Vitamin D-Binding Protein and the Free Hormone Hypothesis for Vitamin D in Bio-Naïve Patients with Psoriasis
Abstract
:1. Introduction
2. Results
2.1. Demographics
2.2. Seasonal Variation of Vitamin D Metabolites and Serum DBP
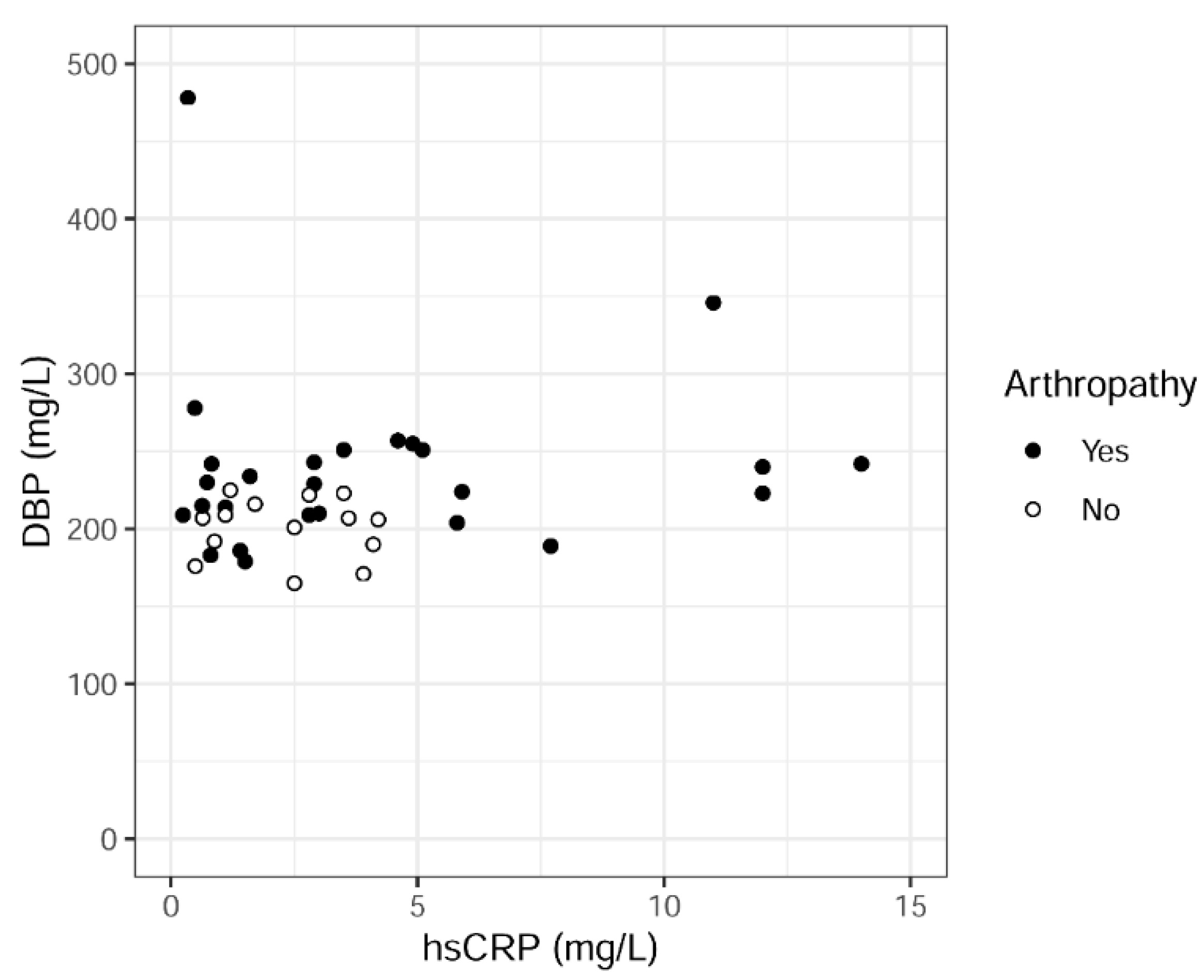
2.3. Serum DBP Levels in Relation to Demographics, Comorbidities, Psoriasis Severity, Vitamin D Metabolites and Other Parameters
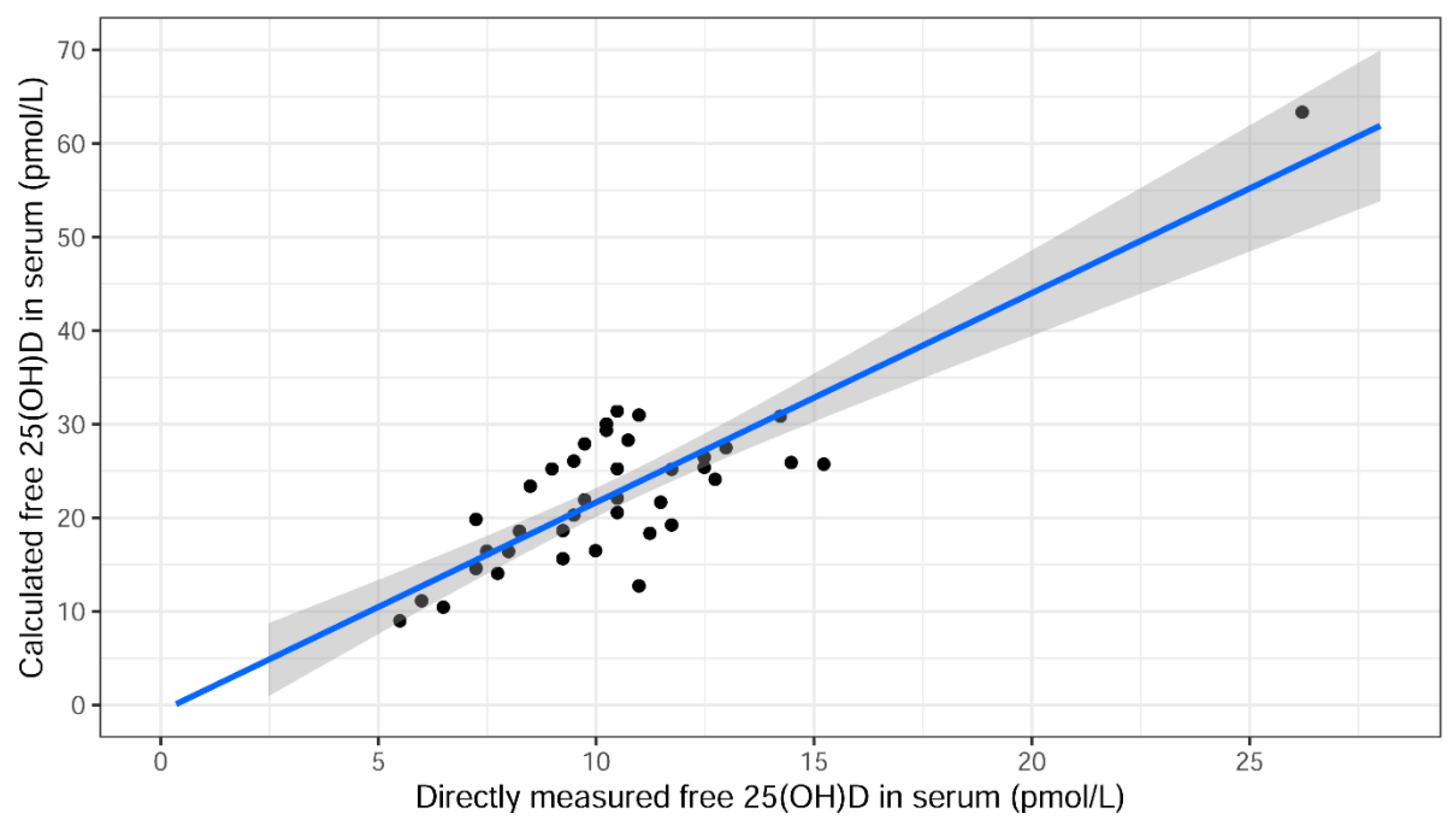
2.4. Association between Total and Directly Measured Free 25(OH)D Serum Levels
2.5. Association between Directly Measured Free 25(OH)D and Calculated Free 25(OH)D Serum Levels According to Bikle
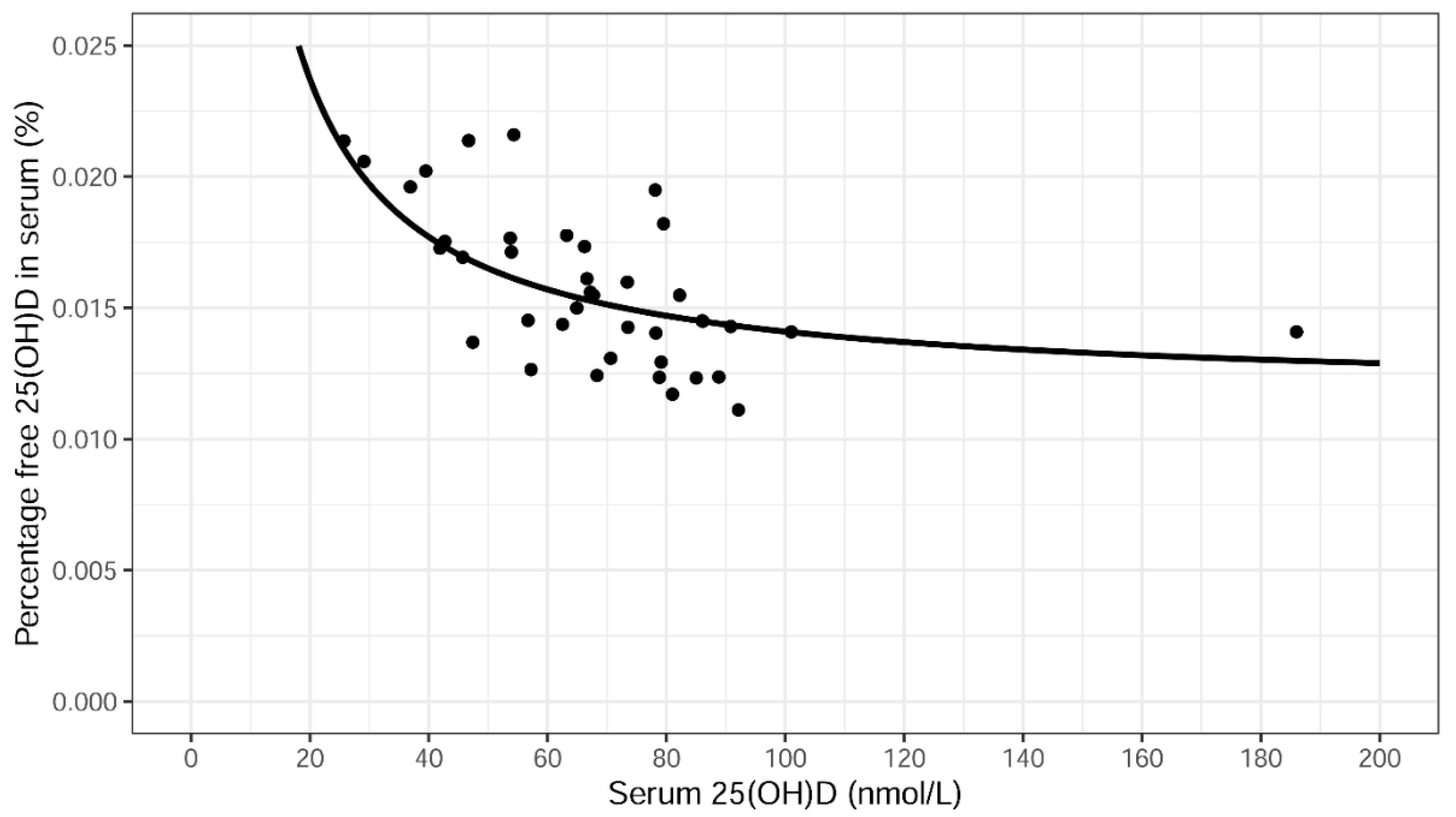
2.6. Total 25(OH)D, Directly Measured Free 25(OH)D and Bioavailable 25(OH)D Levels in Serum in Association with Psoriasis Disease Severity (Free Hormone Hypothesis)
2.7. Other Information
3. Discussion
4. Materials and Methods
4.1. Study Design, Setting and Participants
4.2. Blood Samples and Analyses
4.3. Calculation of Free 25(OH)D, Bioavailable 25(OH)D and the Percentage of Free 25(OH)D
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soleymani, T.; Hung, T.; Soung, J. The role of vitamin D in psoriasis: A review. Int. J. Dermatol. 2015, 54, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Hambly, R.; Kirby, B. The relevance of serum vitamin D in psoriasis: A review. Arch. Dermatol. Res. 2017, 309, 499–517. [Google Scholar] [CrossRef] [PubMed]
- Theodoridis, X.; Grammatikopoulou, M.G.; Stamouli, E.M.; Talimtzi, P.; Pagkalidou, E.; Zafiriou, E.; Haidich, A.B.; Bogdanos, D.P. Effectiveness of oral vitamin D supplementation in lessening disease severity among patients with psoriasis: A systematic review and meta-analysis of randomized controlled trials. Nutrition 2021, 82, 111024. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Bouillon, R.; Binkley, N.; Sempos, C.; Adler, R.A.; Bollerslev, J.; Dawson-Hughes, B.; Ebeling, P.R.; Feldman, D.; Heijboer, A.; et al. Controversies in Vitamin D: A Statement From the Third International Conference. JBMR Plus 2020, 4, e10417. [Google Scholar] [CrossRef]
