Chronic Systemic Dexamethasone Regulates the Mineralocorticoid/Glucocorticoid Pathways Balance in Rat Ocular Tissues
Abstract
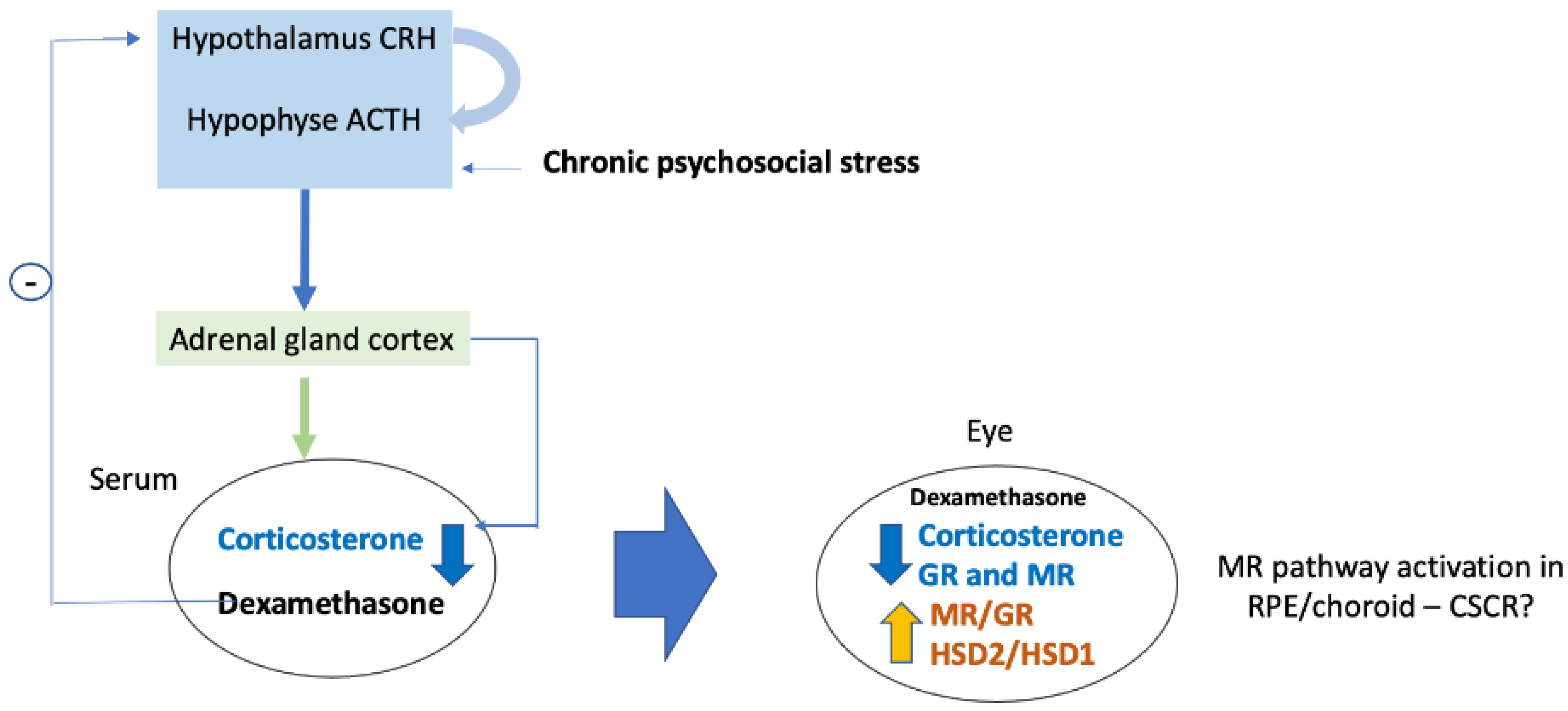
:1. Introduction
2. Results
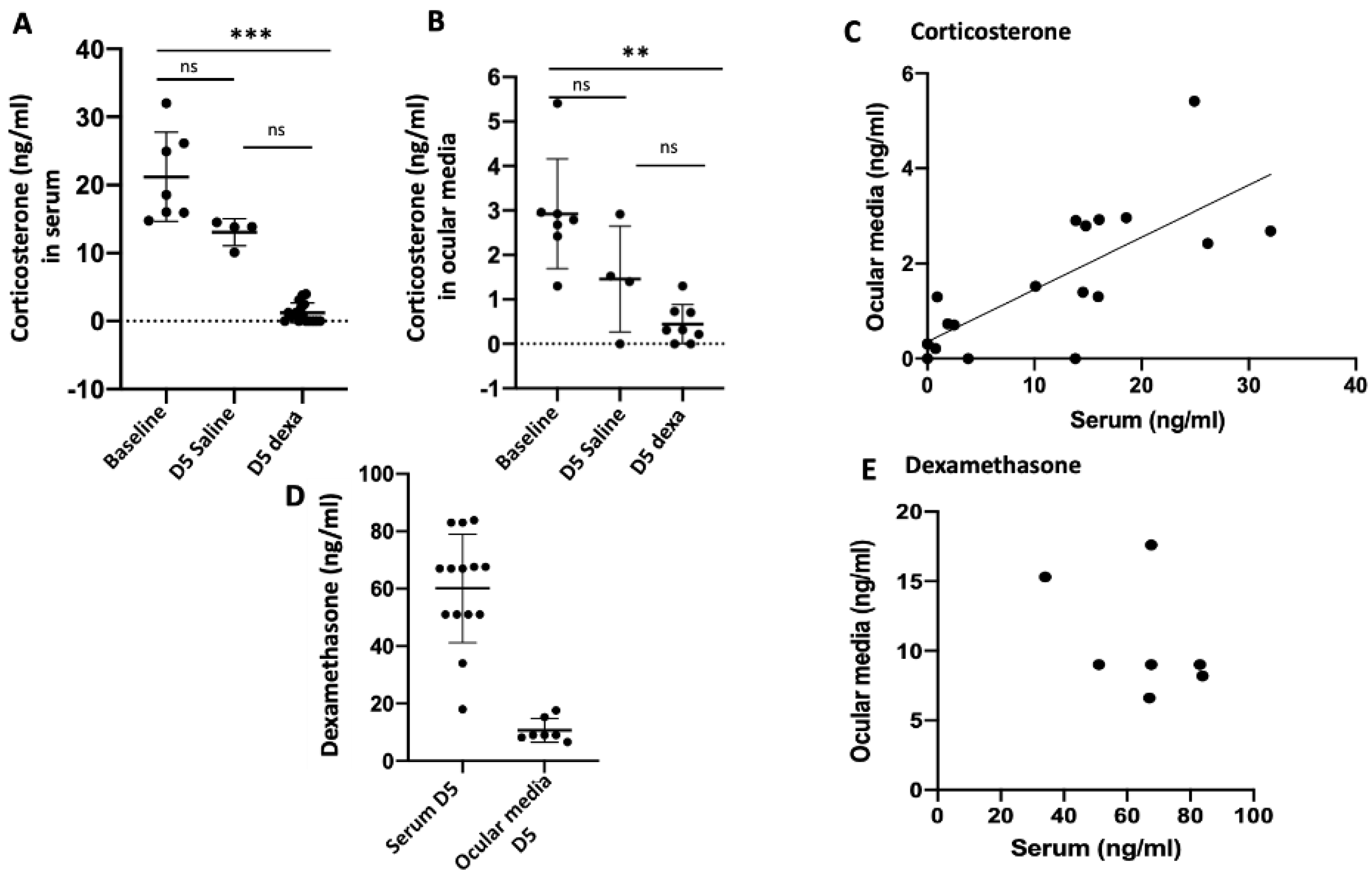
2.1. Correlation between Serum and Ocular Corticosterone and Dexamethasone Levels
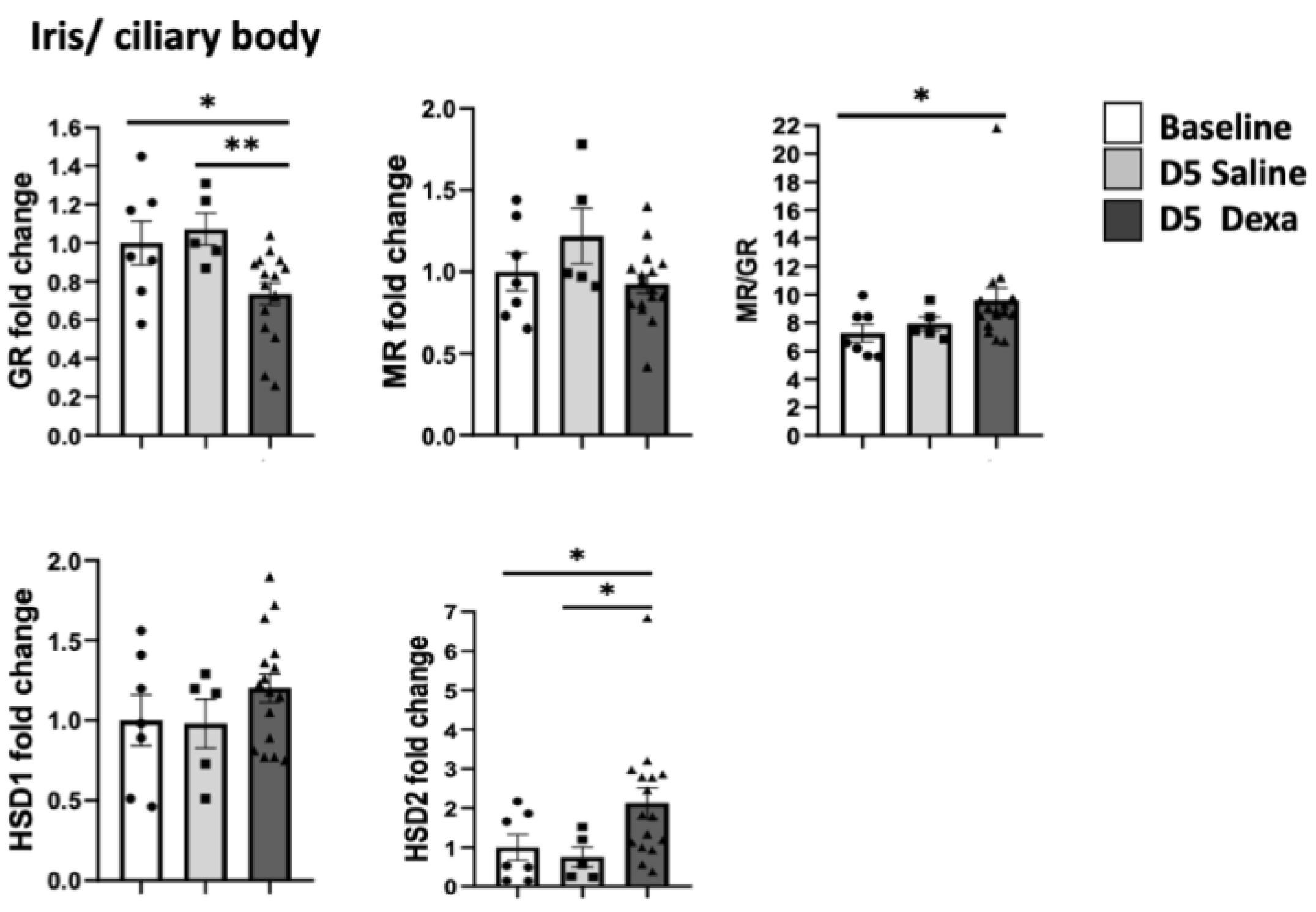
2.2. Corticosteroid Receptors and HSDs Expression in Ocular Tissues after Dexamethasone Treatment
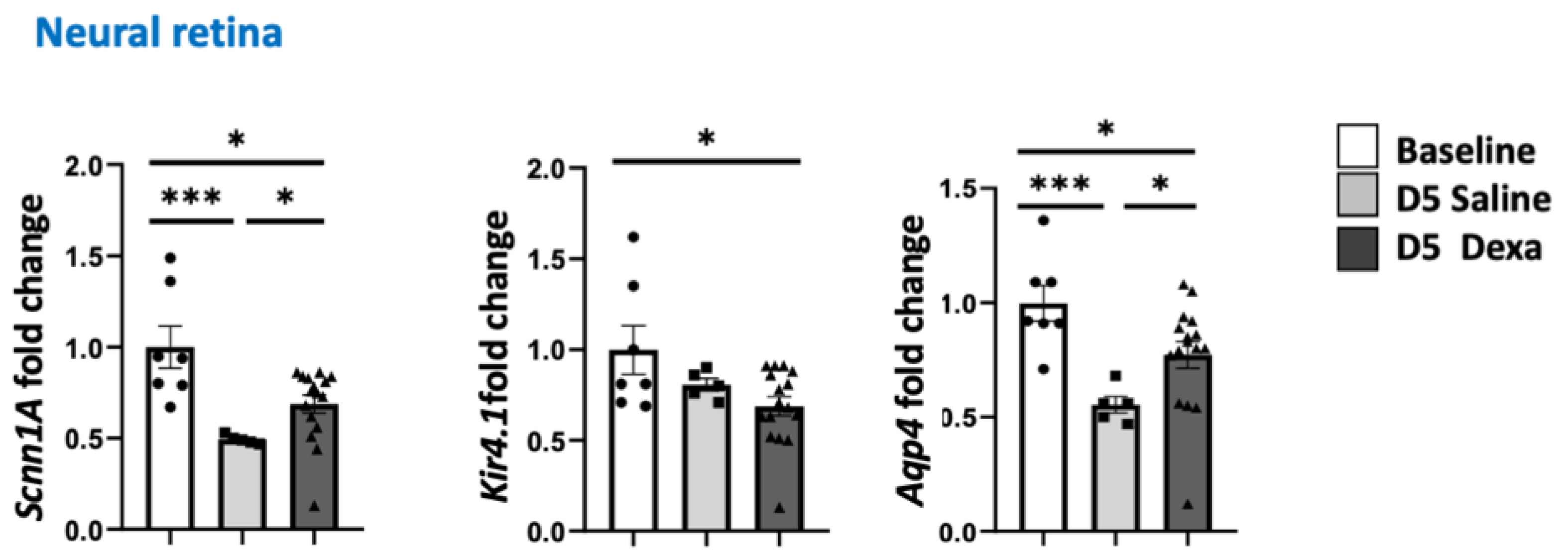
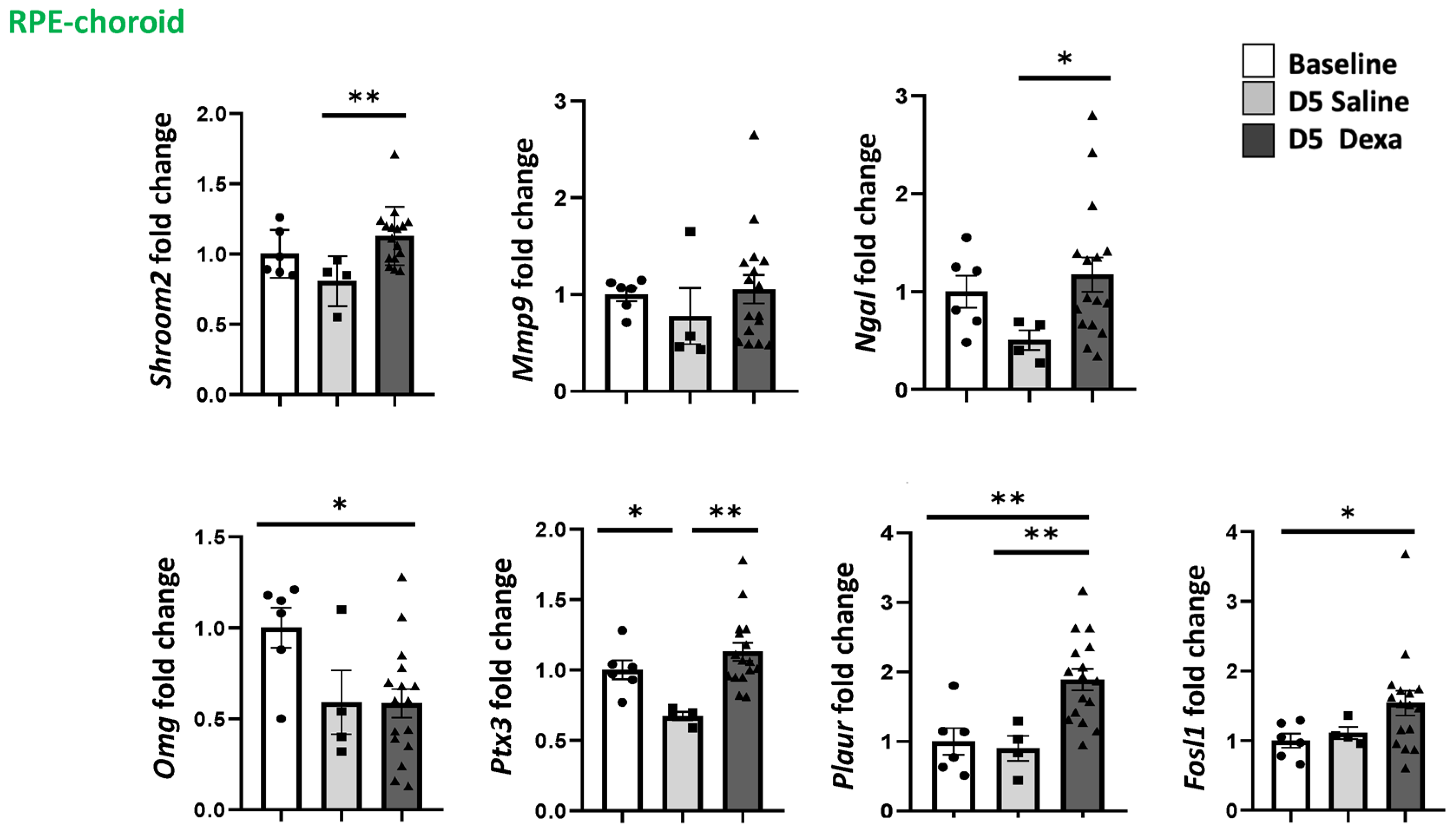
2.3. Regulation of MR-Induced Genes in the Retina and RPE/Choroid
3. Discussion
4. Material and Methods
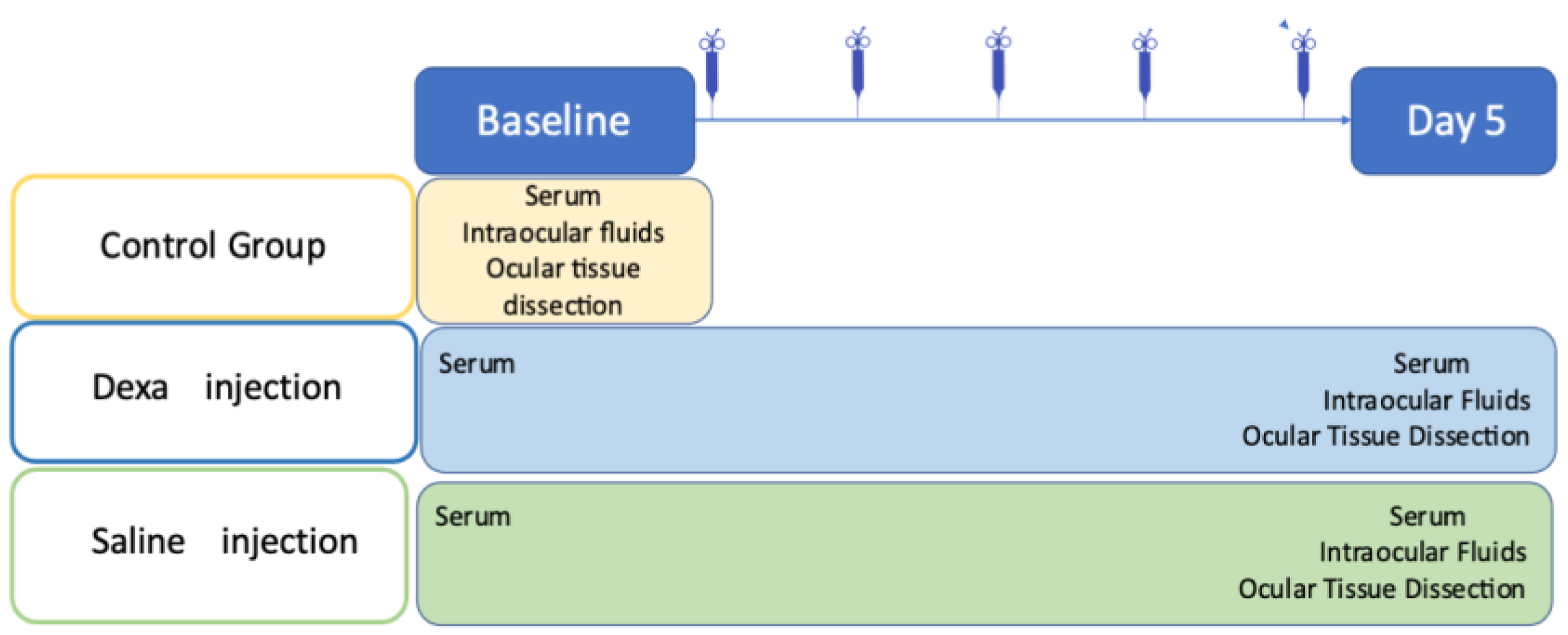
4.1. Animals
4.2. Experimental Scheme
4.3. Corticosterone and Dexamethasone Quantification
4.4. Quantitative PCR
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aliotta, N. ACTH and cortisone, dangerous weapons. Clin. Nuova Rass. Prog. Med. Int. 1952, 14, 1–4. [Google Scholar] [PubMed]
- Lan, N.C.; Graham, B.; Bartter, F.C.; Baxter, J.D. Binding of Steroids to Mineralocorticoid Receptors: Implications for in Vivo Occupancy by Glucocorticoids. J. Clin. Endocrinol. Metab. 1982, 54, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Campen, T.J.; Vaughn, D.A.; Fanestil, D.D. Mineralo- and Glucocorticoid Effects on Renal Excretion of Electrolytes. Pflug. Arch. 1983, 399, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, C.; Scholz, T.; Rochel, M.; Bumke-Vogt, C.; Oelkers, W.; Pfeiffer, A.F.H.; Diederich, S.; Bahr, V. Transactivation via the Human Glucocorticoid and Mineralocorticoid Receptor by Therapeutically Used Steroids in CV-1 Cells: A Comparison of Their Glucocorticoid and Mineralocorticoid Properties. Eur. J. Endocrinol. 2004, 151, 397–406. [Google Scholar] [CrossRef]
- Baxter, J.D.; Funder, J.W.; Apriletti, J.W.; Webb, P. Towards Selectively Modulating Mineralocorticoid Receptor Function: Lessons from Other Systems. Mol. Cell. Endocrinol. 2004, 217, 151–165. [Google Scholar] [CrossRef]
- James, E.R. The Etiology of Steroid Cataract. J. Ocul. Pharmacol. Ther. 2007, 23, 403–420. [Google Scholar] [CrossRef]
- Fini, M.E.; Schwartz, S.G.; Gao, X.; Jeong, S.; Patel, N.; Itakura, T.; Price, M.O.; Price, F.W.; Varma, R.; Stamer, W.D. Steroid-Induced Ocular Hypertension/Glaucoma: Focus on Pharmacogenomics and Implications for Precision Medicine. Prog. Retin. Eye Res. 2017, 56, 58–83. [Google Scholar] [CrossRef] [Green Version]
