GPR18-Mediated Relaxation of Human Isolated Pulmonary Arteries
Abstract
:1. Introduction
2. Results
2.1. General
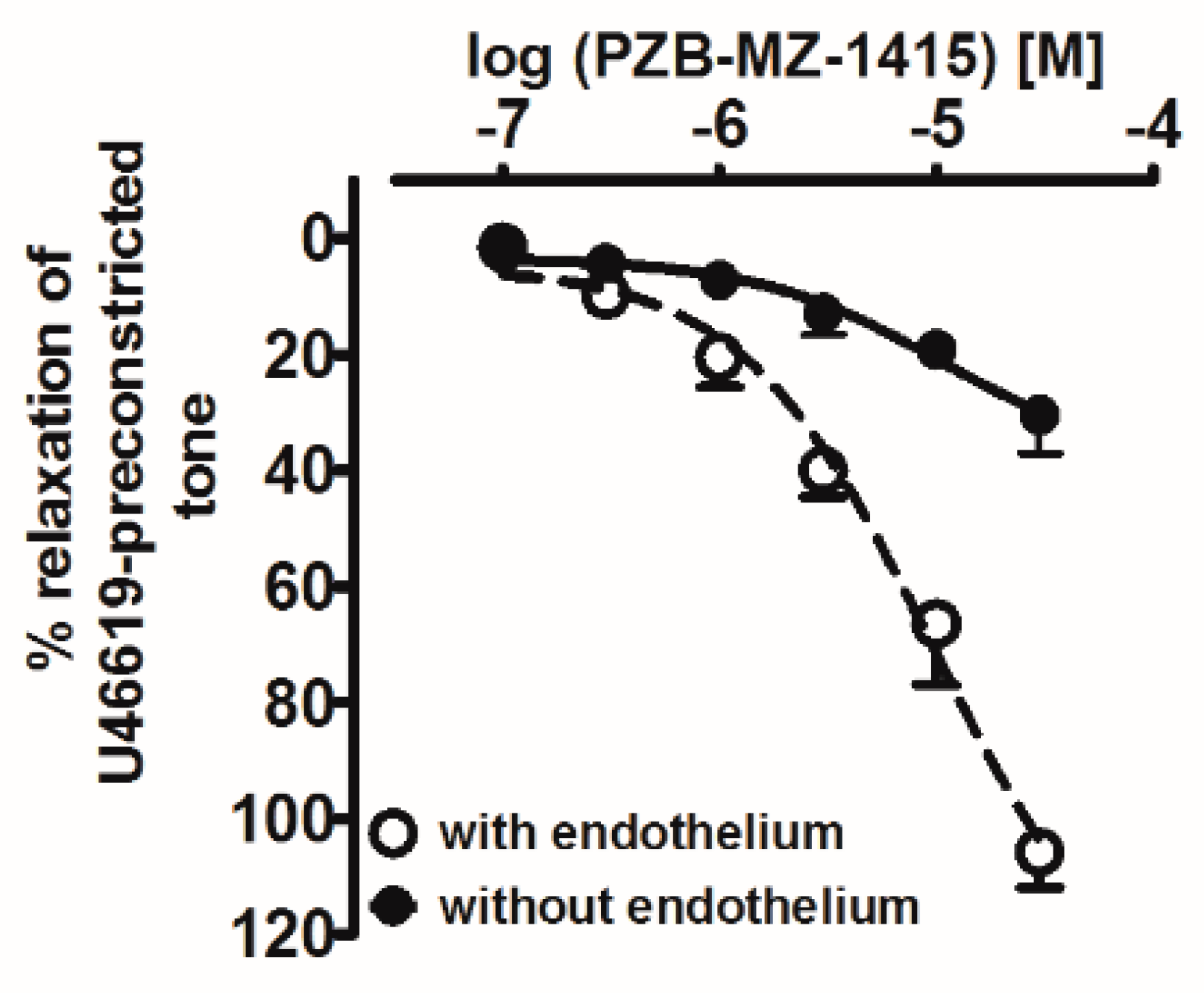
2.2. Influence of Agonists on U46619-Precontracted Human Pulmonary Arteries
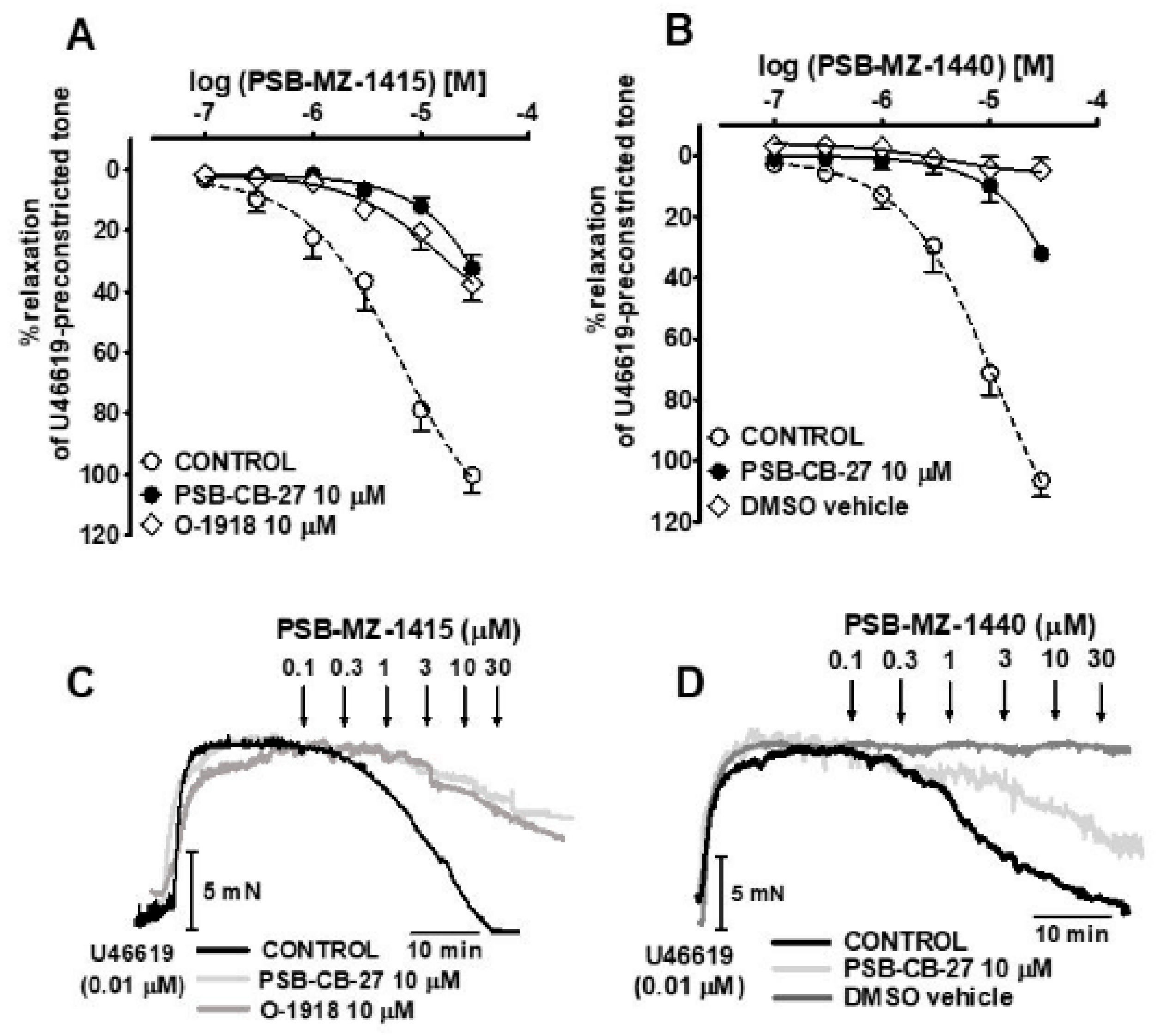
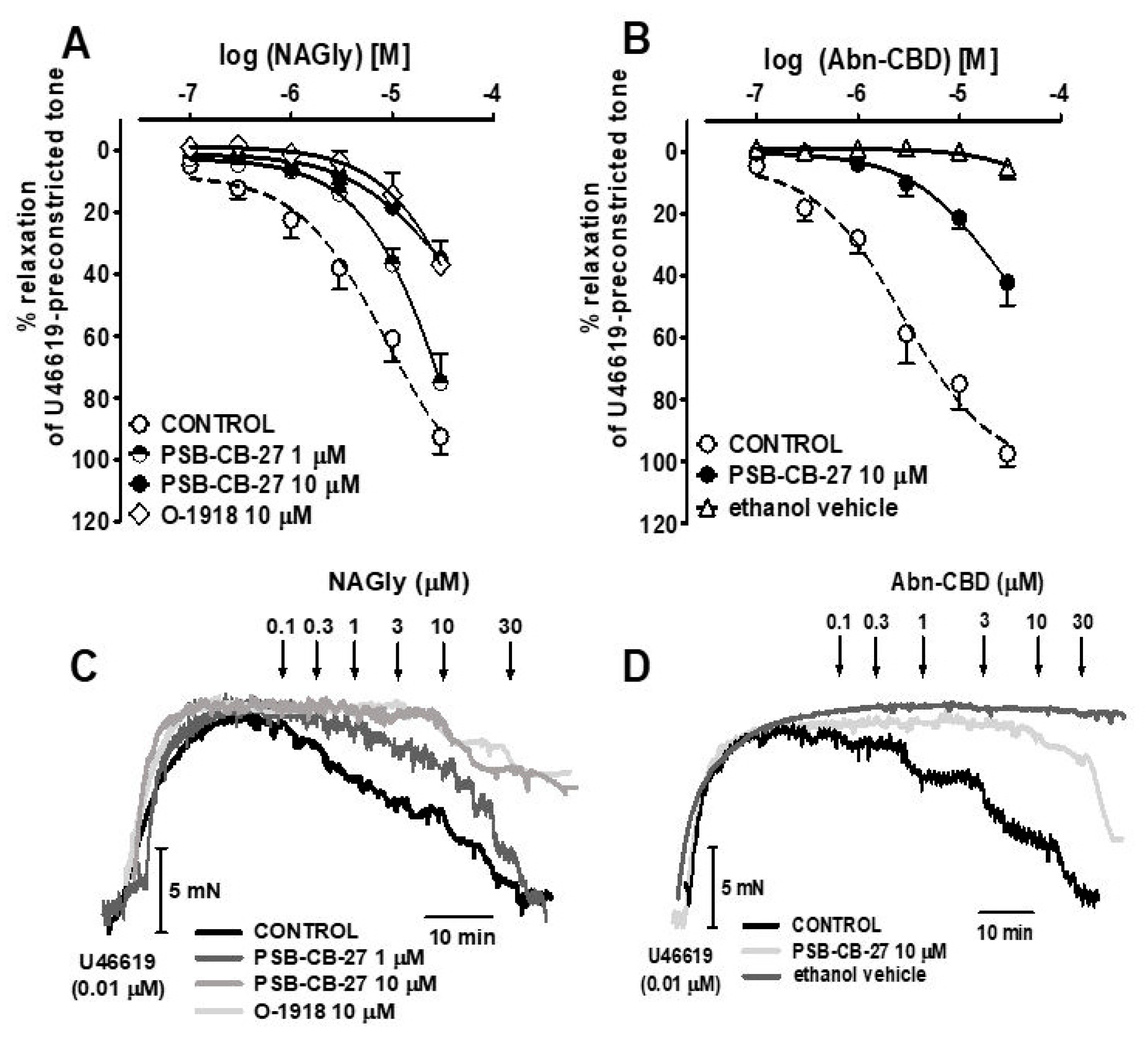
2.3. Interaction of PSB-CB-27 and O-1918 with the Agonist-Induced Relaxation in Human Pulmonary Arteries
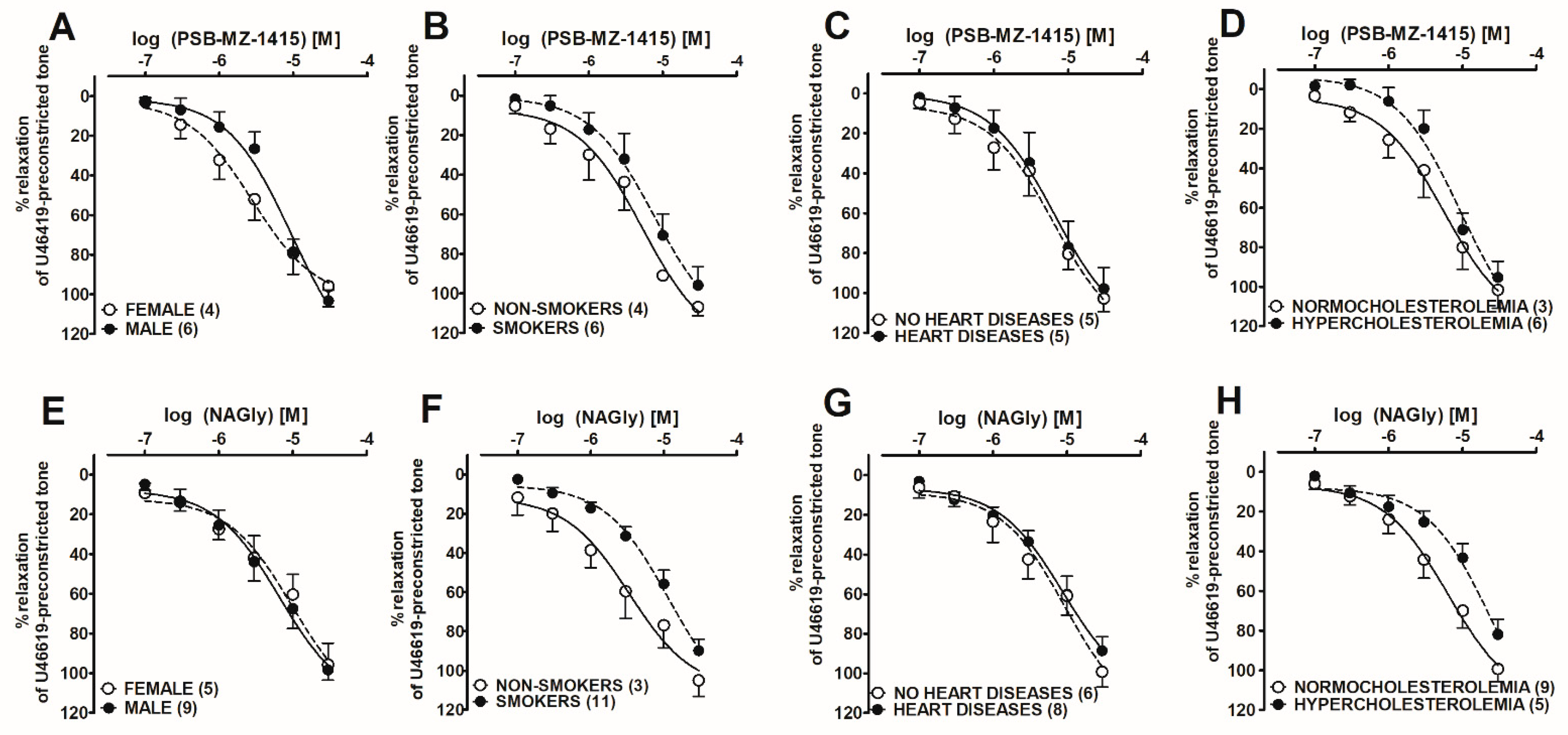
2.4. Post hoc Analysis of the Influence of Co-Morbidities on PSB-MZ-1415- and NAGly-Mediated Vasorelaxation in Human Pulmonary Arteries
2.5. gpr18 mRNA Expression in Human Bronchioles and Pulmonary Arteries
3. Discussion
4. Materials and Methods
4.1. Tissue Preparation
4.2. Vasorelaxation Study
4.3. RNA Isolation and Gene Expression Analysis
4.4. Drugs
4.5. Calculations and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abn-CBD | abnormal cannabidiol |
| AEA | anandamide |
| CRC | concentration-response curve |
| DMSO | dimethyl sulfoxide |
| hPAs | human pulmonary arteries |
| NAGly | N-arachidonyl-glycine |
| PSB-MZ-1415 | GPR18 agonist |
| PSB-MZ-1440 | GPR18 agonist |
| PSB-CB-27 | GPR18 antagonist |
| THC | Δ9-tetrahydrocannabinol |
| RVLM | rostral ventrolateral medulla |
References
- Rajaraman, G.; Simcocks, A.; Hryciw, D.H.; Hutchinson, D.S.; McAinch, A.J. G protein coupled receptor 18: A potential role for endocannabinoid signaling in metabolic dysfunction. Mol. Nutr. Food Res. 2016, 60, 92–102. [Google Scholar] [CrossRef]
- Gantz, I.; Muraoka, A.; Yang, Y.K.; Samuelson, L.C.; Zimmerman, E.M.; Cook, H.; Yamada, T. Cloning and chromosomal localization of a gene (GPR18) encoding a novel seven transmembrane receptor highly expressed in spleen and testis. Genomics 1997, 42, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sumida, H.; Cyster, J.G. GPR18 is required for a normal CD8αα intestinal intraepithelial lymphocyte compartment. J. Exp. Med. 2014, 211, 2351–2359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohno, M.; Hasegawa, H.; Inoue, A.; Muraoka, M.; Miyazaki, T.; Oka, K.; Yasukawa, M. Identification of N-arachidonylglycine as the endogenous ligand for orphan G-protein-coupled receptor GPR18. Biochem. Biophys. Res. Commun. 2006, 347, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Takenouchi, R.; Inoue, K.; Kambe, Y.; Miyata, A. N-arachidonoyl glycine induces macrophage apoptosis via GPR18. Biochem. Biophys. Res. Commun. 2012, 418, 366–371. [Google Scholar] [CrossRef] [PubMed]
