Role of Hydrogen Sulfide and Polysulfides in the Regulation of Lipolysis in the Adipose Tissue: Possible Implications for the Pathogenesis of Metabolic Syndrome
Abstract
:1. Introduction
2. Results
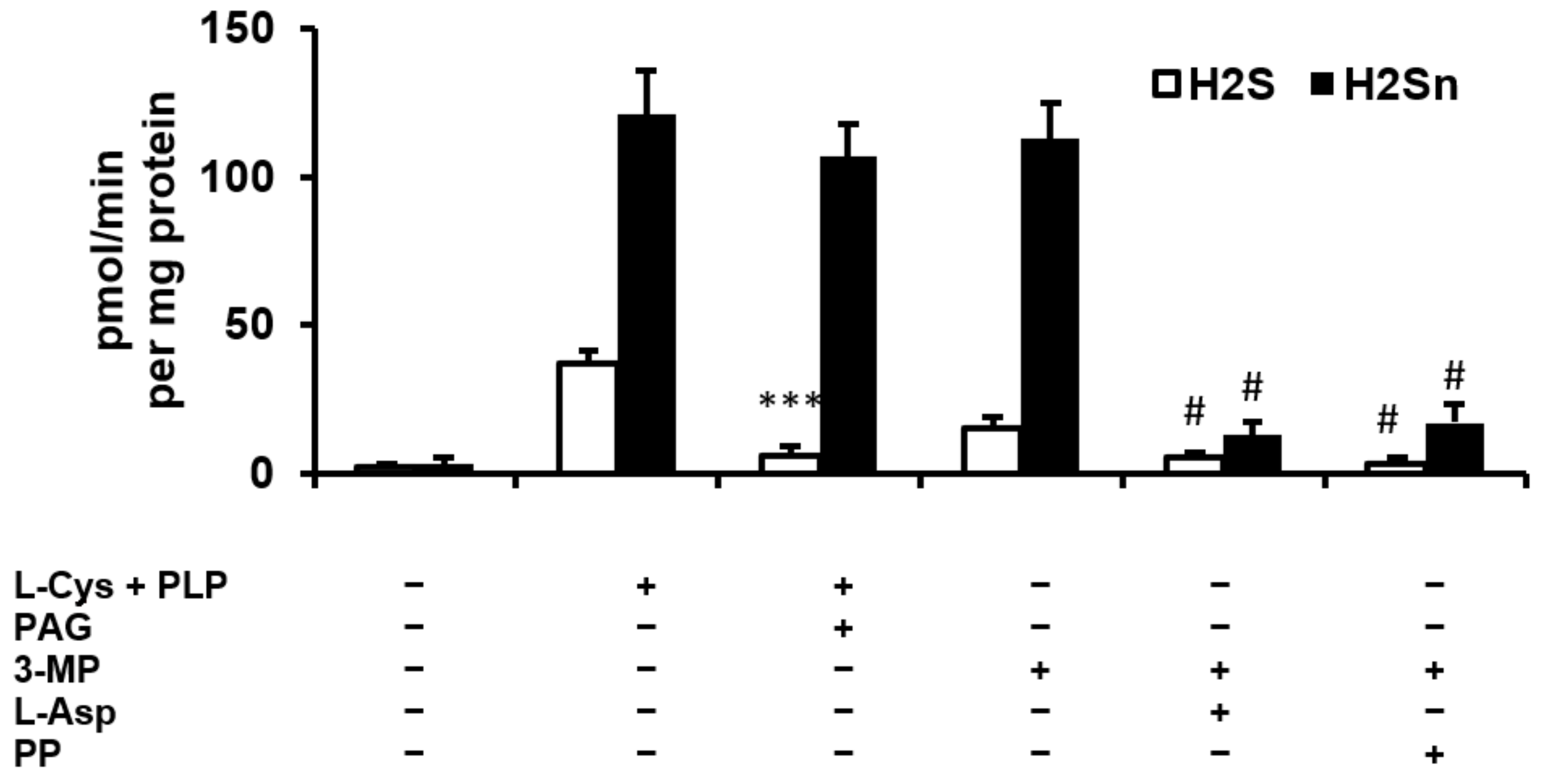
2.1. H2S and Polysulfide Production by Adipose Tissue
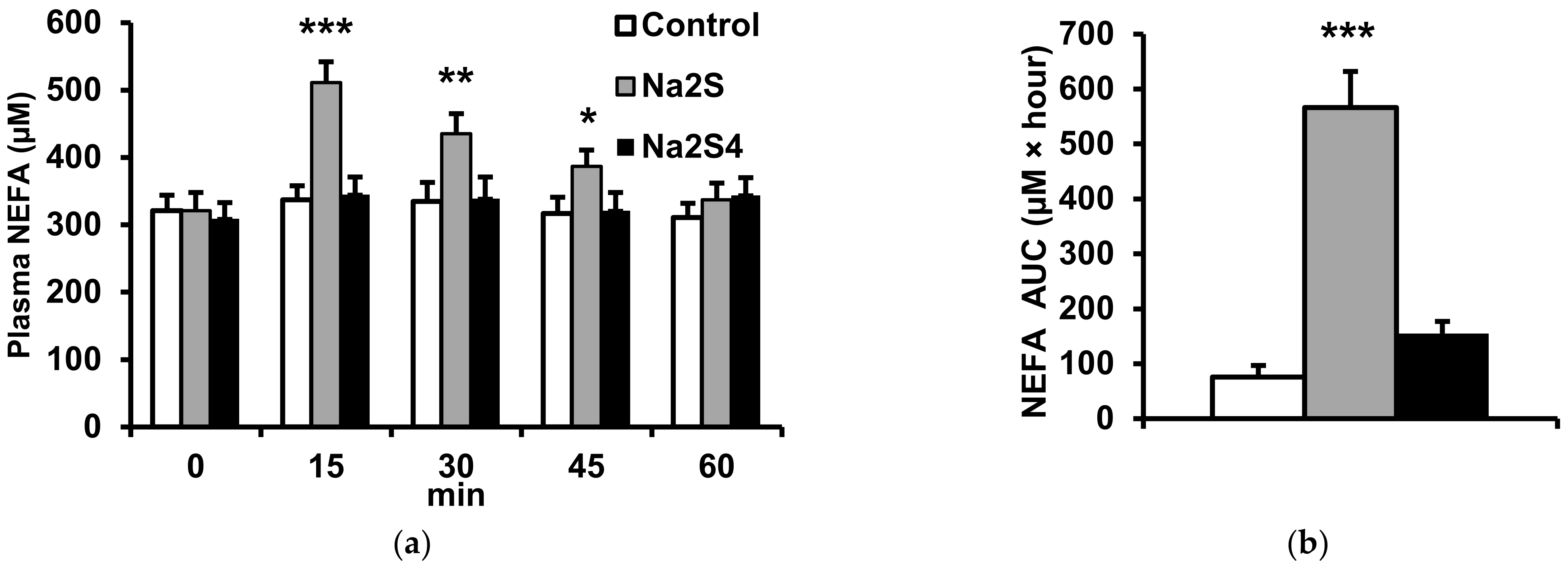
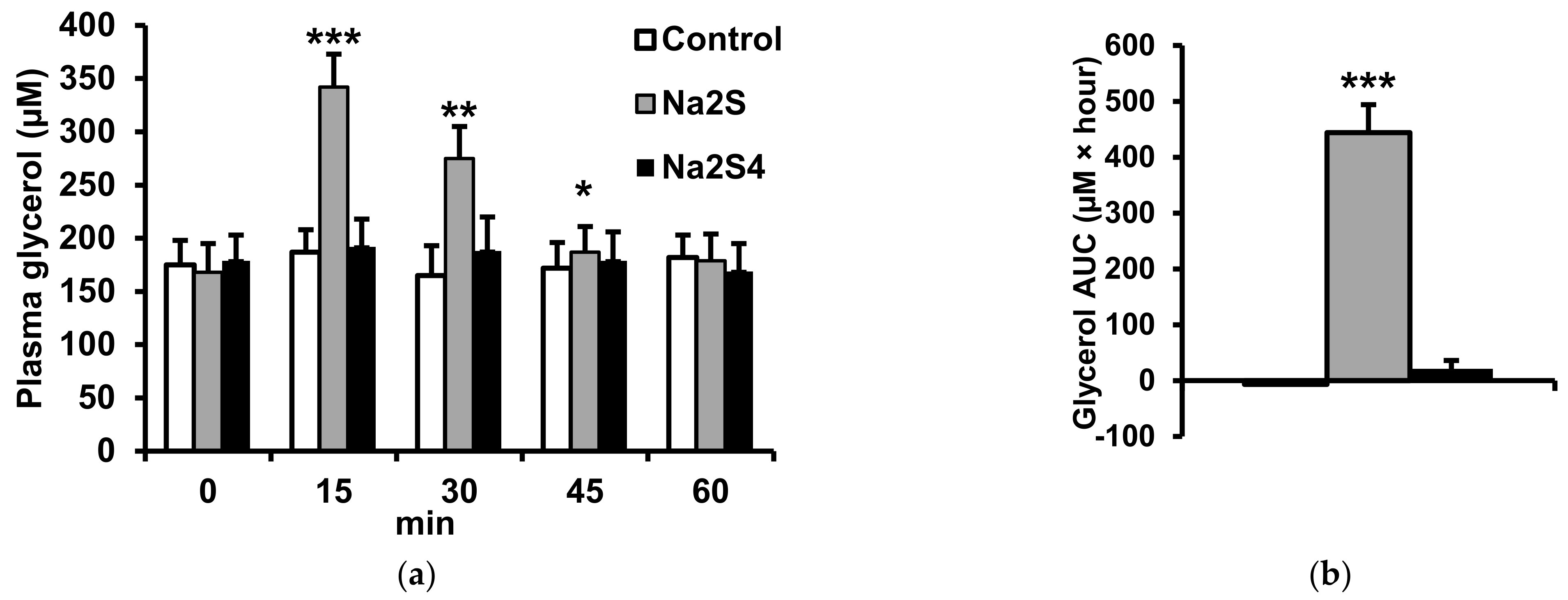
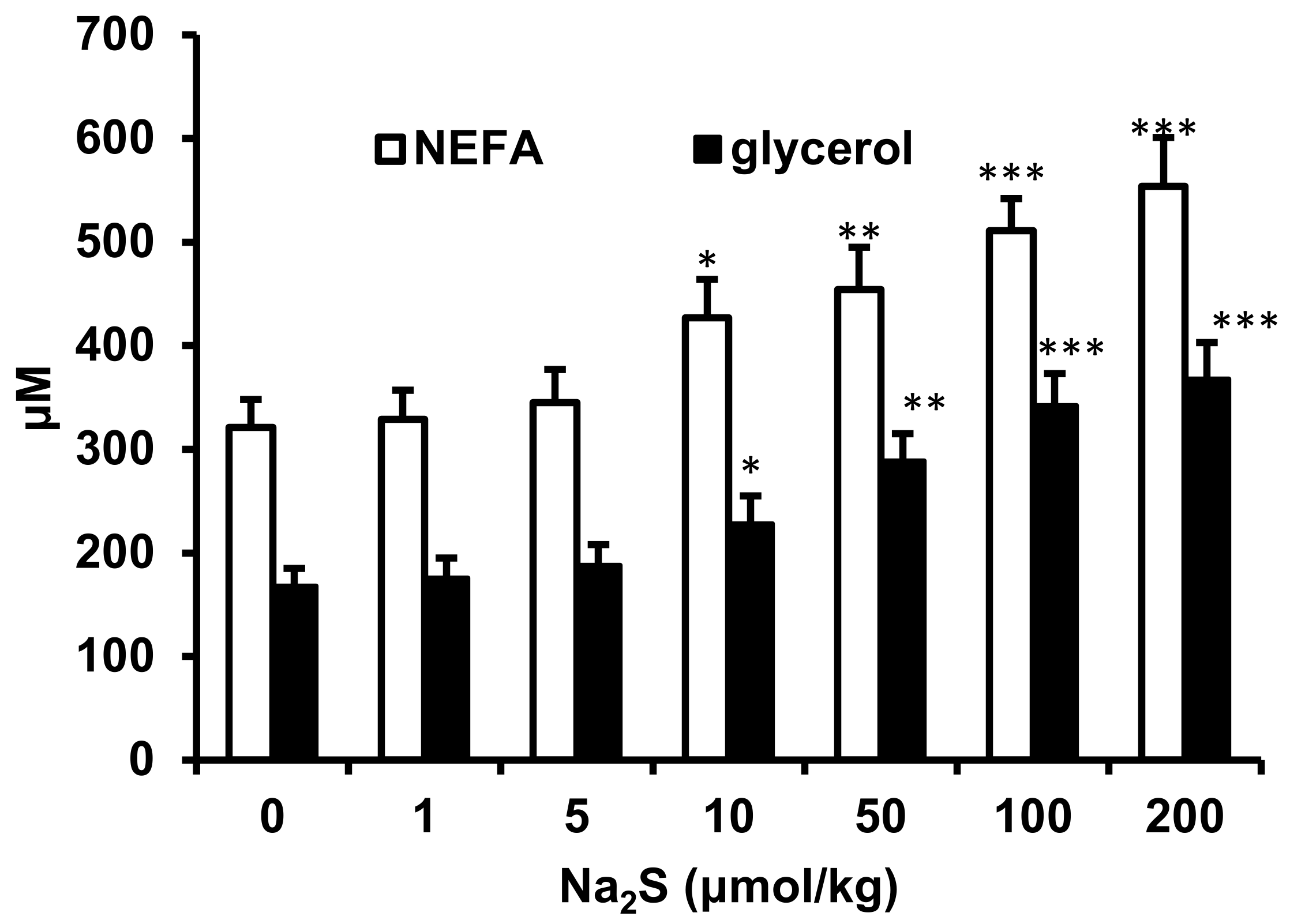
2.2. Effect of Na2S and Na2S4 on Non-Esterified Fatty Acids and Glycerol Concentration
2.3. Role of Cyclic Nucleotides and Cyclic Nucleotide-Dependent Protein Kinases in the Regulation of Lipolysis by Na2S
2.4. Role of the Adrenergic System in the Regulation of Lipolysis by Na2S
2.5. Effect of Na2S on cAMP-Hydrolyzing Phosphodiesterase Activity in Adipose Tissue
2.6. Effect of Obesity on Metabolic Parameters, H2S and Polysulfide Production in Adipose Tissue
3. Discussion
4. Materials and Methods
4.1. Animals and Reagents
4.2. Effect of H2S and Polysulfide Donors on Plasma NEFA, Glycerol, Glucose and Insulin
4.3. Effect of Cyclic Nucleotide-Dependent Protein Kinase Inhibitors on the Lipolytic Response to Na2S
4.4. Role of the Adrenergic System in the Regulation of Lipolysis by Na2S
4.5. Effect of Obesity on H2S and Polysulfide Production by Adipose Tissue
4.6. Measurement of H2S and Polysulfide Production by Adipose Tissue
4.7. Measurement of NEFA Concentrations
4.8. Measurement of Glycerol Concentration
4.9. Measurement of Cyclic Nucleotides in the Adipose Tissue
4.10. Measurement of Phosphodiesterase (PDE) Activity in the Adipose Tissue
4.11. Measurement of Glucose, Lipids and Insulin
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tchernof, A.; Després, J.P. Pathophysiology of human visceral obesity: An update. Physiol. Rev. 2013, 93, 359–404. [Google Scholar] [CrossRef] [PubMed]
