Bee Venom Activates the Nrf2/HO-1 and TrkB/CREB/BDNF Pathways in Neuronal Cell Responses against Oxidative Stress Induced by Aβ1–42
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Cell Culturing
2.3. Cell Viability Assay and Drug Administration
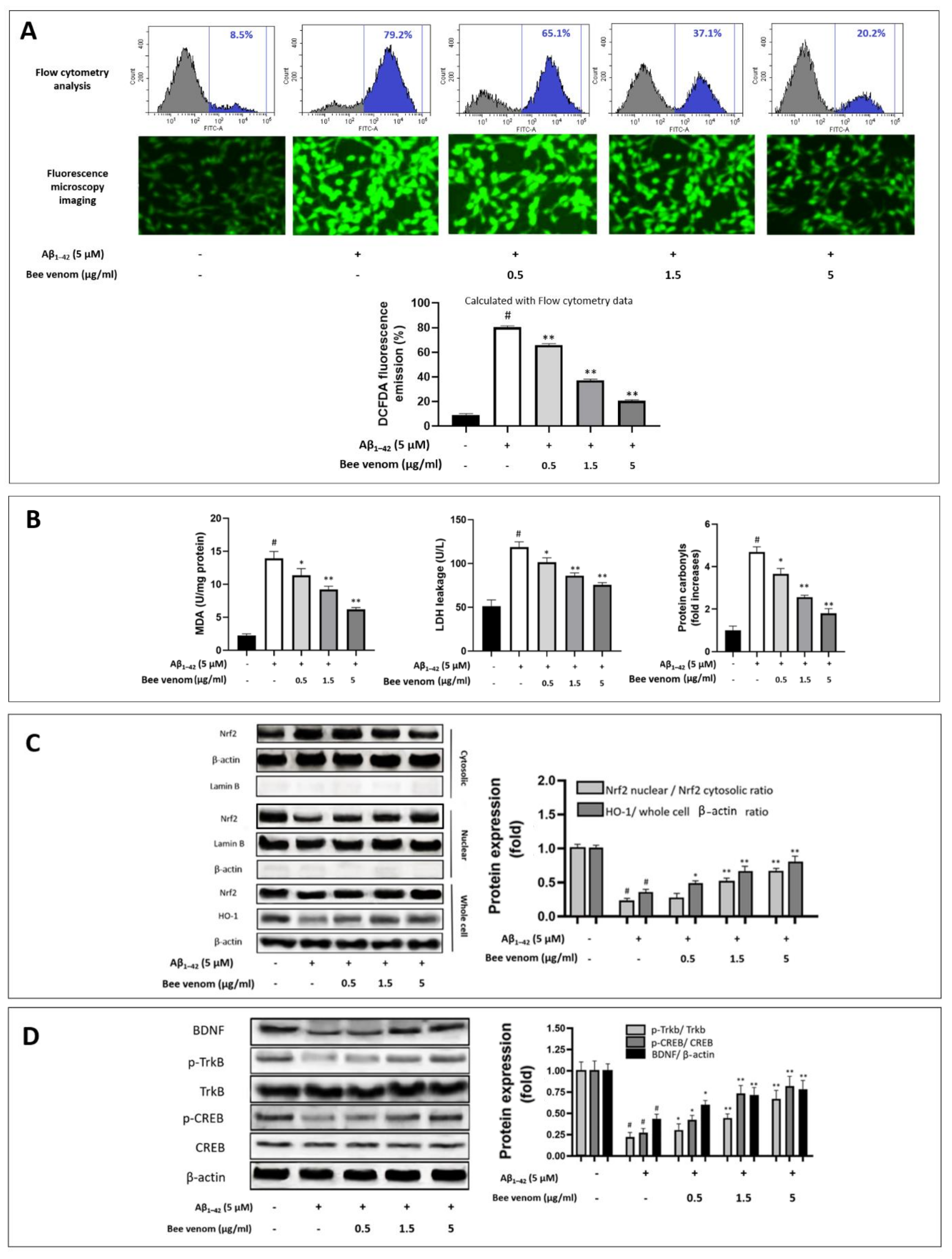
2.4. Cell Apoptosis and ROS Levels Assessment by Flow Cytometry
2.5. Measurements of In Vitro Cell Apoptosis, Cell ROS, SOD, MDA, LDH, and Protein Carbonyl
2.6. Preparation of Whole-Cell, Cytosolic, and Nuclear Proteins
2.7. Animals
2.8. In Vivo Drug Administration
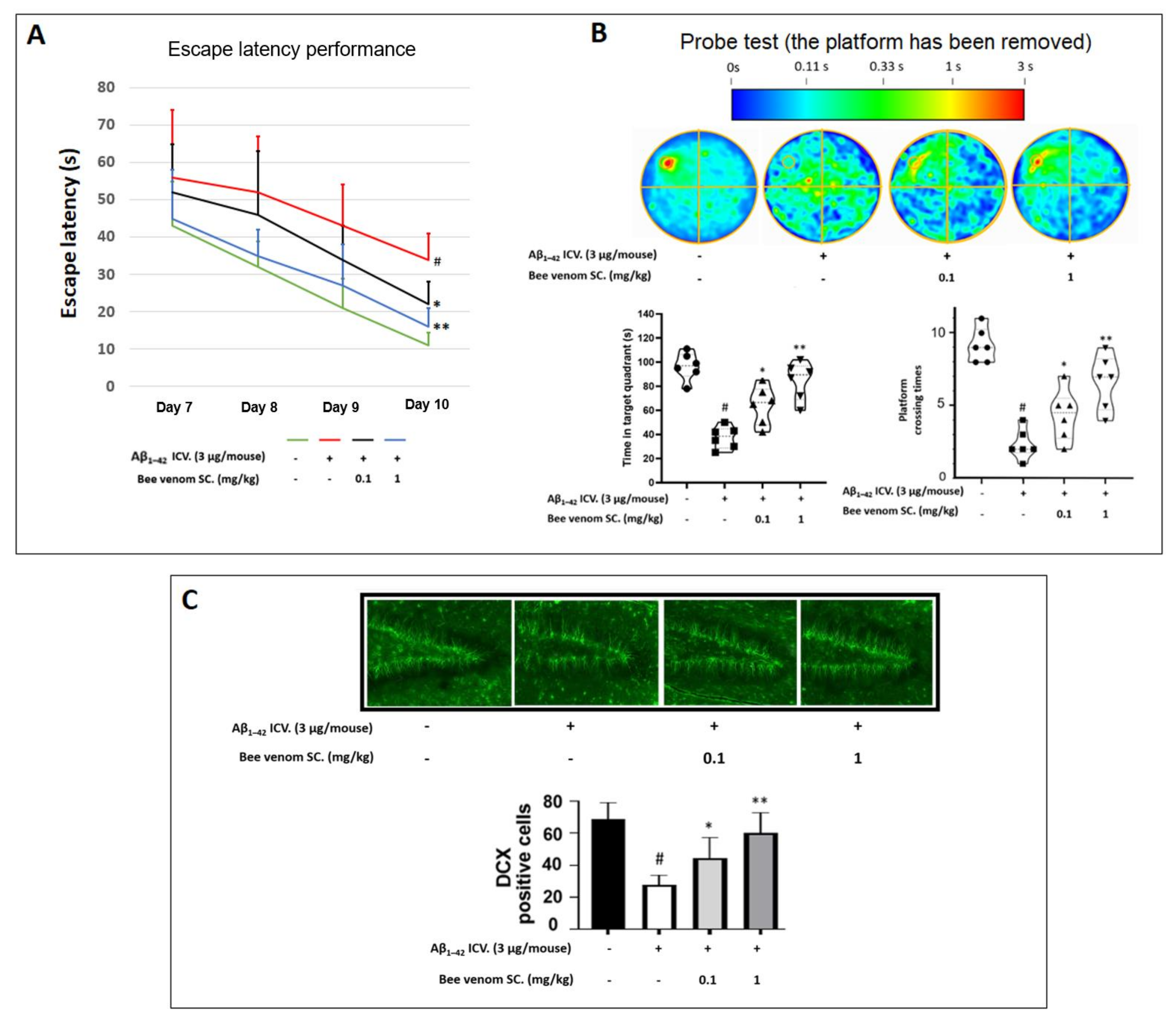
2.9. Morris Water Maze (MWM)
2.10. Collection of Animals Hippocampus and Blood Samples
2.11. Doublecortin Immunofluorescence Staining
2.12. Western Blot Analysis
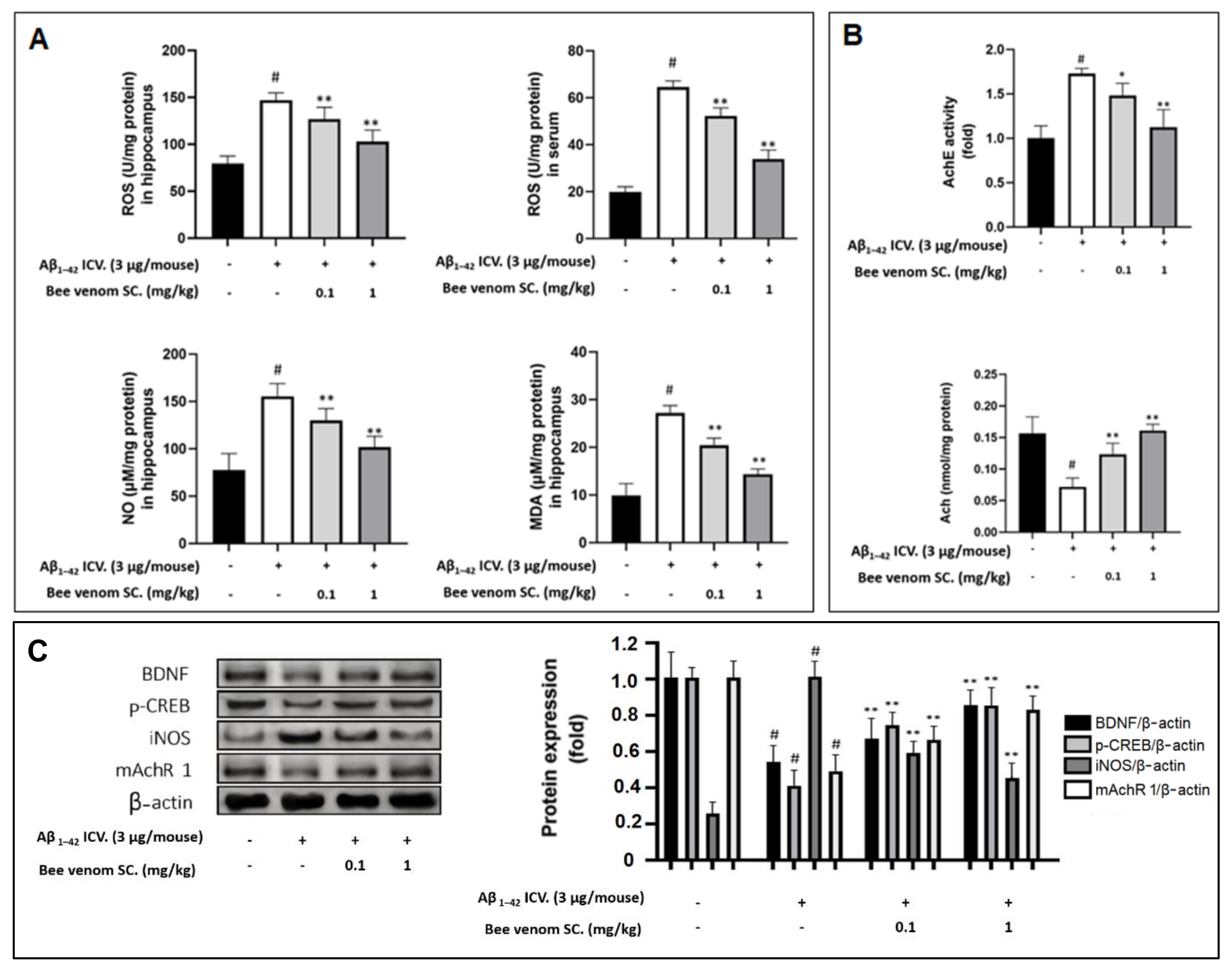
2.13. Measurements of ROS, MDA, NO, AchE, and Ach Levels in In Vivo Samples
2.14. Statistical Analysis
3. Results
3.1. Bee Venom Exhibits Protective Effects on HT22 Cells against Cytotoxicity Induced by Aβ1–42
3.2. Bee Venom Reduces Oxidative Stress Expressions of HT22 Cells during Aβ1–42 Challenge
3.3. Bee Venom Induces Intrinsic Antioxidant Regulation Nrf2/HO-1 of HT22 Cells against Aβ1–42-Induced Oxidative Stress Challenge
3.4. Effect of Bee Venom on the TrkB/CREB/BDNF Pathway
3.5. Bee Venom-Enhanced Learning and Memory Function of Aβ1–42-Induced Cognitive Impairment in Mice Behavior Experiment
3.6. In Vivo Immunohistochemistry Study
3.7. Oxidative and Inflammation Markers in Mouse Hippocampus and Serum
3.8. Study on In Vivo Cholinergic System
3.9. Effect of Bee Venom Treatment on the Expressions of Key Marker Proteins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sun, D.-Z.; Li, S.-D.; Liu, Y.; Zhang, Y.; Mei, R.; Yang, M.-H. Differences in the origin of philosophy between Chinese medicine and western medicine: Exploration of the holistic advantages of Chinese medicine. Chin. J. Integr. Med. 2013, 19, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Ghiso, J.; Frangione, B. Amyloidosis and Alzheimer’s disease. Adv. Drug Deliv. Rev. 2002, 54, 1539–1551. [Google Scholar] [CrossRef]
- García-González, L.; Pilat, D.; Baranger, K.; Rivera, S. Emerging Alternative Proteinases in APP Metabolism and Alzheimer’s Disease Pathogenesis: A Focus on MT1-MMP and MT5-MMP. Front. Aging Neurosci. 2019, 11, 244. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, C.P.; Glass, C.A.; Montgomery, M.B.; Lindl, K.A.; Ritson, G.P.; Chia, L.A.; Hamilton, R.L.; Chu, C.T.; Jordan-Sciutto, K.L. Expression of Nrf2 in Neurodegenerative Diseases. J. Neuropathol. Exp. Neurol. 2007, 66, 75–85. [Google Scholar] [CrossRef]