- Roberti, G.; Oddone, F.; Agnifili, L.; Katsanos, A.; Michelessi, M.; Mastropasqua, L.; Quaranta, L.; Riva, I.; Tanga, L.; Manni, G. Steroid-Induced Glaucoma: Epidemiology, Pathophysiology, and Clinical Management. Surv. Ophthalmol. 2020, 65, 458–472. [Google Scholar] [CrossRef]
- Gordon, D.M.; McLEAN, J.M.; Koteen, H.; Bousquet, F.P.; McCUSKER, W.D.; Baras, I.; Wetzig, P.; Norton, E.W.D. The Use of ACTH and Cortisone in Ophthalmology. Am. J. Ophthalmol. 1951, 34, 1675–1686. [Google Scholar] [CrossRef]
- Semeraro, F.; Morescalchi, F.; Russo, A.; Gambicorti, E.; Pilotto, A.; Parmeggiani, F.; Bartollino, S.; Costagliola, C. Central Serous Chorioretinopathy: Pathogenesis and Management. Clin. Ophthalmol. 2019, 13, 2341–2352. [Google Scholar] [CrossRef] [Green Version]
- Daruich, A.; Matet, A.; Dirani, A.; Bousquet, E.; Zhao, M.; Farman, N.; Jaisser, F.; Behar-Cohen, F. Central Serous Chorioretinopathy: Recent Findings and New Physiopathology Hypothesis. Prog. Retin. Eye Res. 2015, 48, 82–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicholson, B.P.; Atchison, E.; Idris, A.A.; Bakri, S.J. Central Serous Chorioretinopathy and Glucocorticoids: An Update on Evidence for Association. Surv. Ophthalmol. 2018, 63, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ge, G.; Zhang, Y.; Zhang, Y.; Xu, Z.; Zhang, M. Corticosteroids Usage and Central Serous Chorioretinopathy: A Meta-Analysis. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Bouzas, E.A.; Karadimas, P.; Pournaras, C.J. Central Serous Chorioretinopathy and Glucocorticoids. Surv. Ophthalmol. 2002, 47, 431–448. [Google Scholar] [CrossRef]
- Karadimas, P.; Bouzas, E.A. Glucocorticoid Use Represents a Risk Factor for Central Serous Chorioretinopathy: A Prospective, Case-Control Study. Graefes Arch. Clin. Exp. Ophthalmol. 2004, 242, 800–802. [Google Scholar] [CrossRef]
- Cusani, M. Central Serous Chorioretinopathy and Glucocorticoids. Surv. Ophthalmol. 2004, 49, 128–129, author reply 129. [Google Scholar] [CrossRef]
- Chaine, G.; Haouat, M.; Menard-Molcard, C.; Favard, C.; Vignal-Clermont, C.; Campinchi-Tardy, F.; Massin, P.; Gaudric, A.; Badelon, I.; Nicolon, L.; et al. Central serous chorioretinopathy and systemic steroid therapy. J. Fr. Ophtalmol. 2001, 24, 139–146. [Google Scholar]
- Geoffroy, M.; Afriat, M.; Fauconier, M.; Eschard, J.P.; Salmon, J.H. Adverse Effect of Corticosteroid Therapy: Central Serous Chorioretinopathy. Jt. Bone Spine 2018, 85, 127–128. [Google Scholar] [CrossRef]
