Anti-Inflammatory Effect of the Natural H2S-Donor Erucin in Vascular Endothelium
Abstract
1. Introduction
2. Results
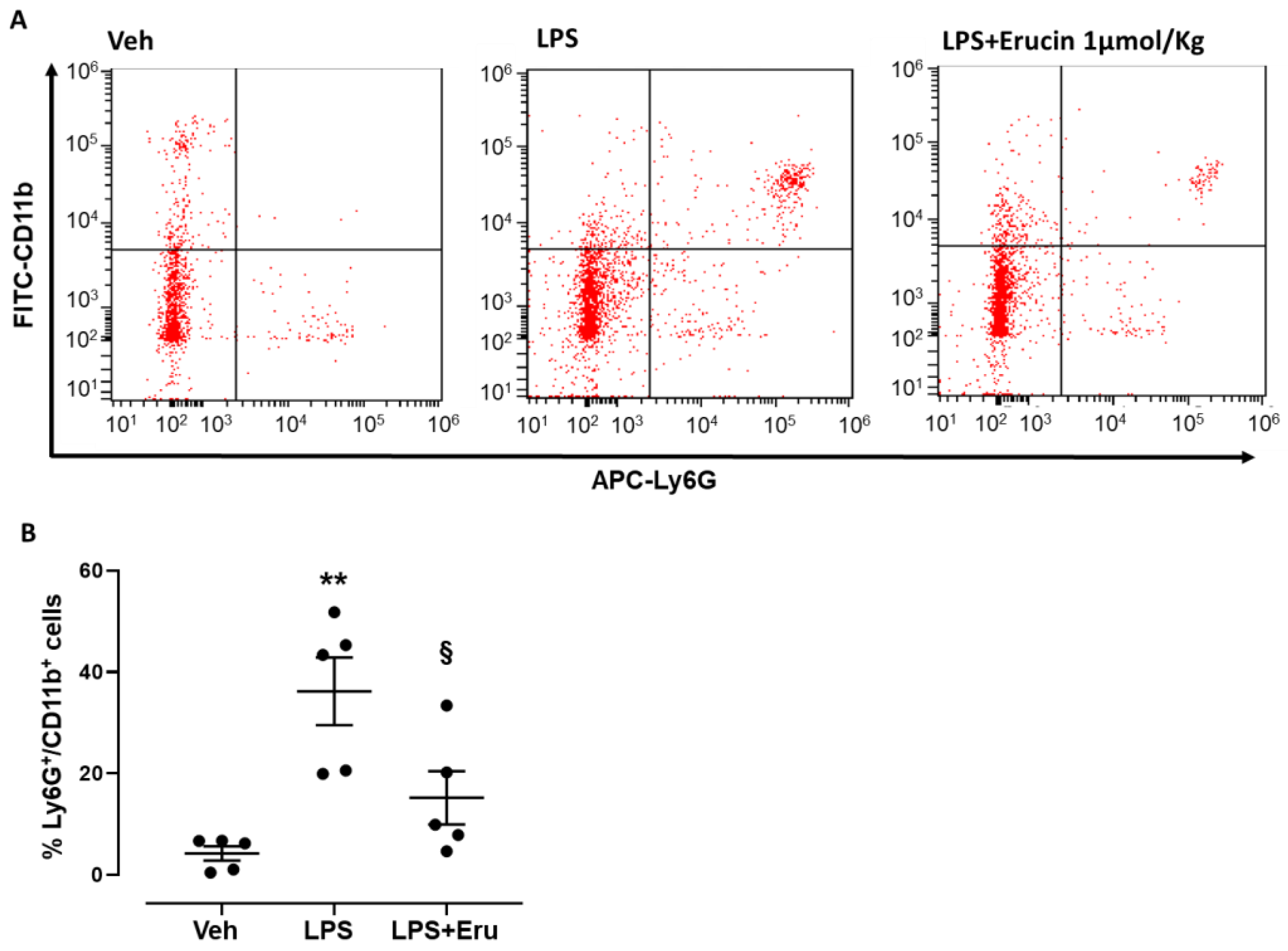
2.1. In Vivo Model of Peritonitis
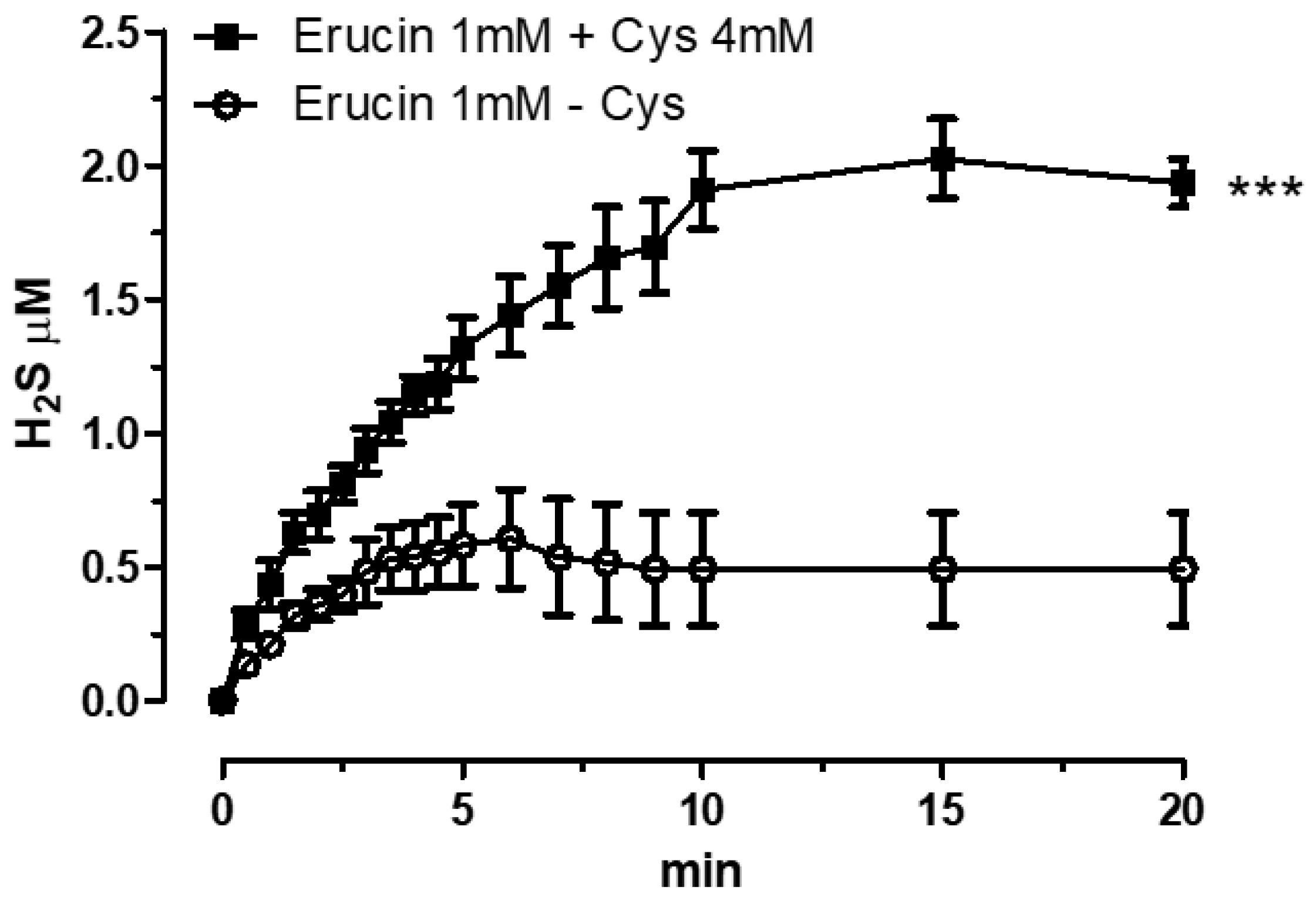
2.2. H2S-Releasing Properties of Erucin via Amperometric Assay
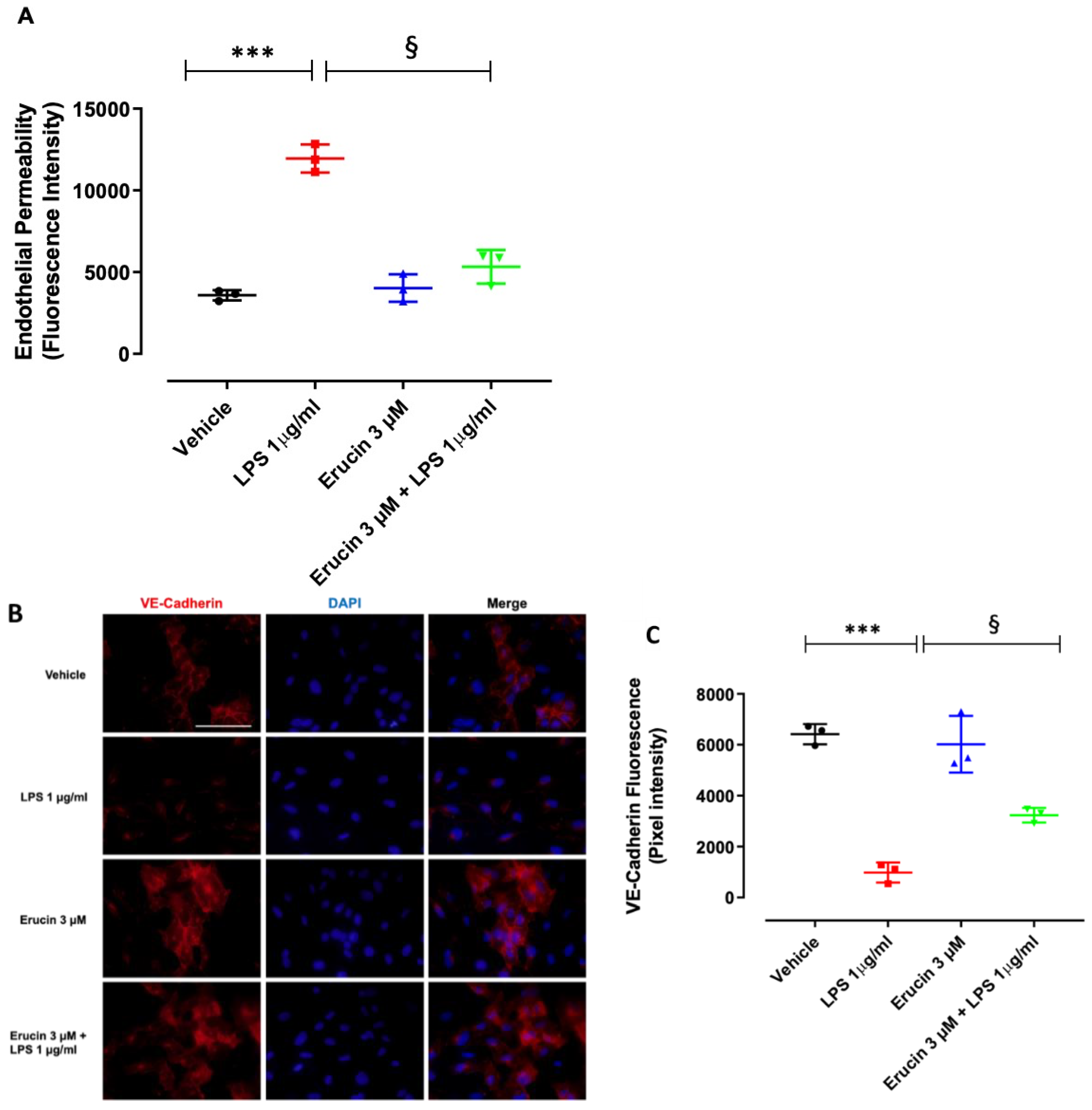
2.3. Effect of Erucin on Endothelial Permeability and VE-Cadherin Changes in Response to LPS
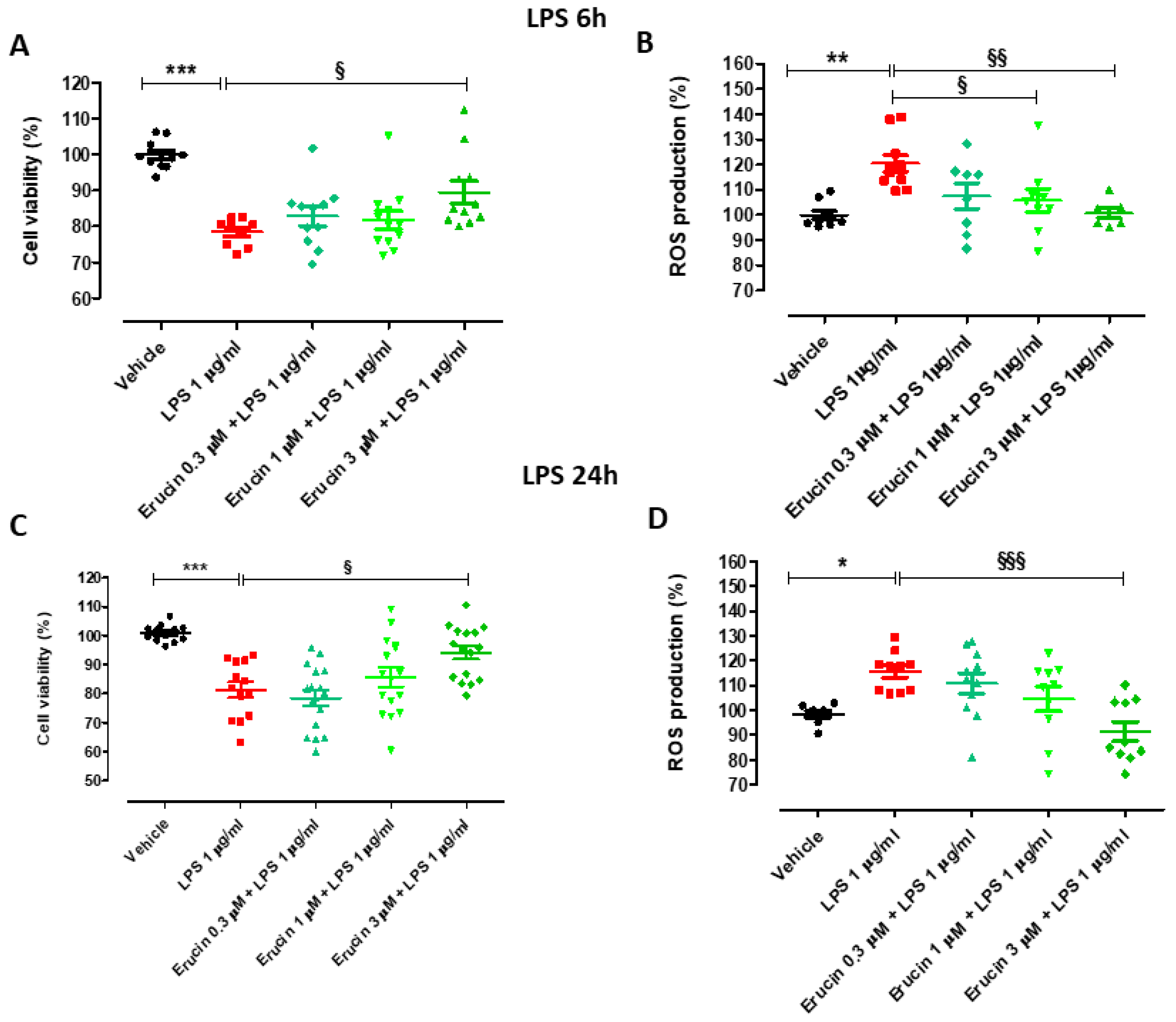
2.4. Preventive Effects of Erucin against Cell Viability Reduction and Intracellular ROS Increase in HUVECs Challenged with LPS
2.5. Evaluation of Erucin Effect on VCAM-1, E-Selectin, COX-2 and mPGES-1 Expression Induced by LPS
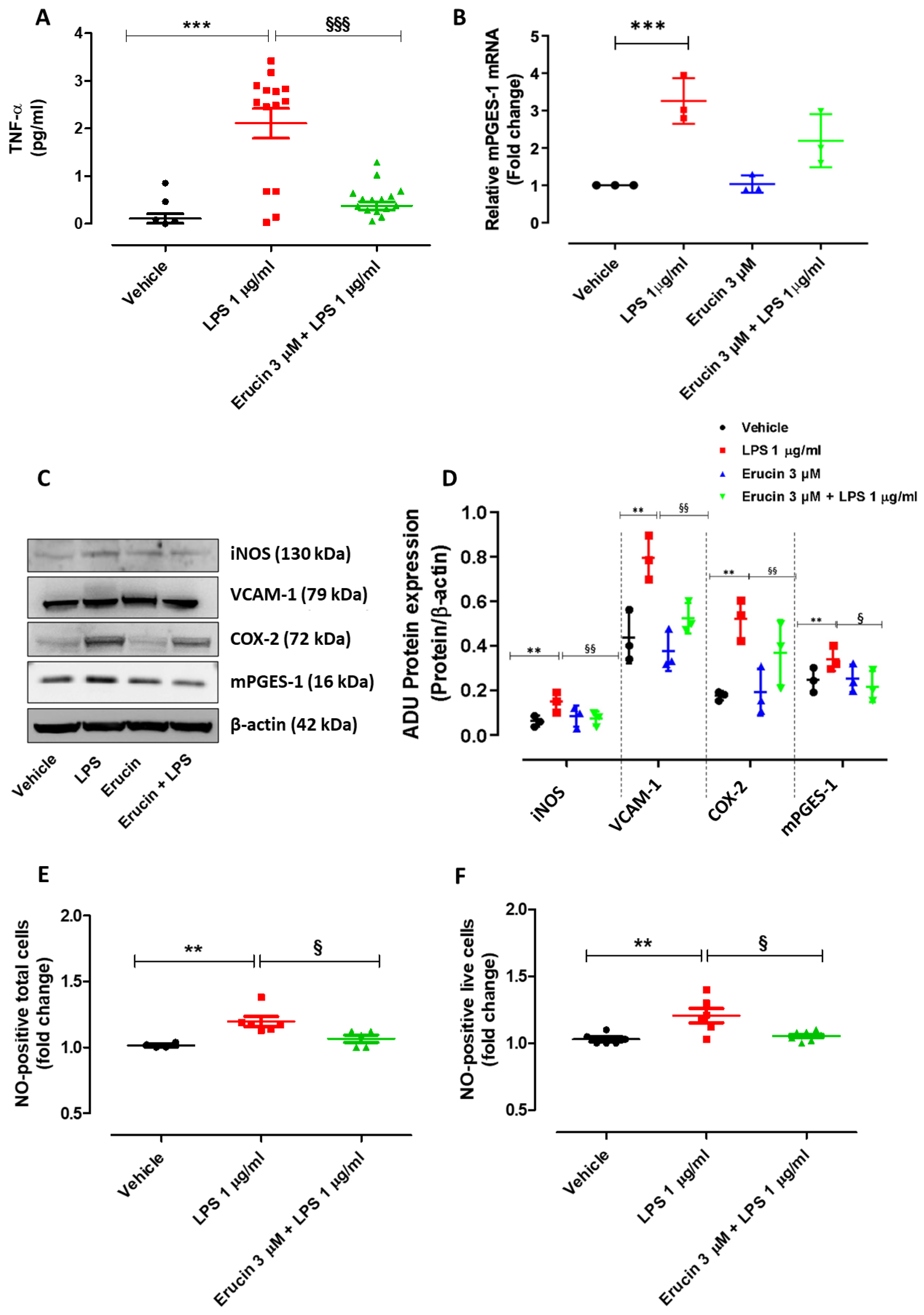
2.6. Erucin Prevents the Increase in TNF-α Levels, Expression of Inflammatory Markers and Enzymes (iNOS, COX-2/mPGES-1 and VCAM-1), and the Number of NO-Positive Cells Induced by Treatment with LPS for 24 h
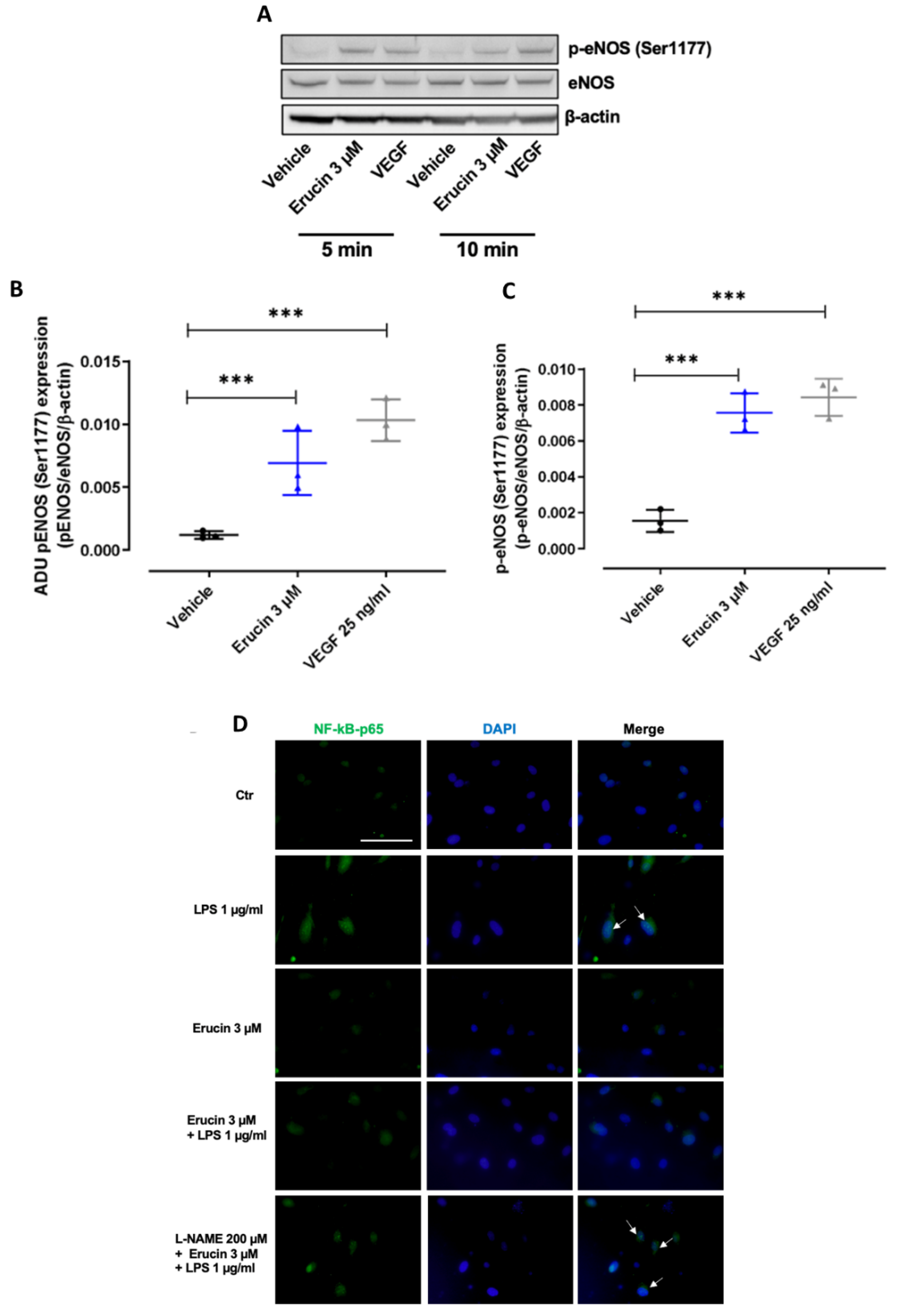
2.7. Erucin Regulates the Nuclear Translocation of NF-kB Induced by LPS through eNOS Activation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals and LPS-Induced Peritonitis
4.3. Flow Cytometry
4.4. Amperometric Evaluation of the H2S-Releasing Properties of Erucin
4.5. Cell Cultures
4.6. Cell Viability
4.7. Measurement of Intracellular Reactive Oxygen Species (ROS) Levels
4.8. Endothelial Permeability
4.9. Western Blot
4.10. Immunofluorescence Analysis
4.11. RNA Isolation and Reverse Transcription-Quantitative (RT-q) PCR
4.12. ELISA Assay for TNF-α
4.13. Assay to Measure NO Levels
4.14. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
Appendix A


References