- MacIntyre, J.; Dong, A.; Straiker, A.; Zhu, J.; Howlett, S.E.; Bagher, A.; Denovan-Wright, E.; Yu, D.Y.; Kelly, M.E. Cannabinoid and lipid-mediated vasorelaxation in retinal micro vasculature. Eur. J. Pharmacol. 2014, 735, 105–114. [Google Scholar] [CrossRef]
- Miller, S.; Leishman, E.; Oehler, O.; Daily, L.; Murataeva, N.; Wager-Miller, J.; Bradshaw, H.; Straiker, A. Evidence for a GPR18 role in diurnal regulation of intraocular pressure. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6419–6426. [Google Scholar] [CrossRef] [Green Version]
- Simcocks, A.C.; O’Keefe, L.; Jenkin, K.A.; Cornall, L.M.; Grinfeld, E.; Mathai, M.L.; Hryciw, D.H.; McAinch, A.J. The role of atypical cannabinoid ligands O-1602 and O-1918 on skeletal muscle homeostasis with a focus on obesity. Int. J. Mol. Sci. 2020, 21, 5922. [Google Scholar] [CrossRef]
- Penumarti, A.; Abdel-Rahman, A.A. The novel endocannabinoid receptor GPR18 is expressed in the rostral ventrolateral medulla and exerts tonic restraining influence on blood pressure. J. Pharmacol. Exp. Ther. 2014, 349, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Fabisiak, A.; Fabisiak, N.; Mokrowiecka, A.; Małecka-Panas, E.; Jacenik, D.; Kordek, R.; Zielińska, M.; Kieć-Kononowicz, K.; Fichna, J. Novel selective agonist of GPR18, PSB-KK-1415 exerts potent anti-inflammatory and anti-nociceptive activities in animal models of intestinal inflammation and inflammatory pain. Neurogastroenterol. Motil. 2020, 33, e14003. [Google Scholar] [CrossRef]
- McHugh, D. GPR18 in microglia: Implications for the CNS and endocannabinoid system signalling. Br. J. Pharmacol. 2012, 167, 1575–1582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McHugh, D.; Hu, S.S.; Rimmerman, N.; Juknat, A.; Vogel, Z.; Walker, J.M.; Bradshaw, H.B. N-arachidonoyl glycine, an abundant endogenous lipid, potently drives directed cellular migration through GPR18, the putative abnormal cannabidiol receptor. BMC Neurosci. 