- Osama, A.; Zhang, J.; Yao, J.; Yao, X.; Fang, J. Nrf2: A dark horse in Alzheimer’s disease treatment. Ageing Res. Rev. 2020, 64, 101206. [Google Scholar] [CrossRef]
- Hannan, M.A.; Dash, R.; Sohag, A.A.M.; Haque, M.N.; Moon, I.S. Neuroprotection Against Oxidative Stress: Phytochemicals Targeting TrkB Signaling and the Nrf2-ARE Antioxidant System. Front. Mol. Neurosci. 2020, 13, 116. [Google Scholar] [CrossRef]
- Sivandzade, F.; Bhalerao, A.; Cucullo, L. Cerebrovascular and Neurological Disorders: Protective Role of NRF2. Int. J. Mol. Sci. 2019, 20, 3433. [Google Scholar] [CrossRef]
- Von Arnim, C.A.; Herbolsheimer, F.; Nikolaus, T.; Peter, R.; Biesalski, H.K.; Ludolph, A.C.; Riepe, M.; Nagel, G. Dietary Antioxidants and Dementia in a Population-Based Case-Control Study among Older People in South Germany. J. Alzheimer’s Dis. 2012, 31, 717–724. [Google Scholar] [CrossRef]
- Yu, W.; Ma, M.; Chen, X.; Min, J.; Li, L.; Zheng, Y.; Li, Y.; Wang, J.; Wang, Q. Traditional Chinese Medicine and Constitutional Medicine in China, Japan and Korea: A Comparative Study. Am. J. Chin. Med. 2017, 45, 1–12. [Google Scholar] [CrossRef]
- Hwang, D.-S.; Kim, S.K.; Bae, H. Therapeutic Effects of Bee Venom on Immunological and Neurological Diseases. Toxins 2015, 7, 2413–2421. [Google Scholar] [CrossRef]
- Bobba, A.; Petragallo, V.A.; Marra, E.; Atlante, A. Alzheimer’s Proteins, Oxidative Stress, and Mitochondrial Dysfunction Interplay in a Neuronal Model of Alzheimer’s Disease. Int. J. Alzheimers Dis. 2010, 2010, 621870. [Google Scholar] [CrossRef][Green Version]
- Butterfield, D.A.; Swomley, A.M.; Sultana, R. Amyloid β-Peptide (1–42)-Induced Oxidative Stress in Alzheimer Disease: Importance in Disease Pathogenesis and Progression. Antioxid. Redox Signal. 2013, 19, 823–835. [Google Scholar] [CrossRef]
- Kasza, Á.; Penke, B.; Frank, Z.; Bozsó, Z.; Szegedi, V.; Hunya, Á.; Németh, K.; Kozma, G.; Fülöp, L. Studies for Improving a Rat Model of Alzheimer’s Disease: Icv Administration of Well-Characterized β-Amyloid 1-42 Oligomers Induce Dysfunction in Spatial Memory. Molecules 2017, 22, 2007. [Google Scholar] [CrossRef]
- Sachdeva, A.K.; Chopra, K. Lycopene abrogates Aβ(1–42)-mediated neuroinflammatory cascade in an experimental model of Alzheimer’s disease. J. Nutr. Biochem. 2015, 26, 736–744. [Google Scholar] [CrossRef]