- Sanchez-Madrid, F.; Sessa, W.C. Spotlight on mechanisms of vascular inflammation. Cardiovasc. Res. 2010, 86, 171–173. [Google Scholar] [CrossRef][Green Version]
- Sardu, C.; Gambardella, J.; Morelli, M.B.; Wang, X.; Marfella, R.; Santulli, G. Hypertension, Thrombosis, Kidney Failure, and Diabetes: Is COVID-19 an Endothelial Disease? A Comprehensive Evaluation of Clinical and Basic Evidence. J. Clin. Med. 2020, 9, 1417. [Google Scholar] [CrossRef]
- Martelli, A.; Citi, V.; Calderone, V. Recent efforts in drug discovery on vascular inflammation and consequent atherosclerosis. Expert Opin. Drug Discov. 2021, 16, 411–427. [Google Scholar] [CrossRef] [PubMed]
- Martelli, A.; Flori, L.; Gorica, E.; Piragine, E.; Saviano, A.; Annunziata, G.; Di Minno, M.N.D.; Ciampaglia, R.; Calcaterra, I.; Maione, F.; et al. Vascular Effects of the Polyphenolic Nutraceutical Supplement Taurisolo((R)): Focus on the Protection of the Endothelial Function. Nutrients 2021, 13, 1540. [Google Scholar] [CrossRef]
- Citi, V.; Martelli, A.; Gorica, E.; Brogi, S.; Testai, L.; Calderone, V. Role of hydrogen sulfide in endothelial dysfunction: Pathophysiology and therapeutic approaches. J. Adv. Res. 2021, 27, 99–113. [Google Scholar] [CrossRef]
- Citi, V.; Martelli, A.; Brancaleone, V.; Brogi, S.; Gojon, G.; Montanaro, R.; Morales, G.; Testai, L.; Calderone, V. Anti-inflammatory and antiviral roles of hydrogen sulfide: Rationale for considering H2S donors in COVID-19 therapy. Br. J. Pharmacol. 2020, 177, 4931–4941. [Google Scholar] [CrossRef]
- Ciccone, V.; Genah, S.; Morbidelli, L. Endothelium as a Source and Target of H2S to Improve Its Trophism and Function. Antioxidants 2021, 10, 486. [Google Scholar] [CrossRef]
