Xenobiotic-Induced Aggravation of Metabolic-Associated Fatty Liver Disease
Abstract
1. Introduction
2. Main Features of MAFLD
2.1. Clinical Features, Liver Pathology and Blood Chemistry
2.2. Physiopathology of MAFLD
2.3. MAFLD and Changes in Xenobiotic Metabolism
3. Xenobiotics Able to Aggravate MAFLD
3.1. Drugs
3.1.1. Corticosteroids
3.1.2. Thiazolidinediones
3.1.3. Other Drugs
3.2. Environmental Toxicants
3.2.1. Bisphenol A
3.2.2. Perfluorooctanoic Acid
- (1)
- Direct binding to PPARγ [111,159], whose activation increases the expression of FAT/CD36 and different genes involved in DNL, such as SREBP1, acetyl-coenzyme A carboxylase (ACC) and FASN [90,152]. Of note, SREBP1 can activate PPARγ via the production of fatty acid derivatives acting as endogenous ligand(s) [160]. Hence, it would be interesting to determine whether PFOA can directly activate SREBP1 in a PPARγ-independent manner, which might reinforce DNL stimulation secondary to PFOA-induced activation of PPARγ.
- (2)
- PXR activation. On one hand, previous studies showed that PFOA can activate PXR, in particular the human ortholog [111,161], although this has not been confirmed in other investigations [162,163]. On the other hand, many investigations showed that PXR can trigger a steatogenic response in liver [159,164,165]. Hence, it would be interesting to assess the metabolic effects of PFOA in PXR-knockout mice and in human hepatocytes with PXR silencing.
- (3)
- (4)
- (5)
- (6)
- Mobilization of lipids from the adipose tissue due to a loss of fat mass [168]. Indeed, some investigations of mice reported that PFOA exposure significantly reduced body weight and adiposity, even when the animals were fed a HFD [154,155,157,168]. PFOA-induced reduction in fat mass in mice might be mainly due to a lower food intake [168,169], possibly via an uncoupling protein 1-dependent mechanism [169].
3.2.3. Other Environmental Toxicants
3.3. Ethanol
4. Key Mechanisms Involved in Xenobiotic-Induced Aggravation of MAFLD
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eslam, M.; Sanyal, A.J.; George, J.; Sanyal, A.; Neuschwander-Tetri, B.; Tiribelli, C.; Kleiner, D.E.; Brunt, E.; Bugianesi, E.; Yki-Järvinen, H.; et al. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef]
- Tschöp, M.H.; Finan, B.; Clemmensen, C.; Gelfanov, V.; Perez-Tilve, D.; Müller, T.D.; DiMarchi, R.D. Unimolecular Polypharmacy for Treatment of Diabetes and Obesity. Cell Metab. 2016, 24, 51–62. [Google Scholar] [CrossRef]
- Assari, S.; Wisseh, C.; Bazargan, M. Obesity and Polypharmacy among African American Older Adults: Gender as the Moderator and Multimorbidity as the Mediator. Int. J. Environ. Res. Public. Health 2019, 16, 2181. [Google Scholar] [CrossRef] [PubMed]
- Joshi-Barve, S.; Kirpich, I.; Cave, M.C.; Marsano, L.S.; McClain, C.J. Alcoholic, Nonalcoholic, and Toxicant-Associated Steatohepatitis: Mechanistic Similarities and Differences. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.M.; Brunt, E.M. Pathological Features of Fatty Liver Disease. Gastroenterology 2014, 147, 754–764. [Google Scholar] [CrossRef]
- Begriche, K.; Massart, J.; Robin, M.-A.; Borgne-Sanchez, A.; Fromenty, B. Drug-Induced Toxicity on Mitochondria and Lipid Metabolism: Mechanistic Diversity and Deleterious Consequences for the Liver. J. Hepatol. 2011, 54, 773–794. [Google Scholar] [CrossRef]
- Hegarty, R.; Deheragoda, M.; Fitzpatrick, E.; Dhawan, A. Paediatric Fatty Liver Disease (PeFLD): All Is Not NAFLD—Pathophysiological Insights and Approach to Management. J. Hepatol. 2018, 68, 1286–1299. [Google Scholar] [CrossRef]
- Ibrahim, S.H.; Hirsova, P.; Gores, G.J. Non-Alcoholic Steatohepatitis Pathogenesis: Sublethal Hepatocyte Injury as a Driver of Liver Inflammation. Gut 2018, 67, 963–972. [Google Scholar] [CrossRef]
- Fierbinteanu-Braticevici, C. Noninvasive Investigations for Non Alcoholic Fatty Liver Disease and Liver Fibrosis. World J. Gastroenterol. 2010, 16, 4784. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Hashimoto, E.; Ikejima, K.; Uto, H.; Ono, M.; Sumida, Y.; Seike, M.; Takei, Y.; Takehara, T.; Tokushige, K.; et al. Evidence-Based Clinical Practice Guidelines for Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis: Clinical Practice Guidelines. Hepatol. Res. 2015, 45, 363–377. [Google Scholar] [CrossRef]
- Regev, A.; Palmer, M.; Avigan, M.I.; Dimick-Santos, L.; Treem, W.R.; Marcinak, J.F.; Seekins, D.; Krishna, G.; Anania, F.A.; Freston, J.W.; et al. Consensus: Guidelines: Best Practices for Detection, Assessment and Management of Suspected Acute Drug-Induced Liver Injury during Clinical Trials in Patients with Nonalcoholic Steatohepatitis. Aliment. Pharmacol. Ther. 2019, 49, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Farrell, G.C.; Haczeyni, F.; Chitturi, S. Pathogenesis of NASH: How Metabolic Complications of Overnutrition Favour Lipotoxicity and Pro-Inflammatory Fatty Liver Disease. In Obesity, Fatty Liver and Liver Cancer; Yu, J., Ed.; Springer Singapore; Advances in Experimental Medicine and Biology; Singapore, 2018; Volume 1061, pp. 19–44. ISBN 978-981-10-8683-0. [Google Scholar]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular Mechanisms of Hepatic Lipid Accumulation in Non-Alcoholic Fatty Liver Disease. Cell. Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J. Past, Present and Future Perspectives in Nonalcoholic Fatty Liver Disease. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.S.; Bril, F.; Cusi, K.; Newsome, P.N. Modulation of Insulin Resistance in Nonalcoholic Fatty Liver Disease. Hepatology 2019, hep.30429. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, S.G. Endoplasmic Reticulum Stress and Autophagy Dysregulation in Alcoholic and Non-Alcoholic Liver Diseases. Clin. Mol. Hepatol. 2020, 26, 715–727. [Google Scholar] [CrossRef]
- Sookoian, S.; Pirola, C.J.; Valenti, L.; Davidson, N.O. Genetic Pathways in Nonalcoholic Fatty Liver Disease: Insights From Systems Biology. Hepatology 2020, 72, 330–346. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Kawada, N.; Japan Study Group of NAFLD (JSG-NAFLD) Japan Study Group of NAFLD (JSG-NAFLD). The Role of Insulin Resistance and Diabetes in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2020, 21, 3863. [Google Scholar] [CrossRef]
- Abdul-Wahed, A.; Guilmeau, S.; Postic, C. Sweet Sixteenth for ChREBP: Established Roles and Future Goals. Cell Metab. 2017, 26, 324–341. [Google Scholar] [CrossRef]
- Khambu, B.; Yan, S.; Huda, N.; Liu, G.; Yin, X.-M. Autophagy in Non-Alcoholic Fatty Liver Disease and Alcoholic Liver Disease. Liver Res. 2018, 2, 112–119. [Google Scholar] [CrossRef]
- Lebeaupin, C.; Vallée, D.; Hazari, Y.; Hetz, C.; Chevet, E.; Bailly-Maitre, B. Endoplasmic Reticulum Stress Signalling and the Pathogenesis of Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2018, 69, 927–947. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of Oxidative Stress in the Pathogenesis of Nonalcoholic Fatty Liver Disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef] [PubMed]
- Begriche, K.; Massart, J.; Robin, M.-A.; Bonnet, F.; Fromenty, B. Mitochondrial Adaptations and Dysfunctions in Nonalcoholic Fatty Liver Disease. Hepatology 2013, 58, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Allard, J.; Bucher, S.; Massart, J.; Ferron, P.-J.; Le Guillou, D.; Loyant, R.; Daniel, Y.; Launay, Y.; Buron, N.; Begriche, K.; et al. Drug-Induced Hepatic Steatosis in Absence of Severe Mitochondrial Dysfunction in HepaRG Cells: Proof of Multiple Mechanism-Based Toxicity. Cell Biol. Toxicol. 2021, 37, 151–175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bharathi, S.S.; Beck, M.E.; Goetzman, E.S. The Fatty Acid Oxidation Enzyme Long-Chain Acyl-CoA Dehydrogenase Can Be a Source of Mitochondrial Hydrogen Peroxide. Redox Biol. 2019, 26, 101253. [Google Scholar] [CrossRef] [PubMed]
