Active Materials for 3D Printing in Small Animals: Current Modalities and Future Directions for Orthopedic Applications
Abstract
1. Introduction
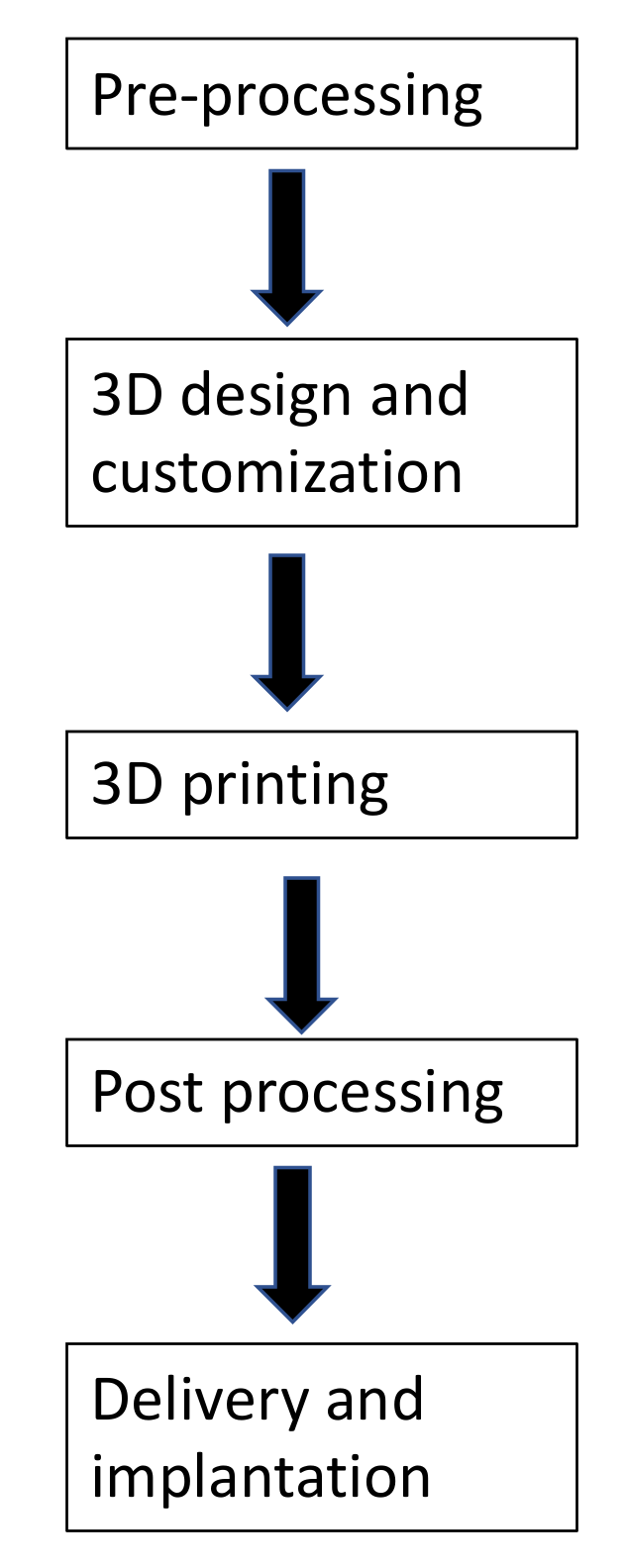
2. 3D Printing Process
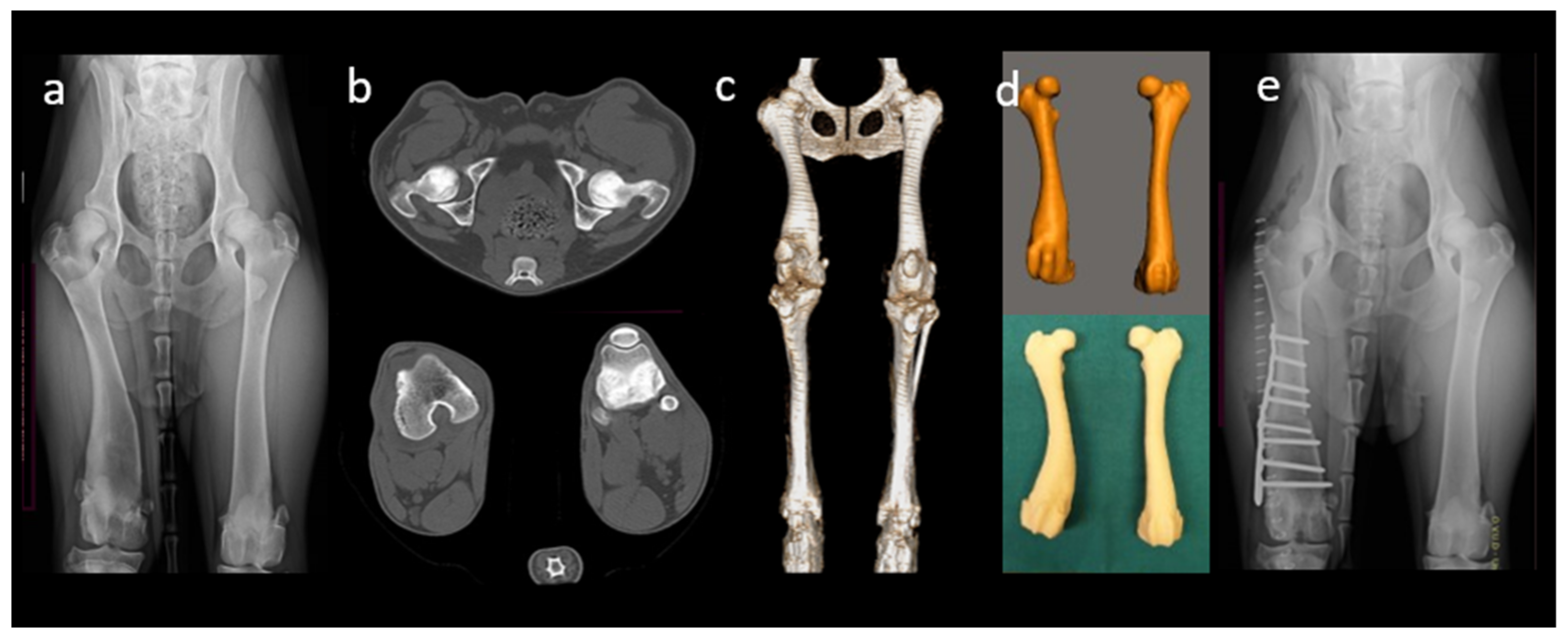
2.1. Image Data Acquisition
2.2. Image Processing
2.2.1. Image Segmentation
2.2.2. Manipulation and Analysis
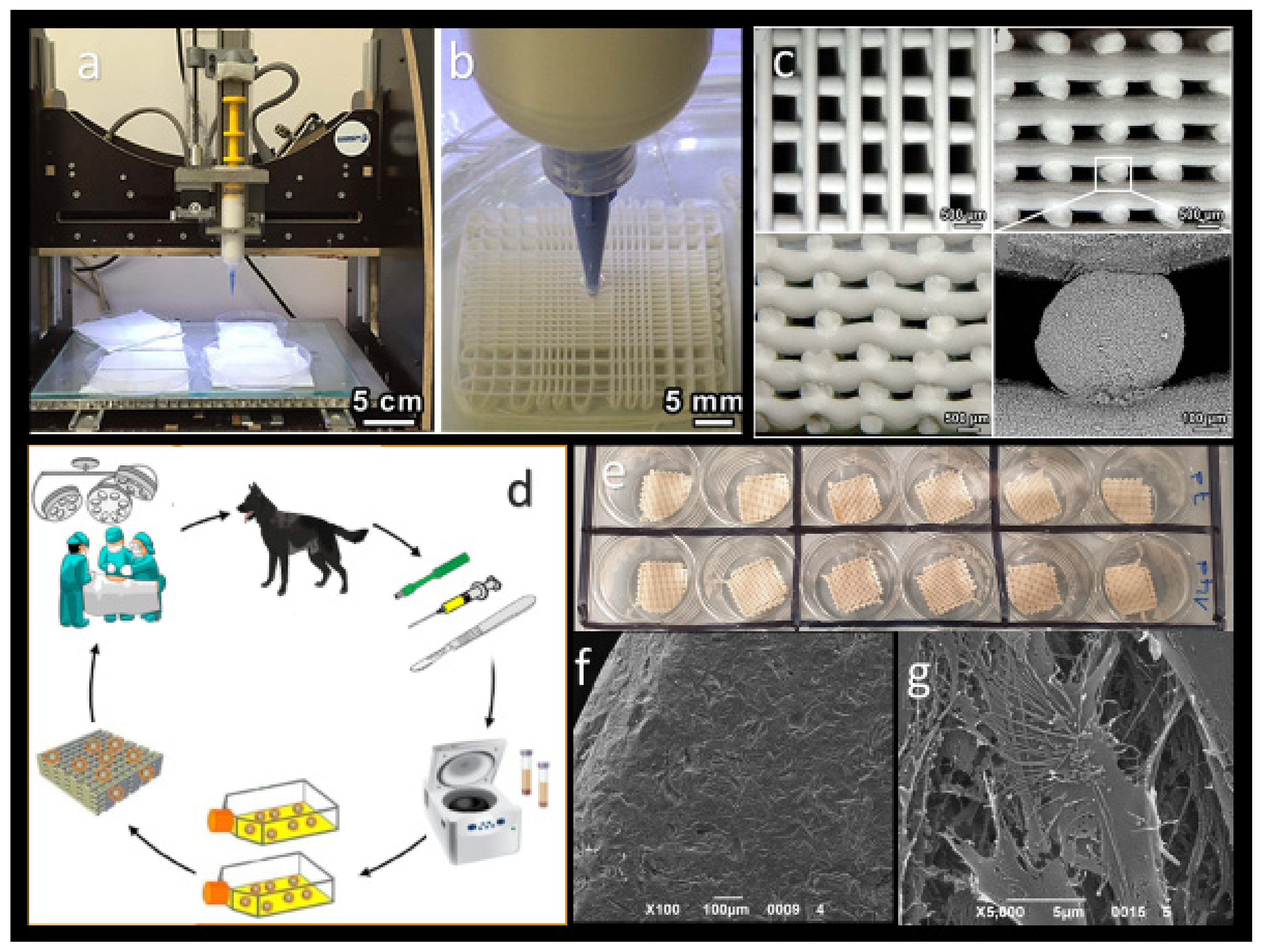
2.3. Object Fabrication
3. Materials for 3D Printing
3.1. Metals
3.2. Ceramics and Glasses
3.3. Polymers
3.4. Composites
4. 3D Printing Techniques
4.1. Stereolithography Apparatus
4.2. Binder Jetting
4.3. Extrusion-Based Printing
4.3.1. Fused Deposition Modeling
4.3.2. Direct Ink Writing
4.4. Powder Bed Fusion
4.4.1. Electron Beam Melting
4.4.2. Selective Laser Sintering
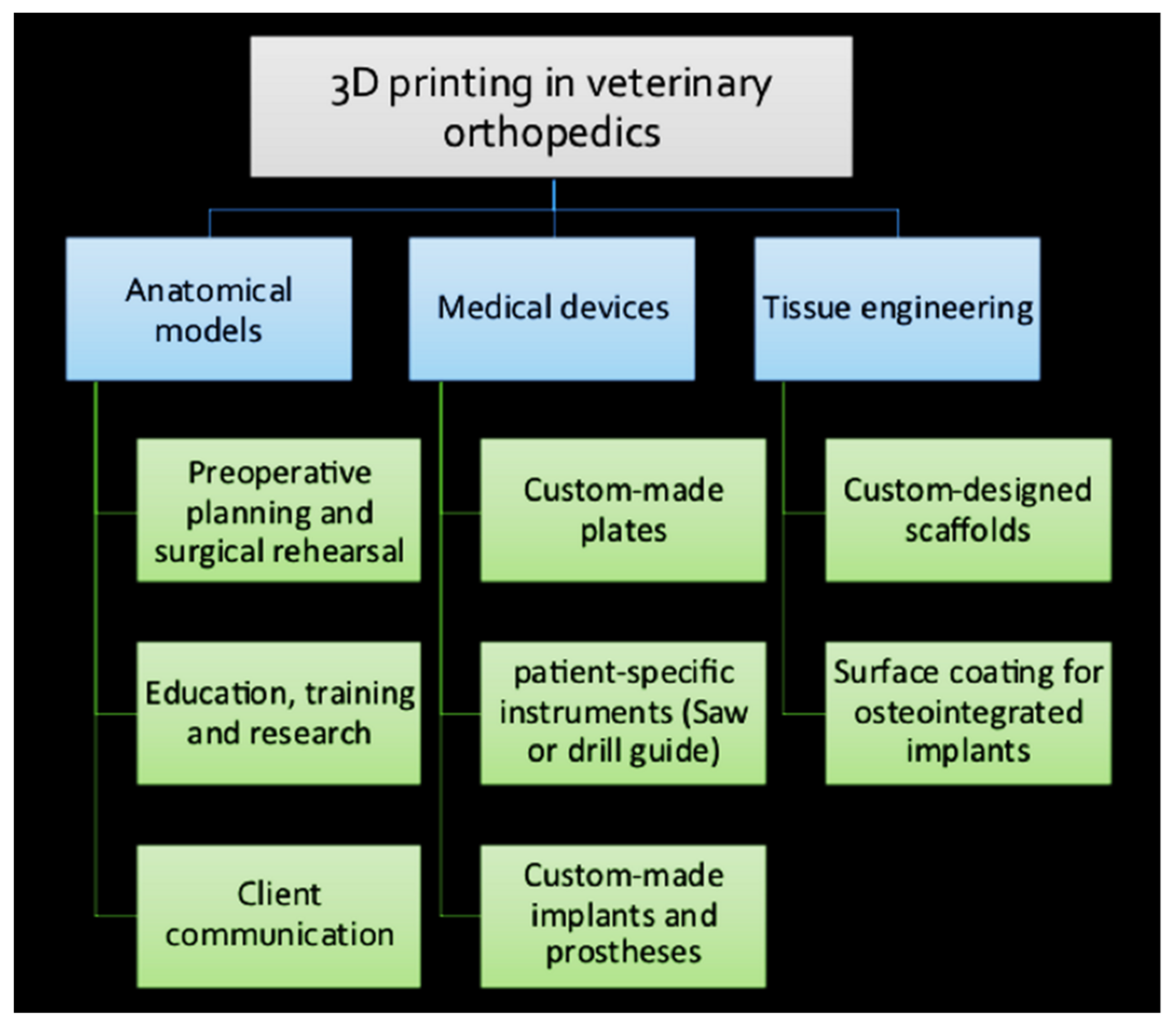
5. Application of 3D Printing in Veterinary Orthopedics
5.1. 3D-Printed Anatomical Models
5.2. Pre-Operative Planning and Rehearsal
5.3. Veterinary Research and Education
5.4. Client Communication and Education
5.5. Receiver-Specific Orthopedic Instruments
5.6. Custom-Made Orthopedic Implants
5.6.1. Total Joint Replacement
5.6.2. Total Hip Replacement
5.6.3. Total Knee Replacement
5.6.4. Patellar Groove Replacement
5.6.5. Total Elbow Replacement
5.6.6. Limb-Sparing Surgery
5.6.7. Corrective Osteotomies
5.6.8. Arthrodesis
5.7. Customized Scaffolds
6. Limitations and Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BTE | bone tissue engineering |
| AM | additive manufacturing |
| CAD | computer-aided design |
| MRI | magnetic resonance |
| MPR | multiplanar reformation or reconstruction |
| CoCr | cobalt–chrome |
| PEEK | polyetheretherketone |
| CaP | Calcium phosphate |
| TCP | Tricalcium phosphate |
| PCL | polycaprolactone |
| FDM | fused deposition modeling |
| SLS | selective laser sintering |
| PDL | Poly-D,L-lactide |
| PGA | polyglycolic acid |
| PCL | Polycaprolactone |
| THR | Total hip replacement |
| BJ | binder jetting |
| SLM | selective laser melting |
| EBM | electron beam melting |
| OBDNT | open but do not touch |
| TJR | total joint replacement |
| TER | total elbow replacement |
| CAP | custom acetabular prosthesis |
| PMMA | polymethylmethacrylate |
| CUE | canine unicompartmental elbow |
| OSA | osteosarcoma |
| 3D | three-dimensional |
| RP | rapid prototyping |
| CT | computer tomography |
| DICOM | digital imaging and communications in medicine |
| Ti | Titanium |
| SS | stainless-steel |
| UHMWPE | ultra-high molecular weight polyethylene |
| HA | hydroxyapatites |
| BCP | biphasic calcium phosphate |
| cAD-MSCs | canine adipose-derived mesenchymal stem cells |
| SLA | stereolithography apparatus |
| DIW | direct ink writing |
| PLA | polylactic acid |
| PLGA | polylactic-co-glycolic acid |
| HDPE | high-density polyethylene |
| ABS | acrylonitrile butadiene styrene |
| FFF | fused filament fabrication |
| SLS | selective laser sintering |
| MIPO | minimally invasive plate osteosynthesis |
| PSIs | receiver-specific instruments |
| TKR | total knee replacement |
| OA | osteoarthritis |
| DMLS | direct metal laser sintering |
| PGR | patellar groove replacement |
| CORA | centers of rotation of angulation |
| TTA | tibial tuberosity advancement |
References
- Fitzpatrick, N.; Guthrie, J.W. Hemipelvic and proximal femoral limb salvage endoprosthesis with tendon ongrowth in a dog. Vet. Surg. 2018, 47, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Castelli, E.; Schmierer, P.A.; Pozzi, A. Custom acetabular prosthesis for total hip replacement: A case report in a dog with acetabular bone loss after femoral head and neck ostectomy. Vet. Surg. 2019, 48, 1520–1529. [Google Scholar] [CrossRef]