2010, 11, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Y.; Verdegaal, E.M.; Siderius, M.; Bebelman, J.P.; Smit, M.J.; Leurs, R.; Willemze, R.; Tensen, C.P.; Osanto, S. Quantitative expression profiling of G-protein-coupled receptors (GPCRs) in metastatic melanoma: The consti tutively active orphan GPCR GPR18 as novel drug target. Pigment Cell Melanoma Res. 2011, 24, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Burstein, S.H. N-Acyl amino acids (elmiric acids): Endogenous signaling molecules with therapeutic potential. Mol. Pharmacol. 2018, 93, 228–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldwell, M.D.; Hu, S.S.; Viswanathan, S.; Bradshaw, H.; Kelly, M.E.; Straiker, A.A. GPR18-based signalling system regulates IOP in murine eye. Br. J. Pharmacol. 2013, 169, 834–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matouk, A.I.; Taye, A.; El-Moselhy, M.A.; Heeba, G.H.; Abdel-Rahman, A.A. The effect of chronic activation of the novel endocannabinoid receptor GPR18 on myocardial func tion and blood pressure in conscious rats. J. Cardiovasc. Pharmacol. 2017, 69, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Parmar, N.; Ho, W.S. N-arachidonoyl glycine, an endogenous lipid that acts as a vasorelaxant via nitric oxide and large conductance calcium-activated potassium channels. Br. J. Pharmacol. 2010, 160, 594–603. [Google Scholar] [CrossRef] [Green Version]
- Baranowska-Kuczko, M.; Kozłowska, H.; Kloza, M.; Sadowska, O.; Kozłowski, M.; Kusaczuk, M.; Kasacka, I.; Malinowska, B. Vasodilatory effects of cannabidiol in human pulmonary and rat small mesenteric arteries: Modification by hypertension and the potential pharmacological opportunities. J. Hypertens. 2020, 38, 896–911. [Google Scholar] [CrossRef]
- Ulu, A.; Sahoo, P.K.; Yuil-Valdes, A.G.; Mukherjee, M.; Van Ormer, M.; Muthuraj, P.G.; Thompson, M.; Anderson Berry, A.; Hanson, C.K.; Natarajan, S.K.; et al. Omega-3 fatty acid-derived resolvin D2 regulates human placental vascular smooth muscle and extravillous trophoblast activities. Int. J. Mol. Sci. 2019, 20, 4402. [Google Scholar] [CrossRef] [Green Version]
- Console-Bram, L.; Brailoiu, E.; Brailoiu, G.C.; Sharir, H.; Abood, M.E. Activation of GPR18 by cannabinoid compounds: A tale of biased agonism. Br. J. Pharmacol. 2014, 171, 3908. [Google Scholar] [CrossRef] [Green Version]
