Metabolites of Cannabis Induce Cardiac Toxicity and Morphological Alterations in Cardiac Myocytes
Abstract
1. Introduction
2. Results
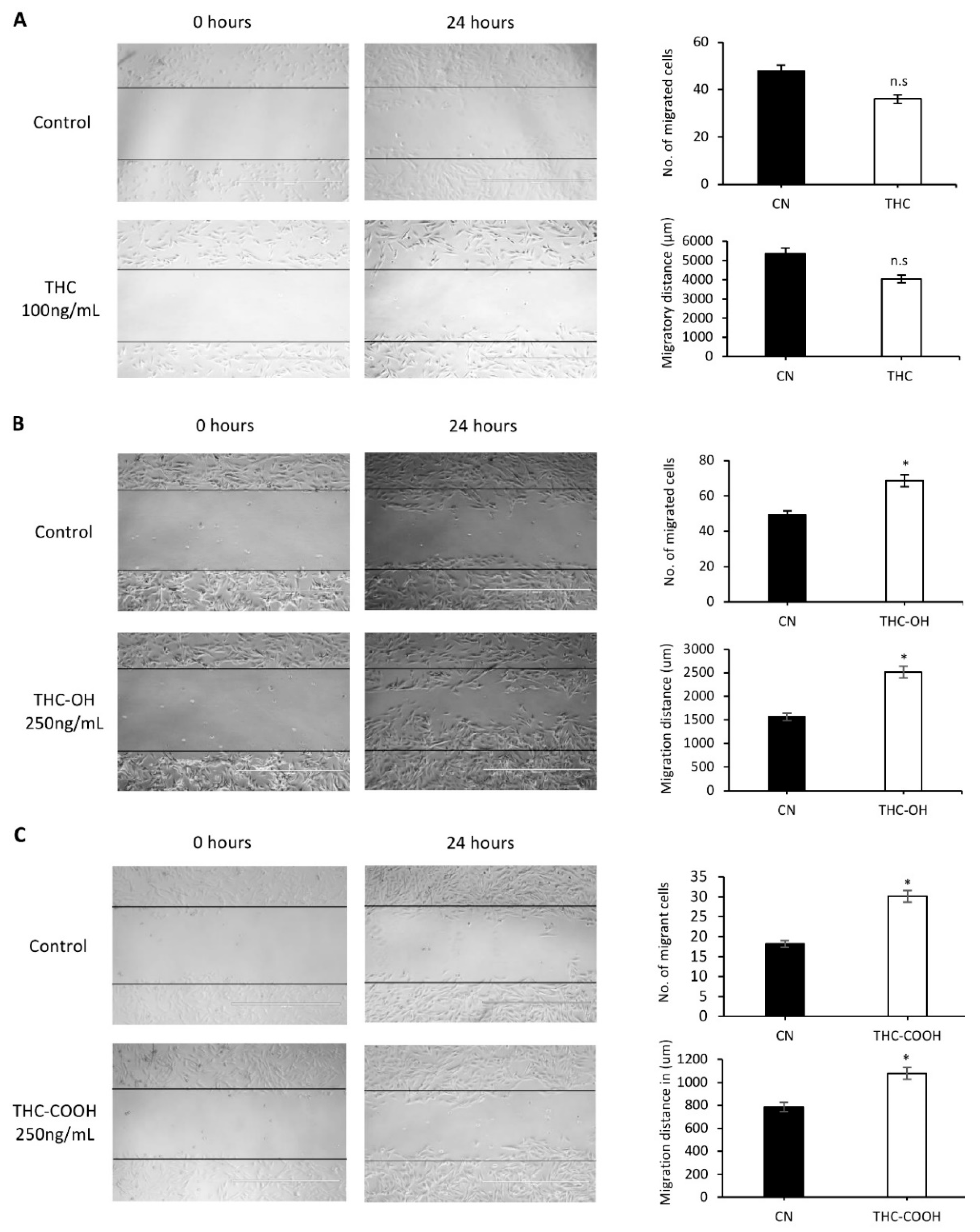
2.1. Metabolites of THC Mediate Increased Cell Migration and Wound Closure in Cardiac Cells
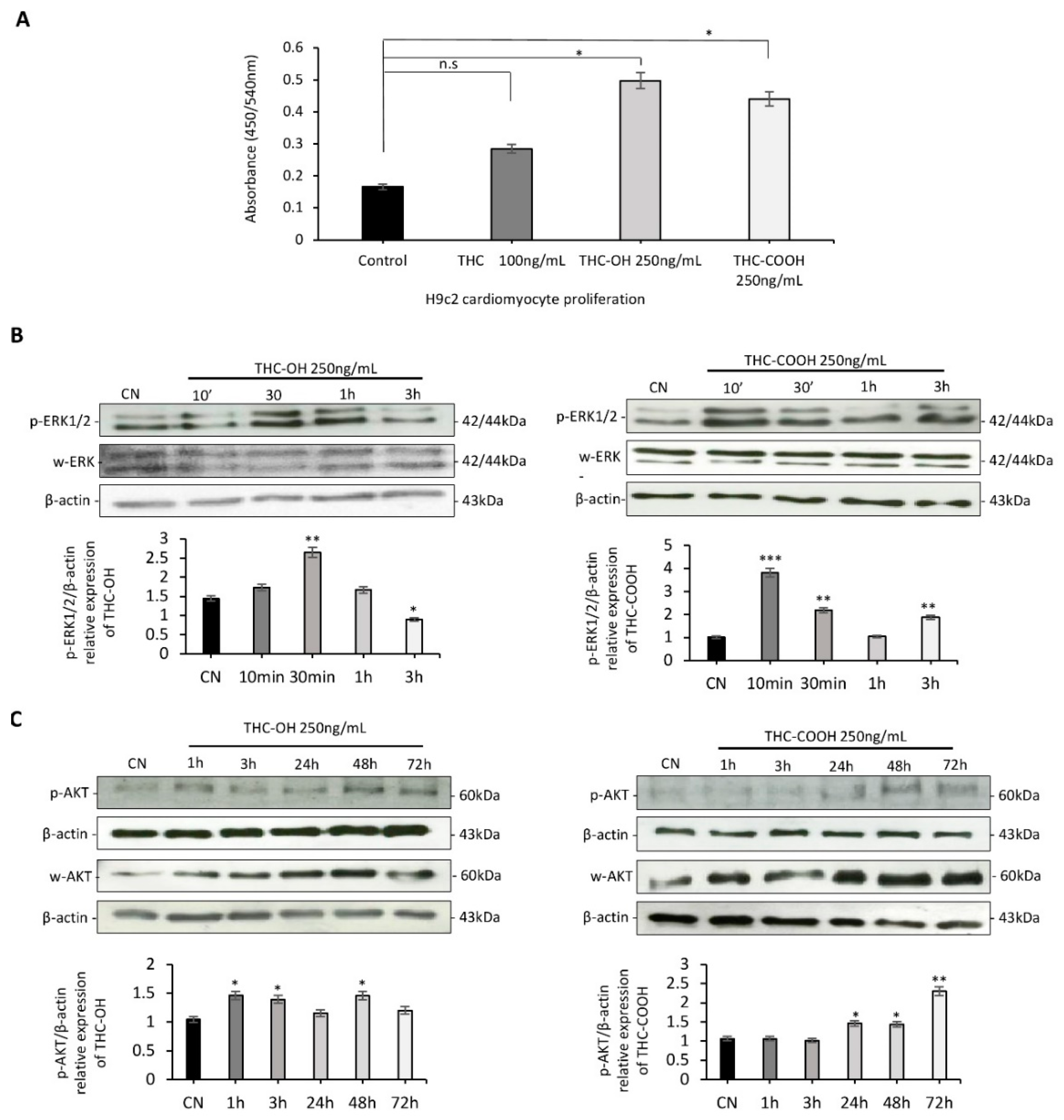
2.2. Cannabinoids Promote Alterations in Cell Proliferation in H9c2 Cells
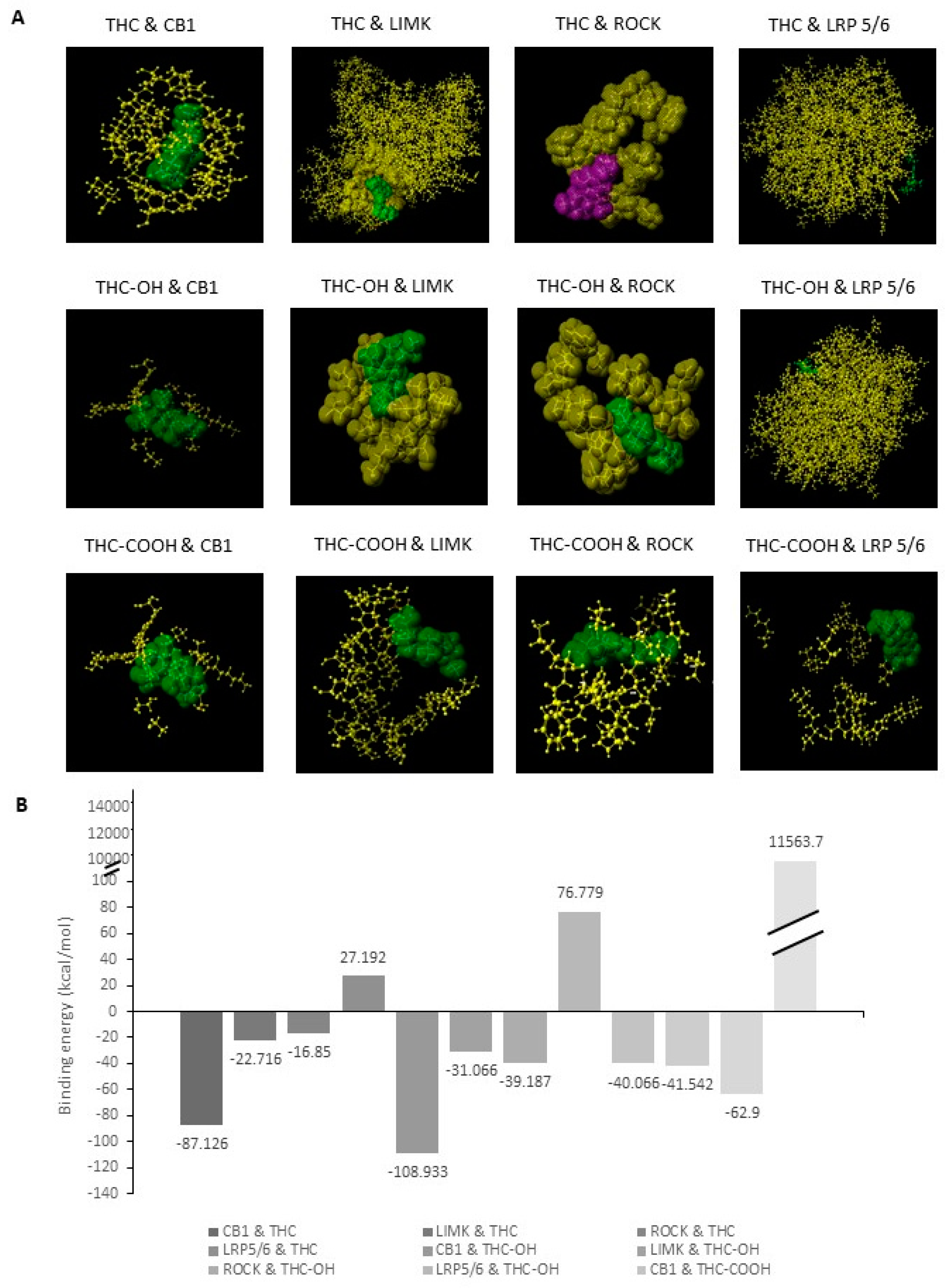
2.3. Bioinformatic Analysis of Binding Affinities between Key Regulators of Cytoskeletal Dynamics and THC Metabolites
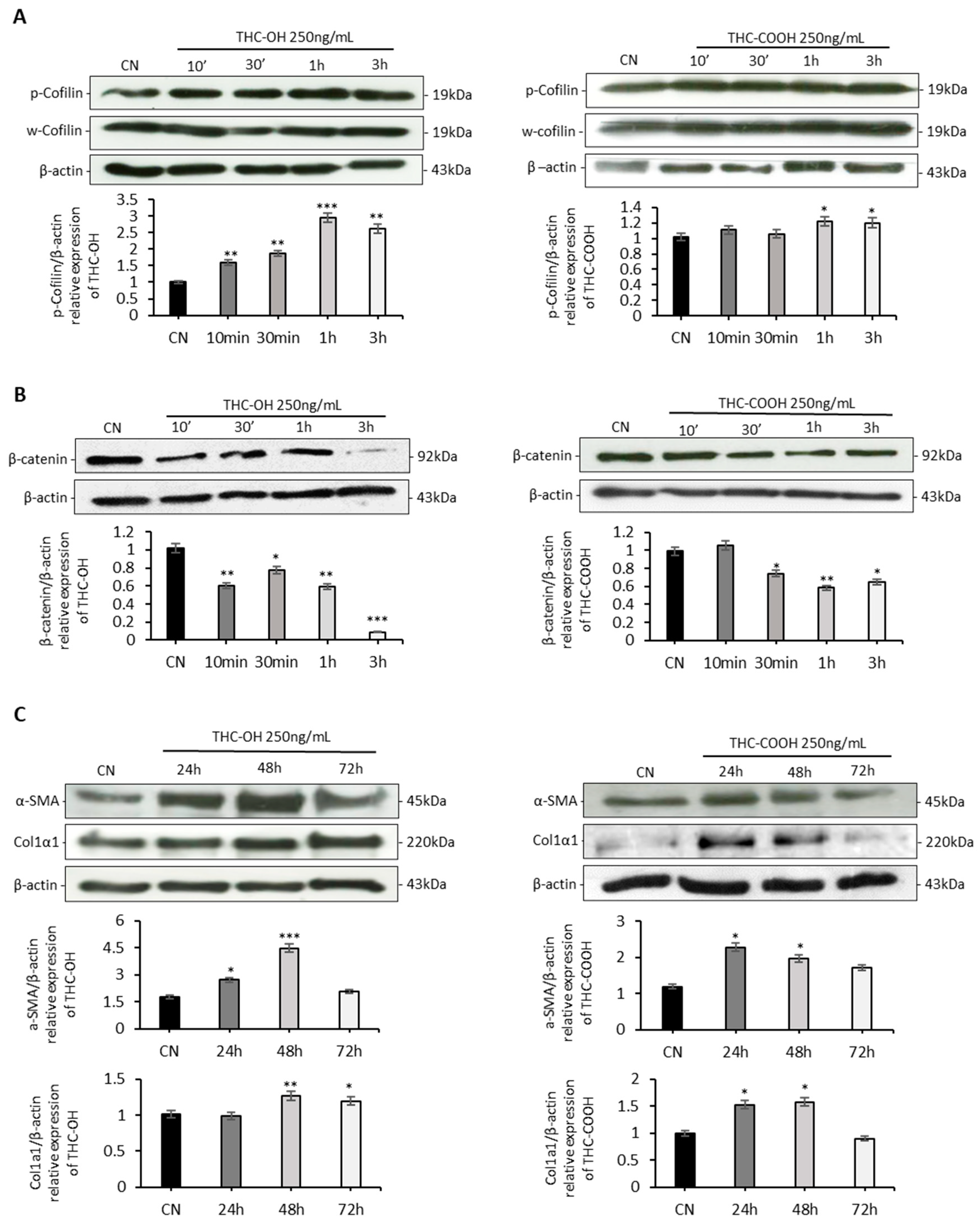
2.4. Cannabinoids Upregulated Matrix Protein Expression in Cardiomyocytes
2.5. In-Vitro Analysis of Cannabinoid Metabolism in Cardiomyocytes
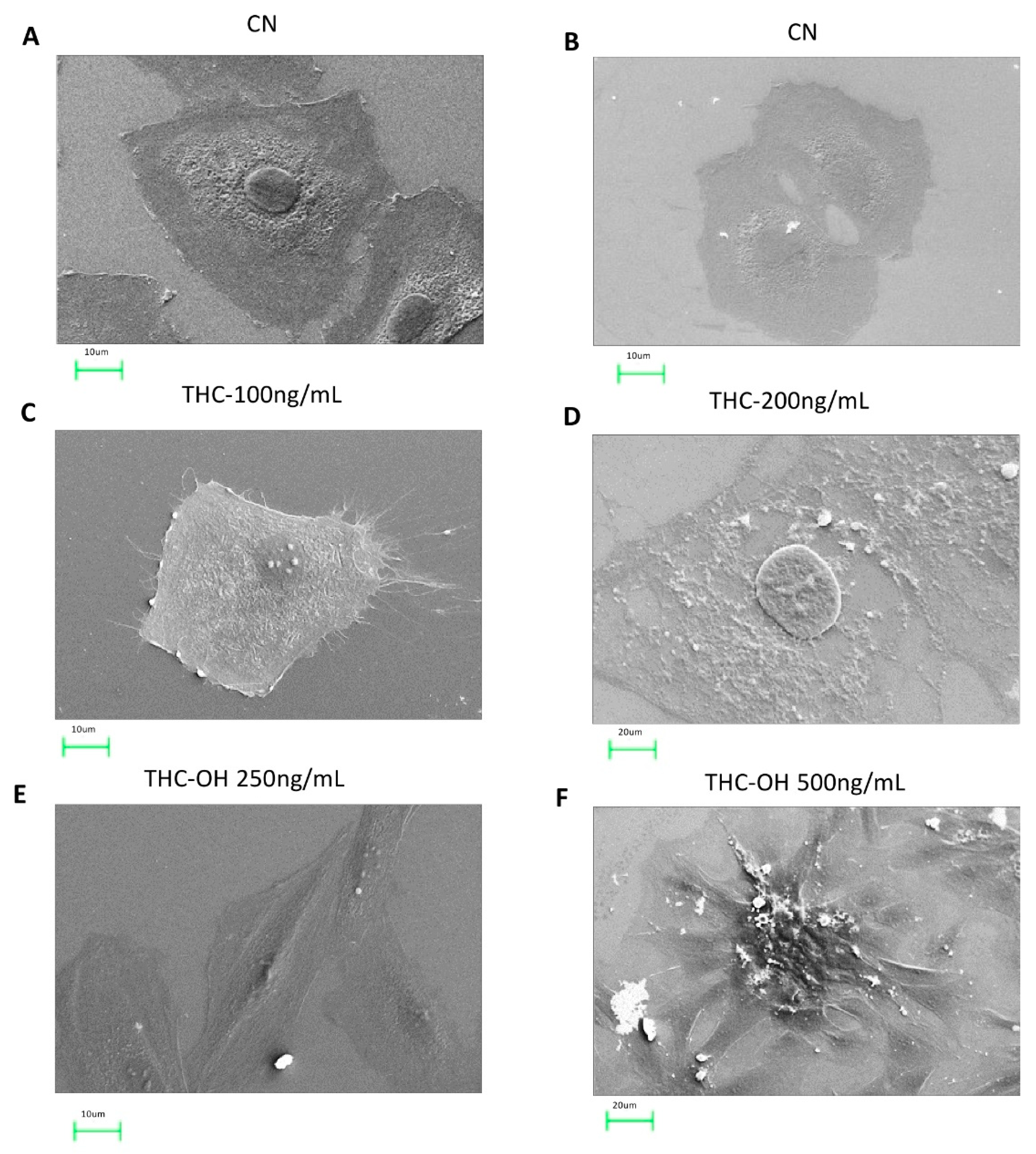
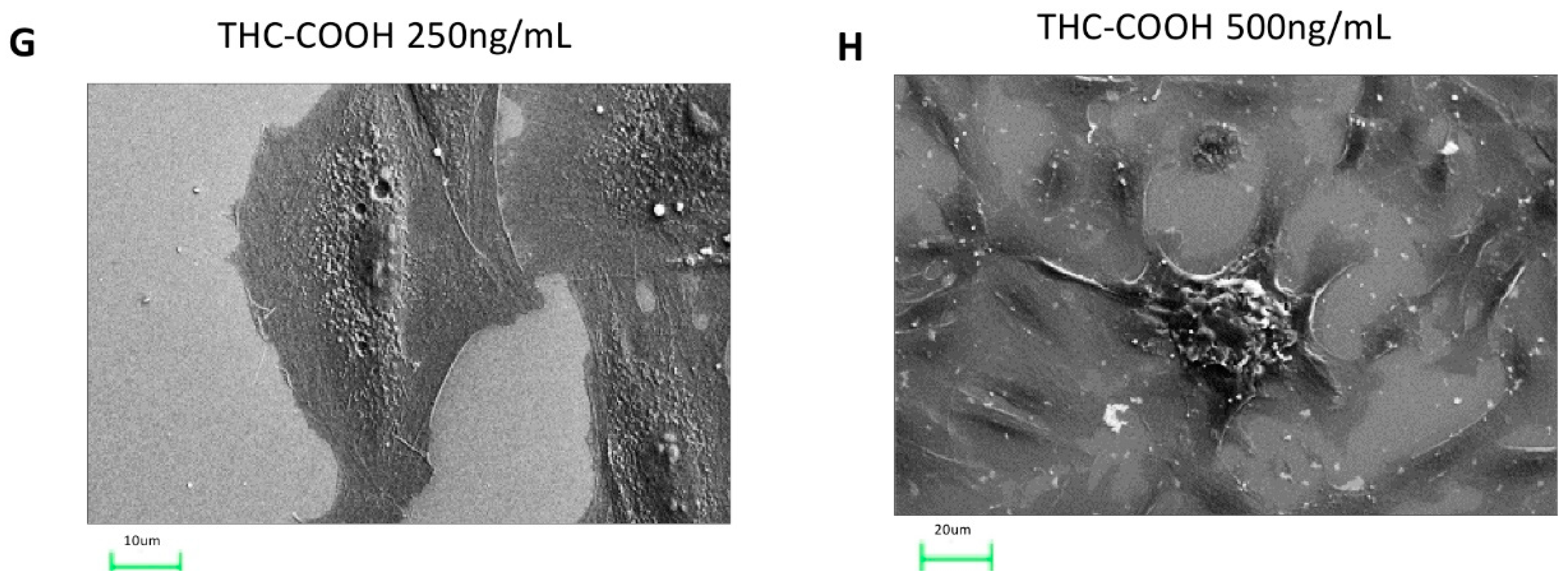
2.6. Cannabinoids Induce Alteration in the Microstructural of H9c2 Cells
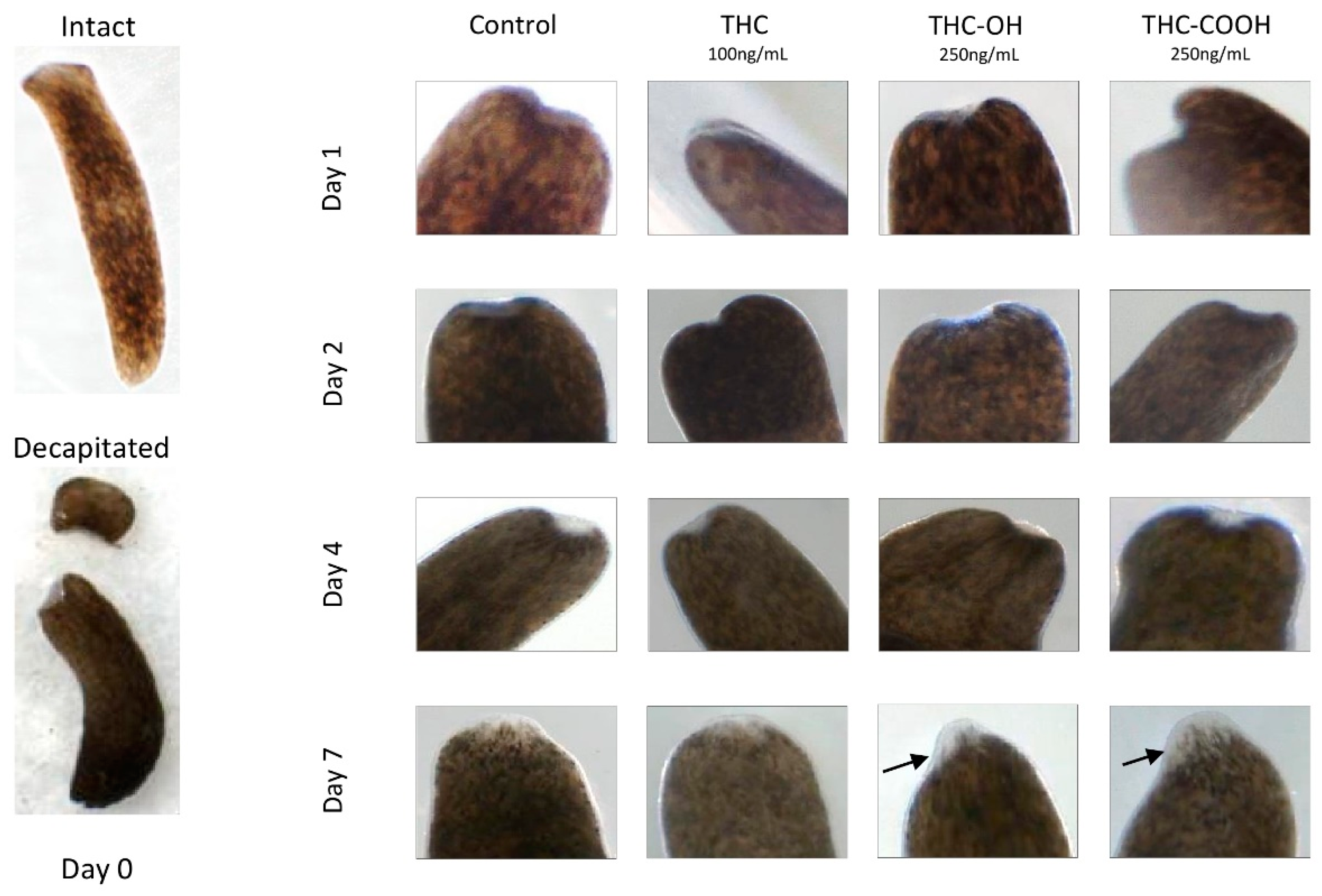
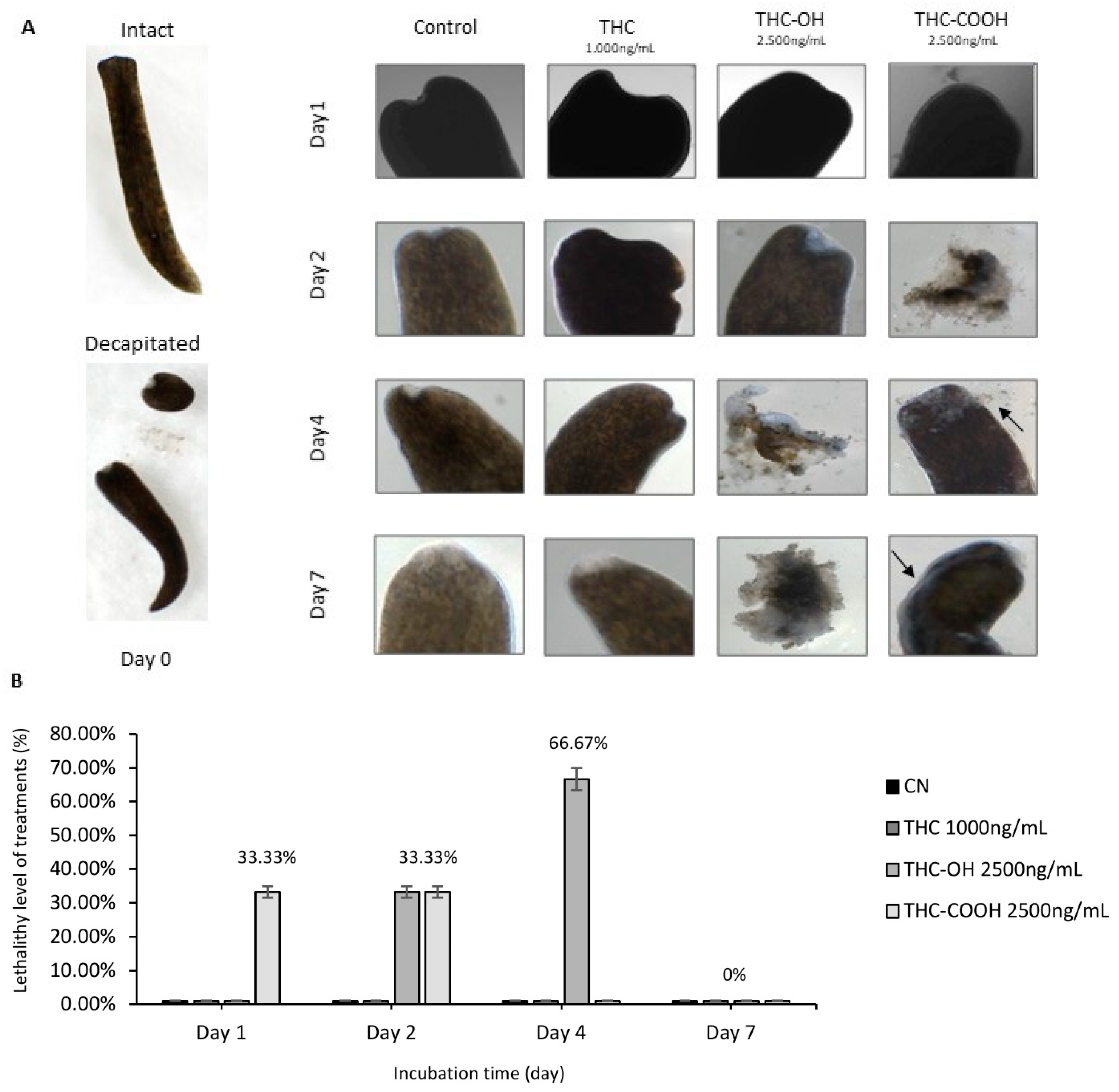
2.7. Cannabinoids Modulate Regenerative Capacity of Polycelis nigra in a Dose Dependant Manner
3. Discussion
4. Materials and Methods
4.1. Chemical and Reagents
4.2. Experimental Methods
4.2.1. Scratch Wound Assay
4.2.2. Cell Proliferation Assay
4.2.3. In-Silico Bioinformatic Docking Studies
4.2.4. Western Blotting
4.2.5. LC-MS Analysis
4.2.6. Scanning Electron Microscopy
4.2.7. Planaria Studies
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABP | Actin binding protein |
| ACS | Acute Coronary Syndrome |
| AKT | Protein Kinase B |
| αSMA | Alpha Smooth Muscle Actin |
| ATP | Adenosine triphosphate |
| BrdU | Bromo-2′-deoxy-uridine |
| CB | Cannabinoid |
| DPBS | Dulbecco’s Phosphate Buffered-Saline |
| DMEM | Dulbecco’s modified eagle medium |
| ECM | Extracellular matrix |
| ERK | Extracellular regulated kinase |
| FASTA | Format DNA and Protein Sequence Alignment |
| FBS | Fetal bovine serum |
| FN | Fibronectin |
| LC-MS | Liquid Chromatography Mass Spectrometry |
| LIMK | LIM-kinase |
| MAPK | Mitogen-Activated Protein Kinase |
| PI3K | Phosphatidylinositol 3-kinases |
| RIPA | Radioimmunoprecipitation assay |
| ROCK | Rho- associated kinase |
| SEM | Scanning Electron Microscopy |
| THC | Δ9-tetrahydrocannabinol |
| THC-COOH | 11-nor-9-carboxy-Δ⁹-tetrahydrocannabinol |
| THC-OH | 11-Hydroxy Δ9-tetrahydrocannabinol |
References
- Knuth, M.; Temme, O.; Daldrup, T.; Pawlik, E. Analysis of cocaine adulterants in human brain in cases of drug-related death. Forensic Sci. Int. 2018, 285, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Mittleman, M.A.; Mintzer, D.; Maclure, M.; Tofler, G.H.; Sherwood, J.B.; Muller, J.E. Triggering of Myocardial Infarction by Cocaine. Circulation 1999, 99, 2737–2741. [Google Scholar] [CrossRef]