- Rim, T.H.; Kim, H.S.; Kwak, J.; Lee, J.S.; Kim, D.W.; Kim, S.S. Association of Corticosteroid Use With Incidence of Central Serous Chorioretinopathy in South Korea. JAMA Ophthalmol. 2018, 136, 1164–1169. [Google Scholar] [CrossRef] [Green Version]
- Chan, L.Y.; Adam, R.S.; Adam, D.N. Localized Topical Steroid Use and Central Serous Retinopathy. J. Dermatolog. Treat. 2016, 27, 425–426. [Google Scholar] [CrossRef]
- Tsai, D.-C.; Chen, S.-J.; Huang, C.-C.; Chou, P.; Chung, C.-M.; Chan, W.-L.; Huang, P.-H.; Lin, S.-J.; Chen, J.-W.; Chen, T.-J.; et al. Risk of Central Serous Chorioretinopathy in Adults Prescribed Oral Corticosteroids: A Population-Based Study in Taiwan. Retina 2014, 34, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, D.F.; Mieler, W.F. The Use of Intraocular Corticosteroids. Expert. Opin. Pharmacother. 2009, 10, 2511–2525. [Google Scholar] [CrossRef] [PubMed]
- Cáceres-del-Carpio, J.; Costa, R.D.; Haider, A.; Narayanan, R.; Kuppermann, B.D. Corticosteroids: Triamcinolone, Dexamethasone and Fluocinolone. Dev. Ophthalmol. 2016, 55, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Knisely, T.L.; Hosoi, J.; Nazareno, R.; Granstein, R.D. The Presence of Biologically Significant Concentrations of Glucocorticoids but Little or No Cortisol Binding Globulin within Aqueous Humor: Relevance to Immune Privilege in the Anterior Chamber of the Eye. Investig. Ophthalmol. Vis. Sci. 1994, 35, 3711–3723. [Google Scholar]
- Benediktsson, R.; Edwards, C.R. 11 Beta-Hydroxysteroid Dehydrogenases: Tissue-Specific Dictators of Glucocorticoid Action. Essays Biochem. 1996, 31, 23–36. [Google Scholar]
- Chapman, K.; Holmes, M.; Seckl, J. 11β-Hydroxysteroid Dehydrogenases: Intracellular Gate-Keepers of Tissue Glucocorticoid Action. Physiol. Rev. 2013, 93, 1139–1206. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Yu, Y.; Qiao, J.; Zhu, L.; Xiao, Z. Mineralocorticoid Receptor Excessive Activation Involved in Glucocorticoid-Related Brain Injury. Biomed. Pharmacother. 2020, 122, 109695. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Farman, N.; Palacios-Ramirez, R.; Sbeih, M.; Behar-Cohen, F.; Aractingi, S.; Jaisser, F. Cutaneous Wound Healing in Diabetic Mice Is Improved by Topical Mineralocorticoid Receptor Blockade. J. Investig. Dermatol. 2020, 140, 223–234.e7. [Google Scholar] [CrossRef] [Green Version]
- Funder, J.W. Mineralocorticoid Receptors: Distribution and Activation. Heart Fail. Rev. 2005, 10, 15–22. [Google Scholar] [CrossRef]