- Shin, E.-J.; Lee, S.H.; Sharma, N.; Nguyen, B.T.; Chung, Y.H.; Kang, S.W.; Nah, S.-Y.; Lee, Y.J.; Nabeshima, T.; Jeong, J.H.; et al. An adenoviral vector encoded with the GPx-1 gene attenuates memory impairments induced by β-amyloid (1–42) in GPx-1 KO mice via activation of M1 mAChR-mediated signalling. Free. Radic. Res. 2021, 55, 11–25. [Google Scholar] [CrossRef]
- Li, P.-P.; Wang, W.-P.; Liu, Z.-H.; Xu, S.-F.; Lu, W.-W.; Wang, L.; Wang, X.-L. Potassium 2-(1-hydroxypentyl)-benzoate promotes long-term potentiation in Aβ1–42-injected rats and APP/PS1 transgenic mice. Acta Pharmacol. Sin. 2014, 35, 869–878. [Google Scholar] [CrossRef]
- Amin, F.U.; Shah, S.A.; Kim, M.O. Vanillic acid attenuates Aβ1-42-induced oxidative stress and cognitive impairment in mice. Sci. Rep. 2017, 7, 40753. [Google Scholar] [CrossRef]
- Han, R.-W.; Zhang, R.-S.; Xu, H.-J.; Chang, M.; Peng, Y.-L.; Wang, R. Neuropeptide S enhances memory and mitigates memory impairment induced by MK801, scopolamine or Aβ1–42 in mice novel object and object location recognition tasks. Neuropharmacology 2013, 70, 261–267. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.H.; Arce, F.T.; Gillman, A.L.; Jang, H.; Kagan, B.L.; Nussinov, R.; Yang, J.; Lal, R. Amyloid β Ion Channels in a Membrane Comprising Brain Total Lipid Extracts. ACS Chem. Neurosci. 2017, 8, 1348–1357. [Google Scholar] [CrossRef]
- Zaretsky, D.V.; Zaretskaia, M.V. Flow cytometry method to quantify the formation of beta-amyloid membrane ion channels. Biochim. Biophys. Acta BBA Biomembr. 2021, 1863, 183506. [Google Scholar] [CrossRef]
- Ekinci, F.J.; Linsley, M.-D.; Shea, T.B. β-Amyloid-induced calcium influx induces apoptosis in culture by oxidative stress rather than tau phosphorylation. Mol. Brain Res. 2000, 76, 389–395. [Google Scholar] [CrossRef]
- Lahmy, V.; Long, R.; Morin, D.; Villard, V.; Maurice, T. Mitochondrial protection by the mixed muscarinic/σ1 ligand ANAVEX2-73, a tetrahydrofuran derivative, in Aβ25–35 peptide-injected mice, a nontransgenic Alzheimer’s disease model. Front. Cell. Neurosci. 2015, 8, 463. [Google Scholar] [CrossRef]
- Luque-Contreras, D.; Carvajal, K.; Toral-Rios, D.; Franco-Bocanegra, D.; Campos-Peña, V. Oxidative Stress and Metabolic Syndrome: Cause or Consequence of Alzheimer’s Disease? Oxid. Med. Cell. Longev. 2014, 2014, 497802. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- He, J.; Zhu, G.; Wang, G.; Zhang, F. Oxidative Stress and Neuroinflammation Potentiate Each Other to Promote Progression of Dopamine Neurodegeneration. Oxid. Med. Cell. Longev. 2020, 2020, 6137521. [Google Scholar] [CrossRef]
