Increased Risky Choice and Reduced CHRNB2 Expression in Adult Male Rats Exposed to Nicotine Vapor
Abstract
:1. Introduction
2. Results
2.1. Training in the Probability Discounting Task
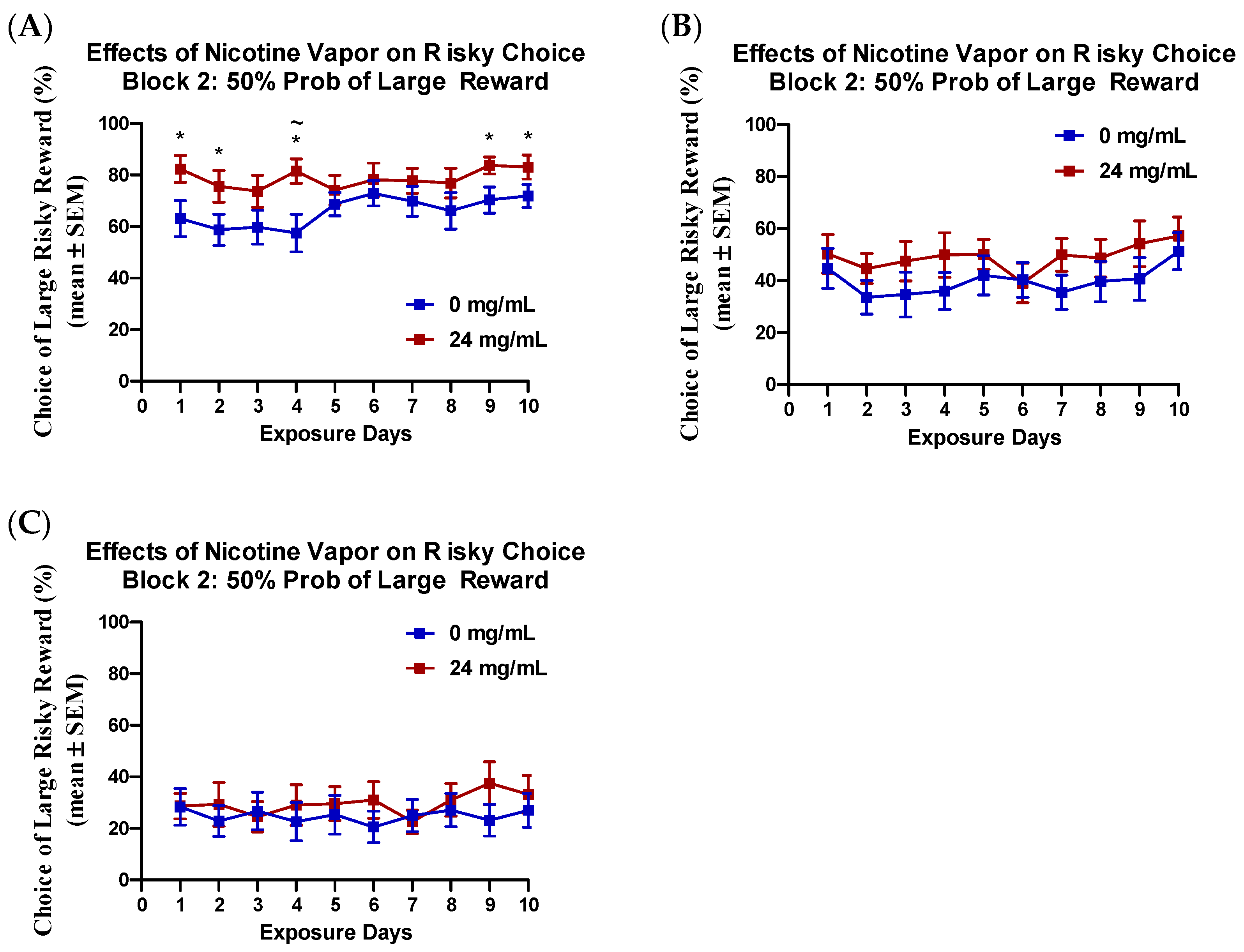
2.2. Effects of Nicotine Vapor Exposure on Risky Choice
2.3. Effects of Nicotine Vapor Exposure on Perseverative Responding
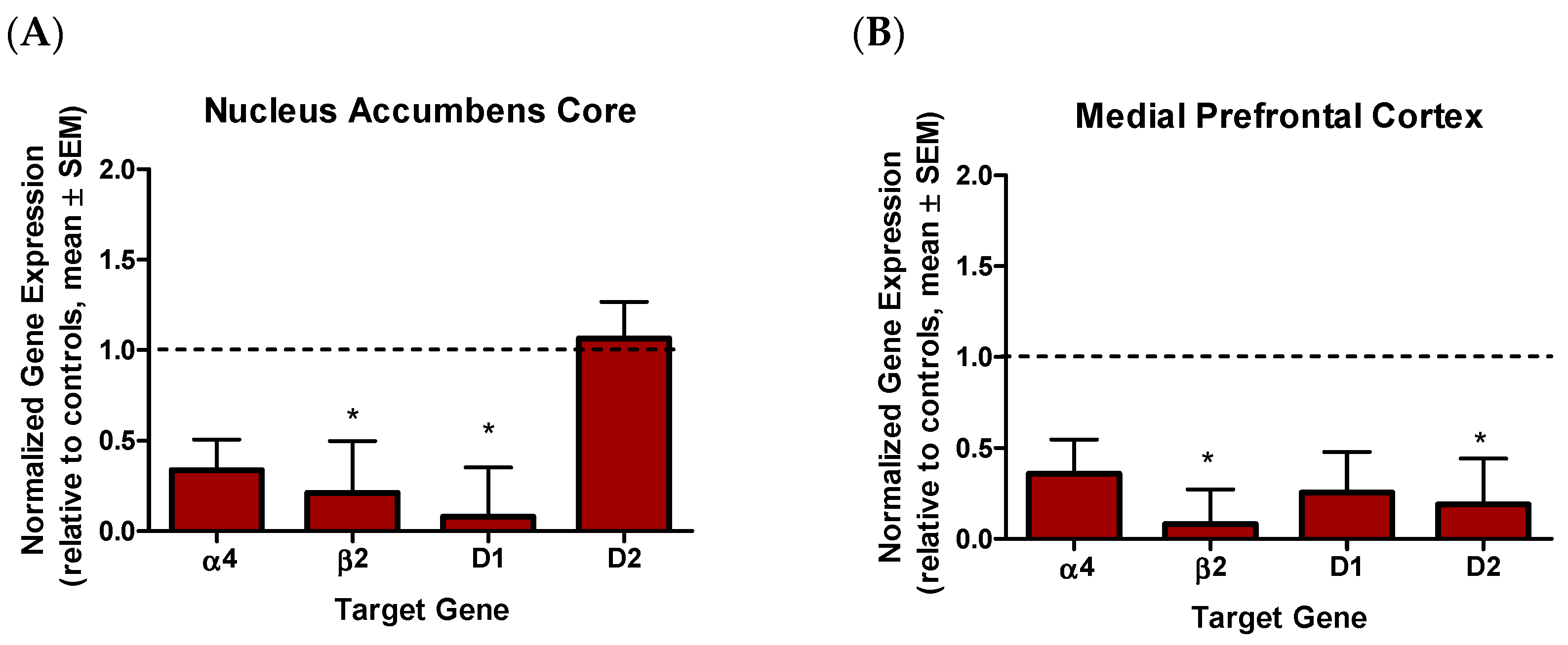
2.4. Effects of Nicotine Vapor Exposure on Gene Expression
3. Discussion
4. Materials and Methods
4.1. Subjects
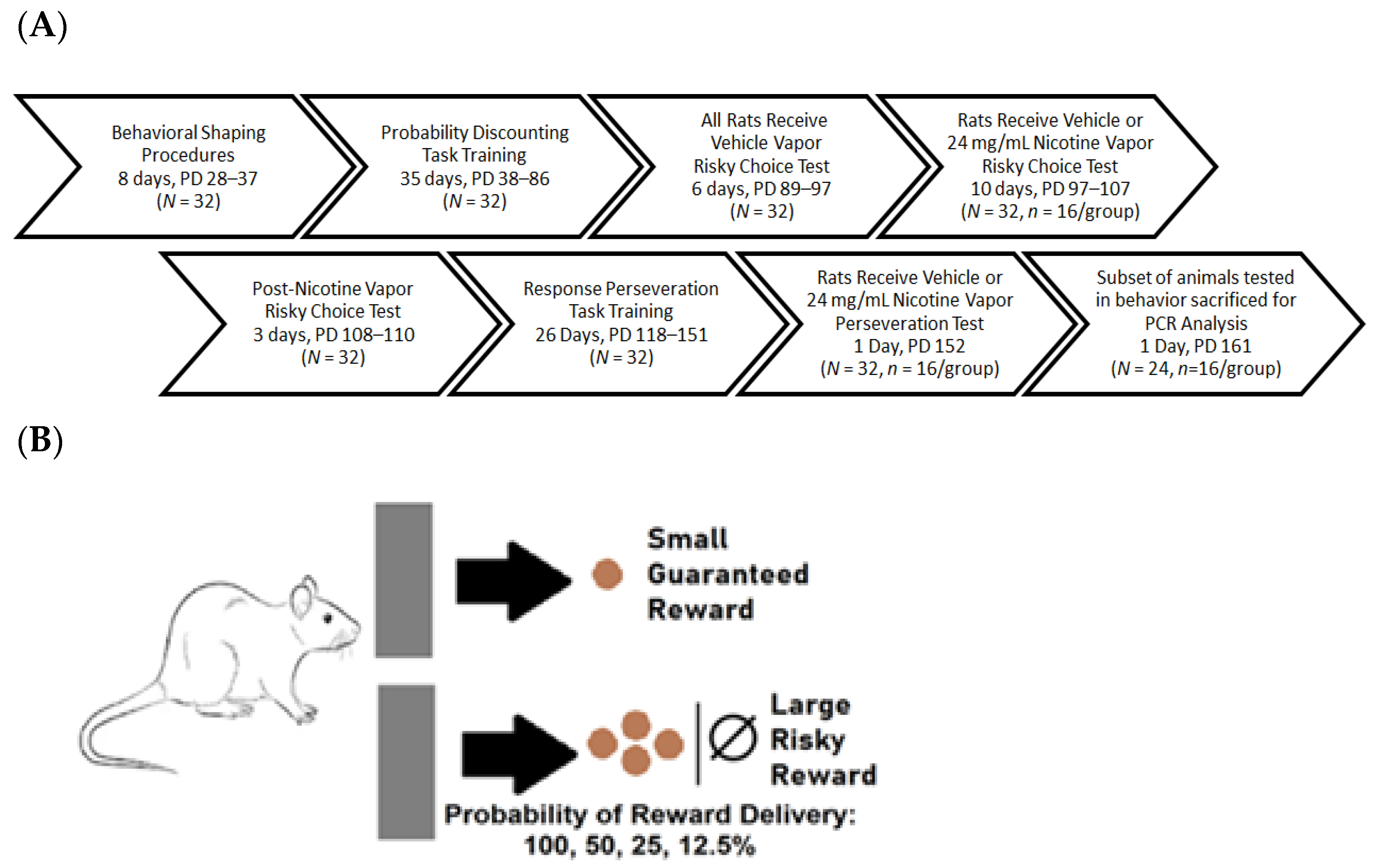
4.2. Experimental Design
4.3. Nicotine Vapor Exposure
4.4. Probability Discounting Task
4.5. Perseverative Responding Task
4.6. Gene Expression
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gentzke, A.S.; Creamer, M.; Cullen, K.A.; Ambrose, B.K.; Willis, G.; Jamal, A.; King, B.A. Vital Signs: Tobacco Product Use Among Middle and High School Students—United States, 2011–2018. Morb. Mortal. Wkly. Rep. 2019, 68, 157–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callahan-Lyon, P. Electronic cigarettes: Human health effects. Tob Control. 2014, 23 (Suppl. 2), 36–40. [Google Scholar] [CrossRef] [PubMed]
- Pisinger, C.; Dossing, M. A systematic review of health effects of electronic cigarettes. Prev. Med. 2014, 69, 248–260. [Google Scholar] [CrossRef] [Green Version]
- Flores, R.J.; Alshbool, F.Z.; Giner, P.; O’Dell, L.E.; Mendez, I.A. Exposure to nicotine vapor produced by an electronic nicotine delivery system causes short-term increases in impulsive choice in adult male rats. Nicotine Tob. Res. 2021, 141. [Google Scholar] [CrossRef] [PubMed]
- Ponzoni, L.; Moretti, M.; Sala, M.; Fasoli, F.; Mucchietto, V.; Lucini, V.; Braida, D. Different physiological and behavioural effects of e-cigarette vapour and cigarette smoke in mice. Eur. Neuropsychopharmacol. 2015, 25, 1775–1786. [Google Scholar] [CrossRef]
- Gilpin, N.W.; Whitaker, A.M.; Baynes, B.; Abdel, A.Y.; Weil, M.T.; George, O. Nicotine vapor inhalation escalates nicotine self-administration. Addict. Biol. 2014, 19, 587–592. [Google Scholar] [CrossRef] [Green Version]
- Primack, B.A.; Soneji, S.; Stoolmiller, M.; Fine, M.J.; Sargent, J.D. Progression to Traditional Cigarette Smoking After Electronic Cigarette Use Among US Adolescents and Young Adults. JAMA Pediatr. 2015, 169, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Morean, M.E.; Butler, E.R.; Bold, K.W.; Kong, G.; Camenga, D.R.; Cavallo, D.A.; Krishnan-Sarin, S. Preferring more e-cigarette flavors is associated with e-cigarette use frequency among adolescents but not adults. PLoS ONE 2018, 13, e0189015. [Google Scholar] [CrossRef] [Green Version]
