Lactobacillus paracasei KW3110 Prevents Inflammatory-Stress-Induced Mitochondrial Dysfunction in Mouse Macrophages
Abstract
1. Introduction
2. Results
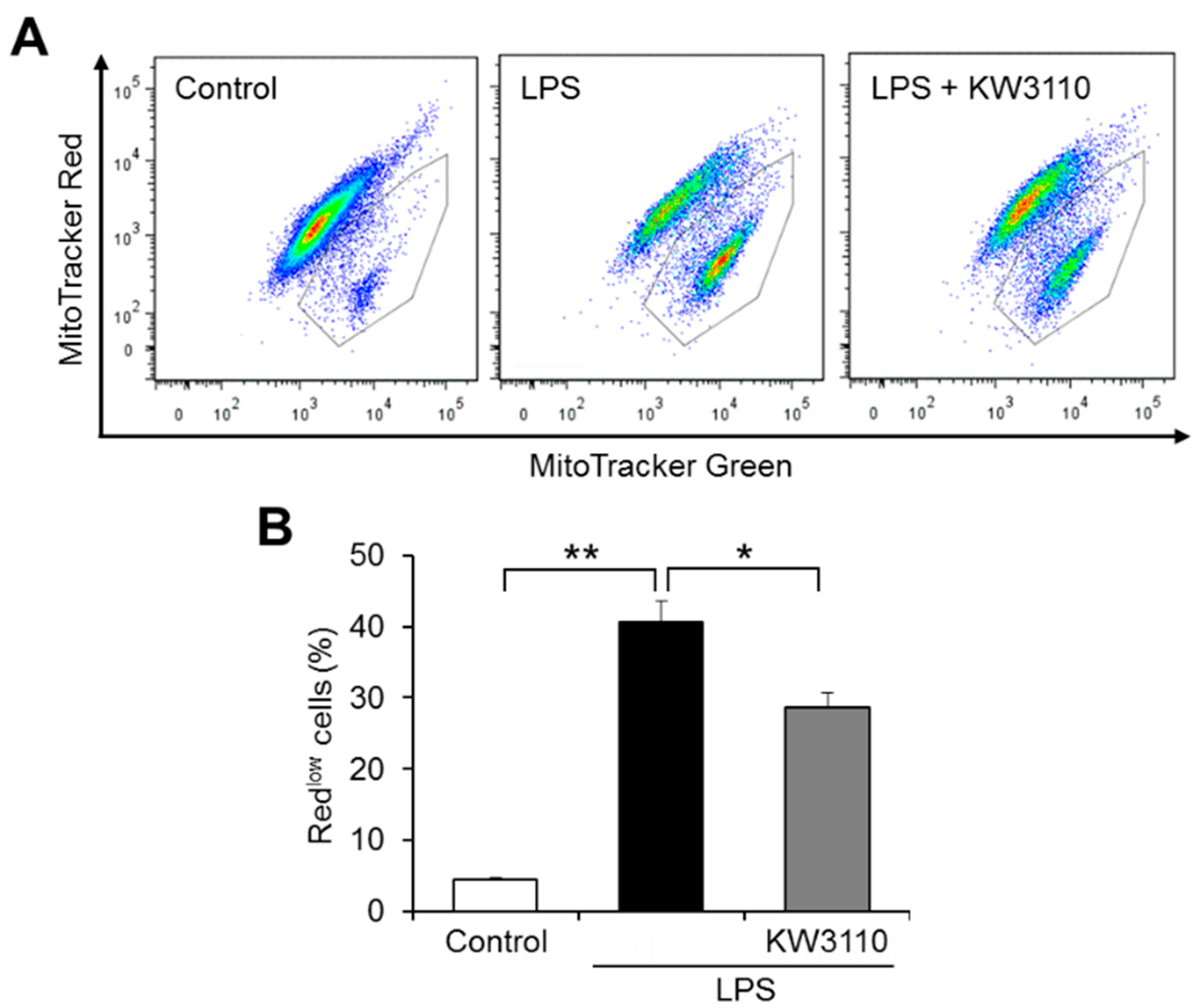
2.1. Effect of KW3110 on Mitochondrial Dysfunction in Inflammatory-Stressed J774A.1 Cells
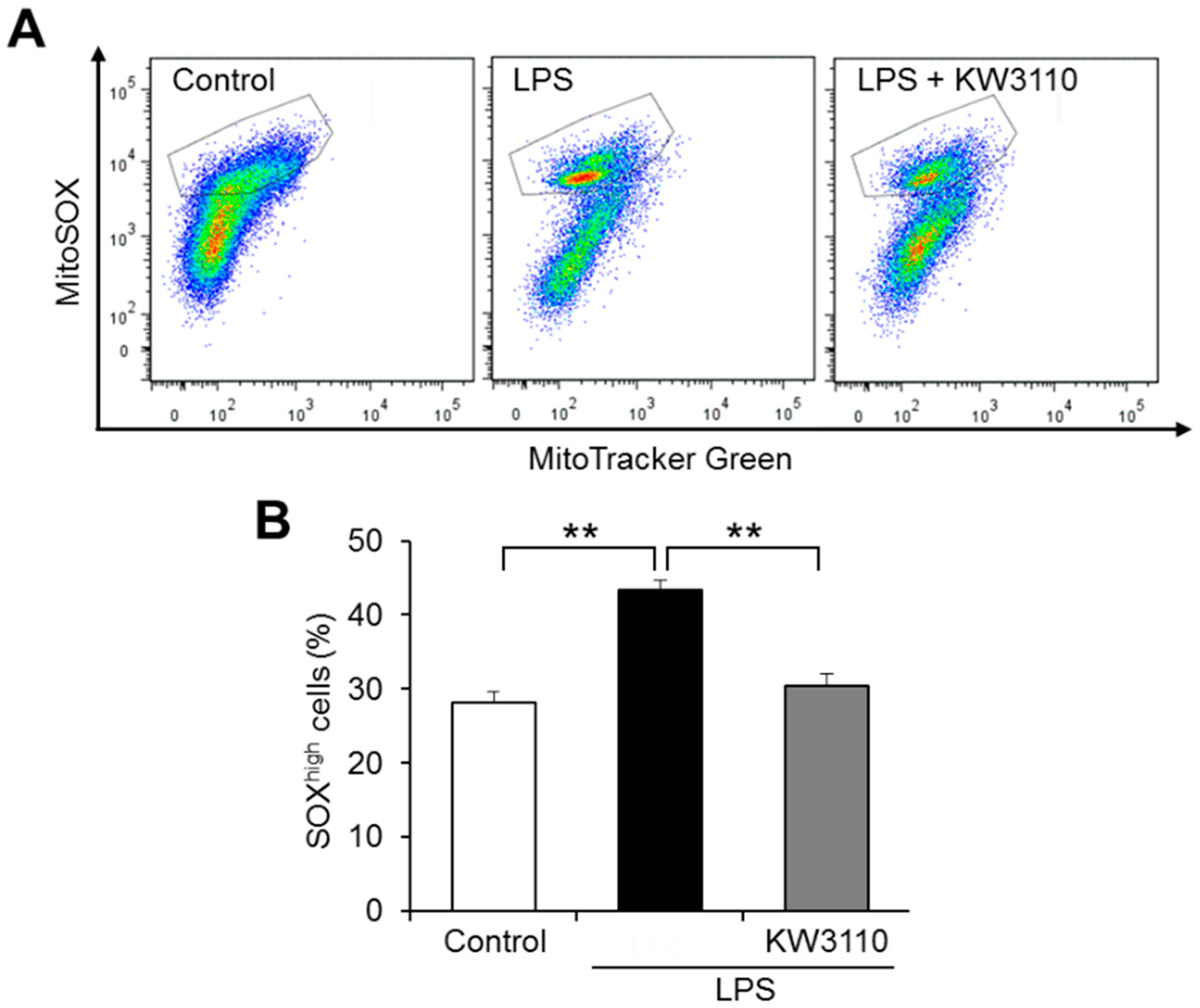
2.2. KW3110 Reduces Mitochondrial Reactive Oxygen Species (ROS) Production in Inflammatory-Stressed J774A.1 Cells
2.3. Effect of KW3110 on Mitochondrial Respiratory Function in Inflammatory-Stressed J774A.1 Cells
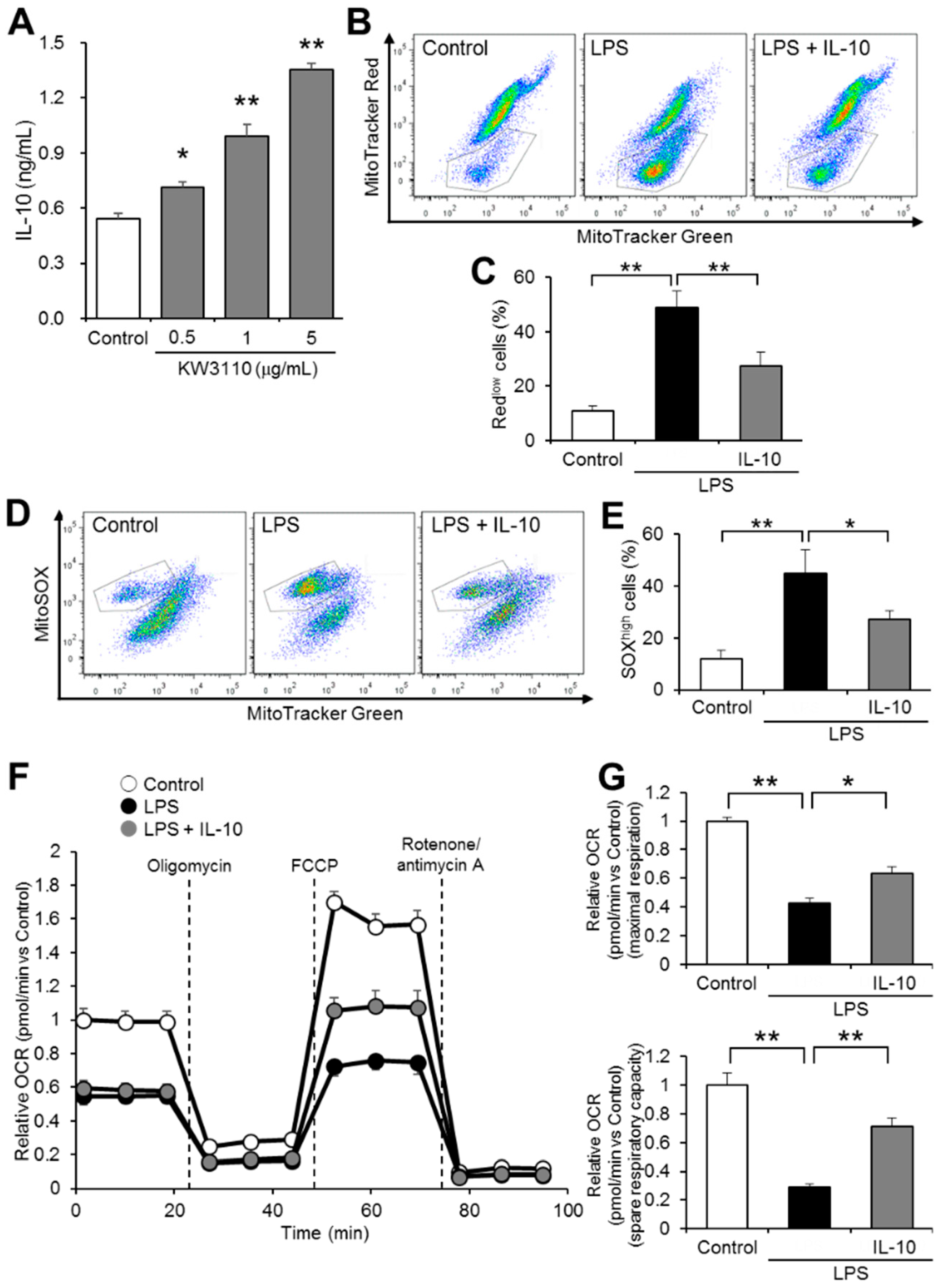
2.4. IL-10 Suppresses Mitochondrial Dysfunction in Inflammatory-Stressed J774A.1 Cells
2.5. Effect of KW3110 and IL-10 on Disruption of Mitochondrial Morphology in Inflammatory-Stressed J774A.1 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Mitochondrial Membrane Potential and ROS Analyses by Flow Cytometry
4.4. Mitochondrial Respiration Analysis
4.5. Determination of Cytokine Production
4.6. Transmission Electron Microscopy
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LAB | lactic acid bacteria |
| KW3110 | Lactobacillus paracasei KW3110 |
| IL | interleukin |
| LPS | lipopolysaccharide |
| ROS | reactive oxygen species |
| DMEM | Dulbecco’s modified Eagle medium |
| PBS | phosphate-buffered saline |
| OCR | oxygen consumption rate |
| ELISA | enzyme-linked immunosorbent assay |
| GA | glutaraldehyde |
| PB | phosphate buffer |
| RT | room temperature |
| ANOVA | analysis of variance |
| ATP | adenosine 5′-triphosphate |
References
- Garlanda, C.; Di Liberto, D.; Vecchi, A.; La Manna, M.P.; Buracchi, C.; Caccamo, N.; Salerno, A.; Dieli, F.; Mantovani, A. Damping excessive inflammation and tissue damage in Mycobacterium tuberculosis infection by Toll IL-1 receptor 8/single Ig IL-1-related receptor, a negative regulator of IL-1/TLR signaling. J. Immunol. 2007, 179, 3119–3125. [Google Scholar] [CrossRef] [PubMed]