- Bikle, D.D.; Schwartz, J. Vitamin D Binding Protein, Total and Free Vitamin D Levels in Different Physiological and Pathophysiological Conditions. Front. Endocrinol. 2019, 10, 317. [Google Scholar] [CrossRef] [Green Version]
- Bouillon, R.; Schuit, F.; Antonio, L.; Rastinejad, F. Vitamin D Binding Protein: A Historic Overview. Front. Endocrinol. 2020, 10, 910. [Google Scholar] [CrossRef]
- Chun, R.F.; Shieh, A.; Gottlieb, C.; Yacoubian, V.; Wang, J.; Hewison, M.; Adams, J.S. Vitamin D Binding Protein and the Biological Activity of Vitamin D. Front. Endocrinol. (Lausanne) 2019, 10, 718. [Google Scholar] [CrossRef]
- Schwartz, J.B.; Lai, J.; Lizaola, B.; Kane, L.; Markova, S.; Weyland, P.; Terrault, N.A.; Stotland, N.; Bikle, D. A comparison of measured and calculated free 25(OH) vitamin D levels in clinical populations. J. Clin. Endocrinol. Metab. 2014, 99, 1631–1637. [Google Scholar] [CrossRef]
- Michaelsson, K.; Rasmusson, A.; Wolk, A.; Byberg, L.; Mitchell, A.; Melhus, H. The Free Hormone Hypothesis: Is Free Serum 25-Hydroxyvitamin D a Better Marker for Bone Mineral Density in Older Women? JBMR Plus 2018, 2, 367–374. [Google Scholar] [CrossRef]
- Yu, C.; Xue, H.; Wang, L.; Chen, Q.; Chen, X.; Zhang, Y.; Hu, G.; Ling, W. Serum Bioavailable and Free 25-Hydroxyvitamin D Levels, but Not Its Total Level, Are Associated With the Risk of Mortality in Patients With Coronary Artery Disease. Circ. Res. 2018, 123, 996–1007. [Google Scholar] [CrossRef]
- Vandikas, M.S.; Landin-Wilhelmsen, K.; Holmäng, A.; Gillstedt, M.; Osmancevic, A. High levels of serum vitamin D-binding protein in patients with psoriasis: A case-control study and effects of ultraviolet B phototherapy. J. Steroid Biochem. Mol. Biol. 2021, 211, 105895. [Google Scholar] [CrossRef] [PubMed]
- Vandikas, M.S.; Landin-Wilhelmsen, K.; Polesie, S.; Gillstedt, M.; Osmancevic, A. Impact of Etanercept on Vitamin D Status and Vitamin D-binding Protein in Bio-naive Patients with Psoriasis. Acta Derm. Venereol. 2021, 101, adv00604. [Google Scholar] [CrossRef] [PubMed]