- Perla, F.; Prelati, M.; Lavorato, M.; Visicchio, D.; Anania, C. The Role of Lipid and Lipoprotein Metabolism in Non-Alcoholic Fatty Liver Disease. Children 2017, 4, 46. [Google Scholar] [CrossRef] [PubMed]
- Sunny, N.E.; Bril, F.; Cusi, K. Mitochondrial Adaptation in Nonalcoholic Fatty Liver Disease: Novel Mechanisms and Treatment Strategies. Trends Endocrinol. Metab. 2017, 28, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Koliaki, C.; Szendroedi, J.; Kaul, K.; Jelenik, T.; Nowotny, P.; Jankowiak, F.; Herder, C.; Carstensen, M.; Krausch, M.; Knoefel, W.T.; et al. Adaptation of Hepatic Mitochondrial Function in Humans with Non-Alcoholic Fatty Liver Is Lost in Steatohepatitis. Cell Metab. 2015, 21, 739–746. [Google Scholar] [CrossRef]
- Francque, S.; Verrijken, A.; Caron, S.; Prawitt, J.; Paumelle, R.; Derudas, B.; Lefebvre, P.; Taskinen, M.-R.; Van Hul, W.; Mertens, I.; et al. PPARα Gene Expression Correlates with Severity and Histological Treatment Response in Patients with Non-Alcoholic Steatohepatitis. J. Hepatol. 2015, 63, 164–173. [Google Scholar] [CrossRef]
- Buechler, C.; S. Weiss, T. Does Hepatic Steatosis Affect Drug Metabolizing Enzymes in the Liver? Curr. Drug Metab. 2011, 12, 24–34. [Google Scholar] [CrossRef]
- Song, B.-J.; Akbar, M.; Jo, I.; Hardwick, J.P.; Abdelmegeed, M.A. Translational Implications of the Alcohol-Metabolizing Enzymes, Including Cytochrome P450-2E1, in Alcoholic and Nonalcoholic Liver Disease. In Advances in Pharmacology; Elsevier: New York, NY, USA, 2015; Volume 74, pp. 303–372. ISBN 978-0-12-803119-3. [Google Scholar]
- Cobbina, E.; Akhlaghi, F. Non-Alcoholic Fatty Liver Disease (NAFLD)—Pathogenesis, Classification, and Effect on Drug Metabolizing Enzymes and Transporters. Drug Metab. Rev. 2017, 49, 197–211. [Google Scholar] [CrossRef]
- Smit, C.; De Hoogd, S.; Brüggemann, R.J.M.; Knibbe, C.A.J. Obesity and Drug Pharmacology: A Review of the Influence of Obesity on Pharmacokinetic and Pharmacodynamic Parameters. Expert Opin. Drug Metab. Toxicol. 2018, 14, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, R.; Barlock, B.J. Nonalcoholic Fatty Liver Disease (NAFLD) and Hepatic Cytochrome P450 (CYP) Enzymes. Pharmaceuticals 2020, 13, 222. [Google Scholar] [CrossRef] [PubMed]
- Brill, M.J.E.; Diepstraten, J.; Rongen, A.; Kralingen, S.; Anker, J.N.; Knibbe, C.A.J. Impact of Obesity on Drug Metabolism and Elimination in Adults and Children. Clin. Pharmacokinet. 2012, 51, 277–304. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, M.; Greller, H.A.; Babu, K.M. A Review of the Toxicologic Implications of Obesity. J. Med. Toxicol. 2015, 11, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Li, D.-Y.; Zhang, B.; Sun, J.-Y.; Sun, F.; Ji, X.; Qiu, J.-C.; Parker, R.B.; Laizure, S.C.; Xu, J. Alterations of Drug-Metabolizing Enzymes and Transporters under Diabetic Conditions: What Is the Potential Clinical Significance? Drug Metab. Rev. 2018, 50, 369–397. [Google Scholar] [CrossRef] [PubMed]
- Ferron, P.-J.; Gicquel, T.; Mégarbane, B.; Clément, B.; Fromenty, B. Treatments in COVID-19 Patients with Pre-Existing Metabolic Dysfunction-Associated Fatty Liver Disease: A Potential Threat for Drug-Induced Liver Injury? Biochimie 2020, 179, 266–274. [Google Scholar] [CrossRef]
- Le Guillou, D.; Bucher, S.; Begriche, K.; Hoët, D.; Lombès, A.; Labbe, G.; Fromenty, B. Drug-Induced Alterations of Mitochondrial DNA Homeostasis in Steatotic and Nonsteatotic HepaRG Cells. J. Pharmacol. Exp. Ther. 2018, 365, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Massart, J.; Begriche, K.; Moreau, C.; Fromenty, B. Role of Nonalcoholic Fatty Liver Disease as Risk Factor for Drug-Induced Hepatotoxicity. J. Clin. Transl. Res. 2017, 3, 212–232. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Razzak, Z.; Loyer, P.; Fautrel, A.; Gautier, J.C.; Corcos, L.; Turlin, B.; Beaune, P.; Guillouzo, A. Cytokines Down-Regulate Expression of Major Cytochrome P-450 Enzymes in Adult Human Hepatocytes in Primary Culture. Mol. Pharmacol. 1993, 44, 707–715. [Google Scholar]
- Aninat, C.; Seguin, P.; Descheemaeker, P.-N.; Morel, F.; Malledant, Y.; Guillouzo, A. Catecholamines Induce an Inflammatory Response in Human Hepatocytes. Crit. Care Med. 2008, 36, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Aubert, J.; Begriche, K.; Knockaert, L.; Robin, M.A.; Fromenty, B. Increased Expression of Cytochrome P450 2E1 in Nonalcoholic Fatty Liver Disease: Mechanisms and Pathophysiological Role. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 630–637. [Google Scholar] [CrossRef]
- Michaut, A.; Le Guillou, D.; Moreau, C.; Bucher, S.; McGill, M.R.; Martinais, S.; Gicquel, T.; Morel, I.; Robin, M.-A.; Jaeschke, H.; et al. A Cellular Model to Study Drug-Induced Liver Injury in Nonalcoholic Fatty Liver Disease: Application to Acetaminophen. Toxicol. Appl. Pharmacol. 2016, 292, 40–55. [Google Scholar] [CrossRef]
- Massart, J.; Begriche, K.; Fromenty, B. Cytochrome P450 2E1 Should Not Be Neglected for Acetaminophen-Induced Liver Injury in Metabolic Diseases with Altered Insulin Levels or Glucose Homeostasis. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101470. [Google Scholar] [CrossRef]
- Michaut, A.; Moreau, C.; Robin, M.-A.; Fromenty, B. Acetaminophen-Induced Liver Injury in Obesity and Nonalcoholic Fatty Liver Disease. Liver Int. 2014, 34, e171–e179. [Google Scholar] [CrossRef] [PubMed]
- Allard, J.; Le Guillou, D.; Begriche, K.; Fromenty, B. Drug-Induced Liver Injury in Obesity and Nonalcoholic Fatty Liver Disease. In Advances in Pharmacology; Elsevier: New York, NY, USA, 2019; Volume 85, pp. 75–107. ISBN 978-0-12-816759-5. [Google Scholar]
- Abdelmegeed, M.A.; Ha, S.-K.; Choi, Y.; Akbar, M.; Song, B.-J. Role of CYP2E1 in Mitochondrial Dysfunction and Hepatic Injury by Alcohol and Non-Alcoholic Substances. Curr. Mol. Pharmacol. 2017, 10, 207–225. [Google Scholar] [CrossRef]
- Seth, R.K.; Das, S.; Dattaroy, D.; Chandrashekaran, V.; Alhasson, F.; Michelotti, G.; Nagarkatti, M.; Nagarkatti, P.; Diehl, A.M.; Bell, P.D.; et al. TRPV4 Activation of Endothelial Nitric Oxide Synthase Resists Nonalcoholic Fatty Liver Disease by Blocking CYP2E1-Mediated Redox Toxicity. Free Radic. Biol. Med. 2017, 102, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Kim, D.; Seo, W.; Gao, B.; Yoo, S.; Song, B. Fructose Promotes Leaky Gut, Endotoxemia, and Liver Fibrosis Through Ethanol-Inducible Cytochrome P450-2E1–Mediated Oxidative and Nitrative Stress. Hepatology 2019, hep.30652. [Google Scholar] [CrossRef] [PubMed]
- Knockaert, L.; Fromenty, B.; Robin, M.-A. Mechanisms of Mitochondrial Targeting of Cytochrome P450 2E1: Physiopathological Role in Liver Injury and Obesity: Mitochondrial CYP2E1. FEBS J. 2011, 278, 4252–4260. [Google Scholar] [CrossRef]
- Guengerich, F.P. Cytochrome P450 2E1 and Its Roles in Disease. Chem. Biol. Interact. 2020, 322, 109056. [Google Scholar] [CrossRef]
- Zeng, T.; Zhang, C.-L.; Song, F.-Y.; Zhao, X.-L.; Xie, K.-Q. CMZ Reversed Chronic Ethanol-Induced Disturbance of PPAR-α Possibly by Suppressing Oxidative Stress and PGC-1α Acetylation, and Activating the MAPK and GSK3β Pathway. PLoS ONE 2014, 9, e98658. [Google Scholar] [CrossRef]
- Mathurin, P. Therapeutic Management of Alcoholic Hepatitis. Clin. Res. Hepatol. Gastroenterol. 2015, 39, S41–S45. [Google Scholar] [CrossRef] [PubMed]
- Woods, C.P.; Hazlehurst, J.M.; Tomlinson, J.W. Glucocorticoids and Non-Alcoholic Fatty Liver Disease. J. Steroid Biochem. Mol. Biol. 2015, 154, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Gutkowski, K.; Chwist, A.; Hartleb, M. Liver Injury Induced by High-Dose Methylprednisolone Therapy: A Case Report and Brief Review of the Literature. Hepat. Mon. 2011, 11, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.B.; White, A.G.; Scarpati, L.M.; Wan, G.; Nelson, W.W. Long-Term Systemic Corticosteroid Exposure: A Systematic Literature Review. Clin. Ther. 2017, 39, 2216–2229. [Google Scholar] [CrossRef]