- Fedorova, M.; Bollineni, R.C.; Hoffmann, R. Protein carbonylation as a major hallmark of oxidative damage: Update of analytical strategies. Mass Spectrom. Rev. 2014, 33, 79–97. [Google Scholar] [CrossRef]
- Hung, C.H.-L.; Cheng, S.S.-Y.; Cheung, Y.-T.; Wuwongse, S.; Zhang, N.Q.; Ho, Y.-S.; Lee, S.M.-Y.; Chang, R.C.-C. A reciprocal relationship between reactive oxygen species and mitochondrial dynamics in neurodegeneration. Redox Biol. 2018, 14, 7–19. [Google Scholar] [CrossRef]
- Zhang, W.; Song, J.-k.; Yan, R.; Li, L.; Xiao, Z.-y.; Zhou, W.-x.; Wang, Z.-z.; Xiao, W.; Du, G.-h. Diterpene ginkgolides protect against cerebral ischemia/reperfusion damage in rats by activating Nrf2 and CREB through PI3K/Akt signaling. Acta Pharm. Sin. 2018, 39, 1259–1272. [Google Scholar] [CrossRef]
- Tu, W.; Wang, H.; Li, S.; Liu, Q.; Sha, H. The Anti-Inflammatory and Anti-Oxidant Mechanisms of the Keap1/Nrf2/ARE Signaling Pathway in Chronic Diseases. Aging Dis. 2019, 10, 637–651. [Google Scholar] [CrossRef]
- Jurcau, A. The Role of Natural Antioxidants in the Prevention of Dementia—Where Do We Stand and Future Perspectives. Nutrients 2021, 13, 282. [Google Scholar] [CrossRef]
- Solleiro-Villavicencio, H.; Rivas-Arancibia, S. Effect of Chronic Oxidative Stress on Neuroinflammatory Response Mediated by CD4+T Cells in Neurodegenerative Diseases. Front. Cell. Neurosci. 2018, 12, 114. [Google Scholar] [CrossRef]
- Victor, P.; Sarada, D.; Ramkumar, K.M. Pharmacological activation of Nrf2 promotes wound healing. Eur. J. Pharmacol. 2020, 886, 173395. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, J.; Xiao, A.; Liu, W.; Shang, Y.; An, J. Melittin ameliorates CVB3-induced myocarditis via activation of the HDAC2-mediated GSK-3β/Nrf2/ARE signaling pathway. Biochem. Biophys. Res. Commun. 2016, 480, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Leem, J.; Hong, H.-L. Melittin Ameliorates Endotoxin-Induced Acute Kidney Injury by Inhibiting Inflammation, Oxidative Stress, and Cell Death in Mice. Oxidative Med. Cell. Longev. 2021, 2021, 8843051. [Google Scholar] [CrossRef]
