Fentanyl but Not Morphine or Buprenorphine Improves the Severity of Necrotizing Acute Pancreatitis in Rats
Abstract
:1. Introduction
2. Results
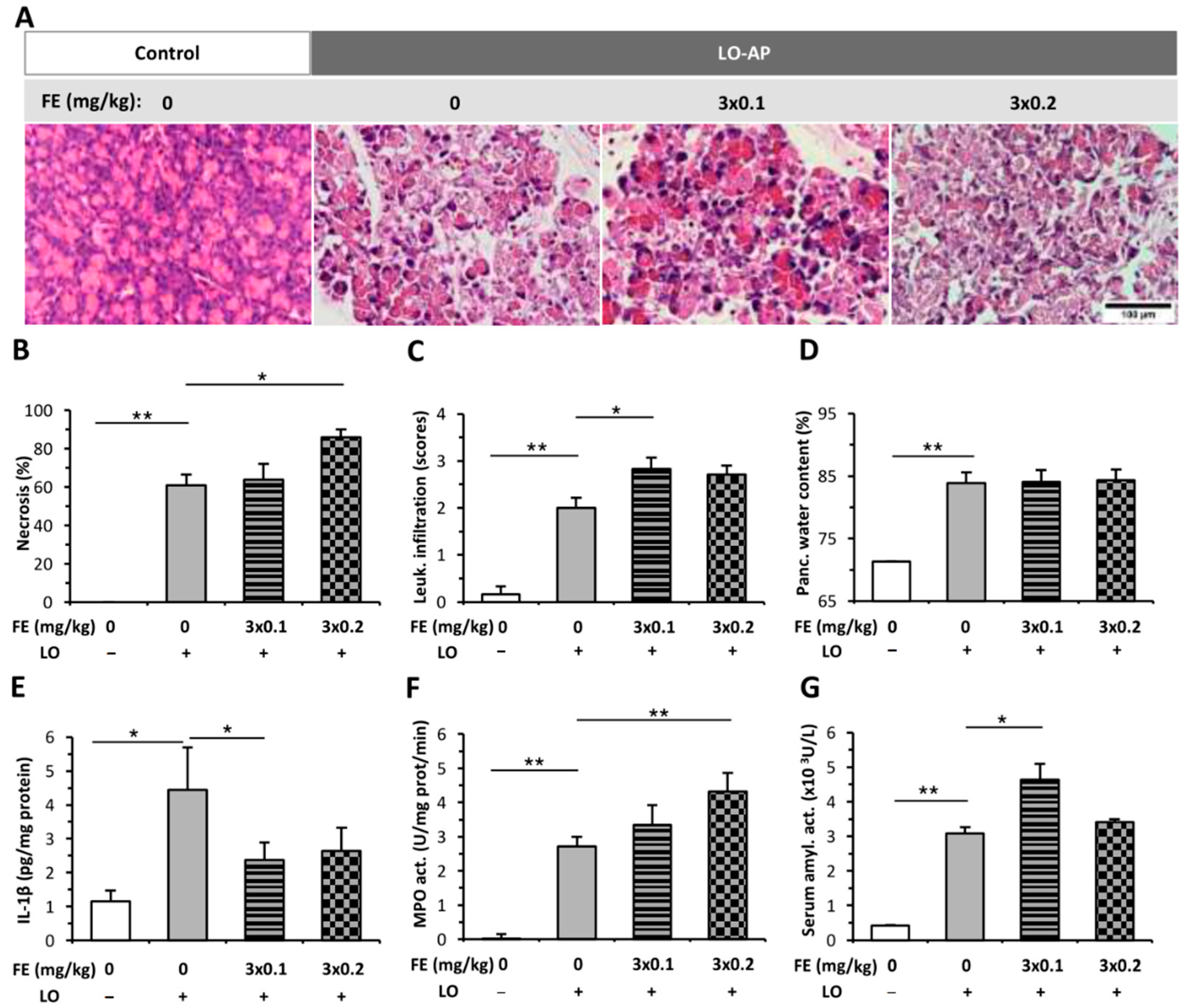
2.1. The Effect of Fentanyl Pre-Treatment on AP Severity
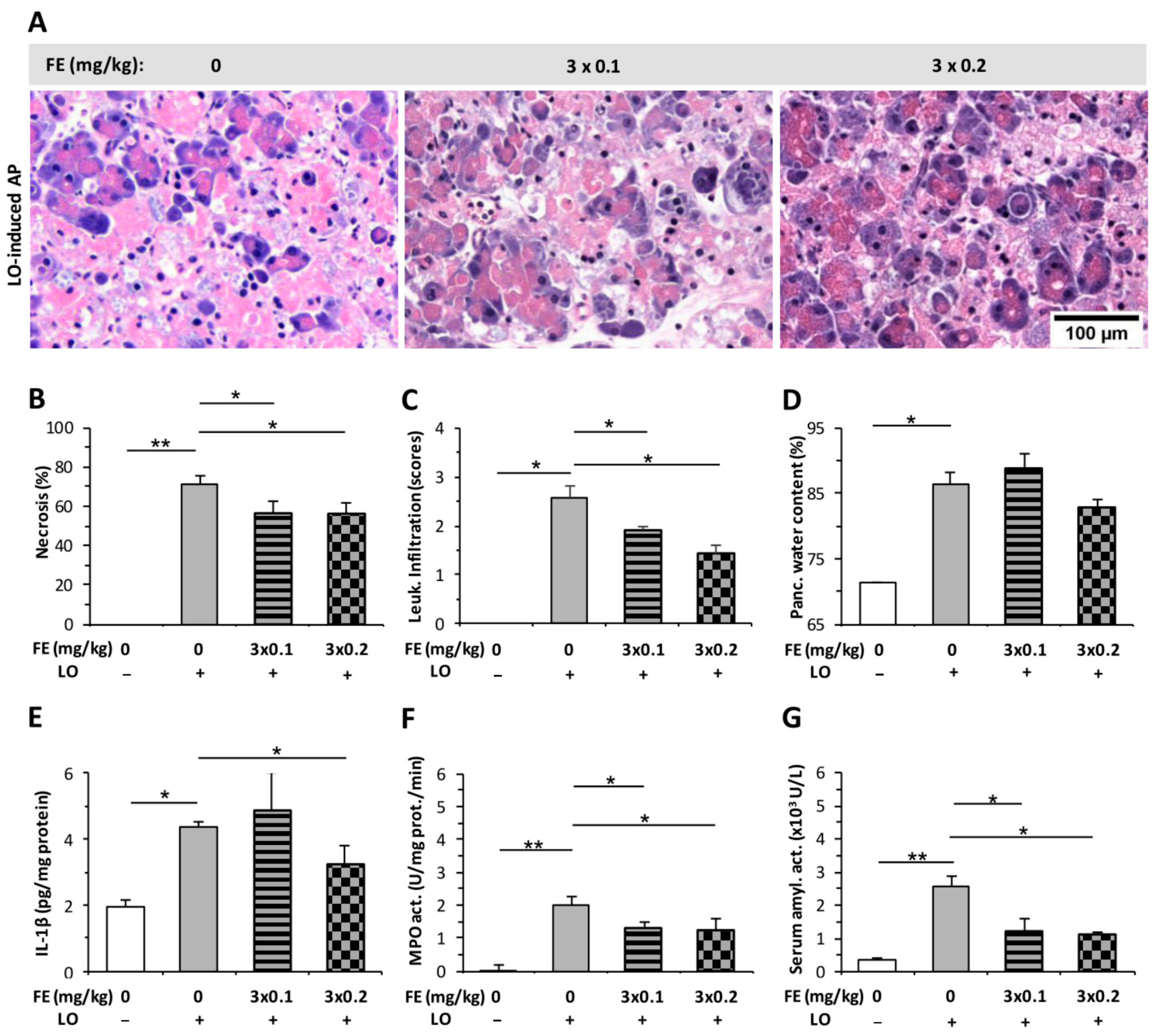
2.2. The Effect of Fentanyl Post-Treatment on AP
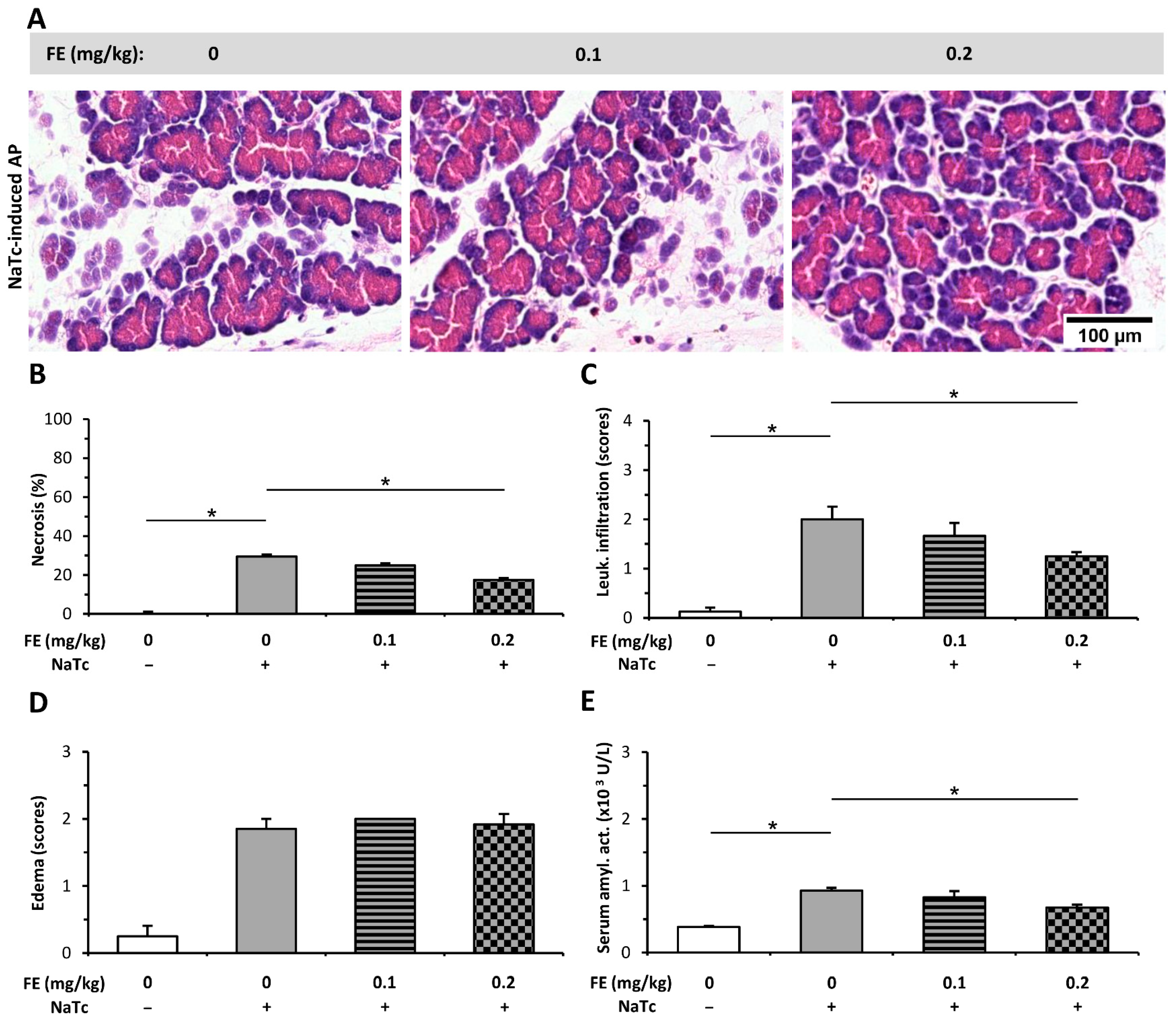
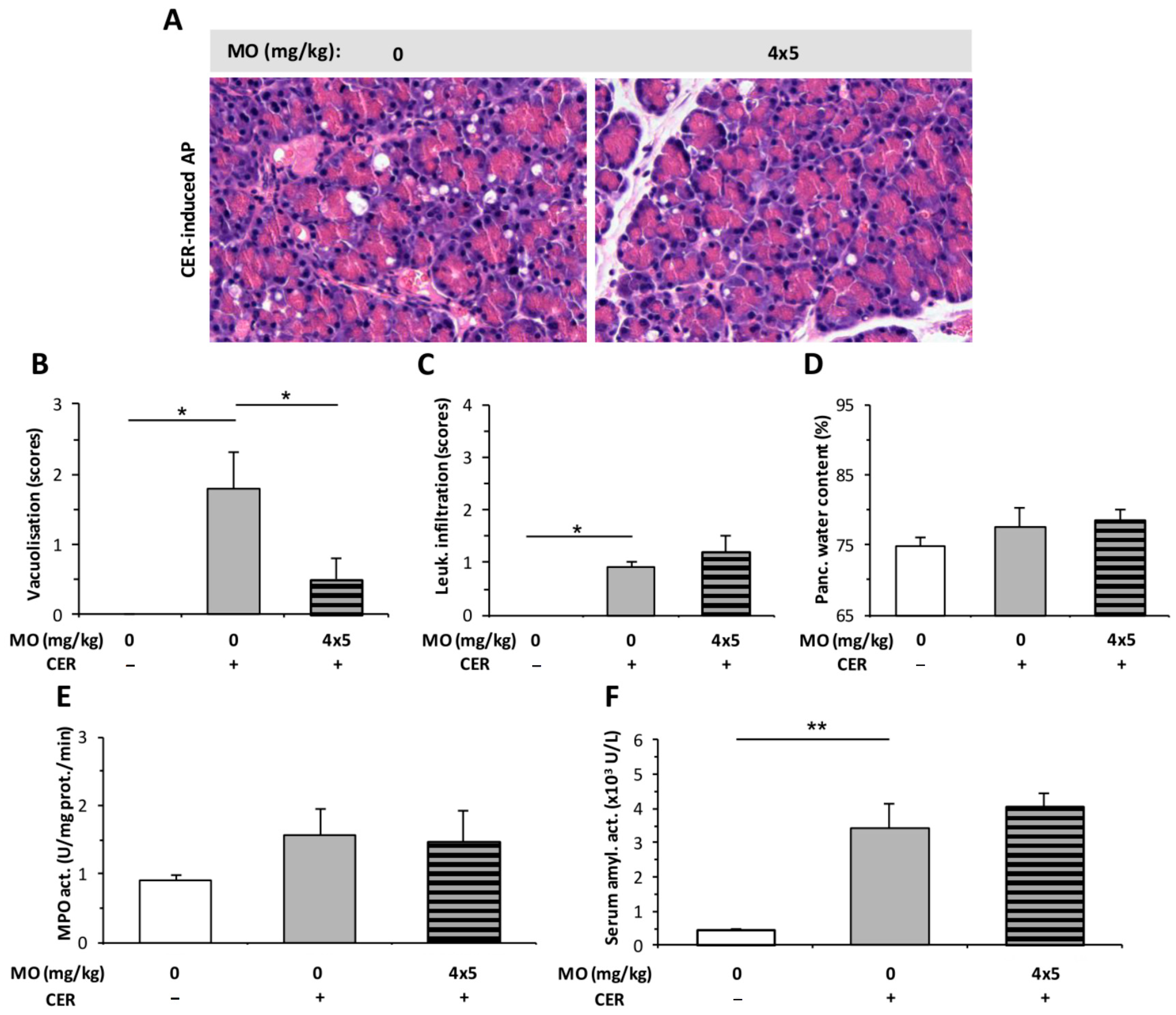
2.3. Morphine Administration Does Not Affect the Severity of AP
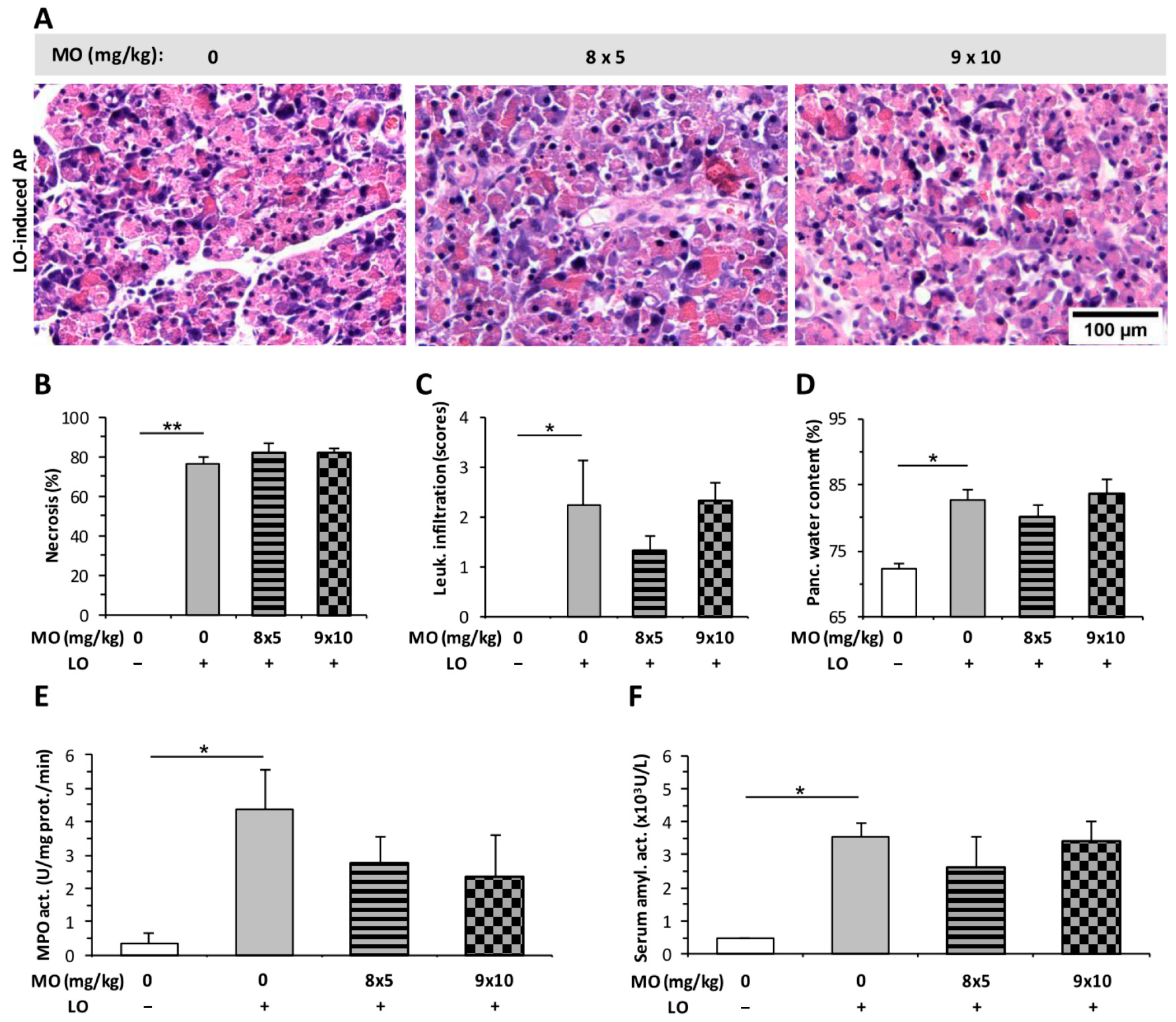
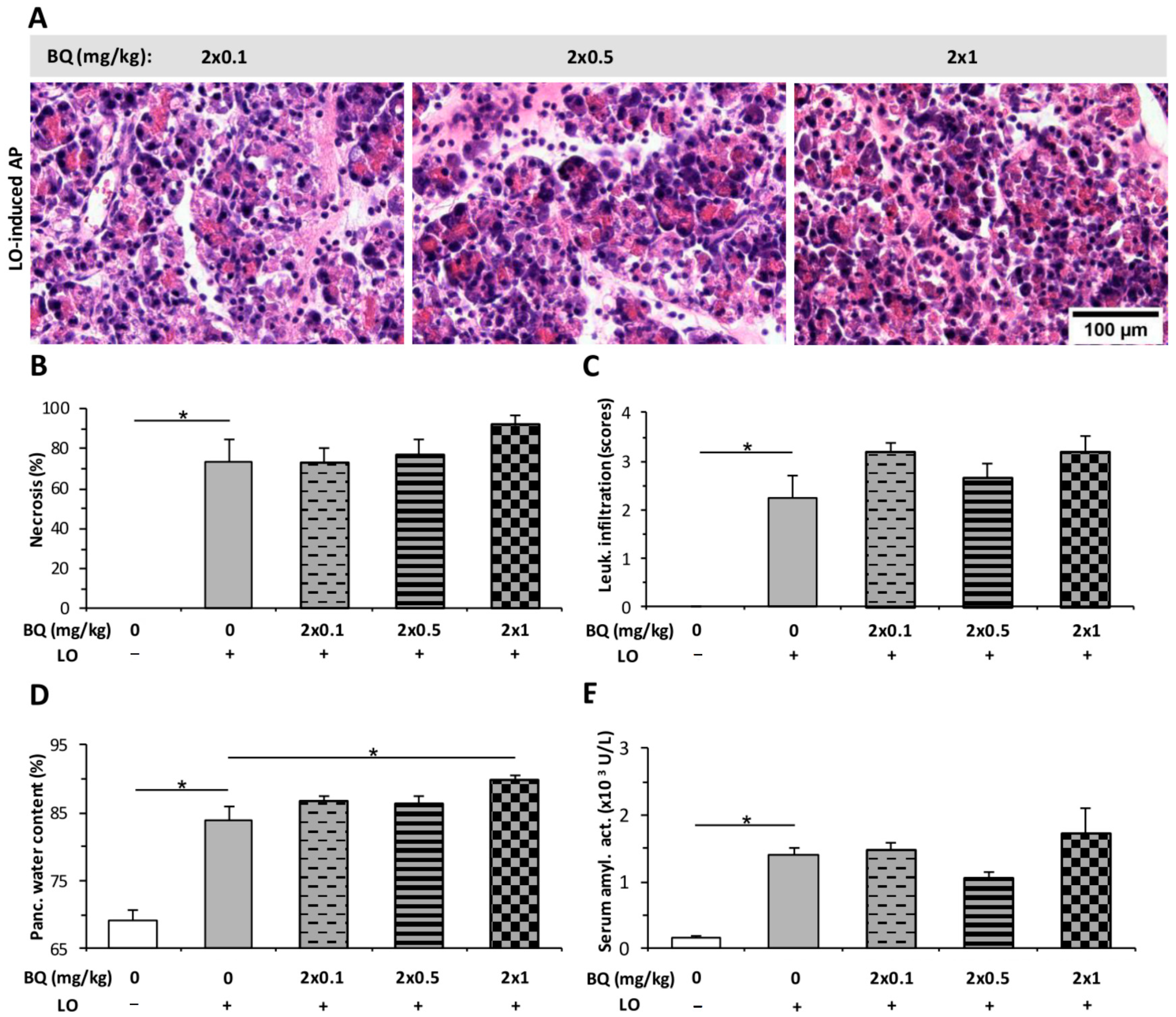
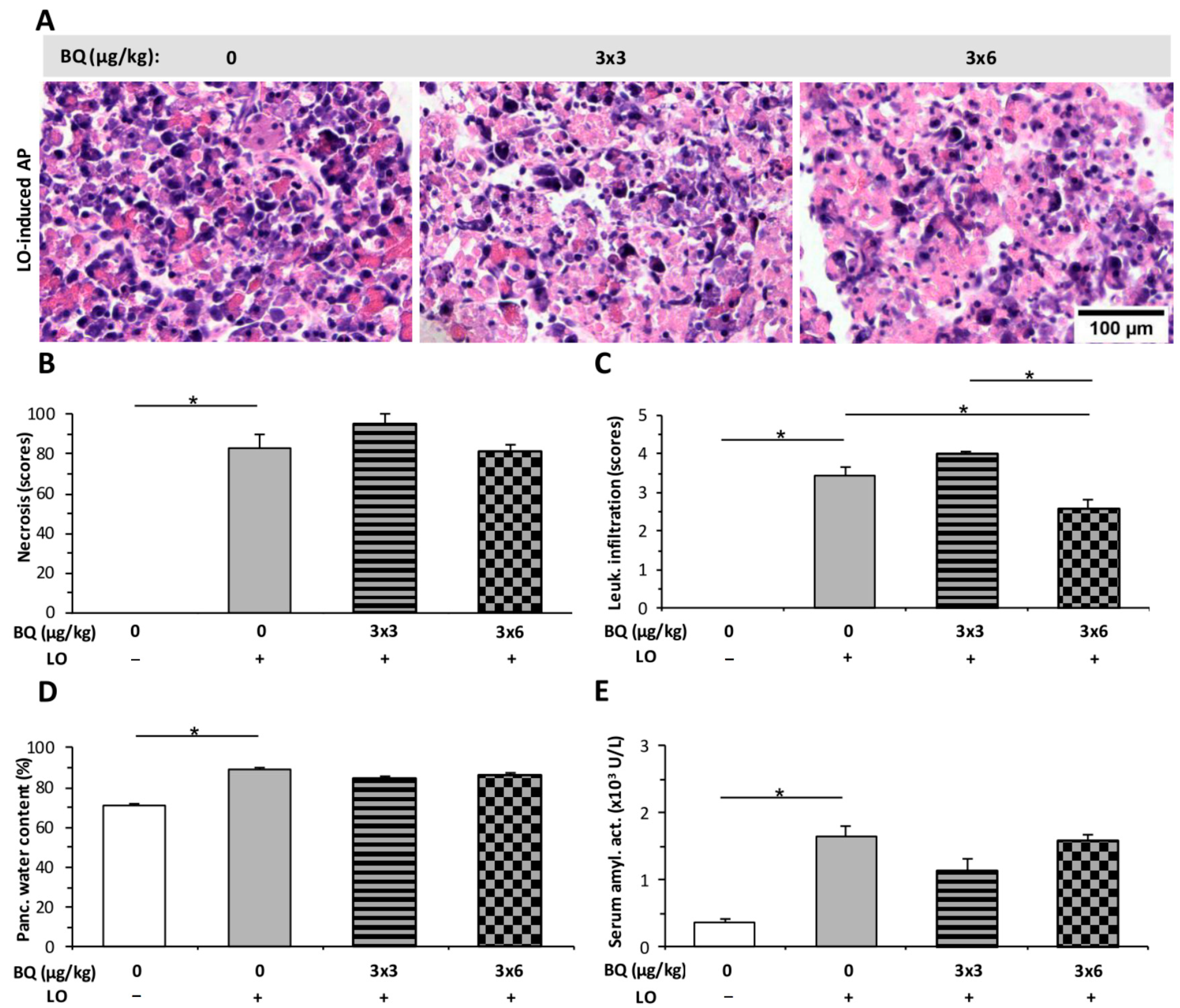
2.4. Buprenorphine Has No Effect on the Severity of LO-Induced AP
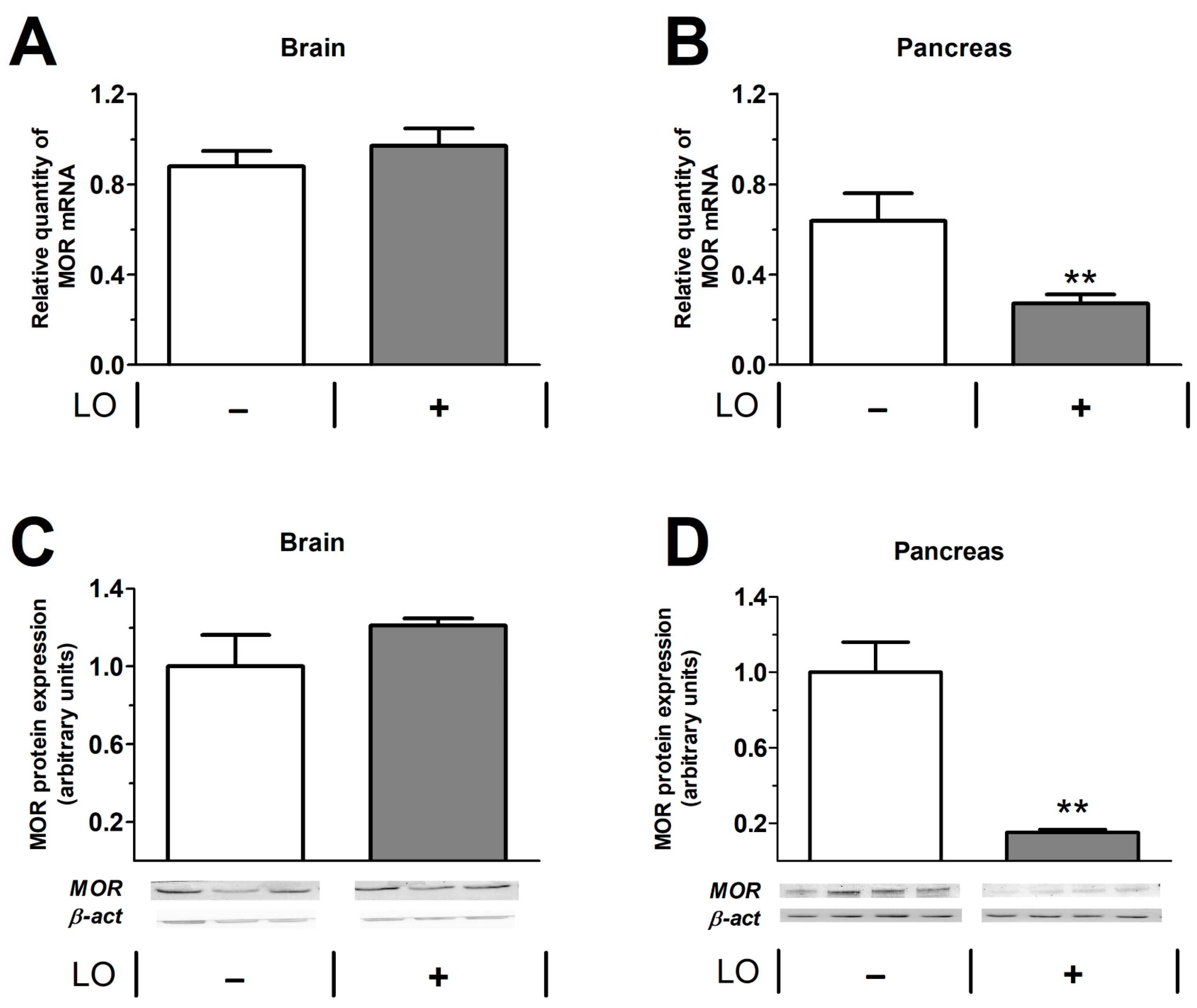
2.5. Pancreatic mu Opioid Receptor Expression Is Decreased in LO-Induced AP
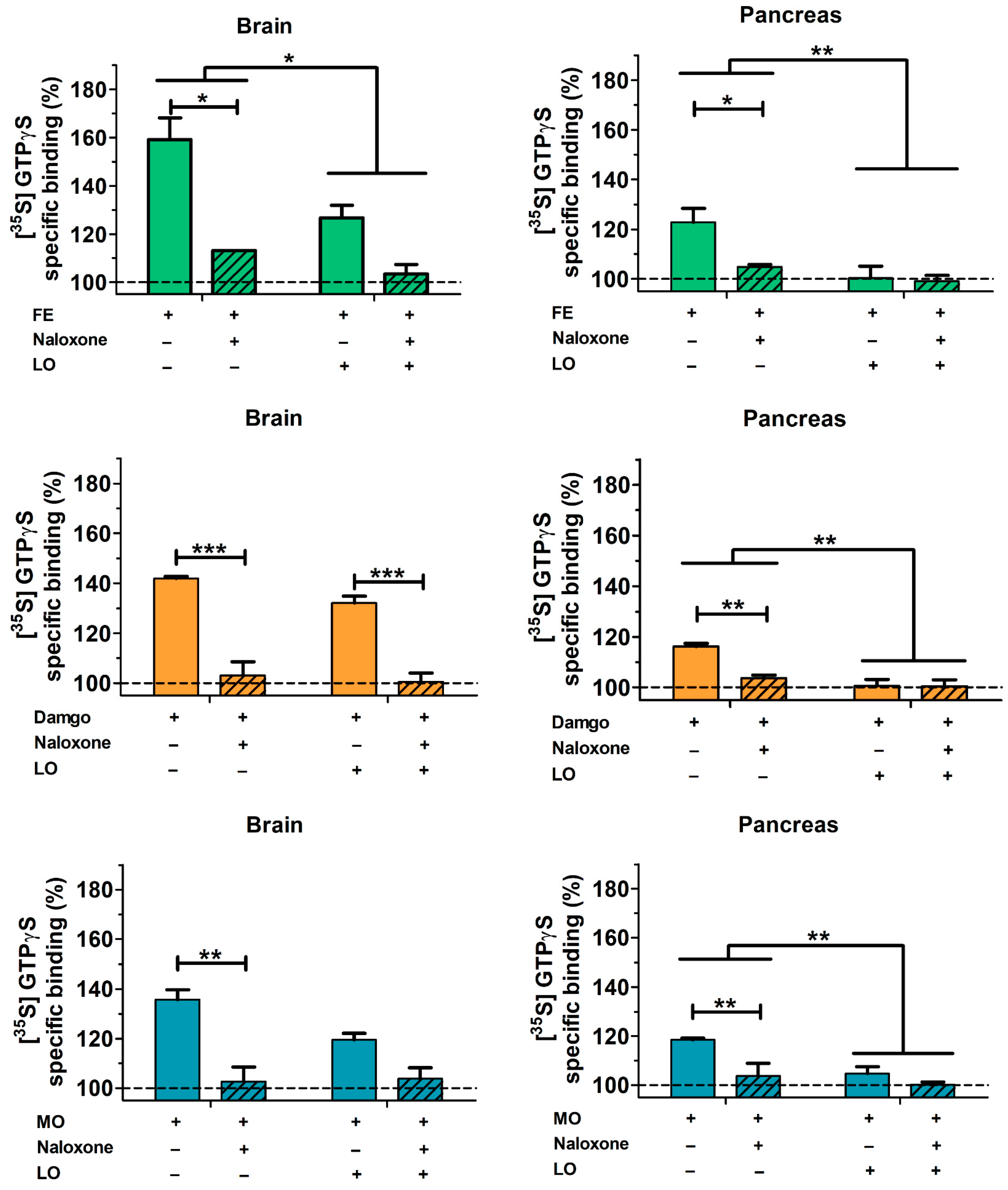
2.6. Pancreatic and Brain mu Opioid Receptor Functions Are Reduced in AP
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Materials
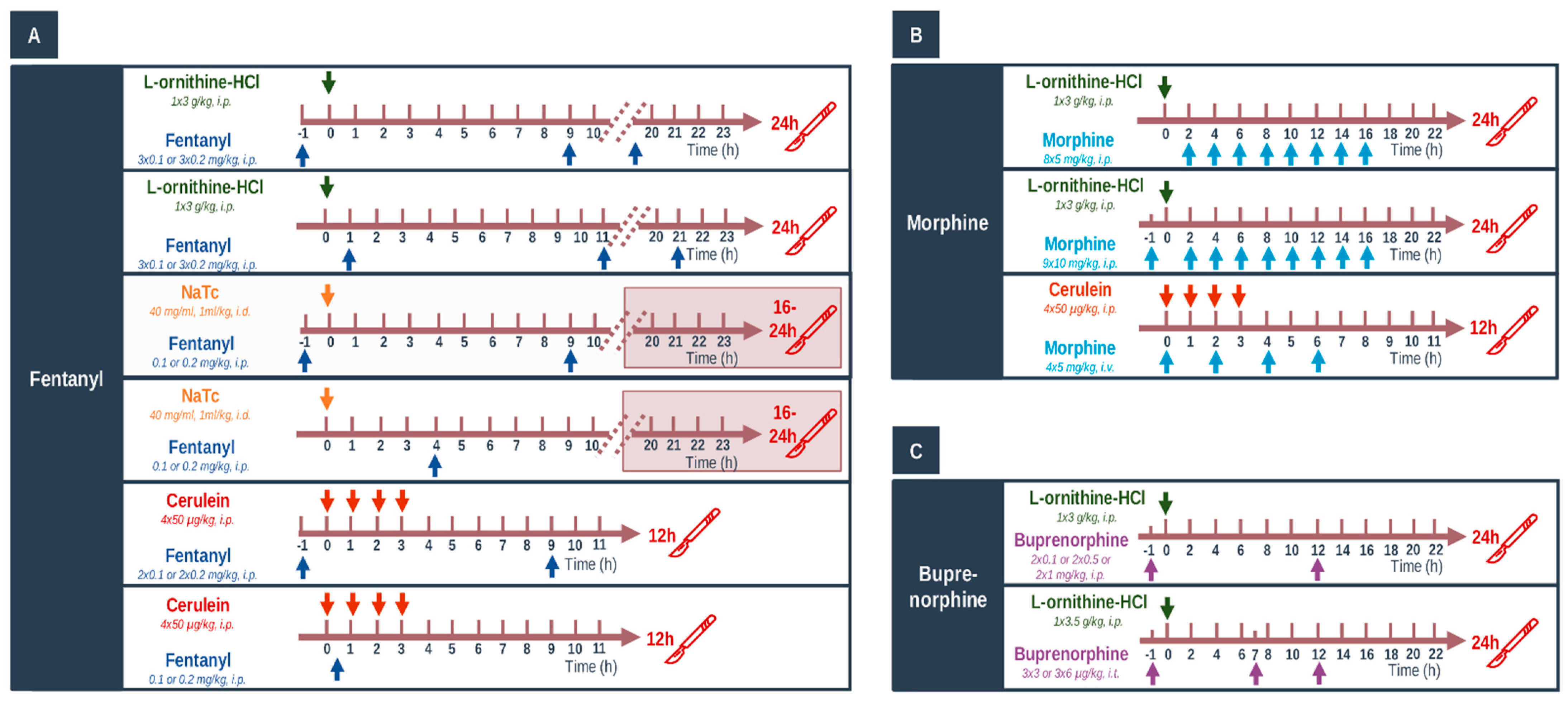
4.3. In Vivo Experiments: Acute Pancreatitis Induction, Opiate Treatments, and Tissue Collection
4.4. Laboratory Measurements
4.5. Histological Examination
4.6. Total RNA Preparation from Tissue
4.7. Real-Time Quantitative Reverse Transcription-PCR (RT-PCR)
4.8. Western Blot Analysis
4.9. Preparation of Brain and Pancreas Samples for Binding Assays
4.10. [35S]GTPγS Functional Binding Assay
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peery, A.F.; Crockett, S.D.; Barritt, A.S.; Dellon, E.S.; Eluri, S.; Gangarosa, L.M.; Jensen, E.T.; Lund, J.L.; Pasricha, S.; Runge, T.; et al. Burden of Gastrointestinal, Liver, and Pancreatic Diseases in the United States. Gastroenterology 2015, 149, 1731–1741.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forsmark, C.E.; Vege, S.S.; Wilcox, C.M. Acute Pancreatitis. N. Engl. J. Med. 2016, 375, 1972–1981. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.E.; Akbari, A.; Thorne, K.; Atkinson, M.; Evans, P.A. The Incidence of Acute Pancreatitis: Impact of Social Deprivation, Alcohol Consumption, Seasonal and Demographic Factors. Aliment. Pharmacol. Ther. 2013, 38, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.E.; Morrison-Rees, S.; John, A.; Williams, J.G.; Brown, T.H.; Samuel, D.G. The Incidence and Aetiology of Acute Pancreatitis across Europe. Pancreatology 2017, 17, 