- Etter, J.F.; Zather, E.; Svensson, S. Analysis of refill liquids for electronic cigarettes. Addiction 2013, 108, 1671–1679. [Google Scholar] [CrossRef]
- Henderson, B.J.; Cooper, S.Y. Nicotine formulations impact reinforcement-related behaviors in a mouse model of vapor self-administration. Drug Alcohol Depend. 2021, 224, 108732. [Google Scholar] [CrossRef]
- Geist, C.; Herrmann, S. A comparison of the psychological characteristics of smokers, ex-smokers, and non-smokers. J. Clin. Psychol. 1990, 46, 102–105. [Google Scholar] [CrossRef]
- Sakurai, Y.; Kanazawa, I. Acute effects of cigarettes in non-deprived smokers on memory, calculation and executive functions. Hum. Psychopharmacol. 2002, 17, 369–373. [Google Scholar] [CrossRef]
- Havermans, R.C.; Debaere, S.; Smulders, F.T.; Wiers, R.W.; Jansen, A.T. Effect of cue exposure, urge to smoke, and nicotine deprivation on cognitive performance in smokers. Psychol. Addict. Behav. 2003, 17, 336–339. [Google Scholar] [CrossRef] [Green Version]
- Bickel, W.; Odum, A.; Madden, G. Impulsivity and cigarette smoking: Delay discounting in current, never, and ex-smokers. Psychopharmacology 1999, 146, 447–454. [Google Scholar] [CrossRef]
- Mitchell, S.H. Measures of impulsivity in cigarette smokers and nonsmokers. Psychopharmacology 1999, 146, 455–464. [Google Scholar] [CrossRef]
- Jenks, R. Attitudes, perceptions, and risk-taking behaviors of smokers, ex-smokers, and non-smokers. J. Soc. Psychol. 1992, 132, 569–575. [Google Scholar] [CrossRef]
- Chivers, L.L.; Hand, D.J.; Priest, J.S.; Higgins, S.T. E-cigarette use among women of reproductive age: Impulsivity, cigarette smoking status, and other risk factors. Prev. Med. 2016, 92, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Bold, K.W.; Morean, M.E.; Kong, G.; Simon, P.; Camenga, D.R.; Cavallo, D.A.; Krishnan-Sarin, S. Early age of e-cigarette use onset mediates the association between impulsivity and e-cigarette use frequency in youth. Drug Alcohol Depend. 2017, 181, 46–151. [Google Scholar] [CrossRef]
- Bialaszek, W.; Marcowski, P.; Cox, D.J. Differences in Delay, but not Probability Discounting, in Current Smokers, E-cigarette Users, and Never Smokers. Psychol. Rec. 2017, 67, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Dallery, J.; Locey, M.L. Effects of acute and chronic nicotine on impulsive choice on rats. Behav. Pharm. 2005, 16, 15–23. [Google Scholar] [CrossRef]