- El-Haleim, E.A.A. Molecular Study on the Potential Protective Effects of Bee Venom against Fructose-Induced Nonalcoholic Steatohepatitis in Rats. Pharmacology 2020, 105, 692–704. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Komosinska-Vassev, K.; Rzepecka-Stojko, A.; Olczyk, P. Bee Venom in Wound Healing. Molecules 2020, 26, 148. [Google Scholar] [CrossRef]
- Hanafi, M.Y.; Zaher, E.L.; El-Adely, S.E.; Sakr, A.; Ghobashi, A.H.; Hemly, M.H.; Kazem, A.H.; Kamel, M.A. The therapeutic effects of bee venom on some metabolic and antioxidant parameters associated with HFD-induced non-alcoholic fatty liver in rats. Exp. Ther. Med. 2018, 15, 5091–5099. [Google Scholar] [CrossRef]
- Wisgrill, L.; Lamm, C.; Hartmann, J.; Preißing, F.; Dragosits, K.; Bee, A.; Hell, L.; Thaler, J.; Ay, C.; Pabinger, I.; et al. Peripheral blood microvesicles secretion is influenced by storage time, temperature, and anticoagulants. Cytom. Part A 2016, 89, 663–672. [Google Scholar] [CrossRef]
- Yoo, J.-M.; Lee, B.D.; Sok, D.-E.; Ma, J.Y.; Kim, M.R. Neuroprotective action of N-acetyl serotonin in oxidative stress-induced apoptosis through the activation of both TrkB/CREB/BDNF pathway and Akt/Nrf2/Antioxidant enzyme in neuronal cells. Redox Biol. 2017, 11, 592–599. [Google Scholar] [CrossRef]
- Hu, D.; Cao, Y.; He, R.-R.; Han, N.; Liu, Z.; Miao, L.; Yin, J. Schizandrin, an Antioxidant Lignan from Schisandra chinensis, Ameliorates Aβ1–42-Induced Memory Impairment in Mice. Oxid. Med. Cell. Longev. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Furukawa-Hibi, Y.; Alkam, T.; Nitta, A.; Matsuyama, A.; Mizoguchi, H.; Suzuki, K.; Moussaoui, S.; Yu, Q.-S.; Greig, N.H.; Nagai, T.; et al. Butyrylcholinesterase inhibitors ameliorate cognitive dysfunction induced by amyloid-β peptide in mice. Behav. Brain Res. 2011, 225, 222–229. [Google Scholar] [CrossRef]
- Fang, F.; Liu, G.-T.; Liu, F.F.-T. Protective effects of compound FLZ on beta-amyloid peptide-(25-35)-induced mouse hippocampal injury and learning and memory impairment1. Acta Pharmacol. Sin. 2006, 27, 651–658. [Google Scholar] [CrossRef]
- Lashein, F.E.-D.M.; Abu Amra, E.-S.; Seleem, A.A.; Badr, A.H. Ameliorative effect of bee venom and its extracted bradykinin-potentiating factor on neurological alteration induced by acrylamide and chips administration. J. Basic Appl. Zool. 2018, 79, 35. [Google Scholar] [CrossRef]
- Cai, M.; Lee, J.H.; Yang, E.J. Bee Venom Ameliorates Cognitive Dysfunction Caused by Neuroinflammation in an Animal Model of Vascular Dementia. Mol. Neurobiol. 2017, 54, 5952–5960. [Google Scholar] [CrossRef]