- Egred, M.; Viswanathan, G.; Davis, G.K. Myocardial infarction in young adults. Postgrad. Med. J. 2005, 81, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Maceira, A.M.; Ripoll, C.; Cosin-Sales, J.; Igual, B.; Gavilan, M.; Salazar, J.; Belloch, V.; Pennell, D.J. Long term effects of cocaine on the heart assessed by cardiovascular magnetic resonance at 3T. J. Cardiovasc. Magn. Reson. 2014, 16, 26. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Martin, J.A.; Gancarz, A.M.; Adank, D.N.; Sim, F.J.; Dietz, D.M. Activin A is increased in the nucleus accumbens following a cocaine binge. Sci. Rep. 2017, 7, 43658. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-M.; Lin, S.-Z.; Chang, N.-C. Membrane ERα attenuates myocardial fibrosis via RhoA/ROCK-mediated actin remodeling in ovariectomized female infarcted rats. J. Mol. Med. 2014, 92, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.; Saez, C.G.; Pallavicini, J.; Pereira-Flores, K.; Mendoza, C.; Hernández, R.; Novoa, U.; Ocaranza, M.P.; Massardo, T.; Mezzano, D.; et al. Cocaine-Induced Endothelial Dysfunction: Role of RhoA/Rho Kinase Pathway Activation. Blood 2012, 120, 2177. [Google Scholar] [CrossRef]
- Pradhan, L.; Mondal, D.; Chandra, S.; Ali, M.; Agrawal, K.C. Molecular Analysis of Cocaine-Induced Endothelial Dysfunction: Role of Endothelin-1 and Nitric Oxide. Cardiovasc. Toxicol. 2008, 8, 161–171. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, G.; Zhao, H.; Wang, Z.; Li, F.; Zhang, P.; Zhang, J.; Wang, X.; Wang, W. Targeted inhibition of Focal Adhesion Kinase Attenuates Cardiac Fibrosis and Preserves Heart Function in Adverse Cardiac Remodeling. Sci. Rep. 2017, 7, srep43146. [Google Scholar] [CrossRef]
- Martins, M.J.; Bravo, R.R.; Enea, M.; Carmo, H.; Carvalho, F.; Bastos, M.D.L.; Dinis-Oliveira, R.J.; Da Silva, D.D. Ethanol addictively enhances the in vitro cardiotoxicity of cocaine through oxidative damage, energetic deregulation, and apoptosis. Arch. Toxicol. 2018, 92, 2311–2325. [Google Scholar] [CrossRef]
- Yang, D.; Liu, W.; Ma, L.; Wang, Y.; Ma, J.; Jiang, M.; Deng, X.; Huang, F.; Yang, T.; Chen, M. Profilin-1 contributes to cardiac injury induced by advanced glycation end-products in rats. Mol. Med. Rep. 2017, 16, 6634–6641. [Google Scholar] [CrossRef] [PubMed]
- Graziani, M.; Sarti, P.; Arese, M.; Magnifico, M.C.; Badiani, A.; Saso, L. Cardiovascular Mitochondrial Dysfunction Induced by Cocaine: Biomarkers and Possible Beneficial Effects of Modulators of Oxidative Stress. Oxidative Med. Cell. Longev. 2017, 2017, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Manninger, M.; Perl, S.; Brussee, H.; Toth, G.G. Sniff of coke breaks the heart: Cocaine-induced coronary vasospasm aggravated by therapeutic hypothermia and vasopressors after aborted sudden cardiac death: A case report. Eur. Hear. J. Case Rep. 2018, 2, yty041-4. [Google Scholar] [CrossRef] [PubMed]
- Calipari, E.; Godino, A.; Salery, M.; Damez-Werno, D.M.; Cahill, M.; Werner, C.T.; Gancarz, A.M.; Peck, E.G.; Jlayer, Z.; Rabkin, J.; et al. Synaptic Microtubule-Associated Protein EB3 and SRC Phosphorylation Mediate Structural and Behavioral Adaptations During Withdrawal From Cocaine Self-Administration. J. Neurosci. 2019, 39, 5634–5646. [Google Scholar] [CrossRef]
