Floating Ricobendazole Delivery Systems: A 3D Printing Method by Co-Extrusion of Sodium Alginate and Calcium Chloride
Abstract
:1. Introduction
2. Results
2.1. Alginate Ink and Crosslinking Ink Preparation
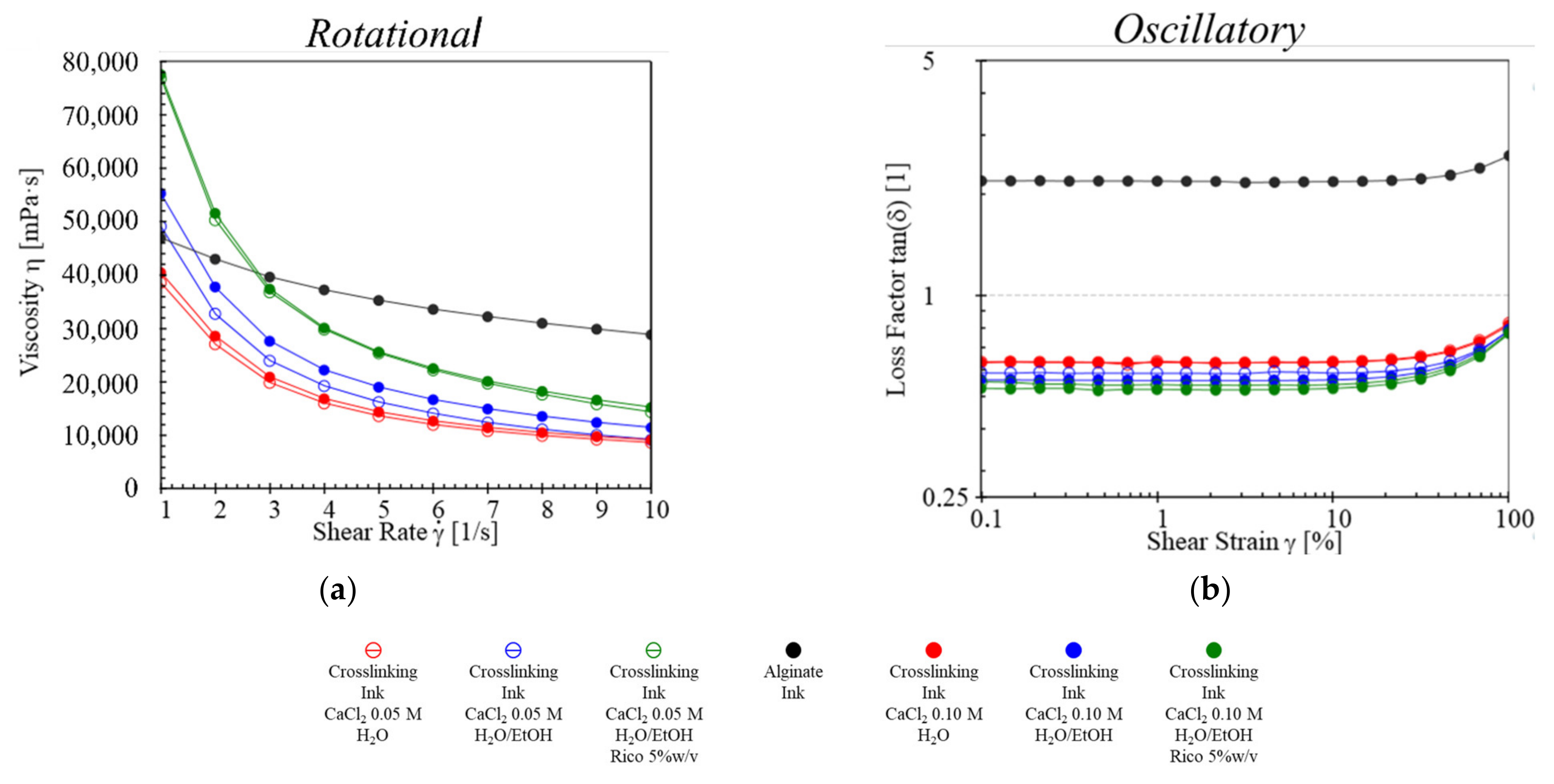
