An Optimized MRM-Based Workflow of the l-Arginine/Nitric Oxide Pathway Metabolites Revealed Disease- and Sex-Related Differences in the Cardiovascular Field
Abstract
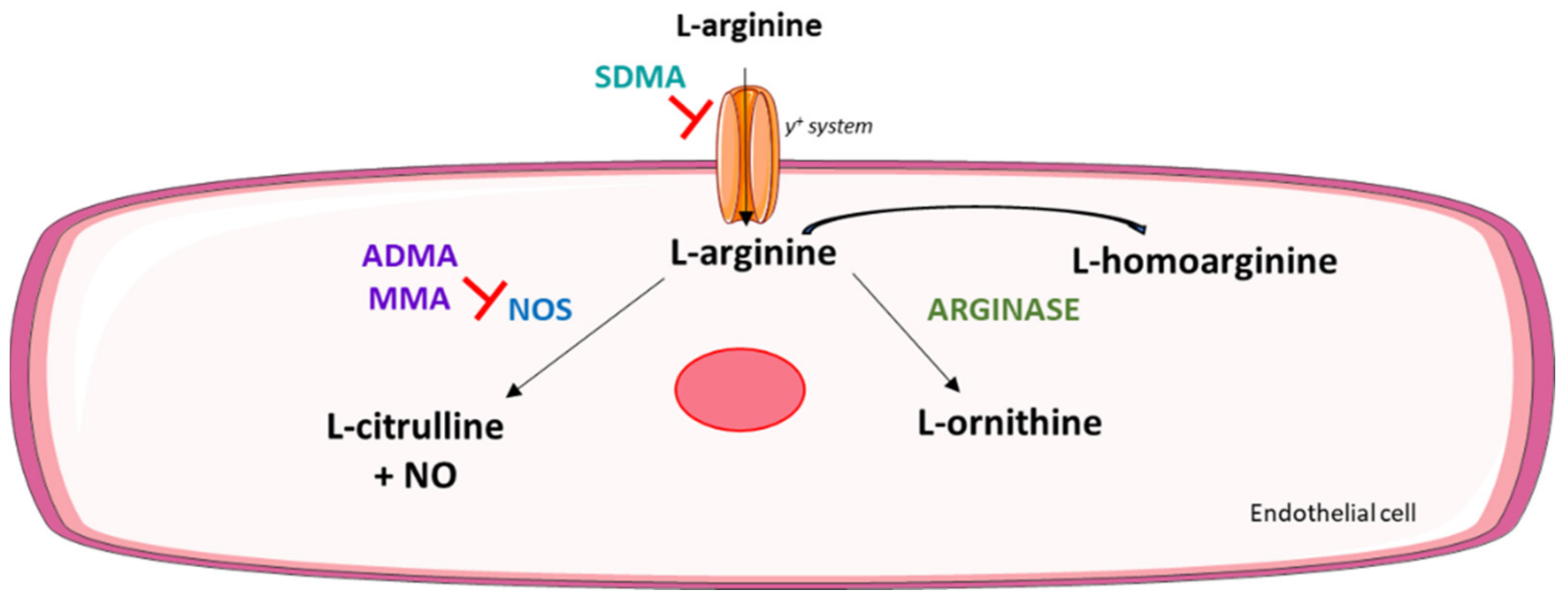
:1. Introduction
2. Results
2.1. Characteristics of the Study Participants
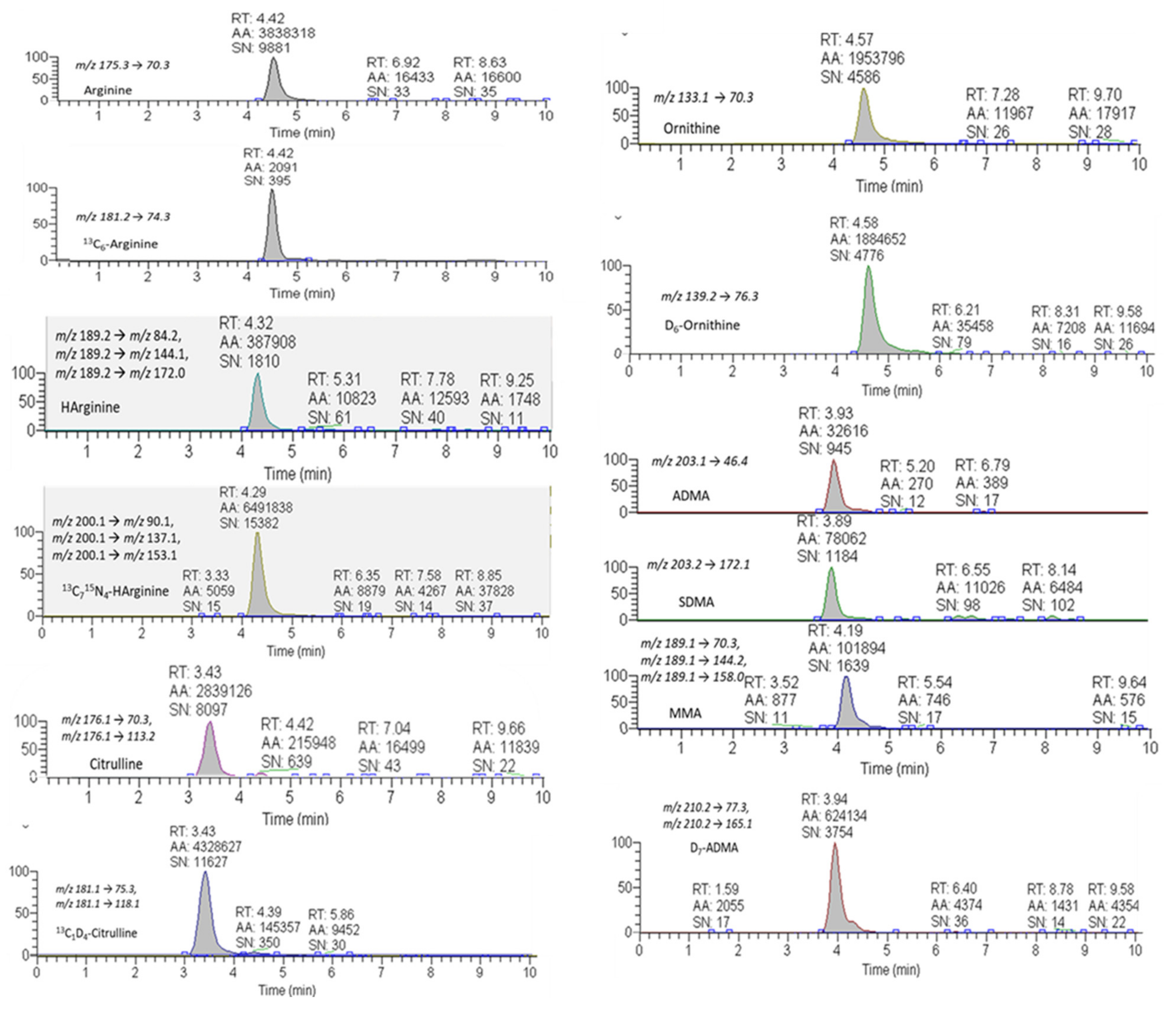
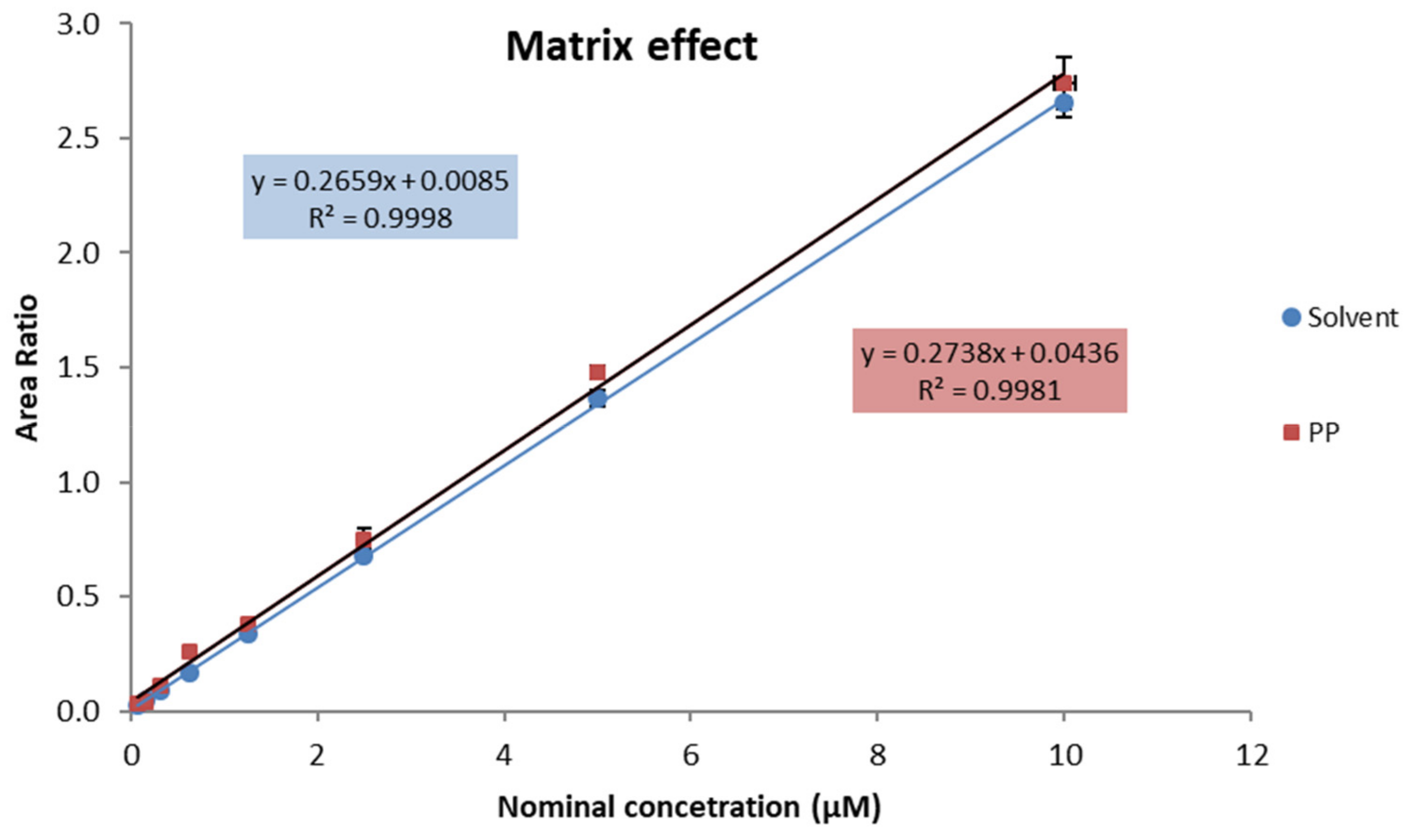
2.2. MRM-Based Analysis of the Arg/NO Pathway Metabolites
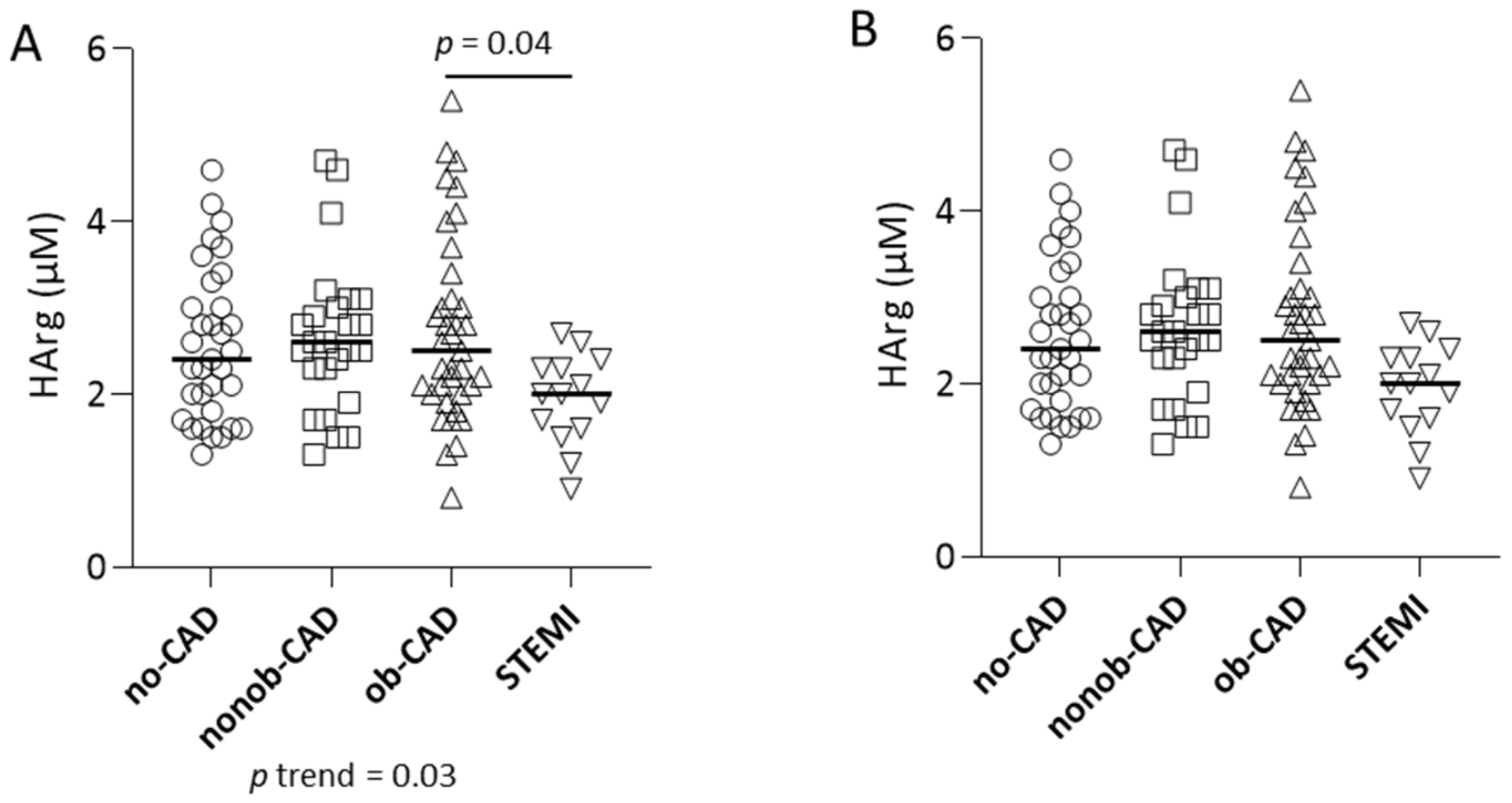
2.3. Arg/NO Metabolic Pathway in Individuals with Different Grades of CAD
2.4. The Effect of Sex on the Arg/NO Metabolic Pathway
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Reagents
5.2. Study Population
5.3. Sample Collection, CCTA and OCT Protocol, Images Reconstruction, and Analysis
5.4. Targeted Metabolomics of the Arg/NO Pathway by Means of an MRM-Based Approach
5.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ryan, W.L.; Johnson, R.J.; Dimari, S. Homoarginine synthesis by rat kidney. Arch. Biochem. Biophys. 1969, 131, 521–526. [Google Scholar] [CrossRef]
- Choe, C.U.; Atzler, D.; Wild, P.S.; Carter, A.M.; Boger, R.H.; Ojeda, F.; Simova, O.; Stockebrand, M.; Lackner, K.; Nabuurs, C.; et al. Homoarginine levels are regulated by L-arginine: Glycine amidinotransferase and affect stroke outcome: Results from human and murine studies. Circulation 2013, 128, 1451–1461. [Google Scholar] [CrossRef] [Green Version]
- Marescau, B.; Nagels, G.; Possemiers, I.; De Broe, M.E.; Becaus, I.; Billiouw, J.M.; Lornoy, W.; De Deyn, P.P. Guanidino compounds in serum and urine of nondialyzed patients with chronic renal insufficiency. Metab. Clin. Exp. 1997, 46, 1024–1031. [Google Scholar] [CrossRef]
- Mizutani, N.; Hayakawa, C.; Ohya, Y.; Watanabe, K.; Watanabe, Y.; Mori, A. Guanidino compounds in hyperargininemia. Tohoku J. Exp. Med. 1987, 153, 197–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moali, C.; Boucher, J.L.; Sari, M.A.; Stuehr, D.J.; Mansuy, D. Substrate specificity of NO synthases: Detailed comparison of L-arginine, homo-L-arginine, their N omega-hydroxy derivatives, and N omega-hydroxynor-L-arginine. Biochemistry 1998, 37, 10453–10460. [Google Scholar] [CrossRef] [PubMed]
- Hrabak, A.; Bajor, T.; Temesi, A. Comparison of substrate and inhibitor specificity of arginase and nitric oxide (NO) synthase for arginine analogues and related compounds in murine and rat macrophages. Biochem. Biophys. Res. Commun. 1994, 198, 206–212. [Google Scholar] [CrossRef]
- Atzler, D.; McAndrew, D.J.; Cordts, K.; Schneider, J.E.; Zervou, S.; Schwedhelm, E.; Neubauer, S.; Lygate, C.A. Dietary Supplementation with Homoarginine Preserves Cardiac Function in a Murine Model of Post-Myocardial Infarction Heart Failure. Circulation 2017, 135, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Dellera, F.; Ganzetti, G.S.; Froio, A.; Manzini, S.; Busnelli, M.; Meinitzer, A.; Sirtori, C.R.; Chiesa, G.; Parolini, C. L-homoarginine administration reduces neointimal hyperplasia in balloon-injured rat carotids. Thromb. Haemost. 2016, 116, 400–402. [Google Scholar] [CrossRef]