- Ibias, J.; Nazarian, A. Sex Differences in nicotine-induced impulsivity and its reversal with bupropion in rats. Psychopharmacology 2020, 34, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Mendez, I.A.; Gilbert, R.J.; Bizon, J.L.; Setlow, B. Effects of acute administration of nicotinic and muscarinic cholinergic agonists and antagonists on performance in different cost-benefit decision-making tasks in rats. Psychopharmacology 2012, 224, 489–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betts, G.D.; Hynes, T.J.; Winstanley, C.A. Pharmacological evidence of a cholinergic contribution to elevated impulsivity and risky decision-making caused by adding win-paired cues to a rat gambling task. J. Psychopharmacol. 2021, 35, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.R.; Vokes, C.M.; Blankenship, A.L.; Simon, N.W.; Setlow, B. Effects of acute administration of nicotine, amphetamine, diazepam, morphine, and ethanol on risky decision-making in rats. Psychopharmacology 2011, 218, 703–712. [Google Scholar] [CrossRef] [Green Version]
- Floresco, S.B.; Onge, J.R.; Ghods-Sharifi, S.; Winstanley, C.A. Cortico-limbic-striatal circuits subserving different forms of cost-benefit decision-making. Cogn. Affect. Behav. Neurosci. 2008, 8, 375–389. [Google Scholar] [CrossRef]
- Gourley, S.L.; Olevska, A.; Zimmermann, K.S.; Ressler, K.J.; Dileone, R.J.; Taylor, J.R. The orbitofrontal cortex regulates outcome-based decision-making via the lateral striatum. Eur. J. Neurosci. 2013, 38, 2382–2388. [Google Scholar] [CrossRef]
- Simon, N.W.; Montgomery, K.S.; Beas, B.S.; Mitchell, M.R.; LaSarge, C.L.; Mendez, I.A.; Setlow, B. Dopaminergic modulation of risky decision-making. J. Neurosci. 2011, 31, 17460–17470. [Google Scholar] [CrossRef] [Green Version]
- Day, J.J.; Jones, J.L.; Wightman, R.M.; Carelli, R.M. Phasic nucleus accumbens dopamine release encodes effort- and delay-related costs. Biol. Psychiatry 2010, 68, 306–309. [Google Scholar] [CrossRef] [Green Version]
- Setlow, B.; Mendez, I.; Mitchell, M.; Simon, N. Effects of chronic administration of drugs of abuse on impulsive choice (delay discounting) in animal models. Behav. Pharmacol. 2009, 20, 380–389. [Google Scholar] [CrossRef] [Green Version]
- Simon, N.W.; Mendez, I.A.; Setlow, B. Cocaine exposure causes long-term increases in impulsive choice. Behav. Neurosci. 2007, 121, 543–549. [Google Scholar] [CrossRef] [Green Version]