- Speeckaert, M.; Huang, G.; Delanghe, J.R.; Taes, Y.E. Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism. Clin. Chim. Acta Int. J. Clin. Chem. 2006, 372, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Delanghe, J.R.; Speeckaert, R.; Speeckaert, M.M. Behind the scenes of vitamin D binding protein: More than vitamin D binding. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 773–786. [Google Scholar] [CrossRef]
- Bikle, D.D.; Gee, E.; Halloran, B.; Kowalski, M.A.; Ryzen, E.; Haddad, J.G. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J. Clin. Endocrinol. Metab. 1986, 63, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Jassil, N.K.; Sharma, A.; Bikle, D.; Wang, X. Vitamin D binding protein and 25-hydroxyvitamin D levels: Emerging clinical applications. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2017, 23, 605–613. [Google Scholar] [CrossRef]
- Yousefzadeh, P.; Shapses, S.A.; Wang, X. Vitamin D Binding Protein Impact on 25-Hydroxyvitamin D Levels under Different Physiologic and Pathologic Conditions. Int. J. Endocrinol. 2014, 2014, 981581. [Google Scholar] [CrossRef]
- Speeckaert, M.M.; Speeckaert, R.; van Geel, N.; Delanghe, J.R. Vitamin D binding protein: A multifunctional protein of clinical importance. Adv. Clin. Chem. 2014, 63, 1–57. [Google Scholar]
- Ghaly, S.; Murray, K.; Baird, A.; Martin, K.; Prosser, R.; Mill, J.; Simms, L.A.; Hart, P.H.; Radford-Smith, G.; Bampton, P.A.; et al. High Vitamin D-Binding Protein Concentration, Low Albumin, and Mode of Remission Predict Relapse in Crohn’s Disease. Inflamm. Bowel Dis. 2016, 22, 2456–2464. [Google Scholar] [CrossRef]
- Robinson-Cohen, C.; Zelnick, L.R.; Hoofnagle, A.N.; Lutsey, P.L.; Burke, G.; Michos, E.D.; Shea, S.J.C.; Tracy, R.; Siscovick, D.S.; Psaty, B.; et al. Associations of Vitamin D-Binding Globulin and Bioavailable Vitamin D Concentrations with Coronary Heart Disease Events: The Multi-Ethnic Study of Atherosclerosis (MESA). J. Clin. Endocrinol. Metab. 