- Calçada, D.; Vianello, D.; Giampieri, E.; Sala, C.; Castellani, G.; de Graaf, A.; Kremer, B.; van Ommen, B.; Feskens, E.; Santoro, A. The role of low-grade inflammation and metabolic flexibility in aging and nutritional modulation thereof: A systems biology approach. Mech. Ageing Dev. 2014, 136, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Serrano, M.; Blasco, M.A. The common biology of cancer and ageing. Nature 2007, 448, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Deleidi, M.; Jäggle, M.; Rubino, G. Immune aging, dysmetabolism, and inflammation in neurological diseases. Front. Neurosci. 2015, 9, 172. [Google Scholar] [CrossRef]
- Krysko, D.V.; Agostinis, P.; Krysko, O.; Garg, A.D.; Bachert, C.; Lambrecht, B.N.; Vandenabeele, P. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 2011, 32, 157–164. [Google Scholar] [CrossRef]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef]
- Green, D.R.; Galluzzi, L.; Kroemer, G. Mitochondria and the autophagy–inflammation–cell death axis in organismal aging. Science 2011, 333, 1109–1112. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Crit. Rev. Food Sci. 2018, 58, 1260–1270. [Google Scholar] [CrossRef]
- Mukherjee, S.; Das, S.K.; Vasudevan, D. Role of polyphenols in diet and nutrition-an updated review. Curr. Nutr. Food Sci. 2009, 5, 149–159. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef]
- Fontana, L.; Bermudez-Brito, M.; Plaza-Diaz, J.; Munoz-Quezada, S.; Gil, A. Sources, isolation, characterisation and evaluation of probiotics. Brit. J. Nutr. 2013, 109, S35–S50. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, D.; Inoue, S.; Wakabayashi, H.; Fujii, T. The anti-allergic effects of lactic acid bacteria are strain dependent and mediated by effects on both Th1/Th2 cytokine expression and balance. Int. Arch. Allergy Immunol. 2004, 135, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Furuzono, T.; Yamakuni, K.; Li, Y.; Kim, Y.I.; Takahashi, H.; Ohue-Kitano, R.; Jheng, H.F.; Takahashi, N.; Kano, Y. 10-oxo-12 (Z)-octadecenoic acid, a linoleic acid metabolite produced by gut lactic acid bacteria, enhances energy metabolism by activation of TRPV1. FASEB J. 2017, 31, 5036–5048. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, S.; Miyake, M.; Fujii, R.; Konishi, Y. Orally administered Lactobacillus paracasei KW3110 induces in vivo IL-12 production. Biosci. Biotechnol. Biochem. 2009, 73, 1561–1565. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Nariai, C.; Takemura, F.; Nakao, W.; Fujiwara, D. Dietary supplementation with lactic acid bacteria attenuates the development of atopic dermatitis-like skin lesions in NC/Nga mice in a strain-dependent manner. Int. Arch. Allergy Immunol. 2008, 145, 141–151. [Google Scholar] [CrossRef]
- Fujiwara, D.; Wakabayashi, H.; Watanabe, H.; Nishida, S.; Iino, H. A double-blind trial of Lactobacillus paracasei strain KW3110 administration for immunomodulation in patients with pollen allergy. Allergol. Int. 2005, 54, 143–149. [Google Scholar] [CrossRef]
- Yamazaki, T.; Ohshio, K.; Sugamata, M.; Morita, Y. Lactic acid bacterium, Lactobacillus paracasei KW3110, suppresses inflammatory stress-induced caspase-1 activation by promoting interleukin-10 production in mouse and human immune cells. PLoS ONE 2020, 15, e0237754. [Google Scholar] [CrossRef]
- Suzuki, H.; Yamazaki, T.; Ohshio, K.; Sugamata, M.; Yoshikawa, M.; Kanauchi, O.; Morita, Y. A specific strain of lactic acid bacteria, lactobacillus paracasei, inhibits inflammasome activation in vitro and prevents inflammation-related disorders. J. Immunol. 2020, 205, 811–821. [Google Scholar] [CrossRef]
- Yamazaki, T.; Suzuki, H.; Yamada, S.; Ohshio, K.; Sugamata, M.; Yamada, T.; Morita, Y. Lactobacillus paracasei KW3110 Suppresses Inflammatory Stress-Induced Premature Cellular Senescence of Human Retinal Pigment Epithelium Cells and Reduces Ocular Disorders in Healthy Humans. Int. J. Mol. Sci. 2020, 21, 5091. [Google Scholar] [CrossRef]