155–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Párniczky, A.; Kui, B.; Szentesi, A.; Balázs, A.; Szűcs, Á.; Mosztbacher, D.; Czimmer, J.; Sarlós, P.; Bajor, J.; Gódi, S.; et al. Prospective, Multicentre, Nationwide Clinical Data from 600 Cases of Acute Pancreatitis. PLoS ONE 2016, 11, e0165309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S. Classification of Acute Pancreatitis—2012: Revision of the Atlanta Classification and Definitions by International Consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef]
- Abu-El-Haija, M.; Gukovskaya, A.S.; Andersen, D.K.; Gardner, T.B.; Hegyi, P.; Pandol, S.J.; Papachristou, G.I.; Saluja, A.K.; Singh, V.K.; Uc, A.; et al. Accelerating the Drug Delivery Pipeline for Acute and Chronic Pancreatitis. Pancreas 2018, 47, 1185–1192. [Google Scholar] [CrossRef]
- Barreto, S.G.; Habtezion, A.; Gukovskaya, A.; Lugea, A.; Jeon, C.; Yadav, D.; Hegyi, P.; Venglovecz, V.; Sutton, R.; Pandol, S.J. Critical Thresholds: Key to Unlocking the Door to the Prevention and Specific Treatments for Acute Pancreatitis. Gut 2021, 70, 194–203. [Google Scholar] [CrossRef]
- Pallagi, P.; Balla, Z.; Singh, A.K.; Dósa, S.; Iványi, B.; Kukor, Z.; Tóth, A.; Riederer, B.; Liu, Y.; Engelhardt, R.; et al. The Role of Pancreatic Ductal Secretion in Protection Against Acute Pancreatitis in Mice. Crit. Care Med. 2014, 42, e177–e188. [Google Scholar] [CrossRef]
- Pallagi, P.; Madácsy, T.; Varga, Á.; Maléth, J. Intracellular Ca2+ Signalling in the Pathogenesis of Acute Pancreatitis: Recent Advances and Translational Perspectives. Int. J. Mol. Sci. 2020, 21, 4005. [Google Scholar] [CrossRef]
- Hritz, I.; Czakó, L.; Dubravcsik, Z.; Farkas, G.; Kelemen, D.; Lásztity, N.; Morvay, Z.; Oláh, A.; Pap, Á.; Párniczky, A.; et al. Acute Pancreatitis: Evidence Based Management Guidelines of the Hungarian Pancreatic Study Group 2014. Orv. Hetil. 2015, 156, 244–261. [Google Scholar] [CrossRef] [Green Version]
- Crockett, S.D.; Wani, S.; Gardner, T.B.; Falck-Ytter, Y.; Barkun, A.N.; Crockett, S.; Falck-Ytter, Y.; Feuerstein, J.; Flamm, S.; Gellad, Z.; et al. American Gastroenterological Association Institute Guideline on Initial Management of Acute Pancreatitis. Gastroenterology 2018, 154, 1096–1101. [Google Scholar] [CrossRef] [Green Version]