- Song, Z.L.; Zhao, L.; Ma, T.; Osama, A.; Shen, T.; He, Y.; Fang, J. Progress and perspective on hydrogen sulfide donors and their biomedical applications. Med. Res. Rev. 2022, 42, 1930–1977. [Google Scholar] [CrossRef] [PubMed]
- Benavides, G.A.; Squadrito, G.L.; Mills, R.W.; Patel, H.D.; Isbell, T.S.; Patel, R.P.; Darley-Usmar, V.M.; Doeller, J.E.; Kraus, D.W. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. USA 2007, 104, 17977–17982. [Google Scholar] [CrossRef]
- Citi, V.; Martelli, A.; Testai, L.; Marino, A.; Breschi, M.C.; Calderone, V. Hydrogen sulfide releasing capacity of natural isothiocyanates: Is it a reliable explanation for the multiple biological effects of Brassicaceae? Planta Med. 2014, 80, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Martelli, A.; Citi, V.; Testai, L.; Brogi, S.; Calderone, V. Organic Isothiocyanates as Hydrogen Sulfide Donors. Antioxid. Redox Signal. 2020, 32, 110–144. [Google Scholar] [CrossRef] [PubMed]
- Piragine, E.; Citi, V.; Lawson, K.; Calderone, V.; Martelli, A. Potential Effects of Natural H2S-Donors in Hypertension Management. Biomolecules 2022, 12, 581. [Google Scholar] [CrossRef] [PubMed]
- Kamal, R.M.; Abdull Razis, A.F.; Mohd Sukri, N.S.; Perimal, E.K.; Ahmad, H.; Patrick, R.; Djedaini-Pilard, F.; Mazzon, E.; Rigaud, S. Beneficial Health Effects of Glucosinolates-Derived Isothiocyanates on Cardiovascular and Neurodegenerative Diseases. Molecules 2022, 27, 624. [Google Scholar] [CrossRef] [PubMed]