- Phan, K.; Smith, S.D. Topical Corticosteroids and Risk of Diabetes Mellitus: Systematic Review and Meta-Analysis. J. Dermatol. Treat. 2019, 1–5. [Google Scholar] [CrossRef]
- Wang, J.-C.; Gray, N.E.; Kuo, T.; Harris, C.A. Regulation of Triglyceride Metabolism by Glucocorticoid Receptor. Cell Biosci. 2012, 2, 19. [Google Scholar] [CrossRef]
- Præstholm, S.M.; Correia, C.M.; Grøntved, L. Multifaceted Control of GR Signaling and Its Impact on Hepatic Transcriptional Networks and Metabolism. Front. Endocrinol. 2020, 11, 572981. [Google Scholar] [CrossRef]
- Wan, J.; Shan, Y.; Song, X.; Chen, S.; Lu, X.; Jin, J.; Su, Q.; Liu, B.; Sun, W.; Li, B. Adipocyte-Derived Periostin Mediates Glucocorticoid-Induced Hepatosteatosis in Mice. Mol. Metab. 2020, 31, 24–35. [Google Scholar] [CrossRef]
- Mendoza-Figueroa, T.; Hernandez, A.; De Lourdes Lopez, M.; Kuri-Harcuch, W. Intracytoplasmic Triglyceride Accumulation Produced by Dexamethasone in Adult Rat Hepatocytes Cultivated on 3T3 Cells. Toxicology 1988, 52, 273–286. [Google Scholar] [CrossRef]
- Harasim-Symbor, E.; Konstantynowicz-Nowicka, K.; Chabowski, A. Additive Effects of Dexamethasone and Palmitate on Hepatic Lipid Accumulation and Secretion. J. Mol. Endocrinol. 2016, 57, 261–273. [Google Scholar] [CrossRef][Green Version]
- Hu, Y.; Feng, Y.; Zhang, L.; Jia, Y.; Cai, D.; Qian, S.-B.; Du, M.; Zhao, R. GR-Mediated FTO Transactivation Induces Lipid Accumulation in Hepatocytes via Demethylation of M6A on Lipogenic MRNAs. RNA Biol. 2020, 17, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Poggioli, R.; Ueta, C.B.; Drigo, R.A.; Castillo, M.; Fonseca, T.L.; Bianco, A.C. Dexamethasone Reduces Energy Expenditure and Increases Susceptibility to Diet-Induced Obesity in Mice. Obesity 2013, 21, E415–E420. [Google Scholar] [CrossRef] [PubMed]
- Harvey, I.; Stephenson, E.J.; Redd, J.R.; Tran, Q.T.; Hochberg, I.; Qi, N.; Bridges, D. Glucocorticoid-Induced Metabolic Disturbances Are Exacerbated in Obese Male Mice. Endocrinology 2018, 159, 2275–2287. [Google Scholar] [CrossRef] [PubMed]
- D’souza, A.M.; Beaudry, J.L.; Szigiato, A.A.; Trumble, S.J.; Snook, L.A.; Bonen, A.; Giacca, A.; Riddell, M.C. Consumption of a High-Fat Diet Rapidly Exacerbates the Development of Fatty Liver Disease That Occurs with Chronically Elevated Glucocorticoids. Am. J. Physiol.-Gastrointest. Liver Physiol. 2012, 302, G850–G863. [Google Scholar] [CrossRef]
- Shpilberg, Y.; Beaudry, J.L.; D’Souza, A.; Campbell, J.E.; Peckett, A.; Riddell, M.C. A Rodent Model of Rapid-Onset Diabetes Induced by Glucocorticoids and High-Fat Feeding. Dis. Model. Mech. 2012, 5, 671–680. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, X.; Jiao, Y.; Xiong, X.; Wang, E.; Wang, X.; Zhang, Z.; Zhang, H.; Pan, L.; Guan, Y.; et al. Periostin Promotes Liver Steatosis and Hypertriglyceridemia through Downregulation of PPARα. J. Clin. Investig. 2014, 124, 3501–3513. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, H.; Niu, Y.; Zhang, W.; Zhu, L.; Li, X.; Lu, S.; Fan, J.; Li, X.; Ning, G.; et al. Circulating Periostin in Relation to Insulin Resistance and Nonalcoholic Fatty Liver Disease among Overweight and Obese Subjects. Sci. Rep. 2016, 6, 37886. [Google Scholar] [CrossRef]
- Zhu, J.-Z.; Zhu, H.-T.; Dai, Y.-N.; Li, C.-X.; Fang, Z.-Y.; Zhao, D.-J.; Wan, X.-Y.; Wang, Y.-M.; Wang, F.; Yu, C.-H.; et al. Serum Periostin Is a Potential Biomarker for Non-Alcoholic Fatty Liver Disease: A Case–Control Study. Endocrine 2016, 51, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T. Drug-Induced Idiosyncratic Hepatotoxicity: Prevention Strategy Developed after the Troglitazone Case. Drug Metab. Pharmacokinet. 2011, 26, 60–70. [Google Scholar] [CrossRef]
- Fromenty, B. Inhibition of Mitochondrial Fatty Acid Oxidation in Drug-Induced Hepatic Steatosis. Liver Res. 2019, 3, 157–169. [Google Scholar] [CrossRef]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Musso, G.; Cassader, M.; Paschetta, E.; Gambino, R. Thiazolidinediones and Advanced Liver Fibrosis in Nonalcoholic Steatohepatitis: A Meta-Analysis. JAMA Intern. Med. 2017, 177, 633. [Google Scholar] [CrossRef]
- Mahjoubin-Tehran, M.; De Vincentis, A.; Mikhailidis, D.P.; Atkin, S.L.; Mantzoros, C.S.; Jamialahmadi, T.; Sahebkar, A. Non-Alcoholic Fatty Liver Disease and Steatohepatitis: State of the Art on Effective Therapeutics Based on the Gold Standard Method for Diagnosis. Mol. Metab. 2020, 101049. [Google Scholar] [CrossRef]
- Ratziu, V.; Charlotte, F.; Bernhardt, C.; Giral, P.; Halbron, M.; LeNaour, G.; Hartmann-Heurtier, A.; Bruckert, E.; Poynard, T.; LIDO Study Group. Long-Term Efficacy of Rosiglitazone in Nonalcoholic Steatohepatitis: Results of the Fatty Liver Improvement by Rosiglitazone Therapy (FLIRT 2) Extension Trial. Hepatology 2010, 51, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, M.; Serfaty, L.; Cervera, P.; Capeau, J.; Ratziu, V. Hepatic Molecular Effects of Rosiglitazone in Human Non-Alcoholic Steatohepatitis Suggest Long-Term pro-Inflammatory Damage. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2014, 44, 1241–1247. [Google Scholar] [CrossRef]
- Bedoucha, M.; Atzpodien, E.; Boelsterli, U.A. Diabetic KKAy Mice Exhibit Increased Hepatic PPARγ1 Gene Expression and Develop Hepatic Steatosis upon Chronic Treatment with Antidiabetic Thiazolidinediones. J. Hepatol. 2001, 35, 17–23. [Google Scholar] [CrossRef]
- Watkins, S.M.; Reifsnyder, P.R.; Pan, H.; German, J.B.; Leiter, E.H. Lipid Metabolome-Wide Effects of the PPARγ Agonist Rosiglitazone. J. Lipid Res. 2002, 43, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Muurling, M.; Hoek, A.M.; Mensink, R.P.; Pijl, H.; Romijn, J.A.; Havekes, L.M.; Voshol, P.J. Overexpression of APOC1 in Obob Mice Leads to Hepatic Steatosis and Severe Hepatic Insulin Resistance. J. Lipid Res. 2004, 45, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.-J.; Reifsnyder, P.; Vance, D.E.; Xiao, Q.; Leiter, E.H. Pharmacogenetic Analysis of Rosiglitazone-Induced Hepatosteatosis in New Mouse Models of Type 2 Diabetes. Diabetes 2005, 54, 1854–1862. [Google Scholar] [CrossRef] [PubMed][Green Version]
- García-Ruiz, I.; Rodríguez-Juan, C.; Díaz-Sanjuán, T.; Martínez, M.Á.; Muñoz-Yagüe, T.; Solís-Herruzo, J.A. Effects of Rosiglitazone on the Liver Histology and Mitochondrial Function in Ob/Ob Mice. Hepatology 2007, 46, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Xu, A.; Lam, K.S.L.; Tam, P.K.H.; Che, C.-M.; Chan, L.; Lee, I.-K.; Wu, D.; Wang, Y. Rosiglitazone Promotes Fatty Acyl CoA Accumulation and Excessive Glycogen Storage in Livers of Mice without Adiponectin. J. Hepatol. 2010, 53, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Rull, A.; Geeraert, B.; Aragonès, G.; Beltrán-Debón, R.; Rodríguez-Gallego, E.; García-Heredia, A.; Pedro-Botet, J.; Joven, J.; Holvoet, P.; Camps, J. Rosiglitazone and Fenofibrate Exacerbate Liver Steatosis in a Mouse Model of Obesity and Hyperlipidemia. A Transcriptomic and Metabolomic Study. J. Proteome Res. 2014, 13, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Ma, Y.; Alsaggar, M.; Liu, D. Dual Outcomes of Rosiglitazone Treatment on Fatty Liver. AAPS J. 2016, 18, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, Z.; Oron-Herman, M.; Pappo, O.; Peleg, E.; Safadi, R.; Schmilovitz-Weiss, H.; Grozovski, M. Hepatic Effects of Rosiglitazone in Rats with the Metabolic Syndrome. Basic Clin. Pharmacol. Toxicol. 2010, 107, 663–668. [Google Scholar] [CrossRef]