- Leppäniemi, A.; Tolonen, M.; Tarasconi, A.; Segovia-Lohse, H.; Gamberini, E.; Kirkpatrick, A.W.; Ball, C.G.; Parry, N.; Sartelli, M.; Wolbrink, D.; et al. 2019 WSES Guidelines for the Management of Severe Acute Pancreatitis. World J. Emerg. Surg. 2019, 14, 27. [Google Scholar] [CrossRef]
- Mandalia, A.; Wamsteker, E.-J.; DiMagno, M.J. Recent Advances in Understanding and Managing Acute Pancreatitis. F1000Research 2019, 7, 959. [Google Scholar] [CrossRef]
- Stigliano, S.; Sternby, H.; de Madaria, E.; Capurso, G.; Petrov, M.S. Early Management of Acute Pancreatitis: A Review of the Best Evidence. Dig. Liver Dis. 2017, 49, 585–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA Evidence-Based Guidelines for the Management of Acute Pancreatitis. Pancreatology 2013, 13, e1–e15. [Google Scholar] [CrossRef] [PubMed]
- Ona, X.B.; Rigau Comas, D.; Urrútia, G. Opioids for Acute Pancreatitis Pain. Cochrane Database Syst. Rev. 2013, 7, CD009179. [Google Scholar] [CrossRef]
- Erbil, Y.; Berber, E.; Seven, R.; Çaliş, A.; Eminoǧlu, L.; Koçak, M.; Bilgiç, L. The Effect of Intestinal Transit Time on Bacterial Translocation. Acta Chir. Belg. 1998, 5458, 245–249. [Google Scholar] [CrossRef]
- Franchi, S.; Moschetti, G.; Amodeo, G.; Sacerdote, P. Do All Opioid Drugs Share the Same Immunomodulatory Properties? A Review From Animal and Human Studies. Front. Immunol. 2019, 10, 2914. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.R. Narcotic Analgesic Effects on the Sphincter of Oddi: A Review of the Data and Therapeutic Implications in Treating Pancreatitis. Am. J. Gastroenterol. 2001, 96, 1266–1272. [Google Scholar] [CrossRef]
- Barlass, U.; Dutta, R.; Cheema, H.; George, J.; Sareen, A.; Dixit, A.; Yuan, Z.; Giri, B.; Meng, J.; Banerjee, S.; et al. Morphine Worsens the Severity and Prevents Pancreatic Regeneration in Mouse Models of Acute Pancreatitis. Gut 2018, 67, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Perides, G.; Van Acker, G.J.D.; Laukkarinen, J.M.; Steer, M.L. Experimental Acute Biliary Pancreatitis Induced by Retrograde Infusion of Bile Acids into the Mouse Pancreatic Duct. Nat. Protoc. 2010, 5, 335–341. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, M. Fentanyl Ameliorates Severe Acute Pancreatitis-Induced Myocardial Injury in Rats by Regulating NF-ΚB Signaling Pathway. Med. Sci. Monit. 