- Mark, G.P.; Shabani, S.; Dobbs, L.K.; Hansen, S. Cholinergic modulation of mesolimbic dopamine function and reward. Physiol. Behav. 2011, 104, 76–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendez, I.A.; Damborsky, J.C.; Winzer-Serhan, U.H.; Bizon, J.L.; Setlow, B. α4β2* and α7 nicotinic acetylcholine receptor binding predicts choice preference in two cost benefit decision-making tasks. Neuroscience 2013, 230, 121–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pittaras, E.C.; Faure, A.; Leray, X.; Moraitopoulou, E.; Cressant, A.; Rabat, A.A.; Meunier, C.; Fossier, P.; Granon, S. Neuronal Nicotinic Receptors Are Crucial for Tuning of E/I Balance in Prelimbic Cortex and for Decision-Making Processes. Front. Psychiatry 2016, 7, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doura, M.B.; Gold, A.B.; Keller, A.B.; Perry, D.C. Adult and periadolescent rats differ in expression of nicotinic cholinergic receptor subtypes and in the response of these subtypes to chronic nicotine exposure. Brain Res. 2008, 1215, 40–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.N.; Rasmussen, B.A.; Perry, D.C. Subtype-selective up-regulation by chronic nicotine of high-affinity nicotinic receptors in rat brain demonstrated by receptor autoradiography. J. Pharm. Exp. 2003, 307, 1090–1097. [Google Scholar] [CrossRef] [Green Version]
- Porcelli, A.J.; Delgado, M.R. Stress and decision making: Effects on valuation, learning, and risk-taking. Curr. Opin. Behav. Sci. 2017, 14, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Palamarchouk, V.; Smagin, G.; Goeders, N.E. Self-administered and passive cocaine infusions produce different effects on corticosterone concentrations in the medial prefrontal cortex (mpc) of rats. Pharm. Biochem. Behav. 2009, 94, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Marsot, A.; Simon, N. Nicotine and Cotinine Levels with Electronic Cigarette: A Review. Int. J. Toxicol. 2016, 35, 179–185. [Google Scholar] [CrossRef]
- Vieira-Brock, P.L.; Andrenyak, D.M.; Nielsen, S.M.; Fleckenstein, A.E.; Wilkins, D.G. Age-related differences in the disposition of nicotine and metabolites in rat brain and plasma. Nicotine Tob. Res. 2013, 15, 1839–1848. [Google Scholar] [CrossRef] [Green Version]
- Allen, S.I.; Wasserman, E.; Veldheer, S.; Yingst, J.; Hrabovsky, S.; Liao, J.; Krebs, N.M.; Horn, K.; Reinhart, L.; Modesto, J.; et al. Characteristics of Adult Cigarette Smokers Who “Relight” and the Effects of Exposure to Tobacco Smoke Constituents. Nicotine Tob. Res. 2019, 21, 1206–1212. [Google Scholar] [CrossRef]