- Jones, C.M.; Baldwin, G.T.; Compton, W.M. Recent Increases in Cocaine-Related Overdose Deaths and the Role of Opioids. Am. J. Public Health 2017, 107, 430–432. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-N.; Chang, H.-D.; Sung, R.J. Cocaine-Induced Inhibition of ATP-Sensitive K+ Channels in Rat Ventricular Myocytes and in Heart-Derived H9c2 Cells. Basic Clin. Pharmacol. Toxicol. 2006, 98, 510–517. [Google Scholar] [CrossRef]
- Fan, L.; Sawbridge, D.; George, V.; Teng, L.; Bailey, A.; Kitchen, I.; Li, J.-M. Chronic Cocaine-Induced Cardiac Oxidative Stress and Mitogen-Activated Protein Kinase Activation: The Role of Nox2 Oxidase. J. Pharmacol. Exp. Ther. 2008, 328, 99–106. [Google Scholar] [CrossRef]
- Lattanzio, F.A.; Tiangco, D.; Osgood, C.; Beebe, S.; Kerry, J.; Hargrave, B.Y. Cocaine Increases Intracellular Calcium and Reactive Oxygen Species, Depolarizes Mitochondria, and Activates Genes Associated With Heart Failure and Remodeling. Cardiovasc. Toxicol. 2005, 5, 377–390. [Google Scholar] [CrossRef]
- Havakuk, O.; Rezkalla, S.H.; Kloner, R.A. The Cardiovascular Effects of Cocaine. J. Am. Coll. Cardiol. 2017, 70, 101–113. [Google Scholar] [CrossRef]
- Moreira, F.P.; Medeiros, J.R.C.; Lhullier, A.C.; Souza, L.D.D.M.; Jansen, K.; Portela, L.V.; Lara, D.R.; da Silva, R.A.; Wiener, C.D.; Oses, J.P. Cocaine abuse and effects in the serum levels of cytokines IL-6 and IL-10. Drug Alcohol Depend. 2016, 158, 181–185. [Google Scholar] [CrossRef]
- Badisa, R.B.; Wi, S.; Jones, Z.; Mazzio, E.; Zhou, Y.; Rosenberg, J.T.; Latinwo, L.M.; Grant, S.C.; Goodman, C.B. Cellular and molecular responses to acute cocaine treatment in neuronal-like N2a cells: Potential mechanism for its resistance in cell death. Cell Death Discov. 2018, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Engeln, M.; Schiefer, C.; Patton, M.; Martin, J.A.; Werner, C.; Riggs, L.M.; Francis, T.C.; McGlincy, M.; Evans, B.; et al. Drp1 Mitochondrial Fission in D1 Neurons Mediates Behavioral and Cellular Plasticity during Early Cocaine Abstinence. Neuron 2017, 96, 1327–1341.e6. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.T.; Mitra, S.; Auerbach, B.D.; Wang, Z.-J.; Martin, J.A.; Stewart, A.F.; Gobira, P.H.; Iida, M.; An, C.; Cobb, M.M.; et al. Neuroadaptations in the dorsal hippocampus underlie cocaine seeking during prolonged abstinence. Proc. Natl. Acad. Sci. USA 2020, 117, 26460–26469. [Google Scholar] [CrossRef] [PubMed]
- Badisa, R.B.; Kumar, S.S.; Mazzio, E.; Haughbrook, R.D.; Allen, J.R.; Davidson, M.W.; Fitch-Pye, C.A.; Goodman, C.B. N-Acetyl Cysteine Mitigates the Acute Effects of Cocaine-Induced Toxicity in Astroglia-Like Cells. PLoS ONE 2015, 10, e0114285. [Google Scholar] [CrossRef]