- Piragine, E.; Petri, D.; Giometto, S.; Martelli, A.; Lucenteforte, E.; Calderone, V. Potential effects of Alliaceae and Brassicaceae edible plants on blood glucose levels in patients with type 2 diabetes: A systematic review and meta-analysis of clinical trials. Pharmacol. Res. 2022, 185, 106519. [Google Scholar] [CrossRef]
- Piragine, E.; Citi, V.; Lawson, K.; Calderone, V.; Martelli, A. Regulation of blood pressure by natural sulfur compounds: Focus on their mechanisms of action. Biochem. Pharmacol. 2022, 206, 115302. [Google Scholar] [CrossRef]
- Martelli, A.; Piragine, E.; Citi, V.; Testai, L.; Pagnotta, E.; Ugolini, L.; Lazzeri, L.; Di Cesare Mannelli, L.; Manzo, O.L.; Bucci, M.; et al. Erucin exhibits vasorelaxing effects and antihypertensive activity by H2S-releasing properties. Br. J. Pharmacol. 2020, 177, 824–835. [Google Scholar] [CrossRef]
- Martelli, A.; Piragine, E.; Gorica, E.; Citi, V.; Testai, L.; Pagnotta, E.; Lazzeri, L.; Pecchioni, N.; Ciccone, V.; Montanaro, R.; et al. The H2S-Donor Erucin Exhibits Protective Effects against Vascular Inflammation in Human Endothelial and Smooth Muscle Cells. Antioxidants 2021, 10, 961. [Google Scholar] [CrossRef]
- Serhan, C.N.; Brain, S.D.; Buckley, C.D.; Gilroy, D.W.; Haslett, C.; O’Neill, L.A.; Perretti, M.; Rossi, A.G.; Wallace, J.L. Resolution of inflammation: State of the art, definitions and terms. FASEB J. 2007, 21, 325–332. [Google Scholar] [CrossRef]
- Ortega-Gomez, A.; Perretti, M.; Soehnlein, O. Resolution of inflammation: An integrated view. EMBO Mol. Med. 2013, 5, 661–674. [Google Scholar] [CrossRef]
- Sugimoto, M.A.; Sousa, L.P.; Pinho, V.; Perretti, M.; Teixeira, M.M. Resolution of Inflammation: What Controls Its Onset? Front. Immunol. 2016, 7, 160. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, X.; Lu, Y.; Liang, D.; Huang, D. Isothiocyanates as H2S Donors Triggered by Cysteine: Reaction Mechanism and Structure and Activity Relationship. Org. Lett. 2019, 21, 5977–5980. [Google Scholar] [CrossRef] [PubMed]