- Conzemius, M.G.; Aper, R.L.; Corti, L.B. Short-term outcome after total elbow arthroplasty in dogs with severe, naturally occurring osteoarthritis. Vet. Surg. 2003, 32, 545–552. [Google Scholar] [CrossRef]
- Choi, S.; Oh, Y.I.; Park, K.H.; Lee, J.S.; Shim, J.H.; Kang, B.J. New clinical application of three-dimensional-printed polycaprolactone/β-tricalcium phosphate scaffold as an alternative to allograft bone for limb-sparing surgery in a dog with distal radial osteosarcoma. J. Vet. Med. Sci. 2019, 81, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Yeates, J.; Main, D. Assessment of companion animal quality of life in veterinary practice and research. J. Small Anim. Pract. 2009, 50, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Memarian, P.; Sartor, F.; Bernardo, E.; Elsayed, H.; Ercan, B.; Delogu, L.G.; Zavan, B.; Isola, M. Osteogenic properties of 3D-printed silica-carbon-calcite composite scaffolds: Novel approach for personalized bone tissue regeneration. Int. J. Mol. Sci. 2021, 22, 475. [Google Scholar] [CrossRef]
- Vidal, L.; Kampleitner, C.; Brennan, M.; Hoornaert, A.; Layrolle, P. Reconstruction of Large Skeletal Defects: Current Clinical Therapeutic Strategies and Future Directions Using 3D Printing. Front. Bioeng. Biotechnol. 2020, 8, 61. [Google Scholar] [CrossRef]
- Harrysson, O.L.A.; Marcellin-Little, D.J.; Horn, T.J. Applications of Metal Additive Manufacturing in Veterinary Orthopedic Surgery. JOM 2015, 67, 647–654. [Google Scholar] [CrossRef]
- Roffi, A.; Krishnakumar, G.S.; Gostynska, N.; Kon, E.; Candrian, C.; Filardo, G.; Shankar Krishnakumar, G.; Gostynska, N.; Kon, E.; Candrian, C.; et al. The Role of Three-Dimensional Scaffolds in Treating Long Bone Defects: Evidence from Preclinical and Clinical Literature—A Systematic Review. Biomed. Res. Int. 2017, 2017, 8074178. [Google Scholar] [CrossRef]
- Castilho, M.; Dias, M.; Vorndran, E.; Gbureck, U.; Fernandes, P.; Pires, I.; Gouveia, B.; Armes, H.; Pires, E.; Rodrigues, J. Application of a 3D printed customized implant for canine cruciate ligament treatment by tibial tuberosity advancement. Biofabrication 2014, 6, 025005. [Google Scholar] [CrossRef]
- Liaw, C.Y.; Guvendiren, M. Current and emerging applications of 3D printing in medicine. Biofabrication 2017, 9, 204102. [Google Scholar] [CrossRef] [PubMed]
- Popov, V.V.; Muller-Kamskii, G.; Katz-Demyanetz, A.; Kovalevsky, A.; Usov, S.; Trofimcow, D.; Dzhenzhera, G.; Koptyug, A. Additive manufacturing to veterinary practice: Recovery of bony defects after the osteosarcoma resection in canines. Biomed. Eng. Lett. 2019, 9, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.R.; Adam, G.O.; Yang, D.K.; Tungalag, T.; Lee, S.J.; Kim, J.S.; Kang, H.S.; Kim, S.J.; Kim, N.S. An easy and economical way to produce a three-dimensional bone phantom in a dog with antebrachial deformities. Animals 2020, 10, 1445. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.C. 3D-printed receiver-specific applications in orthopedics. Orthop. Res. Rev. 2016, 8, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Hilton, T.L.; Campbell, N.; Hosking, K. Additive manufacturing in orthopaedics: Clinical implications. SA Orthop. J. 2017, 16, 63–67. [Google Scholar] [CrossRef][Green Version]
- Hespel, A.M.; Wilhite, R.; Hudson, J. Invited review-applications for 3D printers in veterinary medicine. Vet. Radiol. Ultrasound 2014, 55, 347–358. [Google Scholar] [CrossRef]