- Walker, B.R. Glucocorticoids and Cardiovascular Disease. Eur. J. Endocrinol. 2007, 157, 545–559. [Google Scholar] [CrossRef] [Green Version]
- Claude, A.K.; Miller, W.W.; Beyer, A.M.; Willeford, K.O.; Ross, M.K. Quantification and Comparison of Baseline Cortisol Levels between Aqueous and Plasma from Healthy Anesthetized Hound Dogs Utilizing Mass Spectrometry. Vet. Ophthalmol. 2014, 17, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, E.; Zhao, M.; Ly, A.; Leroux Les Jardins, G.; Goldenberg, B.; Naud, M.-C.; Jonet, L.; Besson-Lescure, B.; Jaisser, F.; Farman, N.; et al. The Aldosterone-Mineralocorticoid Receptor Pathway Exerts Anti-Inflammatory Effects in Endotoxin-Induced Uveitis. PLoS ONE 2012, 7, e49036. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Valamanesh, F.; Celerier, I.; Savoldelli, M.; Jonet, L.; Jeanny, J.-C.; Jaisser, F.; Farman, N.; Behar-Cohen, F. The Neuroretina Is a Novel Mineralocorticoid Target: Aldosterone up-Regulates Ion and Water Channels in Müller Glial Cells. FASEB J. 2010, 24, 3405–3415. [Google Scholar] [CrossRef] [PubMed]
- Leśniewska, B.; Nowak, K.W.; Malendowicz, L.K. Dexamethasone-Induced Adrenal Cortex Atrophy and Recovery of the Gland from Partial, Steroid-Induced Atrophy. Exp. Clin. Endocrinol. 1992, 100, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Hagley, R.D.; Watlington, C.O. Dexamethasone Downregulates Mineralocorticoid Receptors in A6 Amphibian Kidney Cells in Tissue Culture. Ann. N. Y. Acad. Sci. 1995, 761, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Gelize, E.; Levy, R.; Moulin, A.; Azan, F.; Berdugo, M.; Naud, M.-C.; Guegan, J.; Delaunay, K.; Pussard, E.; et al. Mineralocorticoid Receptor Pathway and Its Antagonism in a Model of Diabetic Retinopathy. Diabetes 2021, db210099. [Google Scholar] [CrossRef]
- Sarapultsev, A.; Sarapultsev, P.; Dremencov, E.; Komelkova, M.; Tseilikman, O.; Tseilikman, V. Low Glucocorticoids in Stress-Related Disorders: The Role of Inflammation. Stress 2020, 23, 651–661. [Google Scholar] [CrossRef]
- Zhao, M.; Célérier, I.; Bousquet, E.; Jeanny, J.-C.; Jonet, L.; Savoldelli, M.; Offret, O.; Curan, A.; Farman, N.; Jaisser, F.; et al. Mineralocorticoid Receptor Is Involved in Rat and Human Ocular Chorioretinopathy. J. Clin. Investig. 2012, 122, 2672–2679. [Google Scholar] [CrossRef]