- Choy, K.W.; Lau, Y.S.; Murugan, D.; Vanhoutte, P.M.; Mustafa, M.R. Paeonol Attenuates LPS-Induced Endothelial Dysfunction and Apoptosis by Inhibiting BMP4 and TLR4 Signaling Simultaneously but Independently. J. Pharmacol. Exp. Ther. 2018, 364, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Dorrington, M.G.; Fraser, I.D.C. NF-kappaB Signaling in Macrophages: Dynamics, Crosstalk, and Signal Integration. Front. Immunol. 2019, 10, 705. [Google Scholar] [CrossRef]
- Castellon, X.; Bogdanova, V. Chronic Inflammatory Diseases and Endothelial Dysfunction. Aging Dis. 2016, 7, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Chen, S.; Tang, C.; Jin, H.; Du, J.; Huang, Y. Hydrogen sulfide and vascular regulation—An update. J. Adv. Res. 2021, 27, 85–97. [Google Scholar] [CrossRef]
- Zanardo, R.C.; Brancaleone, V.; Distrutti, E.; Fiorucci, S.; Cirino, G.; Wallace, J.L. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006, 20, 2118–2120. [Google Scholar] [CrossRef]
- Brancaleone, V.; Mitidieri, E.; Flower, R.J.; Cirino, G.; Perretti, M. Annexin A1 mediates hydrogen sulfide properties in the control of inflammation. J. Pharmacol. Exp. Ther. 2014, 351, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Magli, E.; Perissutti, E.; Santagada, V.; Caliendo, G.; Corvino, A.; Esposito, G.; Esposito, G.; Fiorino, F.; Migliaccio, M.; Scognamiglio, A.; et al. H2S Donors and Their Use in Medicinal Chemistry. Biomolecules 2021, 11, 1899. [Google Scholar] [CrossRef]