- Smith, M.L.; Shimomura, E.; Paul, B.D.; Cone, E.J.; Darwin, W.D.; Huestis, M.A. Urinary excretion of ecgonine and five other cocaine metabolites following controlled oral, intravenous, intranasal, and smoked administration of cocaine. J. Anal. Toxicol. 2010, 34, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.W.; Holmgren, A. Concentrations of Cocaine and Benzoylecgonine in Femoral Blood from Cocaine-Related Deaths Compared with Venous Blood from Impaired Drivers. J. Anal. Toxicol. 2013, 38, 46–51. [Google Scholar] [CrossRef]
- Pandhare, J.; Addai, A.B.; Mantri, C.K.; Hager, C.; Smith, R.M.; Barnett, L.; Villalta, F.; Kalams, S.A.; Dash, C. Cocaine en-hances HIV-1–induced CD4 T-Cell apoptosis: Implications in disease progression in cocaine-abusing HIV-1 patients. Am. J. Pathol. 2014, 184, 927–936. [Google Scholar] [CrossRef]
- Mittleman, R.E.; Wetli, C.V. Death caused by recreational cocaine use: An update. JAMA 1984, 252, 1889–1893. [Google Scholar] [CrossRef]
- Del Olmo-Turrubiarte, A.; Calzada-Torres, A.; Diaz-Rosas, G.; Lara, I.P.; Sánchez-Urbina, R.; Balderrábano-Saucedo, N.; González-Márquez, H.; Garcia-Alonso, P.; Contreras-Ramos, A. Mouse models for the study of postnatal cardiac hypertrophy. IJC Heart Vasc. 2015, 7, 131–140. [Google Scholar] [CrossRef][Green Version]
- Huang, C.L.-H. From channels to systems: Ca2+ -sensitive K+ currents, alternans and cardiac arrhythmia. J. Physiol. 2017, 595, 2299–2300. [Google Scholar] [CrossRef]
- Welder, A.; Smith, M.; Ramos, K.; Acosta, D. Cocaine-induced cardiotoxicity in vitro. Toxicol. In Vitro 1988, 2, 205–213. [Google Scholar] [CrossRef]
- Chen, C.W.R.; Makkiya, M.; Aronow, W.; Spevack, D.M. Heightened risk of cardiac events following percutaneous coronary intervention for cocaine-associated myocardial infarction. Arch. Med. Sci. 2020, 16, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Bachi, K.; Mani, V.; Jeyachandran, D.; Fayad, Z.A.; Goldstein, R.Z.; Alia-Klein, N. Vascular disease in cocaine addiction. Atherosclerosis 2017, 262, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; He, J.; Gilbert, R.D.; Zhang, L. Cocaine induces apoptosis in fetal myocardial cells through a mitochondria-dependent pathway. J. Pharmacol. Exp. Ther. 2000, 292, 8–14. [Google Scholar] [PubMed]
- Sinha-Hikim, I.; Shen, R.; Nzenwa, I.; Gelfand, R.; Mahata, S.K.; Sinha-Hikim, A.P. Minocycline suppresses oxidative stress and attenuates fetal cardiac myocyte apoptosis triggered by in utero cocaine exposure. Apoptosis 2011, 16, 563–573. [Google Scholar] [CrossRef]
- Álvaro-Bartolomé, M.; La Harpe, R.; Callado, L.F.; Meana, J.J.; García-Sevilla, J. Molecular adaptations of apoptotic pathways and signaling partners in the cerebral cortex of human cocaine addicts and cocaine-treated rats. Neuroscience 2011, 196, 1–15. [Google Scholar] [CrossRef]
- Jonkman, J.; Cathcart, J.A.; Xu, F.; Bartolini, M.E.; Amon, J.E.; Stevens, K.M.; Colarusso, P. An introduction to the wound healing assay using live-cell microscopy. Cell Adhes. Migr. 2014, 8, 440–451. [Google Scholar] [CrossRef]