- Wang, L.; Yang, N.-N.; Shi, G.-X.; Wang, L.-Q.; Li, Q.-Q.; Yang, J.-W.; Liu, C.-Z. Acupuncture Attenuates Blood Pressure via Inducing the Expression of nNOS. Evid. Based Complement. Altern. Med. 2021, 2021, 9945277. [Google Scholar] [CrossRef]
- Li, S.-P.; Wang, Y.-W.; Qi, S.-L.; Zhang, Y.-P.; Deng, G.; Ding, W.-Z.; Ma, C.; Lin, Q.-Y.; Guan, H.-D.; Liu, W.; et al. Analogous β-Carboline Alkaloids Harmaline and Harmine Ameliorate Scopolamine-Induced Cognition Dysfunction by Attenuating Acetylcholinesterase Activity, Oxidative Stress, and Inflammation in Mice. Front. Pharmacol. 2018, 9, 346. [Google Scholar] [CrossRef]
- Okamoto, S.; Yamauchi, K.; Sohn, J.; Takahashi, M.; Ishida, Y.; Furuta, T.; Koike, M.; Fujiyama, F.; Hioki, H. Exclusive labeling of direct and indirect pathway neurons in the mouse neostriatum by an adeno-associated virus vector with Cre/lox system. STAR Protoc. 2020, 2, 100230. [Google Scholar] [CrossRef]
- Gu, S.M.; Park, M.H.; Hwang, C.J.; Song, H.S.; Lee, U.S.; Han, S.B.; Oh, K.W.; Ham, Y.W.; Song, M.J.; Son, D.J.; et al. Bee venom ameliorates lipopolysaccharide-induced memory loss by preventing NF-kappaB pathway. J. Neuroinflamm. 2015, 12, 1–15. [Google Scholar] [CrossRef]
- Lee, B.D.; Yoo, J.-M.; Baek, S.Y.; Li, F.Y.; Sok, D.-E.; Kim, M.R. 3,3′-Diindolylmethane Promotes BDNF and Antioxidant Enzyme Formation via TrkB/Akt Pathway Activation for Neuroprotection against Oxidative Stress-Induced Apoptosis in Hippocampal Neuronal Cells. Antioxidants 2019, 9, 3. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Giustarini, D.; Colombo, R.; Rossi, R.; Milzani, A. Protein carbonylation in human diseases. Trends Mol. Med. 2003, 9, 169–176. [Google Scholar] [CrossRef]
- Hashimoto, K. Essential Role of Keap1-Nrf2 Signaling in Mood Disorders: Overview and Future Perspective. Front. Pharmacol. 2018, 9, 1182. [Google Scholar] [CrossRef]
- Bernier, B.E.; Lacagnina, A.F.; Ayoub, A.; Shue, F.; Zemelman, B.V.; Krasne, F.B.; Drew, M.R. Dentate Gyrus Contributes to Retrieval as well as Encoding: Evidence from Context Fear Conditioning, Recall, and Extinction. J. Neurosci. 2017, 37, 6359–6371. [Google Scholar] [CrossRef]
- Francis, P.T. The Interplay of Neurotransmitters in Alzheimer’s Disease. CNS Spectr. 2005, 10, 6–9. [Google Scholar] [CrossRef]