- Oakley, R.; Tharakan, B. Vascular hyperpermeability and aging. Aging Dis. 2014, 5, 114–125. [Google Scholar] [CrossRef]
- Hulsmans, M.; Holvoet, P. The vicious circle between oxidative stress and inflammation in atherosclerosis. J. Cell. Mol. Med. 2010, 14, 70–78. [Google Scholar] [CrossRef]
- Guzik, T.J.; Touyz, R.M. Oxidative Stress, Inflammation, and Vascular Aging in Hypertension. Hypertension 2017, 70, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, F.; Morgenstern, R.; Jakobsson, P.J. A review on mPGES-1 inhibitors: From preclinical studies to clinical applications. Prostaglandins Other Lipid Mediat. 2020, 147, 106383. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Munoz, M.D.; Osma-Garcia, I.C.; Cacheiro-Llaguno, C.; Fresno, M.; Iniguez, M.A. Coordinated up-regulation of cyclooxygenase-2 and microsomal prostaglandin E synthase 1 transcription by nuclear factor kappa B and early growth response-1 in macrophages. Cell. Signal. 2010, 22, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Arias-Salvatierra, D.; Silbergeld, E.K.; Acosta-Saavedra, L.C.; Calderon-Aranda, E.S. Role of nitric oxide produced by iNOS through NF-kappaB pathway in migration of cerebellar granule neurons induced by Lipopolysaccharide. Cell. Signal. 2011, 23, 425–435. [Google Scholar] [CrossRef]
- Cho, H.J.; Lee, K.W.; Park, J.H. Erucin exerts anti-inflammatory properties in murine macrophages and mouse skin: Possible mediation through the inhibition of NFkappaB signaling. Int. J. Mol. Sci. 2013, 14, 20564–20577. [Google Scholar] [CrossRef]
- Forstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]