- Wilhelmsen, K.; Khakpour, S.; Tran, A.; Sheehan, K.; Schumacher, M.; Xu, F.; Hellman, J. The endocannabinoid/endovanilloid N-arachidonoyl dopamine (NADA) and synthetic cannabinoid WIN55,212-2 abate the inflammatory activation of human endothelial cells. J. Biol. Chem. 2014, 289, 13079–13100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matouk, A.I.; Taye, A.; El-Moselhy, M.A.; Heeba, G.H.; Abdel-Rahman, A.A. Abnormal can nabidiol confers cardioprotection in diabetic rats independent of glycemic control. Eur. J. Pharmacol. 2018, 820, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Járai, Z.; Wagner, J.A.; Varga, K.; Lake, K.D.; Compton, D.R.; Martin, B.R.; Zimmer, A.M.; Bonner, T.I.; Buckley, N.E.; Mezey, E.; et al. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc. Natl. Acad. Sci. USA 1999, 96, 14136–14141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Suleimani, Y.M.; Al Mahruqi, A.S. The endogenous lipid N-arachidonoyl glycine is hypotensive and nitric oxide-cGMP-dependent vasorelaxant. Eur. J. Pharmacol. 2017, 794, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, H.; Baranowska, M.; Schlicker, E.; Kozlowski, M.; Laudanski, J.; Malinowska, B. Identification of the vasodilatory endothelial cannabinoid receptor in the human pulmonary artery. J. Hypertens. 2007, 25, 2240–2248. [Google Scholar] [CrossRef] [PubMed]
- Baranowska-Kuczko, M.; Kozlowska, H.; Kozlowski, M.; Schlicker, E.; Kloza, M.; Surazynski, A.; Grzeda, E.; Malinowska, B. Mechanisms of endothelium-dependent relaxation evoked by anandamide in isolated human pulmonary arteries. Naunyn. Schmiedebergs Arch. Pharmacol. 2014, 387, 477–486. [Google Scholar] [CrossRef] [Green Version]
- Offertáler, L.; Mo, F.-M.; Bátkai, S.; Liu, J.; Begg, M.; Razdan, R.K.; Martin, B.R.; Bukoski, R.D.; Kunos, G. Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Mol. Pharmacol. 2003, 63, 699–705. [Google Scholar] [CrossRef] [Green Version]
- Finlay, D.B.; Joseph, W.R.; Grimsey, N.L.; Glass, M. GPR18 undergoes a high degree of constitutive trafficking but is unresponsive to N-Arachidonoyl Glycine. PeerJ 2016, 4, e1835. [Google Scholar] [CrossRef] [Green Version]
- Neumann, A.; Engel, V.; Mahardhika, A.B.; Schoeder, C.T.; Namasivayam, V.; Kieć-Kononowicz, K.; Müller, C.E. Computational investigations on the binding mode of ligands for the cannabinoid-activated G protein-coupled receptor GPR18. Biomolecules 2020, 10, 686. [Google Scholar] [CrossRef]