- Sun, H.; Yang, H.L. Calcium phosphate scaffolds combined with bone morphogenetic proteins or mesenchymal stem cells in bone tissue engineering. Chin. Med. J. 2015, 128, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Kamio, T.; Suzuki, M.; Asaumi, R.; Kawai, T. DICOM segmentation and STL creation for 3D printing: A process and software package comparison for osseous anatomy. 3D Print. Med. 2020, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Melancon, D.; Bagheri, Z.S.; Johnston, R.B.; Liu, L.; Tanzer, M.; Pasini, D. Mechanical characterization of structurally porous biomaterials built via additive manufacturing: Experiments, predictive models, and design maps for load-bearing bone replacement implants. Acta Biomater. 2017, 63, 350–368. [Google Scholar] [CrossRef]
- Tappa, K.; Jammalamadaka, U. Novel biomaterials used in medical 3D printing techniques. J. Funct. Biomater. 2018, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Peck, J.N.; Marcellin-Little, D.J. Advances in Small Animal Total Joint Replacement; John Wiley & Sons: Hoboken, NJ, USA, 2012; Volume 4, ISBN 9780470959619. [Google Scholar]
- Pagani, S.; Liverani, E.; Giavaresi, G.; De Luca, A.; Belvedere, C.; Fortunato, A.; Leardini, A.; Fini, M.; Tomesani, L.; Caravaggi, P. Mechanical and in vitro biological properties of uniform and graded Cobalt-chrome lattice structures in orthopedic implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 2091–2103. [Google Scholar] [CrossRef] [PubMed]
- Veiga, C.; Davim, J.P.; Loureiro, A.J.R. Properties and applications of titanium alloys: A brief review. Rev. Adv. Mater. Sci. 2012, 32, 133–148. [Google Scholar]
- Elsayed, H.; Rebesan, P.; Giacomello, G.; Pasetto, M.; Gardin, C.; Ferroni, L.; Zavan, B.; Biasetto, L. Direct ink writing of porous titanium (Ti6Al4V) lattice structures. Mater. Sci. Eng. C 2019, 103, 109794. [Google Scholar] [CrossRef]
- Levine, B.R.; Sporer, S.; Poggie, R.A.; Della Valle, C.J.; Jacobs, J.J. Experimental and clinical performance of porous tantalum in orthopedic surgery. Biomaterials 2006, 27, 4671–4681. [Google Scholar] [CrossRef]
- Nazarali, A.; Singh, A.; Morrison, S.; Gibson, T.W.G.; Rousseau, J.; Weese, J.S.; Boston, S.E. Comparison of methicillin-resistant Staphylococcus pseudintermedius adherence to 2 canine limb salvage endoprosthesis implants. Pub. Med. Can. Vet. J. 2017, 58, 964–966. [Google Scholar]
- Li, M.; Xiong, P.; Yan, F.; Li, S.; Ren, C.; Yin, Z.; Li, A.; Li, H.; Ji, X.; Zheng, Y.; et al. An overview of graphene-based hydroxyapatite composites for orthopedic applications. Bioact. Mater. 2018, 3, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Scholz, M.S.; Blanchfield, J.P.; Bloom, L.D.; Coburn, B.H.; Elkington, M.; Fuller, J.D.; Gilbert, M.E.; Muflahi, S.A.; Pernice, M.F.; Rae, S.I.; et al. The use of composite materials in modern orthopaedic medicine and prosthetic devices: A review. Compos. Sci. Technol. 2011, 71, 1791–1803. [Google Scholar] [CrossRef]
- Kim, J.W.; Yang, B.E.; Hong, S.J.; Choi, H.G.; Byeon, S.J.; Lim, H.K.; Chung, S.M.; Lee, J.H.; Byun, S.H. Bone regeneration capability of 3D printed ceramic scaffolds. Int. J. Mol. Sci. 2020, 21, 4837. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, Q.; Zhao, W.; Qiao, B.; Cui, H.; Fan, J.; Li, H.; Tu, X.; Jiang, D. In vitro and in vivo biocompatibility and osteogenesis of graphene-reinforced nanohydroxyapatite polyamide66 ternary biocomposite as orthopedic implant material. Int. J. Nanomed. 2016, 11, 3179–3189. [Google Scholar] [CrossRef]