2017, 102, 3075–3084. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Meng, H.; Sun, X.; Xu, L.; Zhang, L.; Shi, D.; Feng, X.; Lu, R.; Chen, Z. Elevation of vitamin D-binding protein levels in the plasma of patients with generalized aggressive periodontitis. J. Periodontal Res. 2013, 48, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Antony, A.; Tillett, W. Diagnosis, classification and assessment. Best Pract. Res. Clin. Rheumatol. 2021, 35, 101669. [Google Scholar] [CrossRef] [PubMed]
- Oleröd, G.; Hultén, L.M.; Hammarsten, O.; Klingberg, E. The variation in free 25-hydroxy vitamin D and vitamin D-binding protein with season and vitamin D status. Endocr. Connect. 2017, 6, 111–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srikanth, P.; Chun, R.F.; Hewison, M.; Adams, J.S.; Bouillon, R.; Vanderschueren, D.; Lane, N.; Cawthon, P.M.; Dam, T.; Barrett-Connor, E.; et al. Associations of total and free 25OHD and 1,25(OH)2D with serum markers of inflammation in older men. Osteoporos. Int. 2016, 27, 2291–2300. [Google Scholar] [CrossRef] [Green Version]
- Sokolova, M.V.; Simon, D.; Nas, K.; Zaiss, M.M.; Luo, Y.; Zhao, Y.; Rech, J.; Schett, G. A set of serum markers detecting systemic inflammation in psoriatic skin, entheseal, and joint disease in the absence of C-reactive protein and its link to clinical disease manifestations. Art. Res. 2020, 22, 26. [Google Scholar] [CrossRef] [Green Version]
- Osmancevic, A.; Landin-Wilhelmsen, K.; Larkö, O.; Krogstad, A.L. Vitamin D status in psoriasis patients during different treatments with phototherapy. J. Photochem. Photobiol. B Biol. 2010, 101, 117–123. [Google Scholar] [CrossRef]
- Osmancevic, A.; Nilsen, L.T.; Landin-Wilhelmsen, K.; Soyland, E.; Abusdal Torjesen, P.; Hagve, T.A.; Nenseter, M.S.; Krogstad, A.L. Effect of climate therapy at Gran Canaria on vitamin D production, blood glucose and lipids in patients with psoriasis. J. Eur. Acad. Dermatol. Venereol. JEADV 2009, 23, 1133–1140. [Google Scholar] [CrossRef]