- Alexander, S.P.; Christopoulos, A.; Davenport, A.P.; Kelly, E.; Mathie, A.; Peters, J.A.; Veale, E.L.; Armstrong, J.F.; Faccenda, E.; Harding, S.D.; et al. The Concise Guide to Pharmacology 2021/22: G protein-coupled receptors. Br. J. Pharmacol. 2021, 178 (Suppl. S1), S27–S156. [Google Scholar] [CrossRef]
- Yin, H.; Chu, A.; Li, W.; Wang, B.; Shelton, F.; Otero, F.; Nguyen, D.G.; Caldwell, J.S.; Chen, Y.A. Lipid G protein-coupled receptor ligand identification using beta-arrestin PathHunter assay. J. Biol. Chem. 2009, 284, 12328–12338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bondarenko, A.I.; Drachuk, K.; Panasiuk, O.; Sagach, V.; Deak, A.T.; Malli, R.; Graier, W.F. N-Arachidonoyl glycine suppresses Na+/Ca2+ exchanger-mediated Ca2+ entry into endothelial cells and activates BKCa channels independently of GPCRs. Br. J. Pharmacol. 2013, 169, 933–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoeder, C.T.; Kaleta, M.; Mahardhika, A.B.; Olejarz-Maciej, A.; Łażewska, D.; Kieć-Kononowicz, K.; Müller, C.E. Structure-activity relationships of imidazothiazinones and analogs as antagonists of the cannabinoid-activated orphan G protein-coupled receptor GPR18. Eur. J. Med. Chem. 2018, 155, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Karpińska, O.; Baranowska-Kuczko, M.; Kloza, M.; Ambrozewicz, E.; Kozłowski, T.; Kasacka, I.; Malinowska, B.; Kozłowska, H. Activation of CB1 receptors by 2-arachidonoylglycerol attenuates vasoconstriction induced by U46619 and angiotensin II in human and rat pulmonary arteries. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R883–R893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozlowska, H.; Baranowska, M.; Schlicker, E.; Kozlowski, M.; Laudanski, J.; Malinowska, B. Virodhamine relaxes the human pulmonary through the endothelial cannabinoid receptor and indirectly through a COX product. Br. J. Pharmacol. 2008, 155, 1034–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoeder, C.T.; Mahardhika, A.B.; Drabczyńska, A.; Kieć-Kononowicz, K.; Müller, C.E. Discovery of tricyclic xanthines as agonists of the cannabinoid-activated orphan G-protein-coupled receptor GPR18. ACS Med. Chem. Lett. 2020, 11, 2024–2031. [Google Scholar] [CrossRef]