- Itou, J.; Oishi, I.; Kawakami, H.; Glass, T.J.; Richter, J.; Johnson, A.; Lund, T.C.; Kawakami, Y. Migration of cardiomyocytes is essential for heart regeneration in zebrafish. Development 2012, 139, 4133–4142. [Google Scholar] [CrossRef]
- Carrillo, X.; Curós, A.; Muga, R.; Serra, J.; Sanvisens, A.; Bayes-Genis, A. Acute coronary syndrome and cocaine use: 8-year prevalence and inhospital outcomes. Eur. Heart J. 2011, 32, 1244–1250. [Google Scholar] [CrossRef]
- Song, S.H.; Park, K.; Kim, S.W.; Paick, J.; Cho, M.C. Involvement of Rho-Kinase/LIM Kinase/Cofilin Signaling Pathway in Corporal Fibrosis after Cavernous Nerve Injury in Male Rats. J. Sex. Med. 2015, 12, 1522–1532. [Google Scholar] [CrossRef]
- Cui, K.; Luan, Y.; Wang, T.; Zhuan, L.; Rao, K.; Wang, S.; Ye, Z.-Q.; Liu, J.-H.; Wang, D.-W. Reduced corporal fibrosis to protect erectile function by inhibiting the Rho-kinase/LIM-kinase/cofilin pathway in the aged transgenic rat harboring human tissue kallikrein. Asian J. Androl. 2016, 19, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Kligys, K.; Claiborne, J.N.; DeBiase, P.J.; Hopkinson, S.B.; Wu, Y.; Mizuno, K.; Jones, J.C.R. The Slingshot Family of Phosphatases Mediates Rac1 Regulation of Cofilin Phosphorylation, Laminin-332 Organization, and Motility Behavior of Keratinocytes. J. Biol. Chem. 2007, 282, 32520–32528. [Google Scholar] [CrossRef]
- Toda, S.; Shen, H.-W.; Peters, J.; Cagle, S.; Kalivas, P.W. Cocaine Increases Actin Cycling: Effects in the Reinstatement Model of Drug Seeking. J. Neurosci. 2006, 26, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Kim, K.; Duan, M.; Hayashi, T.; Guo, M.-L.; Morgello, S.; Prat, A.; Wang, J.; Su, T.-P.; Buch, S. Cocaine Hijacks 1 Receptor to Initiate Induction of Activated Leukocyte Cell Adhesion Molecule: Implication for Increased Monocyte Adhesion and Migration in the CNS. J. Neurosci. 2011, 31, 5942–5955. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Heyob, K.; Tipple, T.; Pryhuber, G.S.; Rogers, L.K. Alterations in VASP phosphorylation and profilin1 and cofilin1 expression in hyperoxic lung injury and BPD. Respir. Res. 2018, 19, 229. [Google Scholar] [CrossRef] [PubMed]
- Okayama, T.; Kikuchi, S.; Ochiai, T.; Ikoma, H.; Kubota, T.; Ichikawa, D.; Fujiwara, H.; Okamoto, K.; Sakakura, C.; Sonoyama, T.; et al. Attenuated response to liver injury in moesin-deficient mice: Impaired stellate cell migration and decreased fibrosis. Biochim. Biophys. Acta -Mol. Basis Dis. 2008, 1782, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Arpin, M.; Chirivino, D.; Naba, A.; Zwaenepoel, I. Emerging role for ERM proteins in cell adhesion and migration. Cell Adhes. Migr. 2011, 5, 199–206. [Google Scholar] [CrossRef]