- Yang, S.J.; Choi, J.M.; Chae, S.W.; Kim, W.J.; Park, S.E.; Rhee, E.J.; Lee, W.Y.; Oh, K.W.; Park, S.W.; Kim, S.W.; et al. Activation of Peroxisome Proliferator-Activated Receptor Gamma by Rosiglitazone Increases Sirt6 Expression and Ameliorates Hepatic Steatosis in Rats. PLoS ONE 2011, 6, e17057. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ni, X.; Xu, Q.; Wang, Q.; Li, X.; Hua, J. Regulation of Lipid-induced Macrophage Polarization through Modulating Peroxisome Proliferator-activated Receptor-gamma Activity Affects Hepatic Lipid Metabolism via a Toll-like Receptor 4/NF-κB Signaling Pathway. J. Gastroenterol. Hepatol. 2020, 35, 1998–2008. [Google Scholar] [CrossRef]
- Schadinger, S.E.; Bucher, N.L.R.; Schreiber, B.M.; Farmer, S.R. PPARγ2 Regulates Lipogenesis and Lipid Accumulation in Steatotic Hepatocytes. Am. J. Physiol.-Endocrinol. Metab. 2005, 288, E1195–E1205. [Google Scholar] [CrossRef]
- Kulkarni, N.M.; Malampati, S.; Mahat, M.Y.A.; Chandrasekaran, S.; Raghul, J.; Khan, A.A.; Krishnan, U.M.; Narayanan, S. Altered Pharmacokinetics of Rosiglitazone in a Mouse Model of Non-Alcoholic Fatty Liver Disease. Drug Metab. Pers. Ther. 2016, 31. [Google Scholar] [CrossRef]
- Backman, J.T.; Filppula, A.M.; Niemi, M.; Neuvonen, P.J. Role of Cytochrome P450 2C8 in Drug Metabolism and Interactions. Pharmacol. Rev. 2016, 68, 168–241. [Google Scholar] [CrossRef]
- Puris, E.; Pasanen, M.; Ranta, V.; Gynther, M.; Petsalo, A.; Käkelä, P.; Männistö, V.; Pihlajamäki, J. Laparoscopic Roux-en-Y Gastric Bypass Surgery Influenced Pharmacokinetics of Several Drugs given as a Cocktail with the Highest Impact Observed for CYP1A2, CYP2C8 and CYP2E1 Substrates. Basic Clin. Pharmacol. Toxicol. 2019, bcpt.13234. [Google Scholar] [CrossRef]
- Krogstad, V.; Peric, A.; Robertsen, I.; Kringen, M.K.; Wegler, C.; Angeles, P.C.; Hjelmesæth, J.; Karlsson, C.; Andersson, S.; Artursson, P.; et al. A Comparative Analysis of Cytochrome P450 Activities in Paired Liver and Small Intestinal Samples from Patients with Obesity. Drug Metab. Dispos. 2020, 48, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Wu, C.; Li, Z.; Liu, Y.; Fan, X.; Wang, Q.; Ding, R. Characterizing the Mechanism of Thiazolidinedione-Induced Hepatotoxicity: An in Vitro Model in Mitochondria. Toxicol. Appl. Pharmacol. 2015, 284, 134–141. [Google Scholar] [CrossRef]
- Contreras-Baeza, Y.; Ceballo, S.; Arce-Molina, R.; Sandoval, P.Y.; Alegría, K.; Barros, L.F.; San Martín, A. MitoToxy Assay: A Novel Cell-Based Method for the Assessment of Metabolic Toxicity in a Multiwell Plate Format Using a Lactate FRET Nanosensor, Laconic. PLoS ONE 2019, 14, e0224527. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Huan, Y.; Jiang, Q.; Sun, S.; Jia, C.; Shen, Z. Effects and Potential Mechanisms of Pioglitazone on Lipid Metabolism in Obese Diabetic KKAy Mice. PPAR Res. 2014, 2014, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Huan, Y.; Liu, S.; Hou, S.; Sun, S.; Li, C.; Liu, Q.; Jiang, Q.; Wang, Y.; Shen, Z. Effect of Chronic Pioglitazone Treatment on Hepatic Gene Expression Profile in Obese C57BL/6J Mice. Int. J. Mol. Sci. 2015, 16, 12213–12229. [Google Scholar] [CrossRef] [PubMed]
- Orasanu, G.; Ziouzenkova, O.; Devchand, P.R.; Nehra, V.; Hamdy, O.; Horton, E.S.; Plutzky, J. The Peroxisome Proliferator-Activated Receptor-γ Agonist Pioglitazone Represses Inflammation in a Peroxisome Proliferator-Activated Receptor-α–Dependent Manner In Vitro and In Vivo in Mice. J. Am. Coll. Cardiol. 2008, 52, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Young, P.W.; Buckle, D.R.; Cantello, B.C.; Chapman, H.; Clapham, J.C.; Coyle, P.J.; Haigh, D.; Hindley, R.M.; Holder, J.C.; Kallender, H.; et al. Identification of High-Affinity Binding Sites for the Insulin Sensitizer Rosiglitazone (BRL-49653) in Rodent and Human Adipocytes Using a Radioiodinated Ligand for Peroxisomal Proliferator-Activated Receptor Gamma. J. Pharmacol. Exp. Ther. 1998, 284, 751–759. [Google Scholar] [PubMed]
- Sakamoto, J.; Kimura, H.; Moriyama, S.; Odaka, H.; Momose, Y.; Sugiyama, Y.; Sawada, H. Activation of Human Peroxisome Proliferator-Activated Receptor (PPAR) Subtypes by Pioglitazone. Biochem. Biophys. Res. Commun. 2000, 278, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, M.; Serfaty, L.; Capeau, J. From Nonalcoholic Fatty Liver to Nonalcoholic Steatohepatitis and Cirrhosis in HIV-Infected Patients: Diagnosis and Management. Curr. Opin. Infect. Dis. 2012, 25, 10–16. [Google Scholar] [CrossRef]
- Shetty, A.; Cho, W.; Alazawi, W.; Syn, W.-K. Methotrexate Hepatotoxicity and the Impact of Nonalcoholic Fatty Liver Disease. Am. J. Med. Sci. 2017, 354, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.A.; Zein, C.O.; Angulo, P.; Lindor, K.D. A Pilot Trial of Pentoxifylline in Nonalcoholic Steatohepatitis. Am. J. Gastroenterol. 2004, 99, 2365–2368. [Google Scholar] [CrossRef]
- Massart, J.; Robin, M.A.; Noury, F.; Fautrel, A.; Lettéron, P.; Bado, A.; Eliat, P.A.; Fromenty, B. Pentoxifylline Aggravates Fatty Liver in Obese and Diabetic Ob/Ob Mice by Increasing Intestinal Glucose Absorption and Activating Hepatic Lipogenesis. Br. J. Pharmacol. 2012, 165, 1361–1374. [Google Scholar] [CrossRef] [PubMed]
- Zannikos, P.N.; Bandyopadhyay, A.M.; Robertson, L.W.; Blouin, R.A. Effect of Nutritional Obesity on the Induction of CYP2B Enzymes Following Phenobarbital Treatment. Drug Metab. Dispos. Biol. Fate Chem. 1993, 21, 782–787. [Google Scholar] [PubMed]
- Ito, M.; Suzuki, J.; Sasaki, M.; Watanabe, K.; Tsujioka, S.; Takahashi, Y.; Gomori, A.; Hirose, H.; Ishihara, A.; Iwaasa, H. Development of Nonalcoholic Steatohepatitis Model through Combination of High-Fat Diet and Tetracycline with Morbid Obesity in Mice. Hepatol. Res. 2006, 34, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.A. The Politics of Plastics: The Making and Unmaking of Bisphenol a “Safety”. Am. J. Public Health 2009, 99 Suppl 3, S559–S566. [Google Scholar] [CrossRef]
- Oppeneer, S.J.; Robien, K. Bisphenol A Exposure and Associations with Obesity among Adults: A Critical Review. Public Health Nutr. 2015, 18, 1847–1863. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, H.; Wu, J.; Yuan, L.; Wang, Y.; Du, X.; Wang, R.; Marwa, P.W.; Petlulu, P.; Chen, X.; et al. The Adverse Health Effects of Bisphenol A and Related Toxicity Mechanisms. Environ. Res. 2019, 176, 108575. [Google Scholar] [CrossRef] [PubMed]
- Legler, J.; Zalko, D.; Jourdan, F.; Jacobs, M.; Fromenty, B.; Balaguer, P.; Bourguet, W.; Munic Kos, V.; Nadal, A.; Beausoleil, C.; et al. The GOLIATH Project: Towards an Internationally Harmonised Approach for Testing Metabolism Disrupting Compounds. Int. J. Mol. Sci. 2020, 21, 3480. [Google Scholar] [CrossRef]
- Legeay, S.; Faure, S. Is Bisphenol A an Environmental Obesogen? Fundam. Clin. Pharmacol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Le Magueresse-Battistoni, B.; Multigner, L.; Beausoleil, C.; Rousselle, C. Effects of Bisphenol A on Metabolism and Evidences of a Mode of Action Mediated through Endocrine Disruption. Mol. Cell. Endocrinol. 2018, 475, 74–91. [Google Scholar] [CrossRef]
- Cimmino, I.; Fiory, F.; Perruolo, G.; Miele, C.; Beguinot, F.; Formisano, P.; Oriente, F. Potential Mechanisms of Bisphenol A (BPA) Contributing to Human Disease. Int. J. Mol. Sci. 2020, 21, 5761. [Google Scholar] [CrossRef]
- Shimpi, P.C.; More, V.R.; Paranjpe, M.; Donepudi, A.C.; Goodrich, J.M.; Dolinoy, D.C.; Rubin, B.; Slitt, A.L. Hepatic Lipid Accumulation and Nrf2 Expression Following Perinatal and Peripubertal Exposure to Bisphenol A in a Mouse Model of Nonalcoholic Liver Disease. Environ. Health Perspect. 2017, 125, 087005. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, H.; Zou, J.; Mai, H.; Su, D.; Feng, X.; Feng, D. Bisphenol A Exposure Induces Cholesterol Synthesis and Hepatic Steatosis in C57BL/6 Mice by down-Regulating the DNA Methylation Levels of SREBP-2. Food Chem. Toxicol. 2019, 133, 110786. [Google Scholar] [CrossRef] [PubMed]