- Grassi, T.; Panico, A.; Bagordo, F.; Imbriani, G.; Gambino, I.; Lobreglio, D.; Lobreglio, G.; Congedo, M.; De Donno, A. Direct detection of free vitamin D as a tool to assess risk conditions associated with chronic plaque psoriasis. J. Prev. Med. Hyg. 2020, 61, E489–E495. [Google Scholar] [CrossRef]
- Filoni, A.; Congedo, M.; Lobreglio, D.; Caldarola, G.; Lobreglio, G.; De Simone, C.; Bonamonte, D. Free and total vitamin d in psoriatic patients treated with biological drugs. Exp. Dermatol. 2021, 30, 995–996. [Google Scholar] [CrossRef]
- Ganzetti, G.; Campanati, A.; Scocco, V.; Brugia, M.; Tocchini, M.; Liberati, G.; Giuliodori, K.; Brisigotti, V.; Offidani, A. The potential effect of the tumour necrosis factor-α inhibitors on vitamin D status in psoriatic patients. Acta Derm. Venereol. 2014, 94, 715–717. [Google Scholar] [CrossRef] [Green Version]
- Bikle, D.D. The Free Hormone Hypothesis: When, Why, and How to Measure the Free Hormone Levels to Assess Vitamin D, Thyroid, Sex Hormone, and Cortisol Status. JBMR Plus 2021, 5, e10418. [Google Scholar] [CrossRef] [PubMed]
- Batista, M.C.; Menegat, F.D.; Ferreira, C.E.S.; Faulhaber, A.C.L.; Campos, D.; Mangueira, C.L.P. Analytical and clinical validation of the new Roche Elecsys Vitamin D Total II assay. Clin. Chem. Lab. Med. 2018, 56, e298–e301. [Google Scholar] [CrossRef] [PubMed]

| Patients with Psoriasis | |||||||
|---|---|---|---|---|---|---|---|
| n = 40 | |||||||
| Median | IQR | Mean | SD | 95% CI | n | ||
| Lower | Upper | ||||||
| Age (Years) | |||||||
| Men | 46 | [41–58] | 48 | 13 | 43 | 54 | 25 |
| Women | 48 | [29–58] | 46 | 17 | 37 | 55 | 15 |
| All | 47 | [37–59] | 47 | 15 | 43 | 52 | 40 |
| Duration of psoriasis (years) | 25 | [12–33] | 24 | 14 | 19 | 28 | 39 |
| PASI † | 9.7 | [7.8–13.9] | 11.2 | 4.9 | 9.7 | 12.8 | 40 |
| Mild psoriasis (<5) | 1/40 (3%) | ||||||
| Moderate psoriasis (≥5, <10) | 19/40 (48%) | ||||||
| Severe psoriasis (≥10) | 20/40 (50%) | s | |||||
| Median | IQR | Mean | SD | 95% CI | n | ||