- Sugioka, K.; Kodama, A.; Okada, K.; Iwata, M.; Yoshida, K.; Kusaka, S.; Matsumoto, C.; Kaji, H.; Shimomura, Y. TGF-Β2 Promotes RPE Cell Invasion into a Collagen Gel by Mediating Urokinase-Type Plasminogen Activator (UPA) Expression. Exp. Eye Res. 2013, 115, 13–21. [Google Scholar] [CrossRef]
- Bousquet, E.; Zhao, M.; Daruich, A.; Behar-Cohen, F. Mineralocorticoid Antagonists in the Treatment of Central Serous Chorioetinopathy: Review of the Pre-Clinical and Clinical Evidence. Exp. Eye Res. 2019, 187, 107754. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Huang, L.-Y.; Liao, W.-L.; Chou, P. Association between Central Serous Chorioretinopathy and Risk of Depression: A Population-Based Cohort Study. J. Ophthalmol. 2019, 2019, 2749296. [Google Scholar] [CrossRef] [PubMed]
- Shimojo, M.; Hiroi, N.; Yakushiji, F.; Ueshiba, H.; Yamaguchi, N.; Miyachi, Y. Differences in Down-Regulation of Glucocorticoid Receptor MRNA by Cortisol, Prednisolone and Dexamethasone in HeLa Cells. Endocr. J. 1995, 42, 629–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cidlowski, J.A.; Cidlowski, N.B. Regulation of Glucocorticoid Receptors by Glucocorticoids in Cultured HeLa S3 Cells. Endocrinology 1981, 109, 1975–1982. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.B.; Tan, J.X. Regulation of Glucocorticoid Receptor by Glucocorticoids (II)--The Studies on Rat Liver, Brain and Spleen. Sci. China B 1990, 33, 288–293. [Google Scholar] [PubMed]
- Galvagni, F.; Orlandini, M.; Oliviero, S. Role of the AP-1 Transcription Factor FOSL1 in Endothelial Cells Adhesion and Migration. Cell. Adh. Migr. 2013, 7, 408–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elner, S.G. Human Retinal Pigment Epithelial Lysis of Extracellular Matrix: Functional Urokinase Plasminogen Activator Receptor, Collagenase, and Elastase. Trans. Am. Ophthalmol. Soc. 2002, 100, 273–299. [Google Scholar] [PubMed]
- Das, A.; Boyd, N.; Jones, T.R.; Talarico, N.; McGuire, P.G. Inhibition of Choroidal Neovascularization by a Peptide Inhibitor of the Urokinase Plasminogen Activator and Receptor System in a Mouse Model. Arch. Ophthalmol. 2004, 122, 1844–1849. [Google Scholar] [CrossRef] [Green Version]
- Matet, A.; Jaworski, T.; Bousquet, E.; Canonica, J.; Gobeaux, C.; Daruich, A.; Zhao, M.; Zola, M.; Meester-Smoor, M.; Mohabati, D.; et al. Lipocalin 2 as a Potential Systemic Biomarker for Central Serous Chorioretinopathy. Sci. Rep. 2020, 10, 20175. [Google Scholar] [CrossRef]