- Dallio, M.; Diano, N.; Masarone, M.; Gravina, A.G.; Patanè, V.; Romeo, M.; Di Sarno, R.; Errico, S.; Nicolucci, C.; Abenavoli, L.; et al. Chemical Effect of Bisphenol A on Non-Alcoholic Fatty Liver Disease. Int. J. Environ. Res. Public. Health 2019, 16, 3134. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Zhang, H.; Jiang, X.; Zou, J.; Li, Q.; Mai, H.; Su, D.; Ling, W.; Feng, X. Bisphenol A Exposure Induces Gut Microbiota Dysbiosis and Consequent Activation of Gut-Liver Axis Leading to Hepatic Steatosis in CD-1 Mice. Environ. Pollut. 2020, 265, 114880. [Google Scholar] [CrossRef]
- Franco, M.E.; Fernandez-Luna, M.T.; Ramirez, A.J.; Lavado, R. Metabolomic-Based Assessment Reveals Dysregulation of Lipid Profiles in Human Liver Cells Exposed to Environmental Obesogens. Toxicol. Appl. Pharmacol. 2020, 398, 115009. [Google Scholar] [CrossRef] [PubMed]
- Huc, L.; Lemarié, A.; Guéraud, F.; Héliès-Toussaint, C. Low Concentrations of Bisphenol A Induce Lipid Accumulation Mediated by the Production of Reactive Oxygen Species in the Mitochondria of HepG2 Cells. Toxicol. In Vitro 2012, 26, 709–717. [Google Scholar] [CrossRef]
- Peyre, L.; Rouimi, P.; Sousa, G.; Héliès-Toussaint, C.; Carré, B.; Barcellini, S.; Chagnon, M.-C.; Rahmani, R. Comparative Study of Bisphenol A and Its Analogue Bisphenol S on Human Hepatic Cells: A Focus on Their Potential Involvement in Nonalcoholic Fatty Liver Disease. Food Chem. Toxicol. 2014, 70, 9–18. [Google Scholar] [CrossRef]
- Martella, A.; Silvestri, C.; Maradonna, F.; Gioacchini, G.; Allarà, M.; Radaelli, G.; Overby, D.R.; Di Marzo, V.; Carnevali, O. Bisphenol A Induces Fatty Liver by an Endocannabinoid-Mediated Positive Feedback Loop. Endocrinology 2016, 157, 1751–1763. [Google Scholar] [CrossRef]
- Bucher, S.; Jalili, P.; Le Guillou, D.; Begriche, K.; Rondel, K.; Martinais, S.; Zalko, D.; Corlu, A.; Robin, M.-A.; Fromenty, B. Bisphenol a Induces Steatosis in HepaRG Cells Using a Model of Perinatal Exposure. Environ. Toxicol. 2017, 32, 1024–1036. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ding, D.; Huang, Q.; Liu, Q.; Lu, H.; Lu, Y.; Chi, Y.; Sun, X.; Ye, G.; Zhu, H.; et al. Downregulation of MiR-192 Causes Hepatic Steatosis and Lipid Accumulation by Inducing SREBF1: Novel Mechanism for Bisphenol A-Triggered Non-Alcoholic Fatty Liver Disease. Biochim. Biophys. Acta BBA—Mol. Cell Biol. Lipids 2017, 1862, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, H.; Zou, J.; Feng, X.; Feng, D. Bisphenol A Induces Cholesterol Biosynthesis in HepG2 Cells via SREBP-2/HMGCR Signaling Pathway. J. Toxicol. Sci. 2019, 44, 481–491. [Google Scholar] [CrossRef]
- Liu, Q.; Shao, W.; Weng, Z.; Zhang, X.; Ding, G.; Xu, C.; Xu, J.; Jiang, Z.; Gu, A. In Vitro Evaluation of the Hepatic Lipid Accumulation of Bisphenol Analogs: A High-Content Screening Assay. Toxicol. Vitro Int. J. Publ. Assoc. BIBRA 2020, 68, 104959. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, L.S.; Oliveira, K.M.; Freitas, I.N.; Silva, J.A.; Silva, J.N.; Favero-Santos, B.C.; Bonfleur, M.L.; Carneiro, E.M.; Ribeiro, R.A. Bisphenol-A Exposure Worsens Hepatic Steatosis in Ovariectomized Mice Fed on a High-Fat Diet: Role of Endoplasmic Reticulum Stress and Fibrogenic Pathways. Life Sci. 2020, 256, 118012. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.K.; Jeong, I.-K.; Jung Oh, T.; Ahn, H.Y.; Kim, H.H.; Park, Y.J.; Jang, H.C.; Park, K.S. Long-Term Oral Exposure to Bisphenol A Induces Glucose Intolerance and Insulin Resistance. J. Endocrinol. 2015, 226, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Deng, P.; Lin, M.; Yang, L.; Li, L.; Guo, L.; Zhang, L.; He, M.; Lu, Y.; Pi, H.; et al. Long-Term Bisphenol A Exposure Exacerbates Diet-Induced Prediabetes via TLR4-Dependent Hypothalamic Inflammation. J. Hazard. Mater. 2021, 402, 123926. [Google Scholar] [CrossRef] [PubMed]
- Strakovsky, R.S.; Wang, H.; Engeseth, N.J.; Flaws, J.A.; Helferich, W.G.; Pan, Y.-X.; Lezmi, S. Developmental Bisphenol A (BPA) Exposure Leads to Sex-Specific Modification of Hepatic Gene Expression and Epigenome at Birth That May Exacerbate High-Fat Diet-Induced Hepatic Steatosis. Toxicol. Appl. Pharmacol. 2015, 284, 101–112. [Google Scholar] [CrossRef]
- Yalcin, E.B.; Kulkarni, S.R.; Slitt, A.L.; King, R. Bisphenol A Sulfonation Is Impaired in Metabolic and Liver Disease. Toxicol. Appl. Pharmacol. 2016, 292, 75–84. [Google Scholar] [CrossRef]
- Quesnot, N.; Bucher, S.; Fromenty, B.; Robin, M.-A. Modulation of Metabolizing Enzymes by Bisphenol a in Human and Animal Models. Chem. Res. Toxicol. 2014, 27, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, G.M.; Long, S.M.; Jones, O.A.H. What Are the Effects of PFAS Exposure at Environmentally Relevant Concentrations? Chemosphere 2020, 258, 127340. [Google Scholar] [CrossRef]
- Vieira, V.M.; Hoffman, K.; Shin, H.-M.; Weinberg, J.M.; Webster, T.F.; Fletcher, T. Perfluorooctanoic Acid Exposure and Cancer Outcomes in a Contaminated Community: A Geographic Analysis. Environ. Health Perspect. 2013, 121, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Steenland, K.; Fletcher, T.; Stein, C.R.; Bartell, S.M.; Darrow, L.; Lopez-Espinosa, M.-J.; Barry Ryan, P.; Savitz, D.A. Review: Evolution of Evidence on PFOA and Health Following the Assessments of the C8 Science Panel. Environ. Int. 2020, 145, 106125. [Google Scholar] [CrossRef]
- Steenland, K.; Woskie, S. Cohort Mortality Study of Workers Exposed to Perfluorooctanoic Acid. Am. J. Epidemiol. 2012, 176, 909–917. [Google Scholar] [CrossRef]
- Mancini, F.R.; Rajaobelina, K.; Praud, D.; Dow, C.; Antignac, J.P.; Kvaskoff, M.; Severi, G.; Bonnet, F.; Boutron-Ruault, M.-C.; Fagherazzi, G. Nonlinear Associations between Dietary Exposures to Perfluorooctanoic Acid (PFOA) or Perfluorooctane Sulfonate (PFOS) and Type 2 Diabetes Risk in Women: Findings from the E3N Cohort Study. Int. J. Hyg. Environ. Health 2018, 221, 1054–1060. [Google Scholar] [CrossRef]
- Sun, Q.; Zong, G.; Valvi, D.; Nielsen, F.; Coull, B.; Grandjean, P. Plasma Concentrations of Perfluoroalkyl Substances and Risk of Type 2 Diabetes: A Prospective Investigation among U.S. Women. Environ. Health Perspect. 2018, 126, 037001. [Google Scholar] [CrossRef]
- Liu, P.; Yang, F.; Wang, Y.; Yuan, Z. Perfluorooctanoic Acid (PFOA) Exposure in Early Life Increases Risk of Childhood Adiposity: A Meta-Analysis of Prospective Cohort Studies. Int. J. Environ. Res. Public Health 2018, 15, 2070. [Google Scholar] [CrossRef]
- Braun, J.M.; Eliot, M.; Papandonatos, G.D.; Buckley, J.P.; Cecil, K.M.; Kalkwarf, H.J.; Chen, A.; Eaton, C.B.; Kelsey, K.; Lanphear, B.P.; et al. Gestational Perfluoroalkyl Substance Exposure and Body Mass Index Trajectories over the First 12 Years of Life. Int. J. Obes. 2005 2021, 45, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Rappazzo, K.M.; Coffman, E.; Hines, E.P. Exposure to Perfluorinated Alkyl Substances and Health Outcomes in Children: A Systematic Review of the Epidemiologic Literature. Int. J. Environ. Res. Public Health 2017, 14, 691. [Google Scholar] [CrossRef] [PubMed]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef]