- Ettorre, V.; De Marco, P.; Zara, S.; Perrotti, V.; Scarano, A.; Di Crescenzo, A.; Petrini, M.; Hadad, C.; Bosco, D.; Zavan, B.; et al. In vitro and in vivo characterization of graphene oxide coated porcine bone granules. Carbon 2016, 103, 291–298. [Google Scholar] [CrossRef]
- Prasadh, S.; Suresh, S.; Wong, R. Osteogenic potential of graphene in bone tissue engineering scaffolds. Materials 2018, 11, 1430. [Google Scholar] [CrossRef] [PubMed]
- Shadjou, N.; Hasanzadeh, M.; Khalilzadeh, B. Graphene based scaffolds on bone tissue engineering. Bioengineered 2018, 9, 38–47. [Google Scholar] [CrossRef]
- Brunello, G.; Sivolella, S.; Meneghello, R.; Ferroni, L.; Gardin, C.; Piattelli, A.; Zavan, B.; Bressan, E. Powder-based 3D printing for bone tissue engineering. Biotechnol. Adv. 2016, 34, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.J. Advances in total joint replacement in small animals. J. Small Anim. Pract. 2012, 53, 495–506. [Google Scholar] [CrossRef]
- Pugliese, R.; Beltrami, B.; Regondi, S.; Lunetta, C. Polymeric biomaterials for 3D printing in medicine: An overview. Ann. 3D Print. Med. 2021, 2, 100011. [Google Scholar] [CrossRef]
- Zhai, X.; Ma, Y.; Hou, C.; Gao, F.; Zhang, Y.; Ruan, C.; Pan, H.; Lu, W.W.; Liu, W. 3D-Printed High Strength Bioactive Supramolecular Polymer/Clay Nanocomposite Hydrogel Scaffold for Bone Regeneration. ACS Biomater. Sci. Eng. 2017, 3, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Pishavar, E.; Khosravi, F.; Naserifar, M.; Ghomi, E.R.; Luo, H.; Zavan, B.; Seifalian, A.; Ramakrishna, S. Multifunctional and Self-Healable Intelligent Hydrogels for Cancer Drug Delivery and Promoting Tissue Regeneration In Vivo. Polymers 2021, 13, 2680. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, C.; Kim, H.S.; Moon, C.; Lee, K.Y. 3D Printing of dynamic tissue scaffold by combining self-healing hydrogel and self-healing ferrogel. Colloids Surf. B Biointerfaces 2021, 208, 112108. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, B.; Li, M.; Li, J.; Zhang, C.; Han, Y.; Wang, L.; Wang, K.; Zhou, C.; Liu, L.; et al. 3D printing of PLA/n-HA composite scaffolds with customized mechanical properties and biological functions for bone tissue engineering. Compos. Part B Eng. 2021, 224, 109192. [Google Scholar] [CrossRef]
- Castilho, M.; Dias, M.; Gbureck, U.; Groll, J.; Fernandes, P.; Pires, I.; Gouveia, B.; Rodrigues, J.; Vorndran, E. Fabrication of computationally designed scaffolds by low temperature 3D printing. Biofabrication 2013, 5, 035012. [Google Scholar] [CrossRef]
- Pranzo, D.; Larizza, P.; Filippini, D.; Percoco, G. Extrusion-based 3D printing of microfluidic devices for chemical and biomedical applications: A topical review. Micromachines 2018, 9, 374. [Google Scholar] [CrossRef]
- Gonzalez-Gutierrez, J.; Cano, S.; Schuschnigg, S.; Kukla, C.; Sapkota, J.; Holzer, C. Additive Manufacturing of Metallic and Ceramic Components by the Material Extrusion of Highly-Filled Polymers: A Review and Future Perspectives. Materials 2018, 11, 840. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, H.; Carraro, F.; Agnoli, S.; Bellucci, D.; Cannillo, V.; Ferroni, L.; Gardin, C.; Zavan, B.; Bernardo, E. Direct ink writing of silica-carbon-calcite composite scaffolds from a silicone resin and fillers. J. Eur. Ceram. Soc. 2018, 38, 5200–5207. [Google Scholar] [CrossRef]
- Jeong, B.; Jung, J.; Park, J.; Jeong, S.M.; Lee, H. 3D-printing bone model for surgical planning of corrective osteotomy for treatment of medial patellar luxation in a dog. J. Vet. Clin. 2016, 33, 385–388. [Google Scholar] [CrossRef]