- Pilz, S.; Meinitzer, A.; Gaksch, M.; Grubler, M.; Verheyen, N.; Drechsler, C.; Hartaigh, B.O.; Lang, F.; Alesutan, I.; Voelkl, J.; et al. Homoarginine in the renal and cardiovascular systems. Amino Acids 2015, 47, 1703–1713. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Xhepa, E.; Braun, S.; Cassese, S.; Fusaro, M.; Schunkert, H.; Kastrati, A. Alkaline phosphatase and prognosis in patients with coronary artery disease. Eur. J. Clin. Investig. 2017, 47, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Karetnikova, E.S.; Jarzebska, N.; Markov, A.G.; Weiss, N.; Lentz, S.R.; Rodionov, R.N. Is Homoarginine a Protective Cardiovascular Risk Factor? Arterioscler. Thromb. Vasc. Biol. 2019, 39, 869–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zinellu, A.; Paliogiannis, P.; Carru, C.; Mangoni, A.A. Homoarginine and all-cause mortality: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2018, 48, e12960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atzler, D.; Rosenberg, M.; Anderssohn, M.; Choe, C.U.; Lutz, M.; Zugck, C.; Boger, R.H.; Frey, N.; Schwedhelm, E. Homoarginine—An independent marker of mortality in heart failure. Int. J. Cardiol. 2013, 168, 4907–4909. [Google Scholar] [CrossRef] [PubMed]
- Marz, W.; Meinitzer, A.; Drechsler, C.; Pilz, S.; Krane, V.; Kleber, M.E.; Fischer, J.; Winkelmann, B.R.; Bohm, B.O.; Ritz, E.; et al. Homoarginine, cardiovascular risk, and mortality. Circulation 2010, 122, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Meinitzer, A.; Tomaschitz, A.; Drechsler, C.; Ritz, E.; Krane, V.; Wanner, C.; Boehm, B.O.; Marz, W. Low homoarginine concentration is a novel risk factor for heart disease. Heart 2011, 97, 1222–1227. [Google Scholar] [CrossRef]
- Pilz, S.; Putz-Bankuti, C.; Meinitzer, A.; Marz, W.; Kienreich, K.; Stojakovic, T.; Pieber, T.R.; Stauber, R.E. Association of homoarginine and methylarginines with liver dysfunction and mortality in chronic liver disease. Amino Acids 2015, 47, 1817–1826. [Google Scholar] [CrossRef]
- Pilz, S.; Teerlink, T.; Scheffer, P.G.; Meinitzer, A.; Rutters, F.; Tomaschitz, A.; Drechsler, C.; Kienreich, K.; Nijpels, G.; Stehouwer, C.D.; et al. Homoarginine and mortality in an older population: The Hoorn study. Eur. J. Clin. Investig. 2014, 44, 200–208. [Google Scholar] [CrossRef]
- Pilz, S.; Tomaschitz, A.; Meinitzer, A.; Drechsler, C.; Ritz, E.; Krane, V.; Wanner, C.; Bohm, B.O.; Marz, W. Low serum homoarginine is a novel risk factor for fatal strokes in patients undergoing coronary angiography. Stroke 2011, 42, 1132–1134. [Google Scholar] [CrossRef] [Green Version]
- Drechsler, C.; Meinitzer, A.; Pilz, S.; Krane, V.; Tomaschitz, A.; Ritz, E.; Marz, W.; Wanner, C. Homoarginine, heart failure, and sudden cardiac death in haemodialysis patients. Eur. J. Heart Fail. 2011, 13, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, S.; O’Reilly, D.S.; Talwar, D.K. HPLC analysis of asymmetric dimethylarginine (ADMA) and related arginine metabolites in human plasma using a novel non-endogenous internal standard. Clin. Chim. Acta Int. J. Clin. Chem. 2009, 401, 14–19. [Google Scholar] [CrossRef]
- Jones, C.E.; Darcy, C.J.; Woodberry, T.; Anstey, N.M.; McNeil, Y.R. HPLC analysis of asymmetric dimethylarginine, symmetric dimethylarginine, homoarginine and arginine in small plasma volumes using a Gemini-NX column at high pH. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 8–12. [Google Scholar] [CrossRef]
- Martens-Lobenhoffer, J.; Bode-Boger, S.M. Fast and efficient determination of arginine, symmetric dimethylarginine, and asymmetric dimethylarginine in biological fluids by hydrophilic-interaction liquid chromatography-electrospray tandem mass spectrometry. Clin. Chem. 2006, 52, 488–493. [Google Scholar] [CrossRef] [Green Version]