- Geiger, S.D.; Xiao, J.; Ducatman, A.; Frisbee, S.; Innes, K.; Shankar, A. The Association between PFOA, PFOS and Serum Lipid Levels in Adolescents. Chemosphere 2014, 98, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Convertino, M.; Church, T.R.; Olsen, G.W.; Liu, Y.; Doyle, E.; Elcombe, C.R.; Barnett, A.L.; Samuel, L.M.; MacPherson, I.R.; Evans, T.R.J. Stochastic Pharmacokinetic-Pharmacodynamic Modeling for Assessing the Systemic Health Risk of Perfluorooctanoate (PFOA). Toxicol. Sci. Off. J. Soc. Toxicol. 2018, 163, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Darrow, L.A.; Groth, A.C.; Winquist, A.; Shin, H.-M.; Bartell, S.M.; Steenland, K. Modeled Perfluorooctanoic Acid (PFOA) Exposure and Liver Function in a Mid-Ohio Valley Community. Environ. Health Perspect. 2016, 124, 1227–1233. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Lin, L.-Y.; Chiang, C.-K.; Wang, W.-J.; Su, Y.-N.; Hung, K.-Y.; Chen, P.-C. Investigation of the Associations between Low-Dose Serum Perfluorinated Chemicals and Liver Enzymes in US Adults. Am. J. Gastroenterol. 2010, 105, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.B.; Ducatman, A. Selective Associations of Recent Low Concentrations of Perfluoroalkyl Substances With Liver Function Biomarkers: NHANES 2011 to 2014 Data on US Adults Aged ≥20 Years. J. Occup. Environ. Med. 2019, 61, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; McConnell, R.; Catherine, C.; Xu, S.; Walker, D.I.; Stratakis, N.; Jones, D.P.; Miller, G.W.; Peng, C.; Conti, D.V.; et al. Perfluoroalkyl Substances and Severity of Nonalcoholic Fatty Liver in Children: An Untargeted Metabolomics Approach. Environ. Int. 2020, 134, 105220. [Google Scholar] [CrossRef] [PubMed]
- Kudo, N.; Kawashima, Y. Fish Oil-Feeding Prevents Perfluorooctanoic Acid-Induced Fatty Liver in Mice. Toxicol. Appl. Pharmacol. 1997, 145, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Wang, J.; Dai, J. Activation of Sterol Regulatory Element-Binding Proteins in Mice Exposed to Perfluorooctanoic Acid for 28 Days. Arch. Toxicol. 2015, 89, 1569–1578. [Google Scholar] [CrossRef]
- Das, K.P.; Wood, C.R.; Lin, M.T.; Starkov, A.A.; Lau, C.; Wallace, K.B.; Corton, J.C.; Abbott, B.D. Perfluoroalkyl Acids-Induced Liver Steatosis: Effects on Genes Controlling Lipid Homeostasis. Toxicology 2017, 378, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.E.; Guo, G.L. Understanding Environmental Contaminants’ Direct Effects on Non-Alcoholic Fatty Liver Disease Progression. Curr. Environ. Health Rep. 2019, 6, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Xu, C.; Zhang, X.; Pang, L.; Xu, J.; Liu, Q.; Zhang, L.; Xu, S.; Gu, A. Autophagy Mediates Perfluorooctanoic Acid-Induced Lipid Metabolism Disorder and NLRP3 Inflammasome Activation in Hepatocytes. Environ. Pollut. Barking Essex 1987 2020, 267, 115655. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Klaunig, J.E. The Effects of Perfluorooctanoate on High Fat Diet Induced Non-Alcoholic Fatty Liver Disease in Mice. Toxicology 2019, 416, 1–14. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Liang, Y.; Li, J.; Liu, Y.; Zhang, J.; Zhang, A.; Fu, J.; Jiang, G. Specific Accumulation of Lipid Droplets in Hepatocyte Nuclei of PFOA-Exposed BALB/c Mice. Sci. Rep. 2013, 3, 2174. [Google Scholar] [CrossRef]
- Barbosa, A.D.; Siniossoglou, S. New Kid on the Block: Lipid Droplets in the Nucleus. FEBS J. 2020, 287, 4838–4843. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Zhang, H.; Wang, J.; Zheng, F.; Dai, J. Perfluorooctanoic Acid Exposure Induces Endoplasmic Reticulum Stress in the Liver and Its Effects Are Ameliorated by 4-Phenylbutyrate. Free Radic. Biol. Med. 2015, 87, 300–311. [Google Scholar] [CrossRef]
- Louisse, J.; Rijkers, D.; Stoopen, G.; Janssen, A.; Staats, M.; Hoogenboom, R.; Kersten, S.; Peijnenburg, A. Perfluorooctanoic Acid (PFOA), Perfluorooctane Sulfonic Acid (PFOS), and Perfluorononanoic Acid (PFNA) Increase Triglyceride Levels and Decrease Cholesterogenic Gene Expression in Human HepaRG Liver Cells. Arch. Toxicol. 2020, 94, 3137–3155. [Google Scholar] [CrossRef]
- Foulds, C.E.; Treviño, L.S.; York, B.; Walker, C.L. Endocrine-Disrupting Chemicals and Fatty Liver Disease. Nat. Rev. Endocrinol. 2017, 13, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Wright, H.M.; Wright, M.; Spiegelman, B.M. ADD1/SREBP1 Activates PPARgamma through the Production of Endogenous Ligand. Proc. Natl. Acad. Sci. USA 1998, 95, 4333–4337. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Dong, X.-Y.; Fan, L.-J.; Zhang, Z.-L.; Wang, Q.; Jiang, N.; Yang, X.-S. Poly- and Perfluorinated Compounds Activate Human Pregnane X Receptor. Toxicology 2017, 380, 23–29. [Google Scholar] [CrossRef]
- Bjork, J.A.; Butenhoff, J.L.; Wallace, K.B. Multiplicity of Nuclear Receptor Activation by PFOA and PFOS in Primary Human and Rodent Hepatocytes. Toxicology 2011, 288, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Behr, A.-C.; Plinsch, C.; Braeuning, A.; Buhrke, T. Activation of Human Nuclear Receptors by Perfluoroalkylated Substances (PFAS). Toxicol. Vitro Int. J. Publ. Assoc. BIBRA 2020, 62, 104700. [Google Scholar] [CrossRef] [PubMed]
- Cave, M.C.; Clair, H.B.; Hardesty, J.E.; Falkner, K.C.; Feng, W.; Clark, B.J.; Sidey, J.; Shi, H.; Aqel, B.A.; McClain, C.J.; et al. Nuclear Receptors and Nonalcoholic Fatty Liver Disease. Biochim. Biophys. Acta 2016, 1859, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Staudinger, J.L. Clinical Applications of Small Molecule Inhibitors of Pregnane X Receptor. Mol. Cell. Endocrinol. 2019, 485, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Scharmach, E.; Buhrke, T.; Lichtenstein, D.; Lampen, A. Perfluorooctanoic Acid Affects the Activity of the Hepatocyte Nuclear Factor 4 Alpha (HNF4α). Toxicol. Lett. 2012, 212, 106–112. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, Y.; Hu, S.; Xu, Y.; Stroup, D.; Pan, X.; Bawa, F.C.; Chen, S.; Gopoju, R.; Yin, L.; et al. Hepatocyte Nuclear Factor 4α Prevents the Steatosis-to-NASH Progression by Regulating P53 and Bile Acid Signaling. Hepatol. Baltim. Md 2020. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Xie, G.; Sun, X.; Li, Q.; Zhong, W.; Qiao, P.; Sun, X.; Jia, W.; Zhou, Z. High Fat Diet Feeding Exaggerates Perfluorooctanoic Acid-Induced Liver Injury in Mice via Modulating Multiple Metabolic Pathways. PLoS ONE 2013, 8, e61409. [Google Scholar] [CrossRef]
- Shabalina, I.G.; Kramarova, T.V.; Mattsson, C.L.; Petrovic, N.; Rahman Qazi, M.; Csikasz, R.I.; Chang, S.-C.; Butenhoff, J.; DePierre, J.W.; Cannon, B.; et al. The Environmental Pollutants Perfluorooctane Sulfonate and Perfluorooctanoic Acid Upregulate Uncoupling Protein 1 (UCP1) in Brown-Fat Mitochondria Through a UCP1-Dependent Reduction in Food Intake. Toxicol. Sci. Off. J. Soc. Toxicol. 2015, 146, 334–343. [Google Scholar] [CrossRef]
- White, S.S.; Fenton, S.E.; Hines, E.P. Endocrine Disrupting Properties of Perfluorooctanoic Acid. J. Steroid Biochem. Mol. Biol. 2011, 127, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Schlezinger, J.J.; Puckett, H.; Oliver, J.; Nielsen, G.; Heiger-Bernays, W.; Webster, T.F. Perfluorooctanoic Acid Activates Multiple Nuclear Receptor Pathways and Skews Expression of Genes Regulating Cholesterol Homeostasis in Liver of Humanized PPARα Mice Fed an American Diet. Toxicol. Appl. Pharmacol. 2020, 405, 115204. [Google Scholar] [CrossRef]
- Bougarne, N.; Weyers, B.; Desmet, S.J.; Deckers, J.; Ray, D.W.; Staels, B.; De Bosscher, K. Molecular Actions of PPARα in Lipid Metabolism and Inflammation. Endocr. Rev. 2018, 39, 760–802. [Google Scholar] [CrossRef]
- Filgo, A.J.; Quist, E.M.; Hoenerhoff, M.J.; Brix, A.E.; Kissling, G.E.; Fenton, S.E. Perfluorooctanoic Acid (PFOA)-Induced Liver Lesions in Two Strains of Mice Following Developmental Exposures: PPARα Is Not Required. Toxicol. Pathol. 2015, 43, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Rebholz, S.L.; Jones, T.; Herrick, R.L.; Xie, C.; Calafat, A.M.; Pinney, S.M.; Woollett, L.A. Hypercholesterolemia with Consumption of PFOA-Laced Western Diets Is Dependent on Strain and Sex of Mice. Toxicol. Rep. 2016, 3, 46–54. [Google Scholar] [CrossRef]
- Huck, I.; Beggs, K.; Apte, U. Paradoxical Protective Effect of Perfluorooctanesulfonic Acid Against High-Fat Diet-Induced Hepatic Steatosis in Mice. Int. J. Toxicol. 2018, 37, 383–392. [Google Scholar] [CrossRef]