- Chait, A.; den Hartigh, L.J. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Recinella, L.; Orlando, G.; Ferrante, C.; Chiavaroli, A.; Brunetti, L.; Leone, S. Adipokines: New potential therapeutic target for obesity and metabolic, rheumatic, and cardiovascular diseases. Front. Physiol. 2020, 11, 578966. [Google Scholar] [CrossRef] [PubMed]
- La Russa, D.; Marrone, A.; Mandalà, M.; Macirella, R.; Pellegrino, D. Antioxidant/anti-inflammatory effects of caloric restriction in an aged and obese rat Model: The role of adiponectin. Biomedicines 2020, 8, 532. [Google Scholar] [CrossRef]
- Stanek, A.; Brożyna-Tkaczyk, K.; Myśliński, W. The role of obesity-induced perivascular adipose tissue (PVAT) dysfunction in vascular homeostasis. Nutrients 2021, 13, 3843. [Google Scholar] [CrossRef]
- Hu, H.; Garcia-Barrio, M.; Jiang, Z.S.; Chen, Y.E.; Chang, L. Roles of perivascular adipose tissue in hypertension and atherosclerosis. Antioxid. Redox Signal. 2021, 34, 736–749. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Q.A.; Liu, Y.; Jiang, L. Energy metabolism in brown adipose tissue. FEBS J. 2021, 288, 3647–3662. [Google Scholar] [CrossRef]
- Singh, R.; Barrios, A.; Dirakvand, G.; Pervin, S. Human brown adipose tissue and metabolic health: Potential for therapeutic avenues. Cells 2021, 10, 3030. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- La Russa, D.; Giordano, F.; Marrone, A.; Parafati, M.; Janda, E.; Pellegrino, D. Oxidative imbalance and kidney damage in cafeteria diet-induced rat model of metabolic syndrome: Effect of Bergamot polyphenolic fraction. Antioxidants 2019, 8, 66. [Google Scholar] [CrossRef] [Green Version]
- Bolsoni-Lopes, A.; Alonso-Vale, M.I. Lipolysis and lipases in white adipose tissue—An update. Arch. Endocrinol. Metab. 2015, 59, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Frühbeck, G.; Méndez-Giménez, L.; Fernández-Formoso, J.A.; Fernández, S.; Rodríguez, A. Regulation of adipocyte lipolysis. Nutr. Res. Rev. 2014, 27, 63–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arner, P.; Langin, D. Lipolysis in lipid turnover, cancer cachexia, and obesity-induced insulin resistance. Trends Endocrinol. Metab. 