- Fiocco, L.; Elsayed, H.; Badocco, D.; Pastore, P.; Bellucci, D.; Cannillo, V.; Detsch, R.; Boccaccini, A.R.; Bernardo, E. Direct ink writing of silica-bonded calcite scaffolds from preceramic polymers and fillers. Biofabrication 2017, 9, 025012. [Google Scholar] [CrossRef]
- Worth, A.J.; Crosse, K.R.; Kersley, A. Computer-Assisted Surgery Using 3D Printed Saw Guides for Acute Correction of Antebrachial Angular Limb Deformities in Dogs. Vet. Comp. Orthop. Traumatol. 2019, 32, 241–249. [Google Scholar] [CrossRef]
- Carwardine, D.R.; Gosling, M.J.; Burton, N.J.; O’Malley, F.L.; Parsons, K.J. Three-Dimensional-Printed Receiver-Specific Osteotomy Guides, Repositioning Guides and Titanium Plates for Acute Correction of Antebrachial Limb Deformities in Dogs. Vet. Comp. Orthop. Traumatol. 2021, 34, 43–52. [Google Scholar] [CrossRef] [PubMed]
- DeTora, M.D.; Boudrieau, R.J. Complex angular and torsional deformities (Distal femoral malunions): Preoperative planning using stereolithography and surgical correction with locking plate fixation in four dogs. Vet. Comp. Orthop. Traumatol. 2016, 29, 416–425. [Google Scholar] [CrossRef]
- Lam, G.; Kim, S.K. Three-Dimensional Computer-Assisted Surgical Planning and Use of 3-Dimensional Printing in the Repair of a Complex Articular Femoral Fracture in a Dog. Vet. Orthop. Soc. 2019, 32, 12–18. [Google Scholar] [CrossRef]
- Conzemius, M.G.; Aper, R.L.; Hill, C.M. Evaluation of a canine total-elbow arthroplasty system: A preliminary study in normal dogs. Vet. Surg. 2001, 30, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.J.; Leone, K.A.; Lamonte, K.; Townsend, K.L.; Mann, K.A. Cemented Total Knee Replacement in 24 Dogs_ Surgical Technique, Clinical Results, and Complications. Vet. Surg. 2009, 38, 555–567. [Google Scholar] [CrossRef]
- Liska, W.D.; Marcellin-Little, D.J.; Eskelinen, E.V.; Sidebotham, C.G.; Harrysson, O.L.A.; Hielm-Bjorkman, A.K. Custom total knee replacement in a dog with femoral condylar bone loss. Vet. Surg. 2007, 36, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, N.; Miraldo, D.C.; Meswania, J. Custom-built constrained uniaxial and rotating hinge total knee replacement in cats: Clinical application, design principles, surgical technique, and clinical outcome. Vet. Surg. 2021, 50, 1283–1295. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, Y.; Park, J.; Jeong, S.M.; Lee, H. Bilateral patellar groove replacement in a dog with iatrogenic trochlear groove damage. J. Vet. Clin. 2016, 33, 295–299. [Google Scholar] [CrossRef]
- Dokic, Z.; Lorinson, D.; Weigel, J.P.; Vezzoni, A. Patellar groove replacement in patellar Luxation with severe femoro-patellar osteoarthritis. Vet. Comp. Orthop. Traumatol. 2015, 28, 124–130. [Google Scholar] [CrossRef]
- Conzemius, M. Nonconstrained elbow replacement in dogs. Vet. Surg. 2009, 38, 279–284. [Google Scholar] [CrossRef]
- Cook, J.L.; Schulz, K.S.; Karnes, G.J.; Franklin, S.P.; Canapp, S.O.; Lotsika, P.J.; Fitzpatrick, N.; Wheeler, J.L.; Stiffler, K.S.; Gillick, M.; et al. Clinical outcomes associated with the initial use of the Canine Unicompartmental Elbow (CUE) Arthroplasty System. Can. Vet. J. 2015, 56, 971–977. [Google Scholar] [PubMed]
- Wu, N.; Li, S.; Liu, Y.; Zhang, A.; Chen, B.; Han, Q.; Wang, J. Novel exploration of 3D printed personalized total elbow arthroplasty to solve the severe bone defect after internal fixation failure of comminuted distal humerus fracture. Medicine 2020, 99, e21481. [Google Scholar] [CrossRef] [PubMed]