- Van Eldik, W.; Adel, B.D.; Monshouwer-Kloots, J.; Salvatori, D.; Maas, S.; Van Der Made, I.; Creemers, E.; Frank, D.; Frey, N.; Boontje, N.; et al. Z-disc protein CHAPb induces cardiomyopathy and contractile dysfunction in the postnatal heart. PLoS ONE 2017, 12, e0189139. [Google Scholar] [CrossRef]
- Kim, W.; Shin, S.; Kim, S.; Jeon, S.; Kim, J.-H. Cocaine regulates ezrin–radixin–moesin proteins and RhoA signaling in the nucleus accumbens. Neuroscience 2009, 163, 501–505. [Google Scholar] [CrossRef]
- Sartoretto, J.L.; Jin, B.Y.; Bauer, M.; Gertler, F.B.; Liao, R.; Michel, T. Regulation of VASP phosphorylation in cardiac myocytes: Differential regulation by cyclic nucleotides and modulation of protein expression in diabetic and hypertrophic heart. Am. J. Physiol. Circ. Physiol. 2009, 297, H1697–H1710. [Google Scholar] [CrossRef]
- Schlegel, N.; Waschke, J. VASP is involved in cAMP-mediated Rac 1 activation in microvascular endothelial cells. Am. J. Physiol. Physiol. 2009, 296, C453–C462. [Google Scholar] [CrossRef] [PubMed]
- Brahmbhatt, A.A.; Klemke, R.L. ERK and RhoA Differentially Regulate Pseudopodia Growth and Retraction during Chemotaxis. J. Biol. Chem. 2003, 278, 13016–13025. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.G.H.; Pierotti, V.; Storez, H.; Lindberg, E.; Thuret, A.; Muntaner, O.; Labbé-Jullié, C.; Pitcher, J.A.; Marullo, S. Cooperative Regulation of Extracellular Signal-Regulated Kinase Activation and Cell Shape Change by Filamin A and β-Arrestins. Mol. Cell. Biol. 2006, 26, 3432–3445. [Google Scholar] [CrossRef]
- Mendoza, M.C.; Er, E.E.; Zhang, W.; Ballif, B.A.; Elliott, H.L.; Danuser, G.; Blenis, J. ERK-MAPK Drives Lamellipodia Protrusion by Activating the WAVE2 Regulatory Complex. Mol. Cell 2011, 41, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Schwope, D.M.; Karschner, E.L.; A Gorelick, D.; A Huestis, M. Identification of Recent Cannabis Use: Whole-Blood and Plasma Free and Glucuronidated Cannabinoid Pharmacokinetics following Controlled Smoked Cannabis Administration. Clin. Chem. 2011, 57, 1406–1414. [Google Scholar] [CrossRef]
- Karschner, E.L.; Schwilke, E.W.; Lowe, R.H.; Darwin, W.D.; Herning, R.I.; Cadet, J.L.; Huestis, M.A. Implications of Plasma 9-Tetrahydrocannabinol, 11-Hydroxy-THC, and 11-nor-9-Carboxy-THC Concentrations in Chronic Cannabis Smokers. J. Anal. Toxicol. 2009, 33, 469–477. [Google Scholar] [CrossRef]
- Fabritius, M.; Favrat, B.; Chtioui, H.; Battistella, G.; Annoni, J.-M.; Appenzeller, M.; Dao, K.; Fornari, E.; Lauer, E.; Mall, J.-F.; et al. THCCOOH concentrations in whole blood: Are they useful in discriminating occasional from heavy smokers? Drug Test. Anal. 2013, 6, 155–163. [Google Scholar] [CrossRef]
- Okada, H.; Lai, N.C.; Kawaraguchi, Y.; Liao, P.; Copps, J.; Sugano, Y.; Okada-Maeda, S.; Banerjee, I.; Schilling, J.M.; Gingras, A.R.; et al. Integrins protect cardiomyocytes from ischemia/reperfusion injury. J. Clin. Investig. 2013, 123, 4294–4308. [Google Scholar] [CrossRef]