2014, 25, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.S.; Jessen, N.; Jørgensen, J.O.; Møller, N.; Lund, S. Dissecting adipose tissue lipolysis: Molecular regulation and implications for metabolic disease. J. Mol. Endocrinol. 2014, 52, R199–R222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bełtowski, J.; Jamroz-Wiśniewska, A. Hydrogen sulfide in the adipose tissue-physiology, pathology and a target for pharmacotherapy. Molecules 2016, 22, 63. [Google Scholar] [CrossRef] [Green Version]
- Fang, L.; Zhao, J.; Chen, Y.; Ma, T.; Xu, G.; Tang, C.; Liu, X.; Geng, B. Hydrogen sulfide derived from periadventitial adipose tissue is a vasodilator. J. Hypertens. 2009, 27, 2174–2185. [Google Scholar] [CrossRef]
- Cai, J.; Shi, X.; Wang, H.; Fan, J.; Feng, Y.; Lin, X.; Yang, J.; Cui, Q.; Tang, C.; Xu, G.; et al. Cystathionine γ lyase-hydrogen sulfide increases peroxisome proliferator-activated receptor γ activity by sulfhydration at C139 site thereby promoting glucose uptake and lipid storage in adipocytes. Biochim. Biophys. Acta 2016, 1861, 419–429. [Google Scholar] [CrossRef]
- Feng, X.; Chen, Y.; Zhao, J.; Tang, C.; Jiang, Z.; Geng, B. Hydrogen sulfide from adipose tissue is a novel insulin resistance regulator. Biochem. Biophys. Res. Commun. 2009, 380, 153–169. [Google Scholar] [CrossRef]
- Huang, C.Y.; Yao, W.F.; Wu, W.G.; Lu, Y.L.; Wan, H.; Wang, W. Endogenous CSE/H2 S system mediates TNF-α-induced insulin resistance in 3T3-L1 adipocytes. Cell Biochem. Funct. 2013, 31, 468–475. [Google Scholar] [CrossRef]
- Velmurugan, G.V.; Huang, H.; Sun, H.; Candela, J.; Jaiswal, M.K.; Beaman, K.D.; Yamashita, M.; Prakriya, M.; White, C. Depletion of H2S during obesity enhances store-operated Ca2+ entry in adipose tissue macrophages to increase cytokine production. Sci. Signal. 2015, 8, ra128. [Google Scholar] [CrossRef] [Green Version]
