazzaferro, V.; Massironi, S. Response and Relapse Rates after Treatment with Long-acting Somatostatin Analogs in Multifocal or Recurrent Type-1 Gastric Carcinoids: A Systematic Review and Meta-analysis. United Eur. Gastroenterol. J. 2020, 8, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Mirvis, E.; Mandair, D.; Garcia-Hernandez, J.; Mohmaduvesh, M.; Toumpanakis, C.; Caplin, M. Role of Interferon-Alpha in Patients with Neuroendocrine Tumors: A Retrospective Study. Anticancer Res. 2014, 34, 6601–6607. [Google Scholar]
- Liu, T.; Liao, J.; Dang, J.; Li, G. Treatments for Patients with Advanced Neuroendocrine Tumors: A Network Meta-Analysis. Ther. Adv. Med. Oncol. 2019, 11. [Google Scholar] [CrossRef]
- Chalabi, M.; Duluc, C.; Caron, P.; Vezzosi, D.; Guillermet-Guibert, J.; Pyronnet, S.; Bousquet, C. Somatostatin Analogs: Does Pharmacology Impact Antitumor Efficacy? Trends Endocrinol. Metab. 2014, 25, 115–127. [Google Scholar] [CrossRef]
- Pusceddu, S.; Verzoni, E.; Prinzi, N.; Mennitto, A.; Femia, D.; Grassi, P.; Concas, L.; Vernieri, C.; lo Russo, G.; Procopio, G. Everolimus Treatment for Neuroendocrine Tumors: Latest Results and Clinical Potential. Ther. Adv. Med. Oncol. 2017, 9, 183–188. [Google Scholar] [CrossRef]
- Herrera-Martínez, A.D.; Hofland, J.; Hofland, L.J.; Brabander, T.; Eskens, F.A.L.M.; Gálvez Moreno, M.A.; Luque, R.M.; Castaño, J.P.; de Herder, W.W.; Feelders, R.A. Targeted Systemic Treatment of Neuroendocrine Tumors: Current Options and Future Perspectives. Drugs 2019, 79, 21–42. [Google Scholar] [CrossRef]
- Pham, D.; Koide, K. Discoveries, Target Identifications, and Biological Applications of Natural Products That Inhibit Splicing Factor 3B Subunit 1. Nat. Prod. Rep. 2016, 33, 637–647. [Google Scholar] [CrossRef]
- Valkema, R.; de Jong, M.; Bakker, W.H.; Breeman, W.A.P.; Kooij, P.P.M.; Lugtenburg, P.J.; de Jong, F.H.; Christiansen, A.; Kam, B.L.R.; de Herder, W.W.; et al. Phase I Study of Peptide Receptor Radionuclide Therapy with [111In-DTPA0]Octreotide: The Rotterdam Experience. Semin. Nucl. Med. 2002, 32, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, M.; Eberlein, U. Radiation Dosimetry Aspects of 177Lu. Curr. Radiopharm. 2015, 8, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Krug, S.; Damm, M.; Garbe, J.; König, S.; Schmitz, R.L.; Michl, P.; Schrader, J.; Rinke, A. Finding the Appropriate Therapeutic Strategy in Patients with Neuroendocrine Tumors of the Pancreas: Guideline Recommendations Meet the Clinical Reality. J. Clin. Med. 2021, 10, 3023. [Google Scholar] [CrossRef]
- Strosberg, J.; Wolin, E.; Chasen, B.; Kulke, M.; Bushnell, D.; Caplin, M.; Baum, R.P.; Kunz, P.; Hobday, T.; Hendifar, A.; et al. Health-Related Quality of Life in Patients With Progressive Midgut Neuroendocrine Tumors Treated With 177Lu-Dotatate in the Phase III NETTER-1 Trial. J. Clin. Oncol. 2018, 36, 2578–2584. [Google Scholar] [CrossRef] [PubMed]
- Chin, R.-I.; Wu, F.S.; Menda, Y.; Kim, H. Radiopharmaceuticals for Neuroendocrine Tumors. Semin. Radiat. Oncol. 2021, 31, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; Leeuwenkamp, O.; Siddiqui, M.K. Peptide Receptor Radiotherapy Re-Treatment in Patients with Progressive Neuroendocrine Tumors: A Systematic Review and Meta-Analysis. Cancer Treat. Rev. 2021, 93, 102141. [Google Scholar] [CrossRef] [PubMed]
- Ebbers, S.C.; Braat, A.J.A.T.; Moelker, A.; Stokkel, M.P.M.; Lam, M.G.E.H.; Barentsz, M.W. Intra-Arterial versus Standard Intravenous Administration of Lutetium-177-DOTA-Octreotate in Patients with NET Liver Metastases: Study Protocol for a Multicenter, Randomized Controlled Trial (LUTIA Trial). Trials 2020, 21, 141. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Jacobson, O.; Cheng, Y.; Niu, G.; Li, F.; Bai, C.; Zhu, Z.; Chen, X. Safety, Pharmacokinetics, and Dosimetry of a Long-Acting Radiolabeled Somatostatin Analog 177 Lu-DOTA-EB-TATE in Patients with Advanced Metastatic Neuroendocrine Tumors. J. Nucl. Med. 2018, 59, 1699–1705. [Google Scholar] [CrossRef]