- Martens-Lobenhoffer, J.; Bode-Boger, S.M. Quantification of L-arginine, asymmetric dimethylarginine and symmetric dimethylarginine in human plasma: A step improvement in precision by stable isotope dilution mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 904, 140–143. [Google Scholar] [CrossRef]
- Martens-Lobenhoffer, J.; Schwedhelm, E.; Tsikas, D. Quantification of arginine and its mono- and dimethylated analogs NMMA, ADMA and SDMA in biological fluids by LC-MS/MS: Is LC superfluous? J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 3261–3266. [Google Scholar] [CrossRef]
- Shin, S.; Fung, S.M.; Mohan, S.; Fung, H.L. Simultaneous bioanalysis of L-arginine, L-citrulline, and dimethylarginines by LC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 467–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teerlink, T.; Nijveldt, R.J.; de Jong, S.; van Leeuwen, P.A. Determination of arginine, asymmetric dimethylarginine, and symmetric dimethylarginine in human plasma and other biological samples by high-performance liquid chromatography. Anal. Biochem. 2002, 303, 131–137. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research; Center for Veterinary Medicine. Bioanalytical Method Validation Guidance for Industry; U.S. Department of Health and Human Services: Washington, DC, USA, 2018. [Google Scholar]
- Squellerio, I.; Tremoli, E.; Cavalca, V. Quantification of arginine and its metabolites in human erythrocytes using liquid chromatography-tandem mass spectrometry. Anal. Biochem. 2011, 412, 108–110. [Google Scholar] [CrossRef]
- Brown, C.M.; Becker, J.O.; Wise, P.M.; Hoofnagle, A.N. Simultaneous determination of 6 L-arginine metabolites in human and mouse plasma by using hydrophilic-interaction chromatography and electrospray tandem mass spectrometry. Clin. Chem. 2011, 57, 701–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, F.; Cen, S.Y.; He, H.; Liu, Y.; Yuan, B.F.; Feng, Y.Q. Peptidylation for the determination of low-molecular-weight compounds by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Analyst 2016, 141, 3259–3265. [Google Scholar] [CrossRef]
- Zhang, W.Z.; Kaye, D.M. Simultaneous determination of arginine and seven metabolites in plasma by reversed-phase liquid chromatography with a time-controlled ortho-phthaldialdehyde precolumn derivatization. Anal. Biochem. 2004, 326, 87–92. [Google Scholar] [CrossRef] [PubMed]
- van Dyk, M.; Mangoni, A.A.; McEvoy, M.; Attia, J.R.; Sorich, M.J.; Rowland, A. Targeted arginine metabolomics: A rapid, simple UPLC-QToF-MS(E) based approach for assessing the involvement of arginine metabolism in human disease. Clin. Chim. Acta 2015, 447, 59–65. [Google Scholar] [CrossRef]
- Forstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.H.; Wang, Z.; Cho, L.; Brennan, D.M.; Hazen, S.L. Diminished global arginine bioavailability and increased arginine catabolism as metabolic profile of increased cardiovascular risk. J. Am. Coll. Cardiol. 2009, 53, 2061–2067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tommasi, S.; Elliot, D.J.; Da Boit, M.; Gray, S.R.; Lewis, B.C.; Mangoni, A.A. Homoarginine and inhibition of human arginase activity: Kinetic characterization and biological relevance. Sci. Rep. 2018, 8, 3697. [Google Scholar] [CrossRef] [PubMed]