2.2. Rheological Studies
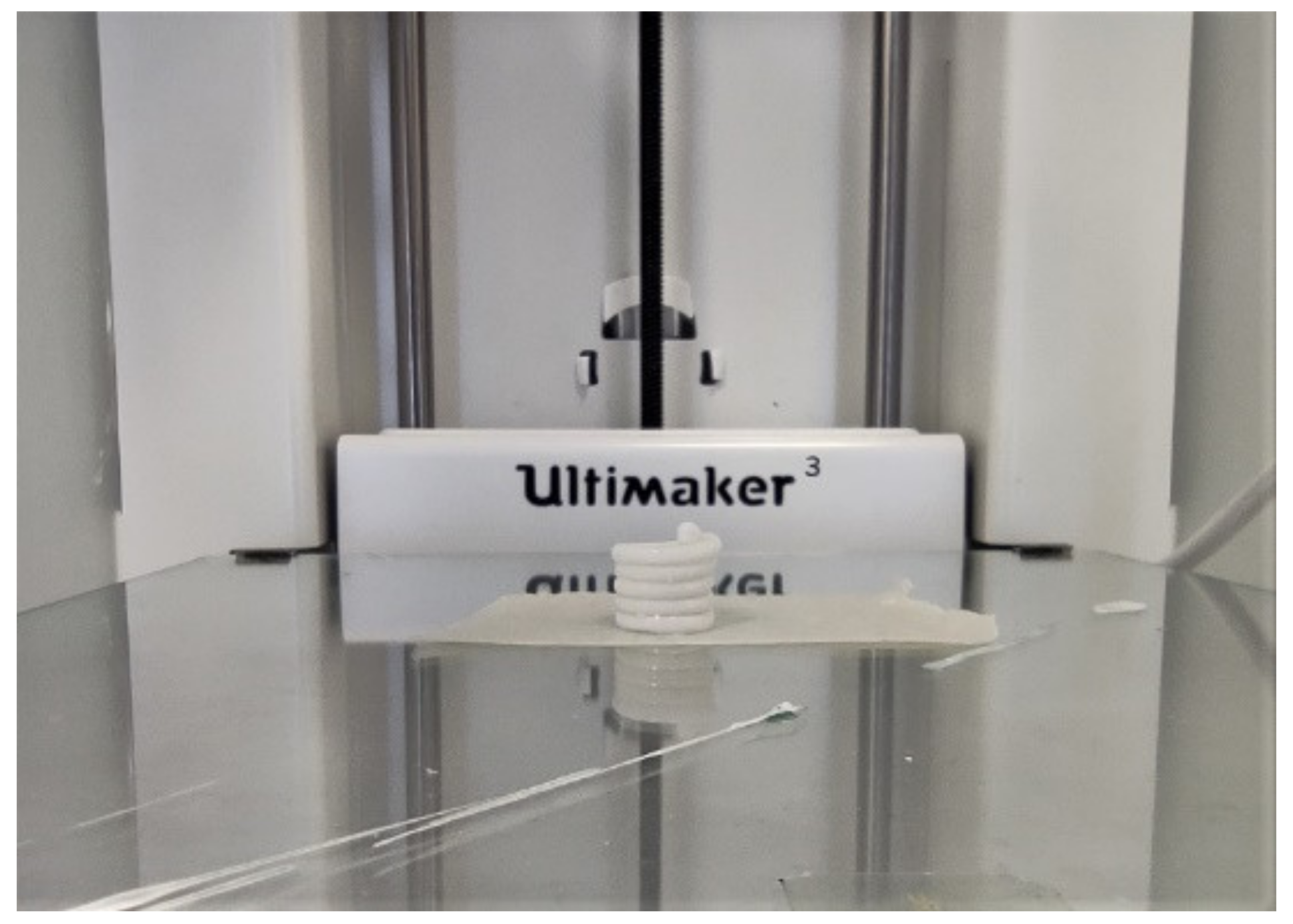
2.3. Design, Development, and Production of DDS