- Lallai, V.; Chen, Y.C.; Roybal, M.M.; Kotha, E.R.; Fowler, J.P.; Staben, A.; Cortez, A.; Fowler, C.D. Nicotine e-cigarette vapor inhalation and self-administration in a rodent model: Sex- and nicotine delivery-specific effects of metabolism and behavior. Addict. Biol. 2021, 26, e13024. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.C.; Kallupi, M.; Tieu, L.; Shankar, K.; Jaquish, A.; Barr, J.; George, O. Validation of a nicotine vapor self-administration model in rats with relevance to electronic cigarette use. Neuropsychopharmacology 2020, 45, 1909–1919. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Uribe, K.P.; Garcia, V.; Lira, O.; Matos-Ocasio, F.; Negishi, K.; Khan, A.M.; O’Dell, L.E.; Mendez, I.A. Withdrawal from repeated nicotine vapor exposure increases somatic signs of physical dependence, anxiety-like behavior, and brain reward thresholds in adult male rats. bioRxiv 2022. [Google Scholar] [CrossRef]
- Geste, J.R.; Levin, B.; Wilks, I.; Pompilus, M.; Zhang, X.; Esser, K.A.; Febo, M.; O’Dell, L.; Bruijnzeel, A.W. Relationship between nicotine intake and reward function in rats with intermittent short versus long access to nicotine. Nicotine Tob. Res. 2020, 22, 213–223. [Google Scholar] [CrossRef]
- Hamilton, K.R.; Perry, M.E.; Berger, S.S.; Grunberg, N.E. Behavioral effects of nicotine withdrawal differ by genetic strain in male and female adolescent rats. Nicotine Tob. Res. 2010, 2, 1236–1245. [Google Scholar] [CrossRef] [Green Version]
- Levin, E.D.; Lawrence, S.; Petro, A.; Horton, K.; Rezvani, A.H.; Seidler, F.J.; Slotkin, T.A. Adolescent vs. adult-onset nicotine self-administration in male rats: Duration of effect and differential nicotinic receptor correlates. Neurotoxicol. Teratol. 2007, 29, 458–465. [Google Scholar] [CrossRef] [Green Version]
- Flores, R.J.; Uribe, K.P.; Swalve, N.; O’Dell, L.E. Sex differences in nicotine intravenous self-administration: A meta-analytic review. Physiol. Behav. 2019, 203, 42–50. [Google Scholar] [CrossRef]
- Park, H.R.; Vallarino, J.; O’Sullivan, M.O.; Wirth, C.; Panganiban, R.A.; Webb, G.; Shumyatcher, M.; Himes, B.E.; Park, J.A.; Christiani, D.C.; et al. Electronic cigarette smoke reduces ribosomal protein gene expression to impair protein synthesis in primary human airway epithelial cells. Sci. Rep. 2021, 11, 17517. [Google Scholar] [CrossRef]