- Hu, W.; Gray, N.W.; Brimijoin, S. Amyloid-beta increases acetylcholinesterase expression in neuroblastoma cells by reducing enzyme degradation. J. Neurochem. 2003, 86, 470–478. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Kim, H.J.; Baek, S.Y.; Sok, D.-E.; Lee, K.J.; Kim, Y.-J.; Kim, M.R. Neuroprotective Activity of Polyphenol-Rich Ribes diacanthum Pall against Oxidative Stress in Glutamate-Stimulated HT-22 Cells and a Scopolamine-Induced Amnesia Animal Model. Antioxidants 2020, 9, 895. [Google Scholar] [CrossRef]
- Jin, W. Regulation of BDNF-TrkB Signaling and Potential Therapeutic Strategies for Parkinson’s Disease. J. Clin. Med. 2020, 9, 257. [Google Scholar] [CrossRef]
- Baek, S.Y.; Kim, M.R. Neuroprotective Effect of Carotenoid-Rich Enteromorpha prolifera Extract via TrkB/Akt Pathway against Oxidative Stress in Hippocampal Neuronal Cells. Mar. Drugs 2020, 18, 372. [Google Scholar] [CrossRef]
- Chen, N.; Wang, J.; He, Y.; Xu, Y.; Zhang, Y.; Gong, Q.; Yu, C.; Gao, J. Trilobatin Protects Against Aβ25–35-Induced Hippocampal HT22 Cells Apoptosis Through Mediating ROS/p38/Caspase 3-Dependent Pathway. Front. Pharmacol. 2020, 11, 584. [Google Scholar] [CrossRef]
- Ishii, T.; Warabi, E.; Mann, G.E. Circadian control of BDNF-mediated Nrf2 activation in astrocytes protects dopaminergic neurons from ferroptosis. Free Radic. Biol. Med. 2019, 133, 169–178. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, B.; Chen, X.; Zhang, N.; Li, G.; Zhang, L.H.; Tan, L.Y. Naringenin Ameliorates Behavioral Dysfunction and Neurological Deficits in a d-Galactose-Induced Aging Mouse Model Through Activation of PI3K/Akt/Nrf2 Pathway. Rejuvenation Res. 2017, 20, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, J.; Lazarovici, P.; Quirion, R.; Zheng, W. cAMP Response Element-Binding Protein (CREB): A Possible Signaling Molecule Link in the Pathophysiology of Schizophrenia. Front. Mol. Neurosci. 2018, 11, 255. [Google Scholar] [CrossRef] [PubMed]
- Bartkowska, K.; Paquin, A.; Gauthier, A.S.; Kaplan, D.R.; Miller, F.D. Trk signaling regulates neural precursor cell proliferation and differentiation during cortical development. Development 2007, 134, 4369–4380. [Google Scholar] [CrossRef] [PubMed]
- Thiele, C.J.; Li, Z.; McKee, A.E. On Trk—The TrkB signal transduction pathway is an increasingly important target in cancer biology. Clin. Cancer Res. 2009, 15, 5962–5967. [Google Scholar] [CrossRef] [PubMed]
- Vorhees, C.V.; Williams, M. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef]