- Kimura, H. Hydrogen sulfide (H2S) and polysulfide (H2Sn) signaling: The first 25 years. Biomolecules 2021, 11, 896. [Google Scholar] [CrossRef] [PubMed]
- Sawa, T.; Motohashi, H.; Ihara, H.; Akaike, T. Enzymatic regulation and biological functions of reactive cysteine persulfides and polysulfides. Biomolecules 2020, 10, 1245. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Radford, M.N.; Yang, C.T.; Chen, W.; Xian, M. Inorganic hydrogen polysulfides: Chemistry, chemical biology and detection. Br. J. Pharmacol. 2019, 176, 616–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.; Ju, Y.; Fu, M.; Zhang, Y.; Pei, Y.; Racine, M.; Baath, S.; Merritt, T.J.S.; Wang, R.; Wu, L. Cystathionine gamma-lyase/hydrogen sulfide system is essential for adipogenesis and fat mass accumulation in mice. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 165–176. [Google Scholar] [CrossRef]
- Nagahara, N.; Koike, S.; Nirasawa, T.; Kimura, H.; Ogasawara, Y. Alternative pathway of H2S and polysulfides production from sulfurated catalytic-cysteine of reaction intermediates of 3-mercaptopyruvate sulfurtransferase. Biochem. Biophys. Res. Commun. 2018, 496, 648–653. [Google Scholar] [CrossRef]
- Geng, B.; Cai, B.; Liao, F.; Zheng, Y.; Zeng, Q.; Fan, X.; Gong, Y.; Yang, J.; Cui, Q.H.; Tang, C.; et al. Increase or decrease hydrogen sulfide exert opposite lipolysis, but reduce global insulin resistance in high fatty diet induced obese mice. PLoS ONE 2013, 8, e73892. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Wang, H.; Geng, B.; Xu, G. Sulfhydration of perilipin 1 is involved in the inhibitory effects of cystathionine gamma lyase/hydrogen sulfide on adipocyte lipolysis. Biochem. Biophys. Res. Commun. 2020, 521, 786–790. [Google Scholar] [CrossRef]
- Hughes, M.N.; Centelles, M.N.; Moore, K.P. Making and working with hydrogen sulfide: The chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo: A review. Free Radic. Biol. Med. 2009, 47, 1346–1353. [Google Scholar] [CrossRef]