- Bucher, S.; Le Guillou, D.; Allard, J.; Pinon, G.; Begriche, K.; Tête, A.; Sergent, O.; Lagadic-Gossmann, D.; Fromenty, B. Possible Involvement of Mitochondrial Dysfunction and Oxidative Stress in a Cellular Model of NAFLD Progression Induced by Benzo[a]Pyrene/Ethanol CoExposure. Oxid. Med. Cell. Longev. 2018, 2018, 4396403. [Google Scholar] [CrossRef] [PubMed]
- Tomaru, M.; Takano, H.; Inoue, K.-I.; Yanagisawa, R.; Osakabe, N.; Yasuda, A.; Shimada, A.; Kato, Y.; Uematsu, H. Pulmonary Exposure to Diesel Exhaust Particles Enhances Fatty Change of the Liver in Obese Diabetic Mice. Int. J. Mol. Med. 2007, 19, 17–22. [Google Scholar] [CrossRef]
- Yanagisawa, R.; Koike, E.; Win-Shwe, T.-T.; Yamamoto, M.; Takano, H. Impaired Lipid and Glucose Homeostasis in Hexabromocyclododecane-Exposed Mice Fed a High-Fat Diet. Environ. Health Perspect. 2014, 122, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Cichocki, J.A.; Furuya, S.; Luo, Y.-S.; Iwata, Y.; Konganti, K.; Chiu, W.A.; Threadgill, D.W.; Pogribny, I.P.; Rusyn, I. Nonalcoholic Fatty Liver Disease Is a Susceptibility Factor for Perchloroethylene-Induced Liver Effects in Mice. Toxicol. Sci. Off. J. Soc. Toxicol. 2017, 159, 481. [Google Scholar] [CrossRef] [PubMed]
- Duval, C.; Teixeira-Clerc, F.; Leblanc, A.F.; Touch, S.; Emond, C.; Guerre-Millo, M.; Lotersztajn, S.; Barouki, R.; Aggerbeck, M.; Coumoul, X. Chronic Exposure to Low Doses of Dioxin Promotes Liver Fibrosis Development in the C57BL/6J Diet-Induced Obesity Mouse Model. Environ. Health Perspect. 2017, 125, 428–436. [Google Scholar] [CrossRef]
- Yu, J.; Yang, X.; Yang, X.; Yang, M.; Wang, P.; Yang, Y.; Yang, J.; Li, W.; Xu, J. Nonylphenol Aggravates Non-Alcoholic Fatty Liver Disease in High Sucrose-High Fat Diet-Treated Rats. Sci. Rep. 2018, 8, 3232. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhu, L.; Kang, Q.; Lee, H.K.; Li, D.; Chung, A.C.K.; Cai, Z. Chronic Exposure to Tetrabromodiphenyl Ether (BDE-47) Aggravates Hepatic Steatosis and Liver Fibrosis in Diet-Induced Obese Mice. J. Hazard. Mater. 2019, 378, 120766. [Google Scholar] [CrossRef]
- Malik, F.; Wickremesinghe, P.; Saverimuttu, J. Case Report and Literature Review of Auto-Brewery Syndrome: Probably an Underdiagnosed Medical Condition. BMJ Open Gastroenterol. 2019, 6, e000325. [Google Scholar] [CrossRef]
- Seitz, H.K.; Bataller, R.; Cortez-Pinto, H.; Gao, B.; Gual, A.; Lackner, C.; Mathurin, P.; Mueller, S.; Szabo, G.; Tsukamoto, H. Alcoholic Liver Disease. Nat. Rev. Dis. Primer 2018, 4, 16. [Google Scholar] [CrossRef]
- Avila, M.A.; Dufour, J.-F.; Gerbes, A.L.; Zoulim, F.; Bataller, R.; Burra, P.; Cortez-Pinto, H.; Gao, B.; Gilmore, I.; Mathurin, P.; et al. Recent Advances in Alcohol-Related Liver Disease (ALD): Summary of a Gut Round Table Meeting. Gut 2020, 69, 764–780. [Google Scholar] [CrossRef]
- Teschke, R. Alcoholic Liver Disease: Alcohol Metabolism, Cascade of Molecular Mechanisms, Cellular Targets, and Clinical Aspects. Biomedicines 2018, 6, 106. [Google Scholar] [CrossRef]
- Begriche, K.; Massart, J.; Fromenty, B. Chapter 15—Mitochondrial Dysfunction Induced by Xenobiotics: Involvement in Steatosis and Steatohepatitis. In Mitochondria in Obesity and Type 2 Diabetes; Morio, B., Pénicaud, L., Rigoulet, M., Eds.; Academic Press: New York, NY, USA, 2019; pp. 347–364. ISBN 978-0-12-811752-1. [Google Scholar]
- Kourkoumpetis, T.; Sood, G. Pathogenesis of Alcoholic Liver Disease: An Update. Clin. Liver Dis. 2019, 23, 71–80. [Google Scholar] [CrossRef]
- Yan, S.; Khambu, B.; Hong, H.; Liu, G.; Huda, N.; Yin, X.-M. Autophagy, Metabolism, and Alcohol-Related Liver Disease: Novel Modulators and Functions. Int. J. Mol. Sci. 2019, 20, 5029. [Google Scholar] [CrossRef] [PubMed]
- Kirpich, I.A.; Warner, D.R.; Feng, W.; Joshi-Barve, S.; McClain, C.J.; Seth, D.; Zhong, W.; Zhou, Z.; Osna, N.A.; Kharbanda, K.K. Mechanisms, Biomarkers and Targets for Therapy in Alcohol-Associated Liver Injury: From Genetics to Nutrition: Summary of the ISBRA 2018 Symposium. Alcohol Fayettev. N 2020, 83, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Seitz, H.K. The Role of Cytochrome P4502E1 in the Pathogenesis of Alcoholic Liver Disease and Carcinogenesis. Chem. Biol. Interact. 2020, 316, 108918. [Google Scholar] [CrossRef]
- Mahli, A.; Hellerbrand, C. Alcohol and Obesity: A Dangerous Association for Fatty Liver Disease. Dig. Dis. Basel Switz. 2016, 34 Suppl 1, 32–39. [Google Scholar] [CrossRef]
- Boyle, M.; Masson, S.; Anstee, Q.M. The Bidirectional Impacts of Alcohol Consumption and the Metabolic Syndrome: Cofactors for Progressive Fatty Liver Disease. J. Hepatol. 2018, 68, 251–267. [Google Scholar] [CrossRef] [PubMed]
- Åberg, F.; Färkkilä, M. Drinking and Obesity: Alcoholic Liver Disease/Nonalcoholic Fatty Liver Disease Interactions. Semin. Liver Dis. 2020, 40, 154–162. [Google Scholar] [CrossRef]
- Hwang, S.; Ren, T.; Gao, B. Obesity and Binge Alcohol Intake Are Deadly Combination to Induce Steatohepatitis: A Model of High-Fat Diet and Binge Ethanol Intake. Clin. Mol. Hepatol. 2020, 26, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lai, K.K.Y.; Verlinsky, A.; Lugea, A.; French, S.W.; Cooper, M.P.; Ji, C.; Tsukamoto, H. Synergistic Steatohepatitis by Moderate Obesity and Alcohol in Mice despite Increased Adiponectin and P-AMPK. J. Hepatol. 2011, 55, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Gyamfi, D.; Everitt, H.E.; Tewfik, I.; Clemens, D.L.; Patel, V.B. Hepatic Mitochondrial Dysfunction Induced by Fatty Acids and Ethanol. Free Radic. Biol. Med. 2012, 53, 2131–2145. [Google Scholar] [CrossRef]
- Grasselli, E.; Voci, A.; Demori, I.; De Matteis, R.; Compalati, A.D.; Gallo, G.; Vergani, L. Effects of Binge Ethanol on Lipid Homeostasis and Oxidative Stress in a Rat Model of Nonalcoholic Fatty Liver Disease. J. Physiol. Biochem. 2014, 70, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Everitt, H.; Hu, M.; Ajmo, J.M.; Rogers, C.Q.; Liang, X.; Zhang, R.; Yin, H.; Choi, A.; Bennett, E.S.; You, M. Ethanol Administration Exacerbates the Abnormalities in Hepatic Lipid Oxidation in Genetically Obese Mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G38–G47. [Google Scholar] [CrossRef]
- Minato, T.; Tsutsumi, M.; Tsuchishima, M.; Hayashi, N.; Saito, T.; Matsue, Y.; Toshikuni, N.; Arisawa, T.; George, J. Binge Alcohol Consumption Aggravates Oxidative Stress and Promotes Pathogenesis of NASH from Obesity-Induced Simple Steatosis. Mol. Med. Camb. Mass 2014, 20, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Mahli, A.; Thasler, W.E.; Patsenker, E.; Müller, S.; Stickel, F.; Müller, M.; Seitz, H.K.; Cederbaum, A.I.; Hellerbrand, C. Identification of Cytochrome CYP2E1 as Critical Mediator of Synergistic Effects of Alcohol and Cellular Lipid Accumulation in Hepatocytes in Vitro. Oncotarget 2015, 6, 41464–41478. [Google Scholar] [CrossRef]
- Yi, H.-W.; Ma, Y.-X.; Wang, X.-N.; Wang, C.-F.; Lu, J.; Cao, W.; Wu, X.-D. Ethanol Promotes Saturated Fatty Acid-Induced Hepatoxicity through Endoplasmic Reticulum (ER) Stress Response. Chin. J. Nat. Med. 2015, 13, 250–256. [Google Scholar] [CrossRef]
- Robin, M.-A.; Demeilliers, C.; Sutton, A.; Paradis, V.; Maisonneuve, C.; Dubois, S.; Poirel, O.; Lettéron, P.; Pessayre, D.; Fromenty, B. Alcohol Increases Tumor Necrosis Factor Alpha and Decreases Nuclear Factor-Kappab to Activate Hepatic Apoptosis in Genetically Obese Mice. Hepatol. Baltim. Md 2005, 42, 1280–1290. [Google Scholar] [CrossRef]
- Carmiel-Haggai, M.; Cederbaum, A.I.; Nieto, N. Binge Ethanol Exposure Increases Liver Injury in Obese Rats. Gastroenterology 2003, 125, 1818–1833. [Google Scholar] [CrossRef]
- Wang, Y.; Seitz, H.K.; Wang, X.-D. Moderate Alcohol Consumption Aggravates High-Fat Diet Induced Steatohepatitis in Rats. Alcohol. Clin. Exp. Res. 2010, 34, 567–573. [Google Scholar] [CrossRef]