- Ledo, A.; Frade, J.G.; Barbosa, R.; Laranjinha, J. Nitric oxide in brain: Diffusion, targets and concentration dynamics in hippocampal subregions. Mol. Asp. Med. 2004, 25, 75–89. [Google Scholar] [CrossRef]
- Ledo, A.; Lourenço, C.; Cadenas, E.; Barbosa, R.; Laranjinha, J. The bioactivity of neuronal-derived nitric oxide in aging and neurodegeneration: Switching signaling to degeneration. Free Radic. Biol. Med. 2021, 162, 500–513. [Google Scholar] [CrossRef]
- Lee, J.-S.; Kim, H.-G.; Lee, H.W.; Han, J.-M.; Lee, S.-K.; Kim, D.W.; Saravanakumar, A.; Son, C.-G. Hippocampal memory enhancing activity of pine needle extract against scopolamine-induced amnesia in a mouse model. Sci. Rep. 2015, 5, 9651. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, R.; Zhang, Y.; Xia, Z.; Hu, Y. Role of CREB in the regulatory action of sarsasapogenin on muscarinic M1receptor density during cell aging. FEBS Lett. 2010, 584, 1549–1552. [Google Scholar] [CrossRef]
- Bramham, C.R.; Messaoudi, E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog. Neurobiol. 2005, 76, 99–125. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, C.D.; Yoo, J.; Hwang, S.-Y.; Cho, S.-Y.; Kim, M.; Jang, H.; No, K.O.; Shin, J.C.; Kim, J.-H.; Lee, G. Bee Venom Activates the Nrf2/HO-1 and TrkB/CREB/BDNF Pathways in Neuronal Cell Responses against Oxidative Stress Induced by Aβ1–42. Int. J. Mol. Sci. 2022, 23, 1193. https://doi.org/10.3390/ijms23031193
Nguyen CD, Yoo J, Hwang S-Y, Cho S-Y, Kim M, Jang H, No KO, Shin JC, Kim J-H, Lee G. Bee Venom Activates the Nrf2/HO-1 and TrkB/CREB/BDNF Pathways in Neuronal Cell Responses against Oxidative Stress Induced by Aβ1–42. International Journal of Molecular Sciences. 2022; 23(3):1193. https://doi.org/10.3390/ijms23031193
Chicago/Turabian StyleNguyen, Cong Duc, Jaehee Yoo, Sun-Young Hwang, Sung-Young Cho, Myeonghun Kim, Hyemin Jang, Kyoung Ok No, Jeong Cheol Shin, Jae-Hong Kim, and Gihyun Lee. 2022. "Bee Venom Activates the Nrf2/HO-1 and TrkB/CREB/BDNF Pathways in Neuronal Cell Responses against Oxidative Stress Induced by Aβ1–42" International Journal of Molecular Sciences 23, no. 3: 1193. https://doi.org/10.3390/ijms23031193
APA StyleNguyen, C. D., Yoo, J., Hwang, S.-Y., Cho, S.-Y., Kim, M., Jang, H., No, K. O., Shin, J. C., Kim, J.-H., & Lee, G. (2022). Bee Venom Activates the Nrf2/HO-1 and TrkB/CREB/BDNF Pathways in Neuronal Cell Responses against Oxidative Stress Induced by Aβ1–42. International Journal of Molecular Sciences, 23(3), 1193. https://doi.org/10.3390/ijms23031193