- Ciccone, V.; Genah, S.; Morbidelli, L. Endothelium as a source and target of H2S to improve its trophism and function. Antioxidants 2021, 10, 486. [Google Scholar] [CrossRef]
- Abramavicius, S.; Petersen, A.G.; Renaltan, N.S.; Prat-Duran, J.; Torregrossa, R.; Stankevicius, E.; Whiteman, M.; Simonsen, U. GYY4137 and sodium hydrogen sulfide relaxations are inhibited by L-Cysteine and KV7 channel blockers in rat small mesenteric arteries. Front. Pharmacol. 2021, 12, 613989. [Google Scholar] [CrossRef]
- Grambow, E.; Mueller-Graf, F.; Delyagina, E.; Frank, M.; Kuhla, A.; Vollmar, B. Effect of the hydrogen sulfide donor GYY4137 on platelet activation and microvascular thrombus formation in mice. Platelets 2014, 25, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Bazhanov, N.; Escaffre, O.; Freiberg, A.N.; Garofalo, R.P.; Casola, A. Broad-range antiviral activity of hydrogen sulfide against highly pathogenic RNA viruses. Sci. Rep. 2017, 7, 41029. [Google Scholar] [CrossRef] [PubMed]
- Drapala, A.; Koszelewski, D.; Tomasova, L.; Ostaszewski, R.; Grman, M.; Ondrias, K.; Ufnal, M. Parenteral Na2S, a fast-releasing H2S donor, but not GYY4137, a slow-releasing H2S donor, lowers blood pressure in rats. Acta Biochim. Pol. 2017, 64, 561–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, H.; Jung, H.; Kim, Y.; Kim, B.; Kim, K.H.; Park, S.J.; Suh, J.G. Aged black garlic extract regulates lipid metabolism by inhibiting lipogenesis and promoting lipolysis in mature 3T3-L1 adipocytes. Food Sci. Biotechnol. 2017, 27, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Ceddia, R.P.; Collins, S. A compendium of G-protein-coupled receptors and cyclic nucleotide regulation of adipose tissue metabolism and energy expenditure. Clin. Sci. 2020, 134, 473–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, X.; Wu, Z.; Xiong, S.; Cao, L.; Sethi, G.; Bian, J.S. The role of hydrogen sulfide in cyclic nucleotide signaling. Biochem. Pharmacol. 2018, 149, 20–28. [Google Scholar] [CrossRef]