2017, 23, 3276–3283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, M.; Esler, R.; Asher, G. Transdermal Fentanyl for the Management of Acute Pancreatitis Pain. Appl. Nurs. Res. 2002, 15, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Demirag, A.; Pastor, C.M.; Morel, P.; Jean-Christophe, C.; Sielenkämper, A.W.; Güvener, N.; Mai, G.; Berney, T.; Frossard, J.L.; Bühler, L.H. Epidural Anaesthesia Restores Pancreatic Microcirculation and Decreases the Severity of Acute Pancreatitis. World J. Gastroenterol. 2006, 12, 915–920. [Google Scholar] [CrossRef] [Green Version]
- Sadowski, S.M.; Andres, A.; Morel, P.; Schiffer, E.; Frossard, J.L.; Platon, A.; Poletti, P.A.; Bühler, L. Epidural Anesthesia Improves Pancreatic Perfusion and Decreases the Severity of Acute Pancreatitis. World J. Gastroenterol. 2015, 21, 12448–12456. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.C.; Ben-Baruch, A.; Taub, D.D.; Howard, O.M.Z.; Resau, J.H.; Wang, J.M.; Ali, H.; Richardson, R.; Snyderman, R.; Oppenheim, J.J. Opiates Transdeactivate Chemokine Receptors: δ and μ Opiate Receptor–Mediated Heterologous Desensitization. J. Exp. Med. 1998, 188, 317–325. [Google Scholar] [CrossRef] [Green Version]
- Peterson, P.K.; Gekker, G.; Brummitt, C.; Pentel, P.; Bullock, M.; Simpson, M.; Hitt, J.; Sharp, B. Suppression of Human Peripheral Blood Mononuclear Cell Function by Methadone and Morphine. J. Infect. Dis. 1989, 159, 480–487. [Google Scholar] [CrossRef]
- Peiró, A.M.; Martínez, J.; Martínez, E.; De Madaria, E.; Llorens, P.; Horga, J.F.; Pérez-Mateo, M. Efficacy and Tolerance of Metamizole versus Morphine for Acute Pancreatitis Pain. Pancreatology 2008, 8, 25–29. [Google Scholar] [CrossRef]
- Meng, W.; Yuan, J.; Zhang, C.; Bai, Z.; Zhou, W.; Yan, J.; Li, X. Parenteral Analgesics for Pain Relief in Acute Pancreatitis: A Systematic Review. Pancreatology 2013, 13, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Wereszczyńska-Siemiatkowska, U.; Nebendahl, K.; Pohl, U.; Otto, J.; Groene, H.J.; Wilms, H.; Lankisch, P.G. Influence of Buprenorphine on Acute Experimental Pancreatitis. Res. Exp. Med. 1987, 187, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Ogden, J.M.; Modlin, I.M.; Gorelick, F.S.; Marks, I.N. Effect of Buprenorphine on Pancreatic Enzyme Synthesis and Secretion in Normal Rats and Rats with Acute Edematous Pancreatitis. Dig. Dis. Sci. 1994, 39, 2407–2415. [Google Scholar] [CrossRef] [PubMed]
- Eisenstein, T.K. The Role of Opioid Receptors in Immune System Function. Front. Immunol. 2019, 10, 2904. [Google Scholar] [CrossRef] [Green Version]