- Morita, Y.; Miwa, Y.; Jounai, K.; Fujiwara, D.; Kurihara, T.; Kanauchi, O. Lactobacillus paracasei KW3110 prevents blue light-induced inflammation and degeneration in the retina. Nutrients 2018, 10, 1991. [Google Scholar] [CrossRef]
- Morita, Y.; Jounai, K.; Sakamoto, A.; Tomita, Y.; Sugihara, Y.; Suzuki, H.; Ohshio, K.; Otake, M.; Fujiwara, D.; Kanauchi, O. Long-term intake of Lactobacillus paracasei KW3110 prevents age-related chronic inflammation and retinal cell loss in physiologically aged mice. Aging 2018, 10, 2723. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-H.; Pan, T.-M.; Wu, Y.-J.; Chang, S.-J.; Chang, M.-S.; Hu, C.-Y. Exopolysaccharide activities from probiotic bifidobacterium: Immunomodulatory effects (on J774A. 1 macrophages) and antimicrobial properties. Int. J. Food Microbiol. 2010, 144, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, G.; Gurley, E.C.; Zhou, H. Flavonoid apigenin inhibits lipopolysaccharide-induced inflammatory response through multiple mechanisms in macrophages. PLoS ONE 2014, 9, e107072. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Li, Y.; Peng, X.; Huang, D.; Gui, L.; Huang, B. Resistance of mitochondrial DNA-depleted cells against oxidized low-density lipoprotein-induced macrophage pyroptosis. Mol. Med. Rep. 2016, 13, 4393–4399. [Google Scholar] [CrossRef] [PubMed]
- Despres, H.; Ckless, K. Effect of Palmitate on Mitochondrial Function and the Implications on Inflammatory Pathway NLRP3/IL-1β in Mouse and Human Myeloid Cell Lines. Free Radical Biol. Med. 2017, 112, 200–201. [Google Scholar] [CrossRef]
- Ralph, P.; Nakoinz, I. Phagocytosis and cytolysis by a macrophage tumour and its cloned cell line. Nature 1975, 257, 393–394. [Google Scholar] [CrossRef]
- Kepp, O.; Galluzzi, L.; Kroemer, G. Mitochondrial control of the NLRP3 inflammasome. Nat. Immunol. 2011, 12, 199–200. [Google Scholar] [CrossRef]
- Hunter, R.L.; Dragicevic, N.; Seifert, K.; Choi, D.Y.; Liu, M.; Kim, H.C.; Cass, W.A.; Sullivan, P.G.; Bing, G. Inflammation induces mitochondrial dysfunction and dopaminergic neurodegeneration in the nigrostriatal system. J. Neurochem. 2007, 100, 1375–1386. [Google Scholar] [CrossRef]