- Katsouda, A.; Szabo, C.; Papapetropoulos, A. Reduced adipose tissue H2S in obesity. Pharmacol. Res. 2018, 128, 190–199. [Google Scholar] [CrossRef]
- Comas, F.; Latorre, J.; Ortega, F.; Arnoriaga Rodríguez, M.; Kern, M.; Lluch, A.; Ricart, W.; Blüher, M.; Gotor, C.; Romero, L.C.; et al. Activation of endogenous H2S biosynthesis or supplementation with exogenous H2S enhances adipose tissue adipogenesis and preserves adipocyte physiology in humans. Antioxid. Redox Signal. 2021, 35, 319–340. [Google Scholar] [CrossRef]
- Alkhouri, N.; Eng, K.; Cikach, F.; Patel, N.; Yan, C.; Brindle, A.; Rome, E.; Hanouneh, I.; Grove, D.; Lopez, R.; et al. Breathprints of childhood obesity: Changes in volatile organic compounds in obese children compared with lean controls. Pediatr. Obes. 2015, 10, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Comas, F.; Latorre, J.; Ortega, F.; Arnoriaga Rodríguez, M.; Lluch, A.; Sabater, M.; Rius, F.; Ribas, X.; Costas, M.; Ricart, W.; et al. Morbidly obese subjects show increased serum sulfide in proportion to fat mass. Int. J. Obes. 2021, 45, 415–426. [Google Scholar] [CrossRef]
- Whiteman, M.; Gooding, K.M.; Whatmore, J.L.; Ball, C.I.; Mawson, D.; Skinner, K.; Tooke, J.E.; Shore, A.C. Adiposity is a major determinant of plasma levels of the novel vasodilator hydrogen sulphide. Diabetologia 2010, 53, 1722–1726. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, O.; Kahn, C.R.; Flier, J.S.; Kahn, B.B. High fat feeding causes insulin resistance and a marked decrease in the expression of glucose transporters (Glut 4) in fat cells of rats. Endocrinology 1991, 129, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Bełtowski, J.; Wójcicka, G.; Jamroz-Wiśniewska, A.; Marciniak, A. Resistance to acute NO-mimetic and EDHF-mimetic effects of leptin in the metabolic syndrome. Life Sci. 2009, 85, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Bełtowski, J.; Guranowski, A.; Jamroz-Wiśniewska, A.; Wolski, A.; Hałas, K. Hydrogen-sulfide-mediated vasodilatory effect of nucleoside 5′-monophosphorothioates in perivascular adipose tissue. Can. J. Physiol. Pharmacol. 2015, 93, 585–595. [Google Scholar] [CrossRef] [PubMed]