- Chida, Y.; Hamer, M. Chronic Psychosocial Factors and Acute Physiological Responses to Laboratory-Induced Stress in Healthy Populations: A Quantitative Review of 30 Years of Investigations. Psychol. Bull. 2008, 134, 829–885. [Google Scholar] [CrossRef]
- D’Agostino, J.; Vaeth, G.F.; Henning, S.J. Diurnal Rhythm of Total and Free Concentrations of Serum Corticosterone in the Rat. Acta Endocrinol. 1982, 100, 85–90. [Google Scholar] [CrossRef]
- Yamazaki, S.; Akira, S.; Sumimoto, H. Glucocorticoid Augments Lipopolysaccharide-Induced Activation of the IκBζ-Dependent Genes Encoding the Anti-Microbial Glycoproteins Lipocalin 2 and Pentraxin 3. J. Biochem. 2015, 157, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.M.G.; Lee, W.K.; Koizumi, H.; Dansingani, K.; Lai, T.Y.Y.; Freund, K.B. Pachychoroid Disease. Eye 2018, 33, 14–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borooah, S.; Sim, P.Y.; Phatak, S.; Moraes, G.; Wu, C.Y.; Cheung, C.M.G.; Pal, B.; Bujarborua, D. Pachychoroid Spectrum Disease. Acta Ophthalmol. 2020, 99, e806–e822. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, E.; Zuccaro, B.; Zucchiatti, I.; Parravano, M.; Querques, L.; Costanzo, E.; Sacconi, R.; Prascina, F.; Scarinci, F.; Bandello, F.; et al. Optical Coherence Tomography Parameters as Predictors of Treatment Response to Eplerenone in Central Serous Chorioretinopathy. J. Clin. Med. 2019, 8, 1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zucchiatti, I.; Sacconi, R.; Parravano, M.C.; Costanzo, E.; Querques, L.; Montorio, D.; Bandello, F.; Querques, G. Eplerenone Versus Observation in the Treatment of Acute Central Serous Chorioretinopathy: A Retrospective Controlled Study. Ophthalmol. Ther. 2018, 7, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Van Haalen, F.M.; van Dijk, E.H.C.; Savas, M.; Brinks, J.; Dekkers, O.M.; Dijkman, G.; van Rossum, E.F.C.; Biermasz, N.R.; Boon, C.J.F.; Pereira, A.M. Hair Cortisol Concentrations in Chronic Central Serous Chorioretinopathy. Acta Ophthalmol. 2020, 98, 390–395. [Google Scholar] [CrossRef]
- Calogero, A.E.; Kamilaris, T.C.; Johnson, E.O.; Tartaglia, M.E.; Chrousos, G. Recovery of the Rat Hypothalamic-Pituitary-Adrenal Axis after Discontinuation of Prolonged Treatment with the Synthetic Glucocorticoid Agonist Dexamethasone. Endocrinology 1990, 127, 1574–1579. [Google Scholar] [CrossRef]
- Marin, M.T.; Cruz, F.C.; Planeta, C.S. Chronic Restraint or Variable Stresses Differently Affect the Behavior, Corticosterone Secretion and Body Weight in Rats. Physiol. Behav. 2007, 90, 29–35. [Google Scholar] [CrossRef]