- Zick, M.; Rabl, R.; Reichert, A.S. Cristae formation—Linking ultrastructure and function of mitochondria. Biochim. Biophys. Acta Mol. Cell. Res. 2009, 1793, 5–19. [Google Scholar] [CrossRef]
- Cogliati, S.; Enriquez, J.A.; Scorrano, L. Mitochondrial cristae: Where beauty meets functionality. Trends Biochem. Sci. 2016, 41, 261–273. [Google Scholar] [CrossRef]
- Du, Z.; Liu, H.; Zhang, Z.; Li, P. Antioxidant and anti-inflammatory activities of Radix Isatidis polysaccharide in murine alveolar macrophages. Int. J. Biol. Macromol. 2013, 58, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-Y.; Lee, K.-C.; Chen, S.-Y.; Chang, H.-H. 6-Gingerol inhibits ROS and iNOS through the suppression of PKC-α and NF-κB pathways in lipopolysaccharide-stimulated mouse macrophages. Biochem. Biophys. Res. Commun. 2009, 382, 134–139. [Google Scholar] [CrossRef]
- Gottlieb, E.; Vander Heiden, M.G.; Thompson, C.B. Bcl-xL prevents the initial decrease in mitochondrial membrane potential and subsequent reactive oxygen species production during tumor necrosis factor alpha-induced apoptosis. Mol. Cell. Biol. 2000, 20, 5680–5689. [Google Scholar] [CrossRef] [PubMed]
- Nhu, Q.M.; Cuesta, N.; Vogel, S.N. Transcriptional regulation of lipopolysaccharide (LPS)-induced Toll-like receptor (TLR) expression in murine macrophages: Role of interferon regulatory factors 1 (IRF-1) and 2 (IRF-2). J. Endotoxin Res. 2006, 12, 285–295. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, L.; Cardinal, J.S.; Bahar, R.; Evankovich, J.; Huang, H.; Nace, G.; Billiar, T.R.; Rosengart, M.R.; Pan, P.; Tsung, A. Interferon regulatory factor-1 regulates the autophagic response in LPS-stimulated macrophages through nitric oxide. Mol. Med. 2012, 18, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.-Y.; Zhang, L.-M.; Ai, Y.-H.; Pan, P.-H.; Zhao, S.-P.; Su, X.-L.; Wu, D.-D.; Tan, H.-Y.; Zhang, L.-N.; Tsung, A. Role of interferon regulatory factor-1 in lipopolysaccharide-induced mitochondrial damage and oxidative stress responses in macrophages. Int. J. Mol. Med. 2017, 40, 1261–1269. [Google Scholar] [CrossRef]