- Vallance, P.; Leone, A.; Calver, A.; Collier, J.; Moncada, S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 1992, 339, 572–575. [Google Scholar]
- Bode-Boger, S.M.; Scalera, F.; Kielstein, J.T.; Martens-Lobenhoffer, J.; Breithardt, G.; Fobker, M.; Reinecke, H. Symmetrical dimethylarginine: A new combined parameter for renal function and extent of coronary artery disease. J. Am. Soc. Nephrol. JASN 2006, 17, 1128–1134. [Google Scholar] [CrossRef]
- Closs, E.I.; Basha, F.Z.; Habermeier, A.; Forstermann, U. Interference of L-arginine analogues with L-arginine transport mediated by the y+ carrier hCAT-2B. Nitric Oxide 1997, 1, 65–73. [Google Scholar] [CrossRef]
- Pope, A.J.; Karuppiah, K.; Cardounel, A.J. Role of the PRMT-DDAH-ADMA axis in the regulation of endothelial nitric oxide production. Pharmacol Res. 2009, 60, 461–465. [Google Scholar] [CrossRef] [Green Version]
- Sydow, K.; Munzel, T. ADMA and oxidative stress. Atheroscler. Suppl. 2003, 4, 41–51. [Google Scholar] [CrossRef]
- Miyazaki, H.; Matsuoka, H.; Cooke, J.P.; Usui, M.; Ueda, S.; Okuda, S.; Imaizumi, T. Endogenous nitric oxide synthase inhibitor: A novel marker of atherosclerosis. Circulation 1999, 99, 1141–1146. [Google Scholar] [CrossRef] [Green Version]
- Schlesinger, S.; Sonntag, S.R.; Lieb, W.; Maas, R. Asymmetric and Symmetric Dimethylarginine as Risk Markers for Total Mortality and Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of Prospective Studies. PLoS ONE 2016, 11, e0165811. [Google Scholar] [CrossRef]
- Johnson, F.K.; Johnson, R.A.; Peyton, K.J.; Durante, W. Arginase inhibition restores arteriolar endothelial function in Dahl rats with salt-induced hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R1057-62. [Google Scholar] [CrossRef]
- Ming, X.F.; Barandier, C.; Viswambharan, H.; Kwak, B.R.; Mach, F.; Mazzolai, L.; Hayoz, D.; Ruffieux, J.; Rusconi, S.; Montani, J.P.; et al. Thrombin stimulates human endothelial arginase enzymatic activity via RhoA/ROCK pathway: Implications for atherosclerotic endothelial dysfunction. Circulation 2004, 110, 3708–3714. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Hein, T.W.; Wang, W.; Miller, M.W.; Fossum, T.W.; McDonald, M.M.; Humphrey, J.D.; Kuo, L. Upregulation of vascular arginase in hypertension decreases nitric oxide-mediated dilation of coronary arterioles. Hypertension 2004, 44, 935–943. [Google Scholar] [CrossRef] [Green Version]
- Heiss, C.; Lauer, T.; Dejam, A.; Kleinbongard, P.; Hamada, S.; Rassaf, T.; Matern, S.; Feelisch, M.; Kelm, M. Plasma nitroso compounds are decreased in patients with endothelial dysfunction. J. Am. Coll. Cardiol. 2006, 47, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.D.; Khan, T.; Zeballos, G.A.; Mathew, L.; Potharlanka, P.; Knecht, M.; Whelan, J. Decreased activity of the L-arginine-nitric oxide metabolic pathway in patients with congestive heart failure. Circulation 1999, 99, 2113–2117. [Google Scholar] [CrossRef] [PubMed]