- Tommasi, S.; Pabustan, N.; Li, M.; Chen, Y.; Siegmund, K.D.; Besaratinia, A. A novel role for vaping in mitochondrial gene dysregulation and inflammation fundamental to disease development. Sci. Rep. 2021, 11, 22773. [Google Scholar] [CrossRef]
- Baldassarri, S.R.; Hillmer, A.T.; Anderson, J.M.; Jatlow, P.; Nabulsi, N.; Labaree, D.; Cosgrove, K.P.; O’Malley, S.S.; Eissenberg, T.; Krishanan-Sarin, S.; et al. Use of electronic cigarettes leads to significant beta2-nicotinic receptor occupancy: Evidence from a PET imagining study. Nicotine Tob. Res. 2018, 20, 425–433. [Google Scholar] [CrossRef]
- Govind, A.P.; Vezina, P.; Green, W.N. Nicotine-induced upregulation of nicotinic receptors: Underlying mechanisms and relevance to nicotine addiction. Biochem. Pharm. 2009, 78, 756–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Counotte, D.S.; Goriounova, N.A.; Moretti, M.; Smoluch, M.T.; Irth, H.; Clementi, F.; Schoffelmeer, A.N.M.; Mansvelder, H.D.; Smit, A.B.; Gotti, C.; et al. Adolescent nicotine exposure transiently increases high-affinity nicotinic receptors and modulates inhibitory synaptic transmission in rat medial prefrontal cortex. FASEB 2012, 26, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Abreu-Villaça, Y.; Seidler, F.J.; Qiao, D.; Tate, C.A.; Cousins, M.M.; Thillai, I.; Slotkin, T.A. Short-term adolescent nicotine exposure has immediate and persistent effects on cholinergic systems: Critical periods, patterns of exposure, dose thresholds. Neuropsychopharmacology 2003, 28, 1935–1949. [Google Scholar] [CrossRef] [PubMed]
- Alasmari, F.; Alexander, L.E.C.; Nelson, J.A.; Schiefer, I.T.; Breen, E.; Drummond, C.A.; Sari, Y. Effects of chronic inhalation of electronic cigarettes containing nicotine on glial glutamate transporters and α-7 nicotine acetylcholine receptor in female CD-1 mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 77, 1–8. [Google Scholar] [CrossRef]
- Huang, L.Z.; Winzer-Serhan, U.H. Chronic neonatal nicotine upregulates heteromeric nicotinic acetylcholine receptor binding without change in subunit mRNA expression. Brain Res. 2006, 1113, 94–109. [Google Scholar] [CrossRef]
- Marks, M.J.; Pauly, J.R.; Gross, S.D.; Deneris, E.S.; Hermans-Borgmeyer, I.; Heinemann, S.F.; Collins, A.C. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J. Neurosci. 1992, 12, 2765–2784. [Google Scholar] [CrossRef] [Green Version]