- Rogers, T.J. Bidirectional Regulation of Opioid and Chemokine Function. Front. Immunol. 2020, 11, 94. [Google Scholar] [CrossRef] [Green Version]
- Trescot, A.M.; Datta, S.; Lee, M.; Hans, H. Opioid Pharmacology. Pain Physician 2008, 11, 133–154. [Google Scholar] [CrossRef]
- Afghani, E.; Lo, S.K.; Covington, P.S.; Cash, B.D.; Pandol, S.J. Sphincter of Oddi Function and Risk Factors for Dysfunction. Front. Nutr. 2017, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuer, J.C.; Dapoigny, M.; Ajmi, S.; Larpent, J.L.; Lunaud, B.; Ferrier, C.; Bommelaer, G. Effects of Buprenorphine on Motor Activity of the Sphincter of Oddi in Man. Eur. J. Clin. Pharmacol. 1989, 36, 203–204. [Google Scholar] [CrossRef] [PubMed]
- Przewlocki, R.; Przewlocka, B. Opioids in Chronic Pain. Eur. J. Pharmacol. 2001, 429, 79–91. [Google Scholar] [CrossRef]
- Delfs, J.M.; Kong, H.; Mestek, A.; Chen, Y.; Yu, L.; Reisine, T.; Chesselet, M.-F. Expression of Mu Opioid Receptor MRNA in Rat Brain: An in Situ Hybridization Study at the Single Cell Level. J. Comp. Neurol. 1994, 345, 46–68. [Google Scholar] [CrossRef] [PubMed]
- Khawaja, X.Z.; Green, I.C.; Thorpe, J.R.; Titheradge, M.A. The Occurrence and Receptor Specificity of Endogenous Opioid Peptides within the Pancreas and Liver of the Rat. Comparison with Brain. Biochem. J. 1990, 267, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Ng, S.L.; Ng, T.N. Materials with Opiate Receptor Binding Activity in Bovine Testis and Ovine Pancreas. Biochem. Int. 1987, 14, 1087–1096. [Google Scholar] [PubMed]
- Peng, J.; Sarkar, S.; Chang, S.L. Opioid Receptor Expression in Human Brain and Peripheral Tissues Using Absolute Quantitative Real-Time RT-PCR. Drug Alcohol Depend. 2012, 124, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Wen, T.; Peng, B.; Pintar, J.E. The MOR-1 Opioid Receptor Regulates Glucose Homeostasis by Modulating Insulin Secretion. Mol. Endocrinol. 2009, 23, 671–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konturek, S.J.; Tasler, J.; Cieszkowski, M.; Jaworek, J.; Coy, D.H.; Schally, A.V. Inhibition of Pancreatic Secretion by Enkephalin and Morphine in Dogs. Gastroenterology 1978, 74, 851–855. [Google Scholar] [CrossRef]