- Kleinbongard, P.; Dejam, A.; Lauer, T.; Jax, T.; Kerber, S.; Gharini, P.; Balzer, J.; Zotz, R.B.; Scharf, R.E.; Willers, R.; et al. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic. Biol. Med. 2006, 40, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, S.; Wang, H.; Wu, W.; Ye, Y. Arginine methylation dysfunction increased risk of acute coronary syndrome in coronary artery disease population: A case-control study. Medicine 2017, 96, e6074. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.L.; Jones, J.; Bolleddu, S.I.; Vanthenapalli, S.; Rodgers, L.E.; Shah, K.; Karia, K.; Panguluri, S.K. Cardiovascular Risks Associated with Gender and Aging. J. Cardiovasc. Dev. Dis. 2019, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- North, B.J.; Sinclair, D.A. The intersection between aging and cardiovascular disease. Circ. Res. 2012, 110, 1097–1108. [Google Scholar] [CrossRef]
- Nettleship, J.; Jones, R.; Channer, K.; Jones, T. Testosterone and coronary artery disease. Front. Horm. Res. 2009, 37, 91–107. [Google Scholar] [PubMed]
- Cattaneo, M.G.; Banfi, C.; Brioschi, M.; Lattuada, D.; Vicentini, L.M. Sex-dependent differences in the secretome of human endothelial cells. Biol. Sex. Differ. 2021, 12, 7. [Google Scholar] [CrossRef]
- Lew, J.; Sanghavi, M.; Ayers, C.R.; McGuire, D.K.; Omland, T.; Atzler, D.; Gore, M.O.; Neeland, I.; Berry, J.D.; Khera, A.; et al. Sex-Based Differences in Cardiometabolic Biomarkers. Circulation 2017, 135, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Evans, M.I. Estrogen modulates the expression of L-arginine: Glycine amidinotransferase in chick liver. Mol. Cell Biochem. 2001, 221, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Atzler, D.; Baum, C.; Ojeda, F.; Keller, T.; Cordts, K.; Schnabel, R.B.; Choe, C.U.; Lackner, K.J.; Munzel, T.; Boger, R.H.; et al. Low Homoarginine Levels in the Prognosis of Patients with Acute Chest Pain. J. Am. Heart Assoc. 2016, 5, e002565. [Google Scholar] [CrossRef] [Green Version]
- Pilz, S.; Edelmann, F.; Meinitzer, A.; Gelbrich, G.; Doner, U.; Dungen, H.D.; Tomaschitz, A.; Kienreich, K.; Gaksch, M.; Duvinage, A.; et al. Associations of methylarginines and homoarginine with diastolic dysfunction and cardiovascular risk factors in patients with preserved left ventricular ejection fraction. J. Card. Fail. 2014, 20, 923–930. [Google Scholar] [CrossRef]
- Jud, P.; Hafner, F.; Verheyen, N.; Meinitzer, A.; Gary, T.; Brodmann, M.; Seinost, G.; Hackl, G. Homoarginine/ADMA ratio and homoarginine/SDMA ratio as independent predictors of cardiovascular mortality and cardiovascular events in lower extremity arterial disease. Sci. Rep. 2018, 8, 14197. [Google Scholar] [CrossRef]
- Rodionov, R.N.; Begmatov, H.; Jarzebska, N.; Patel, K.; Mills, M.T.; Ghani, Z.; Khakshour, D.; Tamboli, P.; Patel, M.N.; Abdalla, M.; et al. Homoarginine Supplementation Prevents Left Ventricular Dilatation and Preserves Systolic Function in a Model of Coronary Artery Disease. J. Am. Heart Assoc. 2019, 8, e012486. [Google Scholar] [CrossRef] [Green Version]
- Porro, B.; Eligini, S.; Veglia, F.; Lualdi, A.; Squellerio, I.; Fiorelli, S.; Giovannardi, M.; Chiorino, E.; Dalla Cia, A.; Crisci, M.; et al. Nitric oxide synthetic pathway in patients with microvascular angina and its relations with oxidative stress. Oxid. Med. Cell Longev. 2014, 2014, 726539. [Google Scholar] [CrossRef]
- Chen, J.W.; Hsu, N.W.; Wu, T.C.; Lin, S.J.; Chang, M.S. Long-term angiotensin-converting enzyme inhibition reduces plasma asymmetric dimethylarginine and improves endothelial nitric oxide bioavailability and coronary microvascular function in patients with syndrome X. Am. J. Cardiol. 2002, 90, 974–982. [Google Scholar] [CrossRef]