- Baranowska-Kuczko, M.; MacLean, M.R.; Kozlowska, H.; Malinowska, B. Endothelium-dependent mechanisms of the vasodilatory effect of the endocannabinoid, anandamide, in the rat pulmonary artery. Pharmacol. Res. 2012, 66, 251–259. [Google Scholar] [CrossRef]
- Kotańska, M.; Kubacka, M.; Bednarski, M.; Nicosia, N.; Szafarz, M.; Jawień, W.; Müller, C.E.; Kieć-Kononowicz, K. The GPR18 agonist PSB-KD-107 exerts endothelium-dependent vasorelaxant effects. Pharmaceuticals 2021, 14, 799. [Google Scholar] [CrossRef]
- Su, J.B. Vascular endothelial dysfunction and pharmacological treatment. World J. Cardiol. 2015, 7, 719–741. [Google Scholar] [CrossRef]
- Galie, N.; Hoeper, M.M.; Humbert, M.; Torbicki, A.; Vachiery, J.L.; Barbera, J.A.; Beghetti, M.; Corris, P.; Gaine, S.; Gibbs, J.S.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [CrossRef]
- Sommer, N.; Ghofrani, H.A.; Pak, O.; Bonnet, S.; Provencher, S.; Sitbon, O.; Rosenkranz, S.; Hoeper, M.M.; Kiely, D.G. Current and future treatments of pulmonary arterial hypertension. Br. J. Pharmacol. 2021, 178, 6–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotańska, M.; Mika, K.; Szafarz, M.; Kubacka, M.; Müller, C.E.; Sapa, J.; Kieć-Kononowicz, K. Effects of GPR18 ligands on body weight and metabolic parameters in a female rat model of excessive eating. Pharmaceuticals 2021, 14, 270. [Google Scholar] [CrossRef] [PubMed]
- Karpińska, O.; Baranowska-Kuczko, M.; Malinowska, B.; Kloza, M.; Kusaczuk, M.; Gęgotek, A.; Golec, P.; Kasacka, I.; Koz łowska, H. Mechanisms of l-alpha-lysophosphatidylinositol-induced relaxation in human pulmonary arteries. Life Sci. 2018, 192, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Kusaczuk, M.; Krętowski, R.; Stypułkowska, A.; Cechowska-Pasko, M. Molecular and cellular effects of a novel hydroxa mate-based HDAC inhibitor-belinostat-in glioblastoma cell lines: A preliminary report. Investig. New Drugs 2016, 34, 552–564. [Google Scholar] [CrossRef] [Green Version]
- Yoo, S.A.; You, S.; Yoon, H.J.; Kim, D.H.; Kim, H.S.; Lee, K.; Ahn, J.H.; Hwang, D.; Lee, A.S.; Kim, K.J.; et al. A novel pathogenic role of the ER chaperone GRP78/BiP in rheumatoid arthritis. J. Exp. Med. 2012, 209, 871–886. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Kenakin, T.P. A Pharmacology Primer: Techniques for More Effective and Strategic Drug Discovery, 5th ed.; Academic Press: San Diego, CA, USA, 2019. [Google Scholar]