- Séguin, B.; O’Donnell, M.D.; Walsh, P.J.; Selmic, L.E. Long-term outcome of dogs treated with ulnar rollover transposition for limb-sparing of distal radial osteosarcoma: 27 limbs in 26 dogs. Vet. Surg. 2017, 46, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Berasi, C.C.; Berend, K.R.; Adams, J.B.; Ruh, E.L.; Lombardi, A.V. Are Custom Triflange Acetabular Components Effective for Reconstruction of Catastrophic Bone Loss? Clin. Orthop. Relat. Res. 2015, 473, 528–535. [Google Scholar] [CrossRef]
- Bray, J.P.; Kersley, A.; Downing, W.; Crosse, K.R.; Worth, A.J.; House, A.K.; Yates, G.; Coomer, A.R.; Brown, I.W.M. Clinical outcomes of receiver-specific porous titanium endoprostheses in dogs with tumors of the mandible, radius, or tibia: 12 cases (2013–2016). J. Am. Vet. Med. Assoc. 2017, 251, 566–579. [Google Scholar] [CrossRef]
- MacDonald, T.L.; Schiller, T.D. Limb-sparing surgery using tantalum metal endoprosthesis in a dog with osteosarcoma of the distal radius. Can. Vet. J. 2010, 51, 497–500. [Google Scholar]
- Timercan, A.; Brailovski, V.; Petit, Y.; Lussier, B.; Séguin, B. Personalized 3D-printed endoprostheses for limb sparing in dogs: Modeling and in vitro testing. Med. Eng. Phys. 2019, 71, 17–29. [Google Scholar] [CrossRef]
- Bordelo, J.P.A.; Dias, M.I.R.; Cardoso, L.M.M.L.; Requicha, J.M.F.; Viegas, C.A.A.; Bardet, J.F. A 3D printed model for radius curvus surgical treatment planning in a dog. Pesqui. Vet. Bras. 2018, 38, 1178–1183. [Google Scholar] [CrossRef]
- Longo, F.; Penelas, A.; Gutbrod, A.; Pozzi, A. Three-dimensional computer-assisted corrective osteotomy with a receiver-specific surgical guide for an antebrachial limb deformity in two dogs. Schweiz. Arch. Tierheilkd. 2019, 161, 473–479. [Google Scholar] [CrossRef] [PubMed]
- McKee, W.M.; May, C.; Macias, C.; Lapish, J.P. Pantarsal arthrodesis with a customised medial or lateral bone plate in 13 dogs. Vet. Rec. 2004, 154, 165–170. [Google Scholar] [CrossRef]
- McCarthy, J.; Comerford, E.J.; Innes, J.F.; Pettitt, R.A. Elbow Arthrodesis Using a Medially Positioned Plate in 6 Dogs. Vet. Comp. Orthop. Traumatol. 2020, 33, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.W.; Heo, S.Y.; Lee, M.H.; Lee, H.B. Evaluation of a poly(lactic-acid) scaffold filled with poly(lactide-co-glycolide)/hydroxyapatite nanofibres for reconstruction of a segmental bone defect in a canine model. Vet. Med. 2019, 64, 531–538. [Google Scholar] [CrossRef]
- Dorea, H.C.; McLaughlin, R.M.; Cantwell, H.D.; Read, R.; Armbrust, L.; Pool, R.; Roush, J.K.; Boyle, C. Evaluation of healing in feline femoral defects filled with cancellous autograft, cancellous allograft or Bioglass. Vet. Comp. Orthop. Traumatol. 2005, 18, 157–168. [Google Scholar] [CrossRef]
- Franch, J.; Barba, A.; Rappe, K.; Maazouz, Y.; Ginebra, M.P. Use of three-dimensionally printed β-tricalcium phosphate synthetic bone graft combined with recombinant human bone morphogenic protein-2 to treat a severe radial atrophic nonunion in a Yorkshire terrier. Vet. Surg. 2020, 49, 1626–1631. [Google Scholar] [CrossRef]
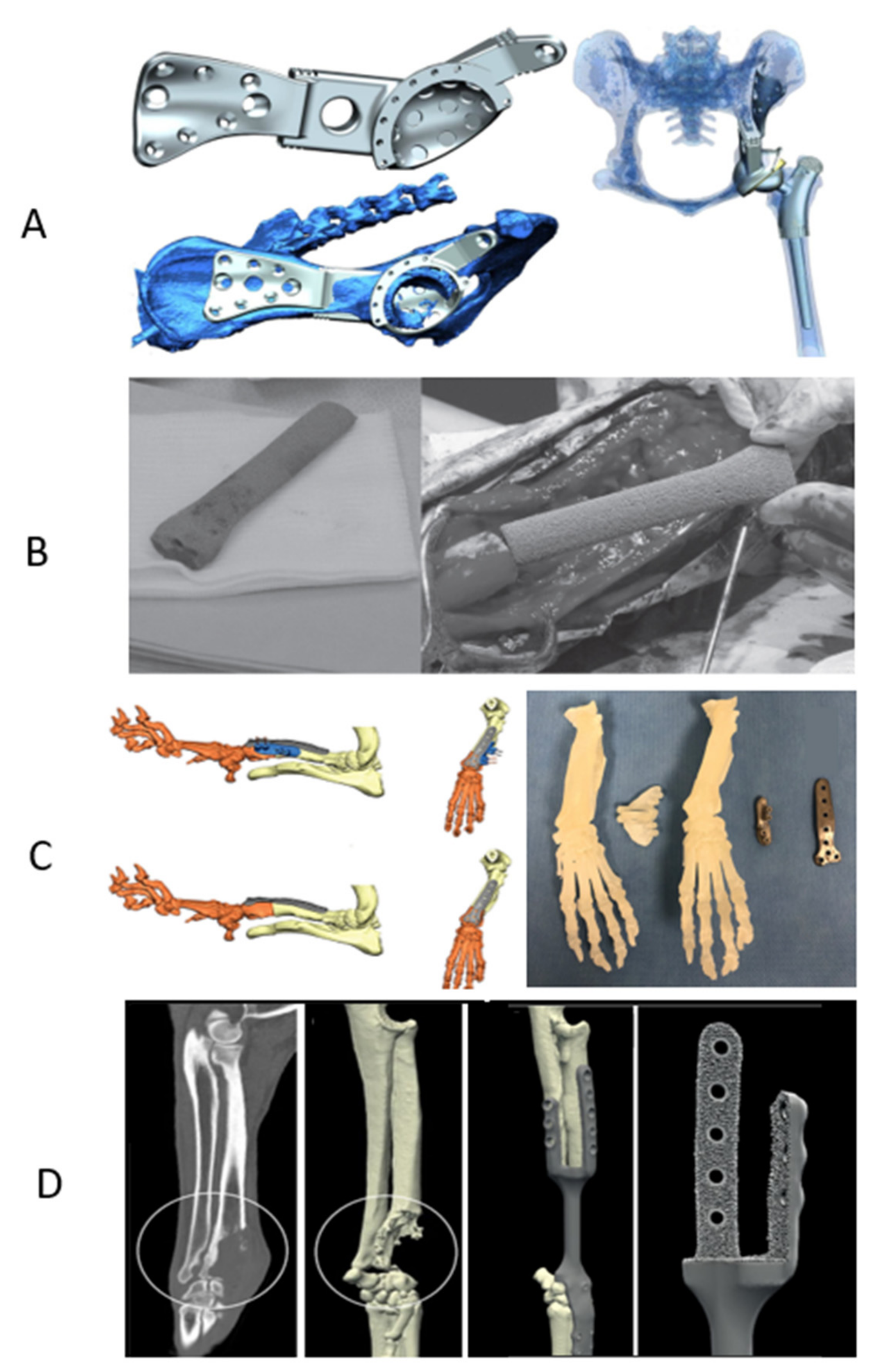
| Group | Material | 3D Printed Object | 3D Printing Technique | Clinical Case or Study | Surgical Intervention | Reference |
|---|---|---|---|---|---|---|
| Metals | CoCr with plasma coating | Femoral and tibial components of Custom-made constrained uniaxial and rotating hinge TKR | Fitzbionics Ltd. (Godalming, Surrey, UK) | 9 cats with traumatic stifle luxation or severe distal femoral deformity | Custom TKR | Fitzpatrik et al., 2021 |