- Linari, G.; Agostini, S.; Broccardo, M.; Petrella, C.; Improta, G. Regulation of Pancreatic Secretion In Vitro by Nociceptin/Orphanin FQ and Opioid Receptors: A Comparative Study. Pharmacol. Res. 2006, 54, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Louie, D.S.; Chen, H.T.; Owyang, C. Inhibition of Exocrine Pancreatic Secretion by Opiates Is Mediated by Suppression of Cholinergic Transmission: Characterization of Receptor Subtypes. J. Pharmacol. Exp. Ther. 1988, 246, 132–136. [Google Scholar] [PubMed]
- Pol, O.; Alameda, F.; Puig, M.M. Inflammation Enhances μ-Opioid Receptor Transcription and Expression in Mice Intestine. Mol. Pharmacol. 2001, 60, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Schäfer, M.; Elde, R.; Stein, C. Effects of Neurotoxins and Hindpaw Inflammation on Opioid Receptor Immunoreactivities in Dorsal Root Ganglia. Neuroscience 1998, 85, 281–291. [Google Scholar] [CrossRef]
- Meyer, J.; Del Vecchio, G.; Seitz, V.; Massaly, N.; Stein, C. Modulation of Μ-opioid Receptor Activation by Acidic pH Is Dependent on Ligand Structure and an Ionizable Amino Acid Residue. Br. J. Pharmacol. 2019, 176, 4510–4520. [Google Scholar] [CrossRef] [Green Version]
- Prossin, A.R.; Zalcman, S.S.; Heitzeg, M.M.; Koch, A.E.; Campbell, P.L.; Phan, K.L.; Stohler, C.S.; Zubieta, J.K. Dynamic Interactions Between Plasma IL-1 Family Cytokines and Central Endogenous Opioid Neurotransmitter Function in Humans. Neuropsychopharmacology 2014, 40, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Satake, K.; Ha, S.S.; Hiura, A. Effects of Bradykinin Receptor Antagonist on the Release of Beta-Endorphin and Bradykinin and on Hemodynamic Changes in a Canine Model of Experimental Acute Pancreatitis. Pancreas 1996, 12, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Llorca-Torralba, M.; Pilar-Cuéllar, F.; Bravo, L.; Bruzos-Cidon, C.; Torrecilla, M.; Mico, J.A.; Ugedo, L.; Garro-Martínez, E.; Berrocoso, E. Opioid Activity in the Locus Coeruleus Is Modulated by Chronic Neuropathic Pain. Mol. Neurobiol. 2019, 56, 4135–4150. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.J.; Traynor, J.R. Endogenous Regulator of g Protein Signaling Proteins Reduce {mu}-Opioid Receptor Desensitization and down-Regulation and Adenylyl Cyclase Tolerance in C6 Cells. J. Pharmacol. Exp. Ther. 2005, 312, 809–815. [Google Scholar] [CrossRef] [Green Version]