| Group | n | Contraction (mN) | pEC25 | pEC50 | Rmax (%) |
|---|---|---|---|---|---|
| NAGly | 13 | 10.8 ± 2.5 | 5.8 ± 0.1 | 5.1 ± 0.1 | 92.5 ± 5.6 |
| +PSB-CB-27 (1 μM) | 7 | 9.9 ± 1.3 | 5.2 ± 0.1 ***a | N.D. | N.D. |
| +PSB-CB-27 (10 μM) | 7 | 10.5 ± 1.8 | 4.9 ± 0.1 ***a | N.D. | N.D. |
| +O-1918 (10 μM) | 7 | 9.8 ± 2.1 | 4.7 ± 0.1 ***a | N.D. | N.D. |
| Abn-CBD | 5 | 10.6 ± 2.5 | 6.2 ± 0.2 | 5.5 ± 0.1 | 97.4 ± 4.1 |
| +PSB-CB-27 (10 μM) | 7 | 9.7 ± 1.9 | 4.8 ± 0.1 ***b | N.D. | N.D. |
| Ethanol | 7 | 8.9 ± 2.6 | N.D. | N.D. | 4.8 ± 4.1 ***b |
| PSB-MZ-1415 | 10 | 9.9 ± 1.2 | 5.8 ± 0.2 | 5.2 ± 0.2 | 100.3 ± 5.9 |
| +PSB-CB-27 (10 μM) | 6 | 10.2 ± 1.3 | 4.6 ± 0.1 ***a | N.D. | N.D. |
| +O-1918 (10 μM) | 6 | 9.8 ± 1.8 | 4.9 ± 0.2 **a | N.D. | N.D. |
| +Endothelium | 3 | 10.3 ± 1.1 | 5.9 ± 0.1 | 5.0 ± 0.1 | N.D. |
| −Endothelium | 3 | 11.3 ± 1.2 | 4.8 ± 0.1 **b | N.D. | N.D. |
| PSB-MZ-1440 | 8 | 10.3 ± 2.0 | 6.2 ± 0.2 | 4.9 ± 0.1 | 106.5 ± 5.3 |
| +PSB-CB-27 (10 μM) | 6 | 9.5 ± 1.2 | 4.8 ± 0.1 ***b | N.D. | N.D. |
| DMSO | 10 | 10.5 ± 1.8 | N.D. | N.D. | 4.9 ± 4.0 ***b |
| NAGly | Abn-CBD | PSB-MZ-1415 | PSB-MZ-1440 | |
|---|---|---|---|---|
| PSB-CB-27 | 6.2 | 6.3 | 6.2 | 6.3 |
| O-1918 | 6.1 | N.D. | 5.8 | N.D. |
| Characteristics | Vasodilatory Effect of: | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| PSB-MZ-1415 | NAGly | ||||||||
| N | Range | Mean ± SEM | n | pEC50 | Rmax (%) | n | pEC50 | Rmax (%) | |
| Ethnicity (Polish white) | 28 | ||||||||
| Male | 19 | 6 | 5.0 ± 0.1 | 103.3 ± 5.4 | 9 | 5.2 ± 0.1 | 98.4 ± 5.2 | ||
| Female | 9 | 4 | 5.5 ± 0.1 ** | 95.9 ± 10.4 | 5 | 5.0 ± 0.1 | 95.7 ± 10.6 | ||
| Age (years) | 28 | 51–79 | 65.2 ± 1.9 | ||||||
| BMI (kg/m2) | 28 | 18.2–34.1 | 25.1 ± 0.8 | ||||||
| Non-smokers | 7 | 4 | 5.3 ± 0.1 | 107.3 ± 4.2 | 3 | 5.5 ± 0.2 | 105.0 ± 8.1 | ||
| Smokers | 21 | 6 | 5.1 ± 0.1 | 95.9 ± 9.4 | 11 | 4.9 ± 0.1 * | 89.9 ± 6.0 | ||
| 0–10 cigarettes per day | 5 | ||||||||
| 10–20 cigarettes per day | 16 | ||||||||
| Systolic blood pressure (mmHg) | 28 | 109–152 | 128.5 ± 2.8 | ||||||
| Diastolic blood pressure (mmHg) | 28 | 64–95 | 73.1 ± 1.8 | ||||||