| Titanium alloy (Ti6Al4V) | Personalized limb-sparing endoprostheses | Laser PBF EOSINT M280 400 W Ytterbium fiber laser system (EOS GmbH, Munich, Germany) | In-vitro testing and modeling of a canine limb | Limb sparing surgery | Timercan et al., 2019 | |
| Custom implant of proximal tibia (with porous features for ligaments and tendons reattachment) in conjunction with commercial TKR | EBM | Large breed dog with OSA of the proximal tibia | Limb sparing surgery | Harrysson et al., 2015 | ||
| Biflanged CAP with a porous surface for long-term biologic fixation | DMLS (Layerwise; 3D Systems, Rock Hill, SC, USA) | Adult Labrador retriever with lameness after femoral head and neck ostectomy | Custom-made THR to restore the acetabular bone loss | Castelli et al., 2019 | ||
| Custom-made limb-sparing implants | PBF including EBM and SLM techniques | Four adult large-breed dogs with OSA | Limb sparing surgery | Vladimir et al., 2019 | ||
| Custom-made plate | EBM (Arcam EBM; Designvägen 2, SE-435 33 Mölnlycke, Sweden) | Four small chondrodystrophic breed dogs with antebrachial limb deformities | Corrective osteotomy (closing wedge ostectomy of the radius) | Carwardine et al., 2020 | ||
| Custom-made hemipelvic and proximal femoral endoprosthesis (coated with HA) | DMLS | Adult flat-coated retriever dog with bone lysis of femoral head and acetabulum due to invasive histiocytic sarcoma | Limb salvage technique | Fitzpatrick et al., 2018 | ||
| Ceramics/Composites | PCL/β-TCP | Custom-designed scaffold | Microextrusion-based 3D printer (3DX Printer, T&R Biofab Co., Siheung, Korea) | Adult Great Pyrenees breed dog with OSA of distal radius and ulna | Limb sparing surgery in a dog with distal radial OSA | Choi et al., 2019 |
| PLA/PLGA/HA | PLA scaffold filled with PLGA/HAp nanofibrous scaffold | FDM 3D printing for PLA (Makerbot, NY, USA) and electrospinning procedure for 3D electrospun nanofibrous scaffold | Bone defects (20 mm) created in radius bone of six beagle dogs bilaterally (in-vivo study) | Bone defect reconstruction surgery | Yun et al., 2019 | |
| Brushite/Monetite/TCP | Customized TTA cage with scaffold structure | Low temperature 3D printing | Adult rottweiler dog with CrCL deficient stifle | Modified TTA | Castilho et al., 2014 | |
| β-TCP (loaded with recombinant human bone morphogenic protein-2) | Custom-designed scaffold | DIW | Adult Yorkshire terrier dog with critical-sized bone defect of left radius | Surgical management of severe, radial atrophic nonunion | Franch et al., 2020 | |
| HA/TCP | Customized scaffold | Digital light processing (DLP) | Twelve healthy adult beagle dogs (in-vivo study); 48 defects were created (two defects on each side of the mandible) | Scaffold placement in defect for bone regeneration | Kim et al., 2020 | |
| Polymers | ABS | Custom-made saw guide | FDM (Dimension Elite; Dimension, Inc., Eden Prairie, MN, USA) | four small- and two large-breed dogs (seven limbs) with antebrachial angular limb deformities | Corrective osteotomy (radial closing wedge ostectomy and ulnar osteotomy) | Worth et al., 2018 |
| Personalized cutting guides | FDM | In-vitro testing and modeling of a canine limb | Limb sparing surgery | Timercan et al., 2019 | ||
| 3D model | FDM | Eight-month-old Azawakh dog with angular limb deformity of right forelimb | Corrective osteotomy | Bordelo et al., 2018 | ||
| PLA | Patient-specific models | FDM (Alpha-i3, Alpha3-D, Seoul, Korea) | Adult Golden Retriever dog with angular limb deformity | Corrective osteotomy | Lee et al., 2020 | |