| Publication | Type and Number of the Studied Tumours | Preoperative Therapy with SSA | Receptors | Method | Correlation with Disease Outcome, Prognosis, or Other Relevant Findings |
|---|---|---|---|---|---|
| Brunner et al., 2016 [79] | PanNETs (n = 150, 53.8%), carcinoids (n = 84, 30.1%), and rare NETs (n = 45, 16.1%) | DOTATOC 41 (24.1%) DOTATOC 84 (49.4%) DOTATOC 45 (26.5%) | SSTR2 | IHC and imaging for all DOTATOC patients | -Patients with higher SSTR2 immunohistochemistry had a longer survival from diagnosis -Patients with G1 tumours survived longer thanks to other tumour grades -SSTR2 expression did not predict a benefit of DOTATOC over alternative treatment |
| Casar-Borota et al., 2013 [80] | Somatotroph PitNETs (n = 65) | Octreotide (n = 28); no preoperative treatment (n = 37) | SSTR1, SSTR2A, SSTR3, SSTR5 | IHC | -SSTR2a expression correlated with response to octreotide -SSTR2a expression was reduced in group of patients pre-treated with octreotide |
| Corleto et al., 2009 [81] | Well-differentiated PanNETs (n = 16, 49%) and well-differentiated GI-NET (n = 17, 51%) | All with SSA (octreotide LAR or lanreotide) | SSTR1, SSTR2, SSTR3, SSTR4, SSTR5 | RT-PCR (SSTR1, SSTR2, SSTR3, SSTR4, and SSTR5), IHC (SSTR2), and somatostatin receptor scintigraphy | -Significantly higher survival rate in tumours expressing SSTR2 and SSTR5 -Five-year survival significantly lower in patients whose tumours did not express SSTR2, SSTR5 (43%) to those who were positive for the expression (91%) |
| Diakatou et al., 2011 [82] | GEP-NETs (n = 76: gastric, small intestine, appendix, large intestine, pancreas, and liver metastasis) | No information | SSTR1, SSTR2A, SSTR2B, SSTR3, SSTR4, SSTR5 | IHC | -No information related to prognosis -SSTR are often co-expressed with D2R |
| Diakatou et al., 2015 [83] | GI-NENs (n = 44, primary and metastasis) and Lu-NENs (n = 16, primary and metastasis) | No information | SSTR2, SSTR3, SSTR5 | SRS, IHC | -No information related to prognosis -IHC positivity correlated with SRS -D2R were always co-expressed with SSTR2, but not only in specific fraction of tumours expressing SSTR2R |
| Fougner et al., 2008 [84] | Somatotroph PitNETs (n = 71) | Twenty-three preoperative octreotide | SSTR2A | IHC, Western blot | -Patients with preoperative octreotide treatment had lower SSTR2a expression in IHC and Western blot -Acute octreotide response was significantly better comparing treatment-naïve and pre-treated patients having higher SSTR2A expression in IHC |
| Franck et al., 2017 [85] | Somatotroph PitNETs (n = 39) | Drug naive (n = 23); pre-treated with LA SSA (n = 9); LA-SSA/PEGV (n = 7) | SSTR2, SSTR5 | IHC | -Treatment-naïve patients had significantly higher SSTR2 expression compared to pre-treated patients -No differences were observed for SSTR5 expression |
| Kaemmerer et al., 2015 [86] | GI-NENs (n = 121) G1 (n = 31), G2 (n = 47), and G3 (n = 43) | No information | SSTR1, SSTR2A, SSTR3, SSTR 5 | IHC | -Expression of SSTR2 was higher in G1 and G2 tumours -SSTR2A expression demonstrated trend towards better overall survival -SSTR1 expression was significantly different in G2 compared to G3b (highly proliferative Ki-67 > 50%) -SSTR3 expression was significantly different in G2 compared to G3a (low proliferative Ki-67 21–49%) |
| Mehta et al., 2015 [87] | PanNETs (n = 99) | No information | SSTR2A, SSTR5 | IHC | -SSTR2A expression correlated with improved overall survival -SSTR5 was not associated with survival |