- Piatti, P.; Fragasso, G.; Monti, L.D.; Setola, E.; Lucotti, P.; Fermo, I.; Paroni, R.; Galluccio, E.; Pozza, G.; Chierchia, S.; et al. Acute intravenous L-arginine infusion decreases endothelin-1 levels and improves endothelial function in patients with angina pectoris and normal coronary arteriograms: Correlation with asymmetric dimethylarginine levels. Circulation 2003, 107, 429–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edvardsen, T.; Asch, F.M.; Davidson, B.; Delgado, V.; DeMaria, A.; Dilsizian, V.; Gaemperli, O.; Garcia, M.J.; Kamp, O.; Lee, D.C.; et al. Non-invasive Imaging in Coronary Syndromes—Recommendations of the European Association of Cardiovascular Imaging and the American Society of Echocardiography, in Collaboration with the American Society of Nuclear Cardiology, Society of Cardiovascular Computed Tomography and Society for Cardiovascular Magnetic Resonance. Eur. Heart J. Cardiovasc. Imaging 2021, 00, 1–28. [Google Scholar]
- Porro, B.; Conte, E.; Zaninoni, A.; Bianchi, P.; Veglia, F.; Barbieri, S.; Fiorelli, S.; Eligini, S.; Di Minno, A.; Mushtaq, S.; et al. Red Blood Cell Morphodynamics: A New Potential Marker in High-Risk Patients. Front. Physiol. 2020, 11, 603633. [Google Scholar] [CrossRef] [PubMed]
- Conte, E.; Annoni, A.; Pontone, G.; Mushtaq, S.; Guglielmo, M.; Baggiano, A.; Volpato, V.; Agalbato, C.; Bonomi, A.; Veglia, F.; et al. Evaluation of coronary plaque characteristics with coronary computed tomography angiography in patients with non-obstructive coronary artery disease: A long-term follow-up study. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Di Vito, L.; Agozzino, M.; Marco, V.; Ricciardi, A.; Concardi, M.; Romagnoli, E.; Gatto, L.; Calogero, G.; Tavazzi, L.; Arbustini, E.; et al. Identification and quantification of macrophage presence in coronary atherosclerotic plaques by optical coherence tomography. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 807–813. [Google Scholar] [CrossRef]
- Eligini, S.; Cosentino, N.; Fiorelli, S.; Fabbiocchi, F.; Niccoli, G.; Refaat, H.; Camera, M.; Calligaris, G.; De Martini, S.; Bonomi, A.; et al. Biological profile of monocyte-derived macrophages in coronary heart disease patients: Implications for plaque morphology. Sci. Rep. 2019, 9, 8680. [Google Scholar] [CrossRef] [PubMed]
- Prati, F.; Regar, E.; Mintz, G.S.; Arbustini, E.; Di Mario, C.; Jang, I.K.; Akasaka, T.; Costa, M.; Guagliumi, G.; Grube, E.; et al. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: Physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur. Heart J. 2010, 31, 401–415. [Google Scholar] [CrossRef]
- Scalone, G.; Niccoli, G.; Refaat, H.; Vergallo, R.; Porto, I.; Leone, A.M.; Burzotta, F.; D’Amario, D.; Liuzzo, G.; Fracassi, F.; et al. Not all plaque ruptures are born equal: An optical coherence tomography study. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1271–1277. [Google Scholar] [CrossRef] [Green Version]
- Tearney, G.J.; Regar, E.; Akasaka, T.; Adriaenssens, T.; Barlis, P.; Bezerra, H.G.; Bouma, B.; Bruining, N.; Cho, J.M.; Chowdhary, S.; et al. Consensus standard.ds for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: A report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J. Am. Coll. Cardiol. 2012, 59, 1058–1072. [Google Scholar] [CrossRef] [Green Version]