| Patient-specific cutting guides | FFF (Alpha-i3, Alpha3-D, Seoul, Korea) | |||||
| Bone models | 3D printing (Drukarka 3D, 3D Gence SP., Przyszowice, Poland) | Two adult dogs with antebrachial limb deformity | Corrective osteotomy | Longo et al., 2019 | ||
| Epoxy resin | 3D Model | 3D printing (Form 2 printer; Formlabs, Somerville, MA, USA) | Adult Labrador retriever with lameness after femoral head and neck ostectomy | Custom-made THR to restore the acetabular bone loss | Castelli et al., 2019 | |
| 3D Model | SLA (Form 2: Formlabs, Somerville, MA, USA) | Adult Golden Retriever dog with severely comminuted fracture of distal femoral supracondylar and bicondylar region | Surgical repair of complex femoral articular fracture | Lam et al., 2019 | ||
| 3D biomodels | SLA | Four dogs (five limbs) with complex distal femoral deformity | Corrective osteotomy | DeTora et al., 2016 | ||
| Polyamide 12 | Custom-made osteotomy guide | 3D printing (Drukarka 3D, 3D Gence SP., Przyszowice, Poland) | Two adult dogs with antebrachial limb deformity | Corrective osteotomy | Longo et al., 2019 | |
| UHMWPE | Cylindrical bearing (bushing) placed medial and lateral in femoral component and then on tibial component | Fitzbionics Ltd. (Godalming, Surrey, UK) | Nine cats with traumatic stifle luxation or severe distal femoral deformity | Custom TKR | Fitzpatrik et al., 2021 | |
| Acetabular cup cemented to the hemipelvic component | (Biomedtrix, Boonton, NJ, USA) | Adult flat-coated retriever dog with bone lysis of femoral head and acetabulum due to invasive histiocytic sarcoma | Limb salvage technique | Fitzpatrick et al., 2018 | ||
| Nextdent Dental SG material | Custom-made cutting and drilling guides | SLA | Four adult large breed dogs with OSA | Limb sparing surgery | Vladimir et al., 2019 | |
| Bone cement | PMMA | Implant fixation and fill bone-implant voids | Liska et al., 2007 | |||
| Calcium carbonate/polyol-based cement | fill bone-implant voids and decrease stress of bone–implant interfaces | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Memarian, P.; Pishavar, E.; Zanotti, F.; Trentini, M.; Camponogara, F.; Soliani, E.; Gargiulo, P.; Isola, M.; Zavan, B. Active Materials for 3D Printing in Small Animals: Current Modalities and Future Directions for Orthopedic Applications. Int. J. Mol. Sci. 2022, 23, 1045. https://doi.org/10.3390/ijms23031045
Memarian P, Pishavar E, Zanotti F, Trentini M, Camponogara F, Soliani E, Gargiulo P, Isola M, Zavan B. Active Materials for 3D Printing in Small Animals: Current Modalities and Future Directions for Orthopedic Applications. International Journal of Molecular Sciences. 2022; 23(3):1045. https://doi.org/10.3390/ijms23031045
Chicago/Turabian StyleMemarian, Parastoo, Elham Pishavar, Federica Zanotti, Martina Trentini, Francesca Camponogara, Elisa Soliani, Paolo Gargiulo, Maurizio Isola, and Barbara Zavan. 2022. "Active Materials for 3D Printing in Small Animals: Current Modalities and Future Directions for Orthopedic Applications" International Journal of Molecular Sciences 23, no. 3: 1045. https://doi.org/10.3390/ijms23031045
APA StyleMemarian, P., Pishavar, E., Zanotti, F., Trentini, M., Camponogara, F., Soliani, E., Gargiulo, P., Isola, M., & Zavan, B. (2022). Active Materials for 3D Printing in Small Animals: Current Modalities and Future Directions for Orthopedic Applications. International Journal of Molecular Sciences, 23(3), 1045. https://doi.org/10.3390/ijms23031045