- Shewchuk, L.J.; Bryan, S.; Ulanova, M.; Khaper, N. Integrin β3 prevents apoptosis of HL-1 cardiomyocytes under conditions of oxidative stress. Can. J. Physiol. Pharmacol. 2010, 88, 324–330. [Google Scholar] [CrossRef]
- Cerretani, D.; Fineschi, V.; Bello, S.; Riezzo, I.; Turillazzi, E.; Neri, M. Role of oxidative stress in cocaine-induced cardiotoxicity and cocaine-related death. Curr. Med. Chem. 2012, 19, 5619–5623. [Google Scholar] [CrossRef]
- Chi, Z.; Melendez, A.J. Role of Cell Adhesion Molecules and Immune-Cell Migration in the Initiation, Onset and Development of Atherosclerosis. Cell Adhes. Migr. 2007, 1, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Patrizi, R.; Pasceri, V.; Sciahbasi, A.; Summaria, F.; Rosano, G.M.; Lioy, E. Evidence of Cocaine-Related Coronary Atherosclerosis in Young Patients With Myocardial Infarction. J. Am. Coll. Cardiol. 2006, 47, 2120–2122. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, J.A.; Singh, M. Pathophysiology of coronary artery disease leading to acute coronary syndromes. F1000Prime Rep. 2015, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Gopalakrishnan, L.; Singh, M.; Singh, S.; Kovacs, D.F.; Benatar, D.; Gibson, C.M.; Khosla, S. Effect of cocaine on coronary microvasculature. J. Am. Coll. Cardiol. 2018, 71, 954–955. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Park, T. Acute and Chronic Effects of Cocaine on Cardiovascular Health. Int. J. Mol. Sci. 2019, 20, 584. [Google Scholar] [CrossRef] [PubMed]
- Witek, P.; Korga, A.; Burdan, F.; Ostrowska-Lesko, M.; Nosowska, B.; Iwan, M.; Dudka, J. The effect of a number of H9C2 rat cardiomyocytes passage on repeatability of cytotoxicity study results. Cytotechnology 2016, 68, 2407–2415. [Google Scholar] [CrossRef] [PubMed]
- Borini, P.; Guimarães, R.C.; Borini, S.B. Possible hepatotoxicity of chronic marijuana usage. Sao Paulo Med. J. 2004, 122, 110–116. [Google Scholar] [CrossRef]
| (A) | |||||||
|---|---|---|---|---|---|---|---|
| Analyte Name | Precursor ion (Amu) | Retention Time (min) | Retention Time Window (min) | Fragmentor (V) | Product Ions (amu) | Collision Energy (V) | Dwell Time (ms) |
| THC | 315.2 | 14.4 | 1.19 | 84 | 193.2 123.0 259.2 | 21 32 18 | 78.64 78.64 78.64 |
| THC-OH | 331.2 | 8.8 | 1.01 | 92 | 313.2 201.0 193.2 | 20 26 15 | 77.48 77.48 77.48 |
| THC-COOH | 345.2 | 9.2 | 0.94 | 96 | 327.2 299.2 193.1 | 13 17 25 | 35.63 35.63 35.63 |
| (B) | |||||||
| QC | Expected Concentration (ng/mL) | Calculated Concentration (ng/mL) | Accuracy (%) | ||||
| THC | 40.00 | 39.7692 | 99.42 | ||||
| THC-OH | 100.00 | 97.3173 | 97.32 | ||||
| THC-COOH | 100.00 | 92.9326 | 92.93 | ||||
| (C) | |||||||
| Data File | THC Conc. (ng/mL) | THC-OH Conc. (ng/mL) | THC-COOH Conc. (ng/mL) | ||||
| THC Control | 0.0000 | ND | 1.1767 | ||||
| THC 10 min | 33.8205 | ND | 1.1408 | ||||
| THC 30 min | 33.8275 | ND | 1.1094 | ||||
| THC 1 H | 37.7523 | ND | 1.1627 | ||||
| THC 3 H | 38.1000 | ND | 1.1176 | ||||
| THC 24 h | 20.8132 | ND | 1.1047 | ||||