- Tearney, G.J.; Yabushita, H.; Houser, S.L.; Aretz, H.T.; Jang, I.K.; Schlendorf, K.H.; Kauffman, C.R.; Shishkov, M.; Halpern, E.F.; Bouma, B.E. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation 2003, 107, 113–119. [Google Scholar] [CrossRef]
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef] [PubMed]
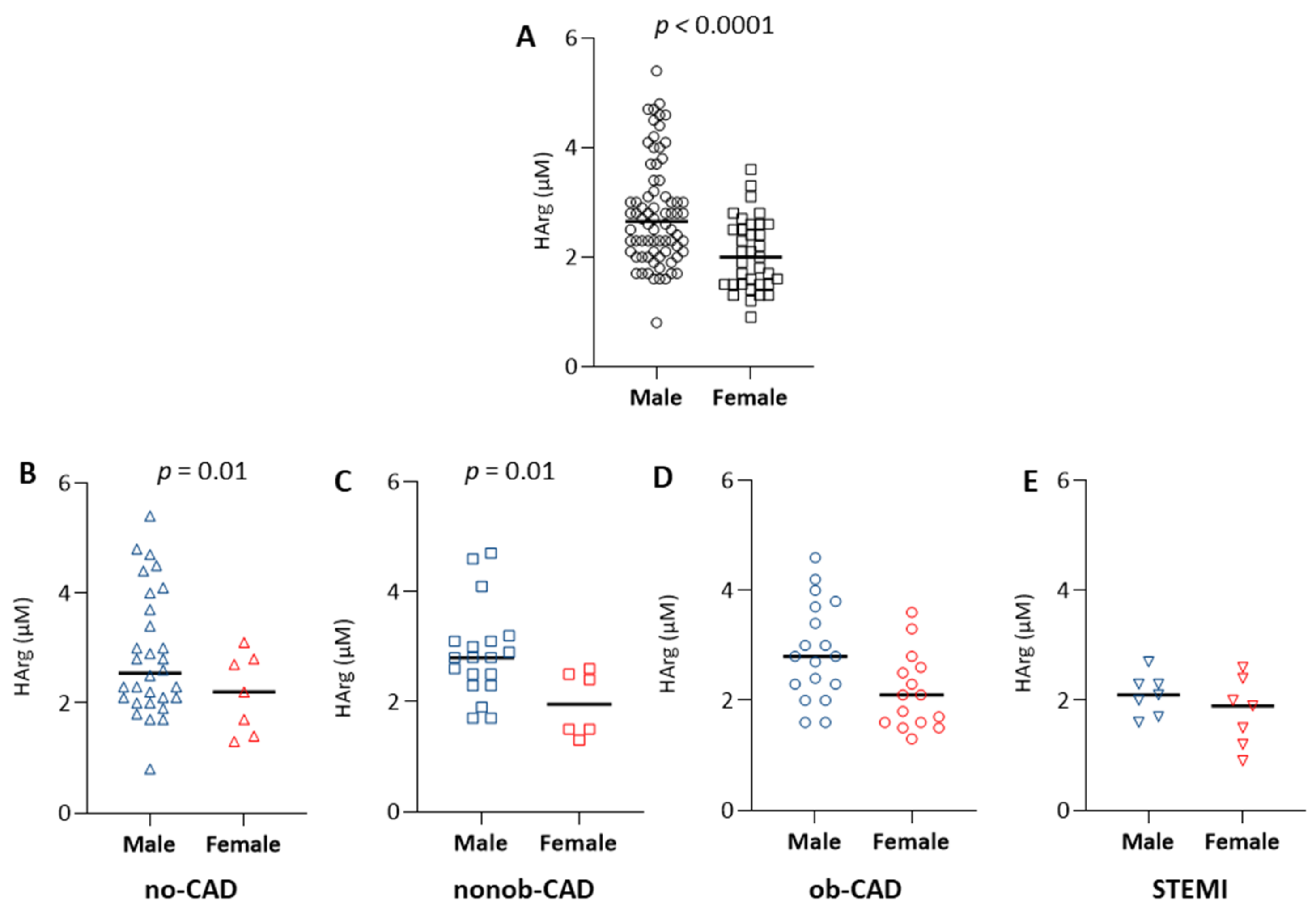
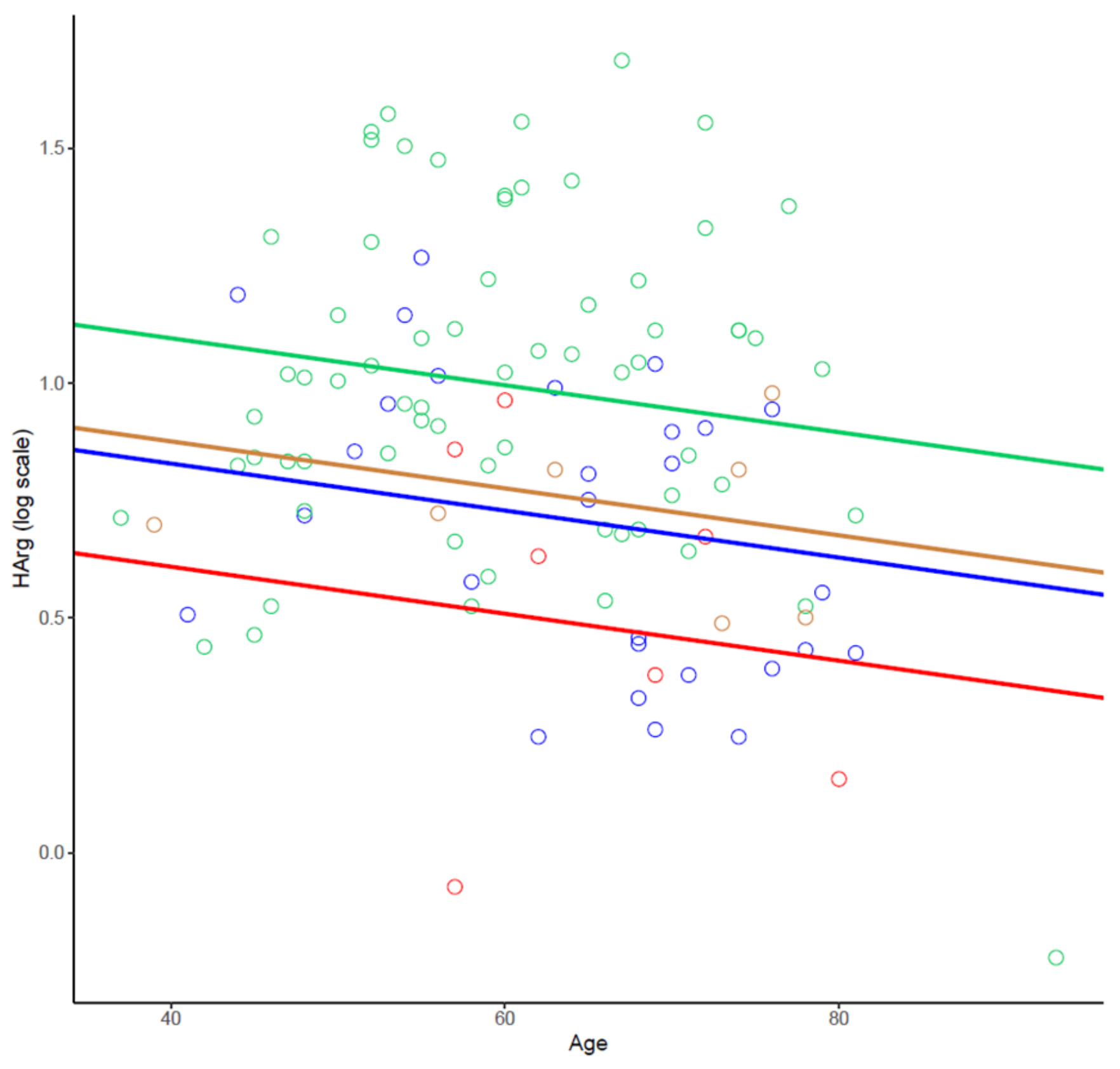
| All Patients (n = 104) | No-CAD (n = 33) | Nonob-CAD (n = 25) | Ob-CAD (n = 32) | STEMI (n = 14) | ANOVA p-Value | |
|---|---|---|---|---|---|---|
| Demographic and clinical characteristics | ||||||
| Age, years | 61.6 ± 10.9 | 57.1 ± 11.5 | 61.3 ± 10.7 | 65.5 ± 9.7 * | 65.4 ± 11.3 | 0.01 |
| Male, n (%) | 69 (66.3) | 18 (54.5) | 19 (76.0) | 24 (75) | 7 (50.0) | 0.12 |
| BMI, kg/m2 | 25.9 ± 3.9 | 25.5 ± 4.5 ◊ | 25.3 ± 3.8 ◊ | 25.7 ± 3.3 ◊ | 29.1 ± 3.0 | 0.01 |
| WBC, 103/μL | 7.5 ± 2.2 | 7.8 ± 2.5 | 7.4 ± 2.2 | 7.1 ± 2.2 | 7.9 ± 2.2 | 0.57 |
| Platelet, 103/μL | 221.2 ± 64.1 | 242.8 ± 70.0 | 220.9 ± 61.9 | 205.5 ± 52.0 | 214.8 ± 80.7 | 0.13 |
| MPV, fL | 10.4 ± 1.3 | 10.5 ± 0.8 | 10.3 ± 1.4 | 10.3 ± 2.0 | 10.7 ± 0.7 | 0.78 |
| RBC, 106/μL | 4.7 ± 0.7 | 4.7 ± 0.5 | 4.8 ± 0.6 | 4.7 ± 0.8 | 4.3 ± 0.8 | 0.15 |
| Hb, g/dL | 14.2 [13.1–15.1] | 13.7 [13–14.6] | 14.4 [13.3–15.2] ◊ | 14.4 [13.4–15.4] ◊ | 12.8 [11.5–13.5] | 0.004 |
| Hct, % | 41.0 ± 5.0 | 40.4 ± 3.2 | 41.6 ± 4.6 | 41.9 ± 6.2 ◊ | 37.6 ± 6.5 | 0.05 |
| MCV, fL | 87.6 ± 4.8 | 86.4 ± 5.5 | 87.6 ± 4.6 | 88.4 ± 3.1 | 87.8 ± 6.3 | 0.40 |
| MCH, pg | 30.2 ± 2.0 | 29.5 ± 2.1 | 30.2 ± 1.9 | 30.7 ± 1.2 | 29.9 ± 2.7 | 0.09 |
| MCHC, % | 34.4 ± 1.0 | 34.1 ± 1 | 34.5 ± 1.0 | 34.8 ± 0.8 * | 34.0 ± 1.1 | 0.01 |
| RDW-CV, % | 13.3 ± 1.0 | 13.2 ± 0.9 | 13.2 ± 0.9 | 13.1 ± 0.9 ◊ | 14.0 ± 1.6 | 0.04 |
| RDW-SD, fL | 41.6 ± 2.9 | 40.8 ± 2.1 ◊ | 41.4 ± 2.8 | 41.6 ± 3.2 | 43.5 ± 2.6 | 0.02 |
| Total cholesterol, mg/dL | 198.6 ± 39.6 | 196.3 ± 35.2 | 199.5 ± 38.2 | 206.8 ± 36.1 | 199.6 ± 43.4 | 0.72 |
| LDL cholesterol, mg/dL | 119.9 ± 35.4 | 116.9 ± 29.1 | 119.9 ± 34.3 | 129.3 ± 31.4 | 128.1 ± 37.0 | 0.40 |
| HDL cholesterol, mg/dL | 56.0 ± 17.2 | 60.2 ± 14.8 ◊ | 57.4 ± 15.5 ◊ | 54.8 ± 12.3 ◊ | 42.9 ± 13.3 | 0.002 |
| Triglycerides, mg/dL | 115.0 ± 55.9 | 97.6 ± 47.6 ◊ | 106.6 ± 48.9 ◊ | 113.4 ± 49.5 ◊ | 178.9 ± 55.4 | <0.0001 |
| Basal glucose, mg/dL | 103.8 ± 22.6 | 103.3 ± 19.7 ◊ | 98.6 ± 15.3 ◊ | 100.8 ± 16.2 ◊ | 131.4 ± 34.3 | <0.0001 |
| Hypertension, n (%) | 19 (18.3) | 14 (42.4) ◊ | 1 (4.0) *,°,◊ | 17 (53.1) ◊ | 12 (85.7) | <0.0001 |
| Family history of CVD, n (%) | 22 (21.1) | 10 (30.3) | 3 (12.0) | 11 (34.4) ◊ | 8 (57.1) | 0.03 |
| Dyslipidaemia, n (%) | 50 (48.1) | 13 (39.4) | 14 (56.0) | 10 (31.2) | 6 (42.8) | 0.30 |
| Diabetes, n (%) | 7 (6.7) | 2 (6.1) ◊ | 0 (0) ◊ | 2 (6.2) ◊ | 5 (35.7) | 0.001 |
| Active smokers, n (%) | 13 (12.5) | 5 (15.2) ◊ | 1 (4.0) ◊ | 6 (18.7) ◊ | 10 (71.4) | <0.0001 |
| Pharmacological treatments | ||||||
| β-blockers, n (%) | 25 (24.0) | 16 (48.5) ◊ | 6 (24.0) | 20 (62.5) °,◊ | 2 (14.3) | 0.003 |
| ACE-inhibitors, n (%) | 21 (20.2) | 6 (18.2) | 4 (16.0) | 6 (18.7) | 4 (28.6) | 0.79 |
| Aspirin, n (%) | 28 (26.9) | 8 (24.4) | 4 (16.0) | 8 (25) | 7 (50.0) | 0.13 |
| Statins, n (%) | 31 (29.8) | 7 (21.1) | 12 (48.0) | 10 (31.2) | 4 (28.6) | 0.16 |
| No-CAD (n = 33) | Nonob-CAD (n = 25) | Ob-CAD (n = 32) | STEMI (n = 14) | p-Value Not Adjusted | p-Value Adjusted for Age | p-Value Adjusted for Age and Sex | |