- López-Armada, M.J.; Riveiro-Naveira, R.R.; Vaamonde-García, C.; Valcárcel-Ares, M.N. Mitochondrial dysfunction and the inflammatory response. Mitochondrion 2013, 13, 106–118. [Google Scholar] [CrossRef]
- Ip, W.E.; Hoshi, N.; Shouval, D.S.; Snapper, S.; Medzhitov, R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science 2017, 356, 513–519. [Google Scholar] [CrossRef]
- Mills, E.L.; Kelly, B.; Logan, A.; Costa, A.S.; Varma, M.; Bryant, C.E.; Tourlomousis, P.; Däbritz, J.H.M.; Gottlieb, E.; Latorre, I. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 2016, 167, 457–470. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamazaki, T.; Yamada, S.; Ohshio, K.; Sugamata, M.; Morita, Y. Lactobacillus paracasei KW3110 Prevents Inflammatory-Stress-Induced Mitochondrial Dysfunction in Mouse Macrophages. Int. J. Mol. Sci. 2022, 23, 1443. https://doi.org/10.3390/ijms23031443
Yamazaki T, Yamada S, Ohshio K, Sugamata M, Morita Y. Lactobacillus paracasei KW3110 Prevents Inflammatory-Stress-Induced Mitochondrial Dysfunction in Mouse Macrophages. International Journal of Molecular Sciences. 2022; 23(3):1443. https://doi.org/10.3390/ijms23031443
Chicago/Turabian StyleYamazaki, Takahiro, Sayuri Yamada, Konomi Ohshio, Miho Sugamata, and Yuji Morita. 2022. "Lactobacillus paracasei KW3110 Prevents Inflammatory-Stress-Induced Mitochondrial Dysfunction in Mouse Macrophages" International Journal of Molecular Sciences 23, no. 3: 1443. https://doi.org/10.3390/ijms23031443
APA StyleYamazaki, T., Yamada, S., Ohshio, K., Sugamata, M., & Morita, Y. (2022). Lactobacillus paracasei KW3110 Prevents Inflammatory-Stress-Induced Mitochondrial Dysfunction in Mouse Macrophages. International Journal of Molecular Sciences, 23(3), 1443. https://doi.org/10.3390/ijms23031443