- Vanhoutte, P.M.; Zhao, Y.; Xu, A.; Leung, S.W. Thirty Years of Saying NO: Sources, Fate, Actions, and Misfortunes of the Endothelium-Derived Vasodilator Mediator. Circ. Res. 2016, 119, 375–396. [Google Scholar] [CrossRef]
- Monti, M.; Terzuoli, E.; Ziche, M.; Morbidelli, L. H(2)S dependent and independent anti-inflammatory activity of zofenoprilat in cells of the vascular wall. Pharmacol. Res. 2016, 113, 426–437. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, D.; Singh, G.; Attri, S.; Singh, D.; Sharma, M.; Buttar, H.S.; Bedi, N.; Singh, B.; Arora, S. Pharmacokinetics and toxicity profiling of 4-(methylthio)butyl isothiocyanate with special reference to pre-clinical safety assessment studies. Toxicon 2022, 212, 19–33. [Google Scholar] [CrossRef]
- Citi, V.; Piragine, E.; Pagnotta, E.; Ugolini, L.; Di Cesare Mannelli, L.; Testai, L.; Ghelardini, C.; Lazzeri, L.; Calderone, V.; Martelli, A. Anticancer properties of erucin, an H2S-releasing isothiocyanate, on human pancreatic adenocarcinoma cells (AsPC-1). Phytother. Res. 2019, 33, 845–855. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Lilley, E.; Stanford, S.C.; Kendall, D.E.; Alexander, S.P.H.; Cirino, G.; Docherty, J.R.; George, C.H.; Insel, P.A.; Izzo, A.A.; Ji, Y.; et al. ARRIVE 2.0 and the British Journal of Pharmacology: Updated guidance for 2020. Br. J. Pharmacol. 2020, 177, 3611–3616. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, V.; Monti, M.; Monzani, E.; Casella, L.; Morbidelli, L. The metal-nonoate Ni(SalPipNONO) inhibits in vitro tumor growth, invasiveness and angiogenesis. Oncotarget 2018, 9, 13353–13365. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, V.; Terzuoli, E.; Ristori, E.; Filippelli, A.; Ziche, M.; Morbidelli, L.; Donnini, S. ALDH1A1 overexpression in melanoma cells promotes tumor angiogenesis by activating the IL8/Notch signaling cascade. Int. J. Mol. Med. 2022, 50, 99. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciccone, V.; Piragine, E.; Gorica, E.; Citi, V.; Testai, L.; Pagnotta, E.; Matteo, R.; Pecchioni, N.; Montanaro, R.; Di Cesare Mannelli, L.; et al. Anti-Inflammatory Effect of the Natural H2S-Donor Erucin in Vascular Endothelium. Int. J. Mol. Sci. 2022, 23, 15593. https://doi.org/10.3390/ijms232415593
Ciccone V, Piragine E, Gorica E, Citi V, Testai L, Pagnotta E, Matteo R, Pecchioni N, Montanaro R, Di Cesare Mannelli L, et al. Anti-Inflammatory Effect of the Natural H2S-Donor Erucin in Vascular Endothelium. International Journal of Molecular Sciences. 2022; 23(24):15593. https://doi.org/10.3390/ijms232415593
Chicago/Turabian StyleCiccone, Valerio, Eugenia Piragine, Era Gorica, Valentina Citi, Lara Testai, Eleonora Pagnotta, Roberto Matteo, Nicola Pecchioni, Rosangela Montanaro, Lorenzo Di Cesare Mannelli, and et al. 2022. "Anti-Inflammatory Effect of the Natural H2S-Donor Erucin in Vascular Endothelium" International Journal of Molecular Sciences 23, no. 24: 15593. https://doi.org/10.3390/ijms232415593
APA StyleCiccone, V., Piragine, E., Gorica, E., Citi, V., Testai, L., Pagnotta, E., Matteo, R., Pecchioni, N., Montanaro, R., Di Cesare Mannelli, L., Ghelardini, C., Brancaleone, V., Morbidelli, L., Calderone, V., & Martelli, A. (2022). Anti-Inflammatory Effect of the Natural H2S-Donor Erucin in Vascular Endothelium. International Journal of Molecular Sciences, 23(24), 15593. https://doi.org/10.3390/ijms232415593