- Moretti, M.; Fasoli, F.; Gotti, C.; Marks, M.J. Reduced α4 subunit expression in α4+− and α4+−/β2+− nicotinic acetylcholine receptors alters α4β2 subtype up-regulation following chronic nicotine treatment. Br. J. Pharm. 2018, 175, 1944–1956. [Google Scholar] [CrossRef] [Green Version]
- Lv, J.; Mao, C.; Zhu, L.; Zhang, H.; Pengpeng, H.; Xu, F.; Liu, Y.; Zhang, L.; Xu, Z. The effect of prenatal nicotine on expression of nicotine receptor subunits in the fetal brain. Neurotoxicology 2008, 29, 722–726. [Google Scholar] [CrossRef] [Green Version]
- Gabriel, D.B.K.; Liley, A.E.; Freels, T.G.; Simon, N.W. Dopamine receptors regulate preference between high-effort and high-risk rewards. Psychopharmacology 2021, 238, 991–1004. [Google Scholar] [CrossRef]
- Onge, J.R.S.; Ahn, S.; Phillips, A.G.; Floresco, S.B. Dynamic fluctuations in dopamine efflux in the prefrontal cortex and nucleus accumbens during risk-based decision-making. J. Neurosci. 2012, 32, 16880–16891. [Google Scholar] [CrossRef] [Green Version]
- Romano, E.; De Angelis, F.; Ulbrich, L.; De Jaco, A.; Fuso, A.; Laviola, G. Nicotine exposure during adolescence: Cognitive performance and brain gene expression in adult heterozygous reeler mice. Psychopharmacology 2014, 231, 1775–1787. [Google Scholar] [CrossRef] [PubMed]
- Faure, A.; Zoratto, F.; Chirico, D.; Romano, E.; Mancinelli, R.; Saso, L.; Callebert, J.; Laviola, G.; Granon, S.; Adriani, W. Reduced adolescent risk-assessment and lower nicotinic beta-2 expression in rats exposed to nicotine through lactation by forcedly drinking dams. Neuroscience 2019, 413, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Gozen, O.; Nesil, T.; Kanit, L.; Koylu, E.O.; Pogun, S. Nicotinic cholinergic and dopaminergic receptor mRNA expression in male and female rats with high or low preference for nicotine. Am. J. Drug Alcohol Abus. 2016, 42, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Orejarena, M.J.; Herrera-Solís, A.; Pons, S.; Maskos, U.; Maldonado, R.; Robledo, P. Selective re-expression of β2 nicotinic acetylcholine receptor subunits in the ventral tegmental area of the mouse restores intravenous nicotine self-administration. Neuropharmacology 2012, 63, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Simmons, S.J.; Gould, T.J. Involvement of neuronal β2 subunit-containing nicotinic acetylcholine receptors in nicotine reward and withdrawal: Implications for pharmacotherapies. J. Clin. Pharm. 2014, 39, 457–467. [Google Scholar] [CrossRef] [Green Version]