- Matsuura, T.; Takimura, R.; Yamaguchi, M.; Ichinose, M. Estimation of Restraint Stress in Rats Using Salivary Amylase Activity. J. Physiol. Sci. 2012, 62, 421–427. [Google Scholar] [CrossRef]
- Christensen, M.V.; Kessing, L.V. The Hypothalamo-Pituitary-Adrenal Axis in Major Affective Disorder: A Review. Nord. J. Psychiatry 2001, 55, 359–363. [Google Scholar] [CrossRef]
- Adam, E.K.; Quinn, M.E.; Tavernier, R.; McQuillan, M.T.; Dahlke, K.A.; Gilbert, K.E. Diurnal Cortisol Slopes and Mental and Physical Health Outcomes: A Systematic Review and Meta-Analysis. Psychoneuroendocrinology 2017, 83, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Paragliola, R.M.; Papi, G.; Pontecorvi, A.; Corsello, S.M. Treatment with Synthetic Glucocorticoids and the Hypothalamus-Pituitary-Adrenal Axis. Int. J. Mol. Sci. 2017, 18, 2201. [Google Scholar] [CrossRef] [PubMed]
- Kemppainen, R.J.; Behrend, E.N. Dexamethasone Rapidly Induces a Novel Ras Superfamily Member-Related Gene in AtT-20 Cells. J Biol Chem 1998, 273, 3129–3131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.; Bousquet, E.; Valamanesh, F.; Farman, N.; Jeanny, J.-C.; Jaisser, F.; Behar-Cohen, F.F. Differential Regulations of AQP4 and Kir4.1 by Triamcinolone Acetonide and Dexamethasone in the Healthy and Inflamed Retina. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6340–6347. [Google Scholar] [CrossRef] [PubMed]
- Canonica, J.; Zhao, M.; Favez, T.; Gelizé, E.; Jonet, L.; Kowalczuk, L.; Guegan, J.; Le Menuet, D.; Viengchareun, S.; Lombès, M.; et al. Pathogenic Effects of Mineralocorticoid Pathway Activation in Retinal Pigment Epithelium. Int. J. Mol. Sci. 2021, 22, 9618. [Google Scholar] [CrossRef]
- Canonica, J.; Mehanna, C.; Bonnard, B.; Jonet, L.; Gelize, E.; Jais, J.-P.; Jaisser, F.; Zhao, M.; Behar-Cohen, F. Effect of Acute and Chronic Aldosterone Exposure on the Retinal Pigment Epithelium-Choroid Complex in Rodents. Exp. Eye Res. 2019, 187, 107747. [Google Scholar] [CrossRef]

| Gene Name | Forward/Reverse | Sequences |
|---|---|---|
| 18S | Forward | TGCAATTATTCCCCATGAACG |
| 18S | Reverse | GCTTATGACCCGCACTTACTGG |
| Ubc | Forward | ATCTAGAAAGAGCCCTTCTTGTGC |
| Ubc | Reverse | ACACCTCCCCATCAAACCC |
| Hprt1 | Forward | GCGAAAGTGGAAAAGCCAAGT |
| Hprt1 | Reverse | GCCACATCAACAGGACTCTTGTAG |
| Nr3c1 | Forward | AACATGTTAGGTGGGCGTCAA |
| Nr3c1 | Reverse | GGTGTAAGTTTCTCAAGCCTAGTATCG |