| Lower | Upper | ||||||
| Total hours spent outdoors during summer | 5.0 | [3.5–8.0] | 5.5 | 3.0 | 4.5 | 6.5 | 37 |
| Fish meals/week | 1.0 | [1.0–2.0] | 1.6 | 0.8 | 1.3 | 1.9 | 35 |
| II | III | IV | |||||
| Skintype (n) | 15 | 24 | 1 | ||||
| Proportion | 37.5% | 60.0% | 2.5% | ||||
| n | % | ||||||
| Self-reported arthropathy | 25 | 64% | |||||
| Current smokers | 17 | 43% | |||||
| Antidyslipidemic use | 5 | 13% | |||||
| Antihypertensive use | 6 | 15% | |||||
| Antidiabetic use | 2 | 5% | |||||
| Antidepressant use | 5 | 13% | |||||
| Painkiller use | 6 | 15% | |||||
| Hypothyroidism medication | 2 | 5% | |||||
| Hormonal contraception | 1 | 3% | |||||
| Aspirin | 1 | 3% | |||||
| Obesity (BMI ‡ ≥ 30 kg/m2) | 9 | 23% | |||||
| Patients with Psoriasis | |||||||
|---|---|---|---|---|---|---|---|
| n = 40 | |||||||
| Median | IQR | Mean | SD | 95% CI | n | ||
| Lower | Upper | ||||||
| Serum 25(OH)D (nmol/L) | 67 | [54–80] | 69 | 26 | 60 | 77 | 40 |
| Directly measured free 25(OH)D in serum (pmol/L) | 10.2 | [8.4–11.5] | 10.4 | 3.4 | 9.3 | 11.5 | 40 |
| Calculated free 25(OH)D in serum (pmol/L) | 22.0 | [16.5–26.2] | 22.6 | 9.0 | 19.8 | 25.5 | 40 |
| Bioavailable 25(OH)D in serum (nmol/L) | 3.7 | [2.9–4.2] | 3.8 | 1.2 | 3.4 | 4.2 | 40 |
| Percentage free 25(OH)D in serum (%) | 0.015 | [0.014–0.018] | 0.016 | 0.003 | 0.015 | 0.017 | 40 |
| Serum 1,25(OH)2D (pmol/L) | 89 | [66–107] | 95 | 43 | 81 | 108 | 40 |
| PTH † (pmol/L) | 3.3 | [2.9–4.1] | 3.7 | 1.3 | 3.2 | 4.1 | 40 |
| DBP ‡ (mg/L) | 216 | [203–241] | 226 | 52 | 209 | 243 | 40 |
| hsCRP § (mg/L) | 2.8 | [1.0–4.3] | 3.5 | 3.5 | 2.4 | 4.6 | 40 |
| Albumin (g/L) | 40.0 | [39.0–42.0] | 40.4 | 2.86 | 39.4 | 41.3 | 40 |
| Creatinine (µmol/L) | 78 | [68–89] | 78 | 15 | 73 | 83 | 40 |
| Calcium (mmol/L) | 2.41 | [2.34–2.46] | 2.41 | 0.086 | 2.38 | 2.44 | 40 |
| ALP # (µkat/L) | 1.20 | [1.00–1.43] | 1.27 | 0.43 | 1.13 | 1.41 | 40 |
| Independent Variable in Linear Regression with DBP as Dependent Variable | Regression Coefficient (95% CI) | Adjusted p-Value * |