|---|---|---|---|---|---|---|---|
| Arg, μM | 87.8 ± 21.8 ◊ | 92.0 ± 18.0 ◊ | 90.4 ± 22.4 | 62.0 ± 23.3 | 0.0002 | 0.0003 | <0.0001 |
| Cit, μM | 33.2 ± 6.3 | 32.5 ± 8.4 | 33.8 ± 7.4 | 27.4 ± 9.3 | 0.70 | 0.06 | 0.048 |
| Orn, μM | 68.0 [61.7;77.2] ◊ | 61.5 [54.1;74.6] ◊ | 69.8 [62.3;77.5] ◊ | 99.9 [69.3;105.1] | 0.006 | 0.003 | 0.003 |
| Harg, μM | 2.4 [1.8;3.0] | 2.6 [2.3;3.0] | 2.5 [2.0;3.1] | 2.0 [1.6;2.3] | 0.03 | 0.04 | 0.16 |
| ADMA, μM | 0.36 ± 0.1 | 0.37 ± 0.1 | 0.36 ± 0.1 | 0.37 ± 0.1 | 0.95 | 0.81 | 0.87 |
| SDMA, μM | 0.7 [0.6;0.9] ◊ | 0.8 [0.6;1.0] ◊ | 0.8 [0.6;1.1] ◊ | 1.0 [0.9;1.3] | 0.01 | 0.002 | 0.002 |
| MMA, μM | 0.1 [0.1;0.1] | 0.1 [0.1;0.1] | 0.1 [0.1;0.1] | 0.1 [0.1;0.1] | 0.05 | 0.18 | 0.20 |
| Arg/Orn + Cit | 0.9 ± 0.2 | 0.9 ± 0.2 ◊ | 0.9 ± 0.3 ◊ | 0.5 ± 0.2 | <0.0001 | <0.0001 | <0.0001 |
| Arg/ADMA + MMA | 188.3 ± 45.8 ◊ | 193.2 ± 50.0 ◊ | 194.5 ± 59.5 ◊ | 125.7 ± 41.6 | <0.0001 | 0.0002 | 0.0002 |
| Arg/SDMA | 119.4 ± 38.8 ◊ | 124.6 ± 54.8 ◊ | 118.6 ± 50.1 ◊ | 60.0 ± 32.5 | <0.0001 | 0.0005 | <0.0001 |
| Arg/ADMA + MMA + SDMA | 71.9 ± 19.5 ◊ | 73.6 ± 23.1 ◊ | 71.9 ± 24.5 ◊ | 39.8 ± 19.1 | <0.0001 | <0.0001 | <0.0001 |
| Orn/Cit | 2.0 [1.9;2.3] ◊ | 1.9 [1.7;2.4] ◊ | 2.1 [1.7;2.5] ◊ | 3.1 [2.8;3.6] | <0.0001 | <0.0001 | <0.0001 |
| Harg/SDMA | 3.4 [1.7;5.2] ◊ | 3.2 [2.6;4.7] ◊ | 3.2 [2.0;5.0] ◊ | 2.1 [1.2;2.6] | 0.004 | 0.002 | 0.004 |
| Harg/ADMA + MMA + SDMA | 2.1 [1.2;3.0] ◊ | 2.1 [1.7;2.5] ◊ | 2.1 [1.3;2.9] ◊ | 1.5 [0.9;1.6] | 0.006 | 0.003 | 0.009 |
| HArg/ADMA + MMA | 5.3 [3.9;7.1] | 5.6 [4.3;6.3] | 5.3 [4.1;7.1] | 4.2 [2.6;5.5] | 0.097 | 0.04 | 0.19 |
| Harg/Orn | 0.04 [0.05;0.05] ◊ | 0.04 [0.03;0.05] ◊ | 0.04 [0.03;0.05] ◊ | 0.02 [0.02;0.03] | 0.001 | 0.003 | 0.001 |
| Harg/Orn + Cit | 0.02 [0.02;0.03] | 0.03 [0.02;0.03] ◊ | 0.02 [0.02;0.03] | 0.02 [0.01;0.02] | 0.03 | 0.02 | 0.051 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porro, B.; Eligini, S.; Conte, E.; Cosentino, N.; Capra, N.; Cavalca, V.; Banfi, C. An Optimized MRM-Based Workflow of the l-Arginine/Nitric Oxide Pathway Metabolites Revealed Disease- and Sex-Related Differences in the Cardiovascular Field. Int. J. Mol. Sci. 2022, 23, 1136. https://doi.org/10.3390/ijms23031136
Porro B, Eligini S, Conte E, Cosentino N, Capra N, Cavalca V, Banfi C. An Optimized MRM-Based Workflow of the l-Arginine/Nitric Oxide Pathway Metabolites Revealed Disease- and Sex-Related Differences in the Cardiovascular Field. International Journal of Molecular Sciences. 2022; 23(3):1136. https://doi.org/10.3390/ijms23031136
Chicago/Turabian StylePorro, Benedetta, Sonia Eligini, Edoardo Conte, Nicola Cosentino, Nicolò Capra, Viviana Cavalca, and Cristina Banfi. 2022. "An Optimized MRM-Based Workflow of the l-Arginine/Nitric Oxide Pathway Metabolites Revealed Disease- and Sex-Related Differences in the Cardiovascular Field" International Journal of Molecular Sciences 23, no. 3: 1136. https://doi.org/10.3390/ijms23031136
APA StylePorro, B., Eligini, S., Conte, E., Cosentino, N., Capra, N., Cavalca, V., & Banfi, C. (2022). An Optimized MRM-Based Workflow of the l-Arginine/Nitric Oxide Pathway Metabolites Revealed Disease- and Sex-Related Differences in the Cardiovascular Field. International Journal of Molecular Sciences, 23(3), 1136. https://doi.org/10.3390/ijms23031136