- Tao, Z.Y.; Wang, P.X.; Wei, S.Q.; Traub, R.J.; Li, J.F.; Cao, D.Y. The Role of Descending Pain Modulation in Chronic Primary Pain: Potential Application of Drugs Targeting Serotonergic System. Neural Plast. 2019, 2019, 1389296. [Google Scholar] [CrossRef]
- Liao, Y.H.; Wang, J.; Wei, Y.Y.; Zhang, T.; Zhang, Y.; Zuo, Z.F.; Teng, X.Y.; Li, Y.Q. Histone Deacetylase 2 Is Involved in Μopioid Receptor Suppression in the Spinal Dorsal Horn in a Rat Model of Chronic Pancreatitis Pain. Mol. Med. Rep. 2018, 17, 2803–2810. [Google Scholar] [CrossRef]
- Patai, Á.; Solymosi, N.; Mohácsi, L.; Patai, Á.V. Indomethacin and Diclofenac in the Prevention of Post-ERCP Pancreatitis: A Systematic Review and Meta-Analysis of Prospective Controlled Trials. Gastrointest. Endosc. 2017, 85, 1144–1156.e1. [Google Scholar] [CrossRef]
- Almousa, A.A.; Ikeda, R.; Wada, M.; Kuroda, N.; Hanajiri, R.K.; Nakashima, K. HPLC-UV Method Development for Fentanyl Determination in Rat Plasma and Its Application to Elucidate Pharmacokinetic Behavior after i.p. Administration to Rats. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 2941–2944. [Google Scholar] [CrossRef] [Green Version]
- Hill, R.; Santhakumar, R.; Dewey, W.; Kelly, E.; Henderson, G. Fentanyl Depression of Respiration: Comparison with Heroin and Morphine. Br. J. Pharmacol. 2020, 177, 254–266. [Google Scholar] [CrossRef]
- Bouw, M.R.; Gårdmark, M.; Hammarlund-Udenaes, M. Modelling of Morphine Transport across the Blood-Brain Barrier as a Cause of the Antinociceptive Effect Delay in Rats—A Microdialysis Study. Pharm. Res. 2000, 17, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Foley, P.L.; Kendall, L.V.; Turner, P.V. Clinical Management of Pain in Rodents. Comp. Med. 2019, 69, 468–489. [Google Scholar] [CrossRef] [PubMed]
- Yaksh, T.L.; Rudy, T.A. Chronic Catheterization of the Spinal Subarachnoid Space. Physiol. Behav. 1976, 17, 1031–1036. [Google Scholar] [CrossRef]
- Dobos, I.; Toth, K.; Kekesi, G.; Joo, G.; Csullog, E.; Klimscha, W.; Benedek, G.; Horvath, G. The Significance of Intrathecal Catheter Location in Rats. Anesth. Analg. 2003, 96, 487–492. [Google Scholar] [CrossRef]