|---|---|---|
| Demographics | ||
| Age (years) | 0.22 (−0.36, 0.80) | 0.46 ** |
| Women vs. Men | −9.3 (−27, 8.1) | 0.30 *** |
| BMI † (kg/m2) | −0.43 (−2.3, 1.5) | 0.66 |
| Smoking (Yes vs. No) | −1.8 (−19, 16) | 0.84 |
| Arthropathy (Yes vs. No) | 24 (7.6, 39) | 0.007 |
| Vitamin D metabolites | ||
| Serum 25(OH)D (nmol/L) | 0.60 (0.15, 1.1) | 0.014 |
| Directly measured free 25(OH)D in serum (pmol/L) | 4.5 (0.81, 8.2) | 0.023 |
| Percentage free 25(OH)D in serum (%) | −2096 (−5051, 860) | 0.17 |
| Serum 1,25(OH)2D (pmol/L) | 0.21 (−0.0038, 0.42) | 0.063 |
| Other variables | ||
| hsCRP ‡ (mg/L) | 1.7 (−1.2, 4.5) | 0.26 |
| Disease duration (years) | −0.52 (−1.3, 0.28) | 0.21 |
| PASI # | 1.4 (−0.29, 3.1) | 0.11 |
| VAS € | 3.4 (−0.14, 6.9) | 0.069 |
| iPTH § (pmol/L) | −2.0 (−8.7, 4.7) | 0.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vandikas, M.S.; Landin-Wilhelmsen, K.; Gillstedt, M.; Osmancevic, A. Vitamin D-Binding Protein and the Free Hormone Hypothesis for Vitamin D in Bio-Naïve Patients with Psoriasis. Int. J. Mol. Sci. 2022, 23, 1302. https://doi.org/10.3390/ijms23031302
Vandikas MS, Landin-Wilhelmsen K, Gillstedt M, Osmancevic A. Vitamin D-Binding Protein and the Free Hormone Hypothesis for Vitamin D in Bio-Naïve Patients with Psoriasis. International Journal of Molecular Sciences. 2022; 23(3):1302. https://doi.org/10.3390/ijms23031302
Chicago/Turabian StyleVandikas, Maria Siekkeri, Kerstin Landin-Wilhelmsen, Martin Gillstedt, and Amra Osmancevic. 2022. "Vitamin D-Binding Protein and the Free Hormone Hypothesis for Vitamin D in Bio-Naïve Patients with Psoriasis" International Journal of Molecular Sciences 23, no. 3: 1302. https://doi.org/10.3390/ijms23031302
APA StyleVandikas, M. S., Landin-Wilhelmsen, K., Gillstedt, M., & Osmancevic, A. (2022). Vitamin D-Binding Protein and the Free Hormone Hypothesis for Vitamin D in Bio-Naïve Patients with Psoriasis. International Journal of Molecular Sciences, 23(3), 1302. https://doi.org/10.3390/ijms23031302