| Control | Obese | |
|---|---|---|
| Body weight (g) | 221 ± 16 | 268 ± 18 ** |
| Triglycerides (mM) | 0.81 ± 0.05 | 0.88 ± 0.08 |
| Total cholesterol (mM) | 2.08 ± 0.23 | 2.15 ± 0.27 |
| NEFA (μM) | 352 ± 25 | 714 ± 46 *** |
| Glycerol (μM) | 172 ± 15 | 252 ± 19 *** |
| Insulin (μU/mL) | 21.3 ± 2.5 | 23.4 ± 2.9 |
| Glucose (mM) | 4.22 ± 0.51 | 4.44 ± 0.58 |
| H2S production (pmol/min per mg protein) | 32.7 ± 3.5 | 72.6 ± 6.1 *** |
| Polysulfide production (pmol/min per mg protein) | 116.4 ± 26.7 | 101.7 ± 19.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bełtowski, J.; Wiórkowski, K. Role of Hydrogen Sulfide and Polysulfides in the Regulation of Lipolysis in the Adipose Tissue: Possible Implications for the Pathogenesis of Metabolic Syndrome. Int. J. Mol. Sci. 2022, 23, 1346. https://doi.org/10.3390/ijms23031346
Bełtowski J, Wiórkowski K. Role of Hydrogen Sulfide and Polysulfides in the Regulation of Lipolysis in the Adipose Tissue: Possible Implications for the Pathogenesis of Metabolic Syndrome. International Journal of Molecular Sciences. 2022; 23(3):1346. https://doi.org/10.3390/ijms23031346
Chicago/Turabian StyleBełtowski, Jerzy, and Krzysztof Wiórkowski. 2022. "Role of Hydrogen Sulfide and Polysulfides in the Regulation of Lipolysis in the Adipose Tissue: Possible Implications for the Pathogenesis of Metabolic Syndrome" International Journal of Molecular Sciences 23, no. 3: 1346. https://doi.org/10.3390/ijms23031346
APA StyleBełtowski, J., & Wiórkowski, K. (2022). Role of Hydrogen Sulfide and Polysulfides in the Regulation of Lipolysis in the Adipose Tissue: Possible Implications for the Pathogenesis of Metabolic Syndrome. International Journal of Molecular Sciences, 23(3), 1346. https://doi.org/10.3390/ijms23031346