- Tejwani, G.A.; Rattan, A.K. The Role of Spinal Opioid Receptors in Antinociceptive Effects Produced by Intrathecal Administration of Hydromorphone and Buprenorphine in the Rat. Anesth. Analg. 2002, 94, 1542–1546. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, M.; Brayton, C.; Detolla, L.; Forbes-Mcbean, N.; Sarabia-Estrada, R.; Zadnik, P. Safety and Efficacy of Buprenorphine for Analgesia in Laboratory Mice and Rats. Lab Anim. 2012, 41, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Kuebler, W.M.; Abels, C.; Schuerer, L.; Goetz, A.E. Measurement of Neutrophil Content in Brain and Lung Tissue by a Modified Myeloperoxidase Assay. Int. J. Microcirc. Clin. Exp. 1996, 16, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Szűcs, E.; Büki, A.; Kékesi, G.; Horváth, G.; Benyhe, S. Mu-Opioid (MOP) Receptor Mediated G-Protein Signaling Is Impaired in Specific Brain Regions in a Rat Model of Schizophrenia. Neurosci. Lett. 2016, 619, 29–33. [Google Scholar] [CrossRef]
- Traynor, J.R.; Nahorski, S.R. Modulation by Mu-Opioid Agonists of Guanosine-5′-O-(3-[35S]Thio)Triphosphate Binding to Membranes from Human Neuroblastoma SH-SY5Y Cells. Mol. Pharmacol. 1995, 47, 848–854. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bálint, E.R.; Fűr, G.; Kui, B.; Balla, Z.; Kormányos, E.S.; Orján, E.M.; Tóth, B.; Horváth, G.; Szűcs, E.; Benyhe, S.; et al. Fentanyl but Not Morphine or Buprenorphine Improves the Severity of Necrotizing Acute Pancreatitis in Rats. Int. J. Mol. Sci. 2022, 23, 1192. https://doi.org/10.3390/ijms23031192
Bálint ER, Fűr G, Kui B, Balla Z, Kormányos ES, Orján EM, Tóth B, Horváth G, Szűcs E, Benyhe S, et al. Fentanyl but Not Morphine or Buprenorphine Improves the Severity of Necrotizing Acute Pancreatitis in Rats. International Journal of Molecular Sciences. 2022; 23(3):1192. https://doi.org/10.3390/ijms23031192
Chicago/Turabian StyleBálint, Emese Réka, Gabriella Fűr, Balázs Kui, Zsolt Balla, Eszter Sára Kormányos, Erik Márk Orján, Brigitta Tóth, Gyöngyi Horváth, Edina Szűcs, Sándor Benyhe, and et al. 2022. "Fentanyl but Not Morphine or Buprenorphine Improves the Severity of Necrotizing Acute Pancreatitis in Rats" International Journal of Molecular Sciences 23, no. 3: 1192. https://doi.org/10.3390/ijms23031192
APA StyleBálint, E. R., Fűr, G., Kui, B., Balla, Z., Kormányos, E. S., Orján, E. M., Tóth, B., Horváth, G., Szűcs, E., Benyhe, S., Ducza, E., Pallagi, P., Maléth, J., Venglovecz, V., Hegyi, P., Kiss, L., & Rakonczay, Z., Jr. (2022). Fentanyl but Not Morphine or Buprenorphine Improves the Severity of Necrotizing Acute Pancreatitis in Rats. International Journal of Molecular Sciences, 23(3), 1192. https://doi.org/10.3390/ijms23031192