| Nr3c2 | Forward | TAAGTTTCCCCACGTGGTTC |
| Nr3c2 | Reverse | ATCCACGTCTCATGGCTTTC |
| 11b-Hsd1 | Forward | GAAGAAGCATGGAGGTCAAC |
| 11b-Hsd1 | Reverse | GCAATCAGAGGTTGGGTCAT |
| 11b-Hsd2 | Forward | TGCTGGCTGGATCGCGTTGTC |
| 11b-Hsd2 | Reverse | CACAGTGGCCAGCACCGTGAA |
| Aqp4 | Forward | CGGTTCATGGAAACCTCACT |
| Aqp4 | Reverse | CATGCTGGCTCCGGTATAAT |
| Kir4.1 | Forward | CAAAGAAGAGGGCTGAGACG |
| Kir4.1 | Reverse | TTGAGCCGAATATCCTCACC |
| Scnn1A | Forward | TCATGCTGCTACGCCGGTTCC |
| Scnn1A | Reverse | TCCATCAGTTTACAAGGGAG |
| Ngal | Forward | TCACCCTGTACGGAAGAACC |
| Ngal | Reverse | GGTGGGAACAGAGAAAACGA |
| Mmp9 | Forward | CTGCCTGCACCACTAAAGG |
| Mmp9 | Reverse | GAAGACGAAGGGGAAGACG |
| Omg | Forward | CAACACAATGCAGCTGAGCA |
| Omg | Reverse | CCATCAAAGCCATAATGTCGTCT |
| Ptx3 | Forward | TACCCGCAGGCTGTGAAAC |
| Ptx3 | Reverse | GGGTTCCACTTGGTGCCATA |
| Plaur | Forward | CTGAAGTGCTGCAACTTCAC |
| Plaur | Reverse | AGCACATCTAAGCCTGTAGC |
| Fosl1 | Forward | GAGAAAATATGTCCCTCTGC |
| Fosl1 | Reverse | TTCTAGGCTAGTTAAAGGGC |
| Shroom2 | Forward | CCTATTATAGCACATCAGCC |
| Shroom2 | Reverse | TGAGCTCTTGCTTCTTAATG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zola, M.; Mejlachowicz, D.; Gregorio, R.; Naud, M.-C.; Jaisser, F.; Zhao, M.; Behar-Cohen, F. Chronic Systemic Dexamethasone Regulates the Mineralocorticoid/Glucocorticoid Pathways Balance in Rat Ocular Tissues. Int. J. Mol. Sci. 2022, 23, 1278. https://doi.org/10.3390/ijms23031278
Zola M, Mejlachowicz D, Gregorio R, Naud M-C, Jaisser F, Zhao M, Behar-Cohen F. Chronic Systemic Dexamethasone Regulates the Mineralocorticoid/Glucocorticoid Pathways Balance in Rat Ocular Tissues. International Journal of Molecular Sciences. 2022; 23(3):1278. https://doi.org/10.3390/ijms23031278
Chicago/Turabian StyleZola, Marta, Dan Mejlachowicz, Raquel Gregorio, Marie-Christine Naud, Frédéric Jaisser, Min Zhao, and Francine Behar-Cohen. 2022. "Chronic Systemic Dexamethasone Regulates the Mineralocorticoid/Glucocorticoid Pathways Balance in Rat Ocular Tissues" International Journal of Molecular Sciences 23, no. 3: 1278. https://doi.org/10.3390/ijms23031278
APA StyleZola, M., Mejlachowicz, D., Gregorio, R., Naud, M.-C., Jaisser, F., Zhao, M., & Behar-Cohen, F. (2022). Chronic Systemic Dexamethasone Regulates the Mineralocorticoid/Glucocorticoid Pathways Balance in Rat Ocular Tissues. International Journal of Molecular Sciences, 23(3), 1278. https://doi.org/10.3390/ijms23031278