| THC-OH control | ND | 1.5706 | 1.1057 | ||||
| THC-OH 10 min | ND | 143.4358 | 1.1159 | ||||
| Hydroxy-THC 30 minl | ND | 129.2503 | 1.1364 | ||||
| THC-OH 1 h | ND | 121.2555 | 1.1277 | ||||
| THC-OH 3 h | ND | 106.0970 | 1.1277 | ||||
| THC-OH 24 h | ND | 91.2759 | 1.1374 | ||||
| Treatment Condition | Disintegration | Wound Healing at Day 1 | Obvious Blastema at Day 4 | Presence of Regenerated Tissue at Day 7 | Regeneration Rate at Day 7 Compared to Control |
|---|---|---|---|---|---|
| Control | No | Yes | Yes | Yes | -- |
| THC 100 ng/mL | No | Yes | Yes | Yes | No difference to control |
| THC-OH 250 ng/mL | No | Yes | Yes | Yes | Increased thickness of regenerated tissues |
| THC-COOH 250 ng/mL | No | Yes | Yes | Yes | Increased thickness of regenerated tissues |
| Treatment Condition | Wound Healing at Day 1 | Disintegration | Obvious Blastema at: | Presence of Regenerated Tissue at: | Regeneration Rate at Day 7 Compared to Control |
|---|---|---|---|---|---|
| Control | Yes | No | Day 4 | Day 7 | --- |
| THC 1000 ng/mL | Yes | No | Day 7 | Day 7 | No difference to control |
| THC-OH 2500 ng/mL | Yes | Disintegrated completely between days 2–4 | Day 1 | Day 2 | N/A; completely disintegrated |
| THC-COOH 2500 ng/mL | Yes | Disintegrated completely at day 1 (n = 1) and day 2 (n = 1) | Day 1 | Day 4 | N/A; completely disintegrated except 1 individual with slow response to stimuli and little regeneration |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merve, A.O.; Sobiecka, P.; Remeškevičius, V.; Taylor, L.; Saskoy, L.; Lawton, S.; Jones, B.P.; Elwakeel, A.; Mackenzie, F.E.; Polycarpou, E.; et al. Metabolites of Cannabis Induce Cardiac Toxicity and Morphological Alterations in Cardiac Myocytes. Int. J. Mol. Sci. 2022, 23, 1401. https://doi.org/10.3390/ijms23031401
Merve AO, Sobiecka P, Remeškevičius V, Taylor L, Saskoy L, Lawton S, Jones BP, Elwakeel A, Mackenzie FE, Polycarpou E, et al. Metabolites of Cannabis Induce Cardiac Toxicity and Morphological Alterations in Cardiac Myocytes. International Journal of Molecular Sciences. 2022; 23(3):1401. https://doi.org/10.3390/ijms23031401
Chicago/Turabian StyleMerve, Ayse Orme, Pola Sobiecka, Vytautas Remeškevičius, Luke Taylor, Lili Saskoy, Scott Lawton, Ben P. Jones, Ahmed Elwakeel, Francesca E. Mackenzie, Elena Polycarpou, and et al. 2022. "Metabolites of Cannabis Induce Cardiac Toxicity and Morphological Alterations in Cardiac Myocytes" International Journal of Molecular Sciences 23, no. 3: 1401. https://doi.org/10.3390/ijms23031401
APA StyleMerve, A. O., Sobiecka, P., Remeškevičius, V., Taylor, L., Saskoy, L., Lawton, S., Jones, B. P., Elwakeel, A., Mackenzie, F. E., Polycarpou, E., Bennett, J., & Rooney, B. (2022). Metabolites of Cannabis Induce Cardiac Toxicity and Morphological Alterations in Cardiac Myocytes. International Journal of Molecular Sciences, 23(3), 1401. https://doi.org/10.3390/ijms23031401