| No heart diseases | 14 | 5 | 5.2 ± 0.1 | 97.9 ± 10.6 | 6 | 5.0 ± 0.2 | 99.2 ± 7.0 | ||
| Heart diseases | 14 | 5 | 5.2 ± 0.1 | 102.8 ± 6.5 | 8 | 5.0 ± 0.1 | 88.6 ± 5.9 | ||
| Normocholesterolemia | 19 | 3 | 5.2 ± 0.1 | 95.3 ± 8.3 | 9 | 5.2 ± 0.1 | 99.4 ± 6.2 | ||
| Hypercholesterolemia | 9 | 6 | 5.0 ± 0.1 | 101.6 ± 9.3 | 5 | 4.6 ± 0.1 ** | 81.9 ± 7.5 | ||
| Arterial hypertension | 9 | ||||||||
| Diabetes mellitus | 4 | ||||||||
| Reason for surgery Operation Tumor staging Medications | Cancer (27 patients), tuberculoma (1) Right upper lobectomy (10), right middle lobectomy (1), right lower lobectomy (7), left upper lobectomy (3), left lower lobectomy (5), left pneumonectomy (1) IA (7), IB (2), IIA (4), IIB (10), IIIA (4) α1-Adrenoceptor antagonist (3), ACE inhibitors (8), β blockers (7), statins (9), calcium channel blocker (4), potassium channel blocker (1), diuretics (5), hypoglycemic medication (4), anticoagulants (4), protein pump inhibitors (1) | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozłowska, H.; Malinowska, B.; Baranowska-Kuczko, M.; Kusaczuk, M.; Nesterowicz, M.; Kozłowski, M.; Müller, C.E.; Kieć-Kononowicz, K.; Schlicker, E. GPR18-Mediated Relaxation of Human Isolated Pulmonary Arteries. Int. J. Mol. Sci. 2022, 23, 1427. https://doi.org/10.3390/ijms23031427
Kozłowska H, Malinowska B, Baranowska-Kuczko M, Kusaczuk M, Nesterowicz M, Kozłowski M, Müller CE, Kieć-Kononowicz K, Schlicker E. GPR18-Mediated Relaxation of Human Isolated Pulmonary Arteries. International Journal of Molecular Sciences. 2022; 23(3):1427. https://doi.org/10.3390/ijms23031427
Chicago/Turabian StyleKozłowska, Hanna, Barbara Malinowska, Marta Baranowska-Kuczko, Magdalena Kusaczuk, Miłosz Nesterowicz, Mirosław Kozłowski, Christa E. Müller, Katarzyna Kieć-Kononowicz, and Eberhard Schlicker. 2022. "GPR18-Mediated Relaxation of Human Isolated Pulmonary Arteries" International Journal of Molecular Sciences 23, no. 3: 1427. https://doi.org/10.3390/ijms23031427
APA StyleKozłowska, H., Malinowska, B., Baranowska-Kuczko, M., Kusaczuk, M., Nesterowicz, M., Kozłowski, M., Müller, C. E., Kieć-Kononowicz, K., & Schlicker, E. (2022). GPR18-Mediated Relaxation of Human Isolated Pulmonary Arteries. International Journal of Molecular Sciences, 23(3), 1427. https://doi.org/10.3390/ijms23031427