| Nasir et al., 2006 [92] | Liver metastasis from patients with small intestinal and Pan-NETs (n = 14) | All with octreotide therapy | SSTR1, SSTR2, SSTR3, SSTR4, SSTR5 | IHC | -No information related to prognosis -In total, 61% of studied metastasis was positive for SSTR1, 83% for SSTR2, 72% for SSTR3, 56% for SSTR4, and 83% for SSTR5 |
| Nielsen et al., 2020 [93] | GEP-NENs (n = 163) | Two patients received peptide receptor radionuclide therapy | SSTR2A | IHC | -Tendency for increased survival in patients with tumour with positive SSTR2a expression |
| Papotti et al., 2002 [88] | Total GEP NET (n = 81: GI (n = 28) and PanNETs (n = 53)) | No information | SSTR1, SSTR2, SSTR3, SSTR4, SSTR5 | RT-PCR (SSTR1, SSTR2, SSTR3, SSTR4, and SSTR5) and immunohistochemistry (SSTR2, SSTR3, and SSTR5) | -No information related to prognosis -Results from RT-PCR and IHC were comparable -Tendency that lower level SSTR expression in poorly differentiated tumours |
| Okuwaki et al., 2013 [94] | PanNETs (n = 79) | Fifty-nine patients’ surgery as first line treatment, and the rest that accepted treatment any of the following regiments (SSA, chemotherapy, and local targeting of liver metastasis) | SSTR2A | IHC | -Poor prognosis was predicted for patients with no SSTR2A expression -Higher SSTR2A expression predicted higher survival rate |
| Popa et al., 2021 [49] | GI-NENs (n = 67) | No information | SSTR2, SSTR5 | IHC | -Tumours with grade G1 and G2 had higher SSTR2 expression compared to G3 -Decreased SSTR2 expression was associated with increased malignancy and tumour stage |
| Righi et al., 2010 [95] | Lu-NETs (n = 218) | Eight patients octreotide or DOTATOC | SSTR2A, SSTR3, | IHC | -Higher grade tumour had lower levels of SSTR expression; -SSTR2A was overexpressed in metastatic typical carcinoid compared to atypical carcinoids |
| Srirajaskanthan et al., 2009 [96] | NETs (n = 56: foregut (n = 20), midgut (n = 25), hindgut (n = 3), ovarian (n = 1), and unknown primary origin (n = 7)) | No information | SSTR2, SSTR5 | IHC | -Lower grade tumours had significantly higher SSTR2 and SSTR5 expression -OctreoScan data were comparable with IHC |
| Wang et al., 2017 [43] | GEP-NETs (n = 143) and GEP-NET patients with octreotide LAR (n = 54) | GEP-NET octreotide LAR (n = 54) | SSTR2, SSTR5 | IHC | -G1 and G2 tumours had higher SSTR2 expression -Well-differentiated pancreatic NETs had higher SSTR5 expression -SSTR2 and SSTR5 expression in GEP-NETs were correlated with improved survival |
| Zamora et al., 2010 [91] | GEP-NETs (n = 100: GI (n = 67), pancreatic (n = 25), and liver metastasis of unknown origin (n = 8) | No information | SSTR1, SSTR2, SSTR3, SSTR4, SSTR5 | IHC | -Well-differentiated NETs had higher SSTR expression; -SSTR expression was less frequent in pancreatic NETs compared to gastrointestinal NETs -SSTR2A was expressed on call membrane, while other subtypes (SSTR 1, 3, 4, 5) stained in cytoplasm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogoza, O.; Megnis, K.; Kudrjavceva, M.; Gerina-Berzina, A.; Rovite, V. Role of Somatostatin Signalling in Neuroendocrine Tumours. Int. J. Mol. Sci. 2022, 23, 1447. https://doi.org/10.3390/ijms23031447
Rogoza O, Megnis K, Kudrjavceva M, Gerina-Berzina A, Rovite V. Role of Somatostatin Signalling in Neuroendocrine Tumours. International Journal of Molecular Sciences. 2022; 23(3):1447. https://doi.org/10.3390/ijms23031447
Chicago/Turabian StyleRogoza, Olesja, Kaspars Megnis, Marija Kudrjavceva, Aija Gerina-Berzina, and Vita Rovite. 2022. "Role of Somatostatin Signalling in Neuroendocrine Tumours" International Journal of Molecular Sciences 23, no. 3: 1447. https://doi.org/10.3390/ijms23031447
APA StyleRogoza, O., Megnis, K., Kudrjavceva, M., Gerina-Berzina, A., & Rovite, V. (2022). Role of Somatostatin Signalling in Neuroendocrine Tumours. International Journal of Molecular Sciences, 23(3), 1447. https://doi.org/10.3390/ijms23031447