- Qasim, H.; Karim, Z.A.; Silva-Espinoza, J.C.; Khasawneh, F.T.; Rivera, J.O.; Ellis, C.C.; Alshbool, F.Z. Short-Term E-Cigarette Exposure Increases the Risk of Thrombogenesis and Enhances Platelet Function in Mice. J. Am. Heart Assoc. 2018, 7, e009264. [Google Scholar] [CrossRef] [Green Version]
- Radonić, A.; Thulke, S.; Mackay, I.M.; Landt, O.; Siegert, W.; Nitsche, A. Guideline to reference gene selection for quantitative real-time PCR. Biochem. Biophys Res. 2004, 313, 856–862. [Google Scholar] [CrossRef]
- Huang, J.; Feng, B.; Weaver, S.R.; Pechacek, T.F.; Slovic, P.; Eriksen, M.P. Changing Perceptions of Harm of e-Cigarette vs Cigarette Use Among Adults in 2 US National Surveys From 2012 to 2017. JAMA 2019, 2, e191047. [Google Scholar] [CrossRef] [Green Version]

| Gene | Forward Primer Sequence (5’ to 3’) | Reverse Primer Sequence (3’ to 5’) |
|---|---|---|
| * GAPDH | CAA CTC CCT CAA GAT TGT CAG CAA | GGC ATG GAC TGT GGT CAT GA |
| CHRNA4 | CAA CTT TCT GCA ACC CCA CG | TGG CAA CGT ATT TCC AGT CC |
| CHRNB2 | AAG TGA GCG ACA CTG GTC TG | GTC TGG AGC CCT CTG AGG TA |
| DRD1 | GAC ACA AGG TTG AGC A | CTG GGC AAT CCT GTA GAT A |
| DRD2 | GTG TGT TCA TCA TCT GCT | GAA CTC GAT GTT GAA GGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giner, P.; Maynez-Anchondo, L.; Liley, A.E.; Uribe, K.P.; Frietze, G.A.; Simon, N.W.; Mendez, I.A. Increased Risky Choice and Reduced CHRNB2 Expression in Adult Male Rats Exposed to Nicotine Vapor. Int. J. Mol. Sci. 2022, 23, 1231. https://doi.org/10.3390/ijms23031231
Giner P, Maynez-Anchondo L, Liley AE, Uribe KP, Frietze GA, Simon NW, Mendez IA. Increased Risky Choice and Reduced CHRNB2 Expression in Adult Male Rats Exposed to Nicotine Vapor. International Journal of Molecular Sciences. 2022; 23(3):1231. https://doi.org/10.3390/ijms23031231
Chicago/Turabian StyleGiner, Priscilla, Liliana Maynez-Anchondo, Anna E. Liley, Kevin P. Uribe, Gabriel A. Frietze, Nicholas W. Simon, and Ian A. Mendez. 2022. "Increased Risky Choice and Reduced CHRNB2 Expression in Adult Male Rats Exposed to Nicotine Vapor" International Journal of Molecular Sciences 23, no. 3: 1231. https://doi.org/10.3390/ijms23031231
APA StyleGiner, P., Maynez-Anchondo, L., Liley, A. E., Uribe, K. P., Frietze, G. A., Simon, N. W., & Mendez, I. A. (2022). Increased Risky Choice and Reduced CHRNB2 Expression in Adult Male Rats Exposed to Nicotine Vapor. International Journal of Molecular Sciences, 23(3), 1231. https://doi.org/10.3390/ijms23031231