- Chang, B.; Xu, M.-J.; Zhou, Z.; Cai, Y.; Li, M.; Wang, W.; Feng, D.; Bertola, A.; Wang, H.; Kunos, G.; et al. Short- or Long-Term High-Fat Diet Feeding plus Acute Ethanol Binge Synergistically Induce Acute Liver Injury in Mice: An Important Role for CXCL1. Hepatol. Baltim. Md 2015, 62, 1070–1085. [Google Scholar] [CrossRef]
- Puri, P.; Xu, J.; Vihervaara, T.; Katainen, R.; Ekroos, K.; Daita, K.; Min, H.-K.; Joyce, A.; Mirshahi, F.; Tsukamoto, H.; et al. Alcohol Produces Distinct Hepatic Lipidome and Eicosanoid Signature in Lean and Obese. J. Lipid Res. 2016, 57, 1017–1028. [Google Scholar] [CrossRef]
- Song, M.; Chen, T.; Prough, R.A.; Cave, M.C.; McClain, C.J. Chronic Alcohol Consumption Causes Liver Injury in High-Fructose-Fed Male Mice Through Enhanced Hepatic Inflammatory Response. Alcohol. Clin. Exp. Res. 2016, 40, 518–528. [Google Scholar] [CrossRef]
- Wang, W.; Xu, M.-J.; Cai, Y.; Zhou, Z.; Cao, H.; Mukhopadhyay, P.; Pacher, P.; Zheng, S.; Gonzalez, F.J.; Gao, B. Inflammation Is Independent of Steatosis in a Murine Model of Steatohepatitis. Hepatol. Baltim. Md 2017, 66, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Zhao, Y.; Tang, Y.; Wei, X.; Shi, X.; Sun, W.; Sun, X.; Yin, X.; Sun, X.; Kim, S.; et al. Chronic Alcohol Exposure Stimulates Adipose Tissue Lipolysis in Mice: Role of Reverse Triglyceride Transport in the Pathogenesis of Alcoholic Steatosis. Am. J. Pathol. 2012, 180, 998–1007. [Google Scholar] [CrossRef]
- Kema, V.H.; Khan, I.; Jamal, R.; Vishwakarma, S.K.; Lakki Reddy, C.; Parwani, K.; Patel, F.; Patel, D.; Khan, A.A.; Mandal, P. Protective Effects of Diallyl Sulfide Against Ethanol-Induced Injury in Rat Adipose Tissue and Primary Human Adipocytes. Alcohol. Clin. Exp. Res. 2017, 41, 1078–1092. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chao, X.; Wang, S.; Williams, J.A.; Ni, H.-M.; Ding, W.-X. Role of Mechanistic Target of Rapamycin and Autophagy in Alcohol-Induced Adipose Atrophy and Liver Injury. Am. J. Pathol. 2020, 190, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Bucher, S.; Tête, A.; Podechard, N.; Liamin, M.; Le Guillou, D.; Chevanne, M.; Coulouarn, C.; Imran, M.; Gallais, I.; Fernier, M.; et al. Co-Exposure to Benzo[a]Pyrene and Ethanol Induces a Pathological Progression of Liver Steatosis in Vitro and in Vivo. Sci. Rep. 2018, 8, 5963. [Google Scholar] [CrossRef] [PubMed]
- Tête, A.; Gallais, I.; Imran, M.; Chevanne, M.; Liamin, M.; Sparfel, L.; Bucher, S.; Burel, A.; Podechard, N.; Appenzeller, B.M.R.; et al. Mechanisms Involved in the Death of Steatotic WIF-B9 Hepatocytes Co-Exposed to Benzo[a]Pyrene and Ethanol: A Possible Key Role for Xenobiotic Metabolism and Nitric Oxide. Free Radic. Biol. Med. 2018, 129, 323–337. [Google Scholar] [CrossRef]
- Imran, M.; Sergent, O.; Tête, A.; Gallais, I.; Chevanne, M.; Lagadic-Gossmann, D.; Podechard, N. Membrane Remodeling as a Key Player of the Hepatotoxicity Induced by Co-Exposure to Benzo[a]Pyrene and Ethanol of Obese Zebrafish Larvae. Biomolecules 2018, 8, 26. [Google Scholar] [CrossRef]
- Luo, Y.; Rana, P.; Will, Y. Palmitate Increases the Susceptibility of Cells to Drug-Induced Toxicity: An in Vitro Method to Identify Drugs with Potential Contraindications in Patients with Metabolic Disease. Toxicol. Sci. Off. J. Soc. Toxicol. 2012, 129, 346–362. [Google Scholar] [CrossRef]
- Ariaans, G.; Jong, S.; Gietema, J.A.; Lefrandt, J.D.; Vries, E.G.E.; Jalving, M. Cancer-Drug Induced Insulin Resistance: Innocent Bystander or Unusual Suspect. Cancer Treat. Rev. 2015, 41, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, N.; Fénichel, P. Endocrine Disruptors: New Players in the Pathophysiology of Type 2 Diabetes? Diabetes Metab. 2015, 41, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Fathallah, N.; Slim, R.; Larif, S.; Hmouda, H.; Ben Salem, C. Drug-Induced Hyperglycaemia and Diabetes. Drug Saf. 2015, 38, 1153–1168. [Google Scholar] [CrossRef] [PubMed]
- Vanni, R.; Bussuan, R.M.; Rombaldi, R.L.; Arbex, A.K. Endocrine Disruptors and the Induction of Insulin Resistance. Curr. Diabetes Rev. 2020. [Google Scholar] [CrossRef]
- Mitra, M.S.; Donthamsetty, S.; White, B.; Mehendale, H.M. High Fat Diet-Fed Obese Rats Are Highly Sensitive to Doxorubicin-Induced Cardiotoxicity. Toxicol. Appl. Pharmacol. 2008, 231, 413–422. [Google Scholar] [CrossRef]
- Guenancia, C.; Lefebvre, A.; Cardinale, D.; Yu, A.F.; Ladoire, S.; Ghiringhelli, F.; Zeller, M.; Rochette, L.; Cottin, Y.; Vergely, C. Obesity As a Risk Factor for Anthracyclines and Trastuzumab Cardiotoxicity in Breast Cancer: A Systematic Review and Meta-Analysis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 3157–3165. [Google Scholar] [CrossRef]
- Skinner, C.M.; Miousse, I.R.; Ewing, L.E.; Sridharan, V.; Cao, M.; Lin, H.; Williams, D.K.; Avula, B.; Haider, S.; Chittiboyina, A.G.; et al. Impact of Obesity on the Toxicity of a Multi-Ingredient Dietary Supplement, OxyELITE ProTM (New Formula), Using the Novel NZO/HILtJ Obese Mouse Model: Physiological and Mechanistic Assessments. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 122, 21–32. [Google Scholar] [CrossRef]
- Corcoran, G.B.; Salazar, D.E. Obesity as a Risk Factor in Drug-Induced Organ Injury. IV. Increased Gentamicin Nephrotoxicity in the Obese Overfed Rat. J. Pharmacol. Exp. Ther. 1989, 248, 17–22. [Google Scholar]
- Rutter, W.C.; Hall, R.G.; Burgess, D.S. Impact of Total Body Weight on Rate of Acute Kidney Injury in Patients Treated with Piperacillin-Tazobactam and Vancomycin. Am. J. Health-Syst. Pharm. AJHP Off. J. Am. Soc. Health-Syst. Pharm. 2019, 76, 1211–1217. [Google Scholar] [CrossRef]
- Tsai, H.-P.; Hou, P.-H.; Mao, F.-C.; Chang, C.-C.; Yang, W.-C.; Wu, C.-F.; Liao, H.-J.; Lin, T.-C.; Chou, L.-S.; Hsiao, L.-W.; et al. Risperidone Exacerbates Glucose Intolerance, Nonalcoholic Fatty Liver Disease, and Renal Impairment in Obese Mice. Int. J. Mol. Sci. 2021, 22, 409. [Google Scholar] [CrossRef] [PubMed]

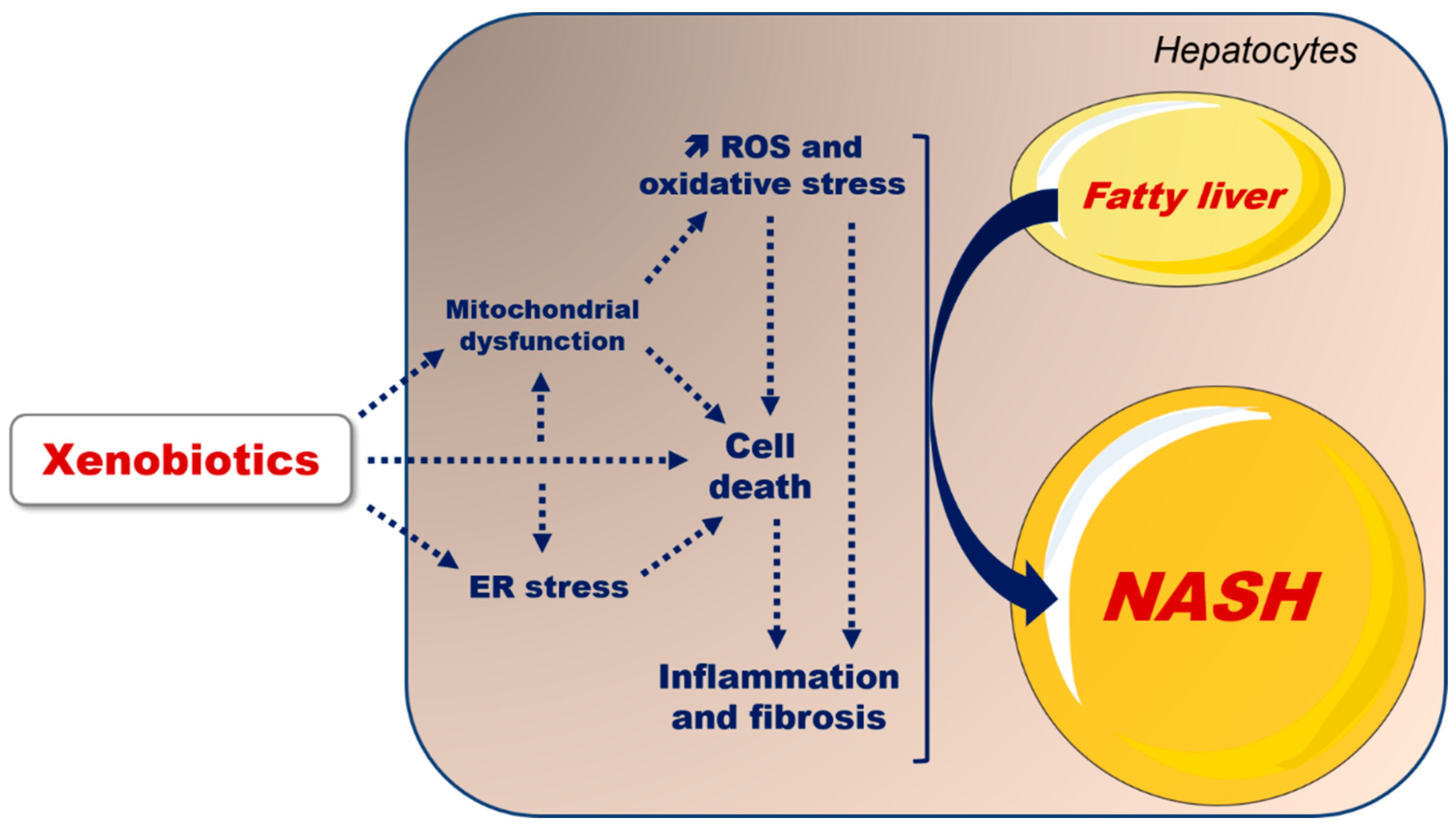
 : increase;
: increase;  : decrease).
: decrease).
 : increase;
: increase; 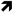 : decrease).
: decrease).
| Drugs | Environmental Contaminants |
|---|---|
| Corticosteroids (Corticosterone, Dexamethasone) | Bisphenol A (BPA) |
| Irinotecan 2 | Diesel exhaust particles |
| Methotrexate 2 | Hexabromocyclododecane (HBCD) |
| Nucleoside reverse transcriptase inhibitors (didanosine, stavudine) 2 | Nonylphenol |
| Pentoxifylline 2 | Perchloroethylene |
| Phenobarbital 2 | Perfluorooctanoic acid (PFOA) |
| Tamoxifen 2 | Tetrabromodiphenyl ether (BDE-47) |
| Tetracycline 2 | 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) |
| Thiazolidinediones (rosiglitazone, troglitazone, pioglitazone) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massart, J.; Begriche, K.; Corlu, A.; Fromenty, B. Xenobiotic-Induced Aggravation of Metabolic-Associated Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 1062. https://doi.org/10.3390/ijms23031062
Massart J, Begriche K, Corlu A, Fromenty B. Xenobiotic-Induced Aggravation of Metabolic-Associated Fatty Liver Disease. International Journal of Molecular Sciences. 2022; 23(3):1062. https://doi.org/10.3390/ijms23031062
Chicago/Turabian StyleMassart, Julie, Karima Begriche, Anne Corlu, and Bernard Fromenty. 2022. "Xenobiotic-Induced Aggravation of Metabolic-Associated Fatty Liver Disease" International Journal of Molecular Sciences 23, no. 3: 1062. https://doi.org/10.3390/ijms23031062
APA StyleMassart, J., Begriche, K., Corlu, A., & Fromenty, B. (2022). Xenobiotic-Induced Aggravation of Metabolic-Associated Fatty Liver Disease. International Journal of Molecular Sciences, 23(3), 1062. https://doi.org/10.3390/ijms23031062