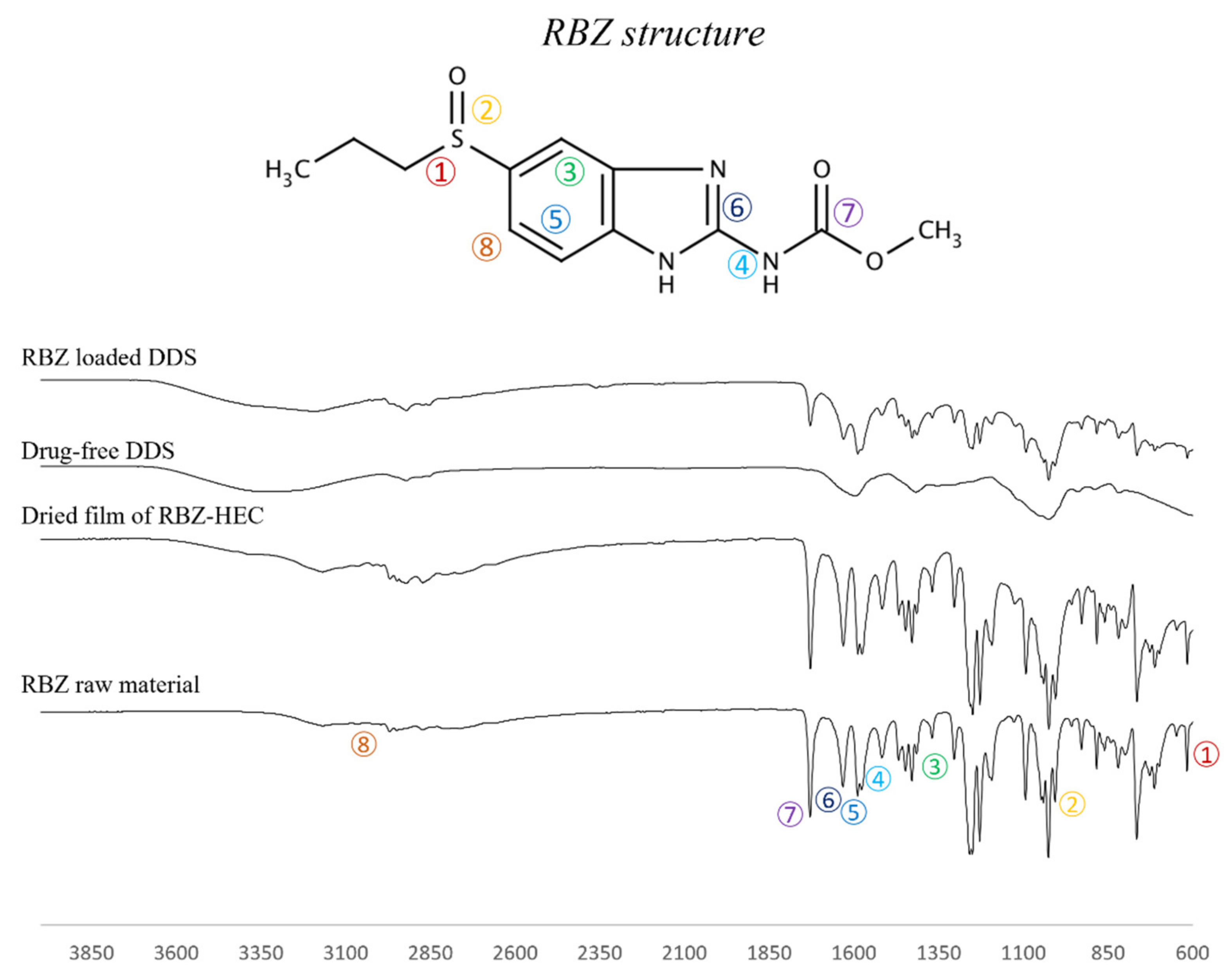
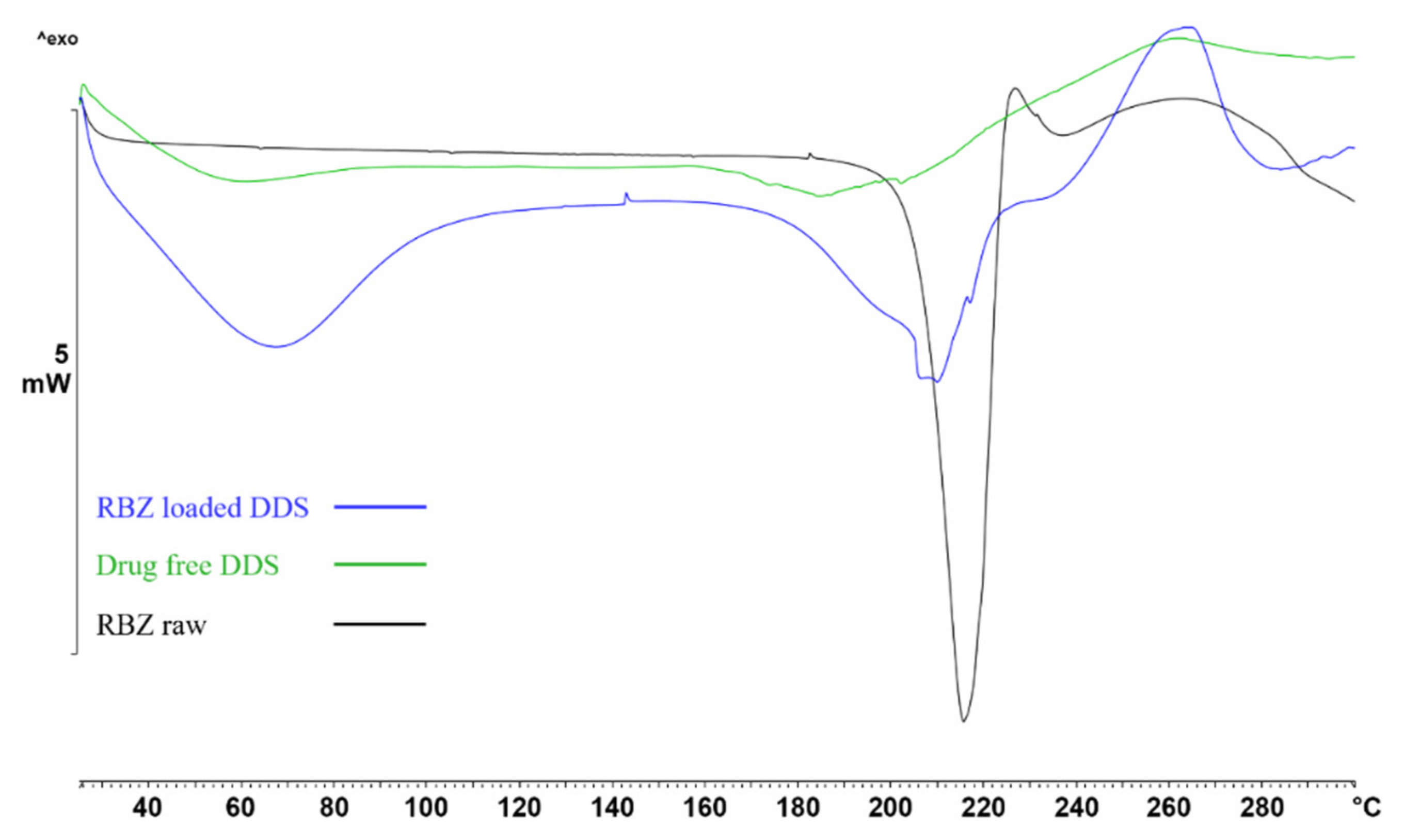
2.4. FT-IR and DSC Analysis
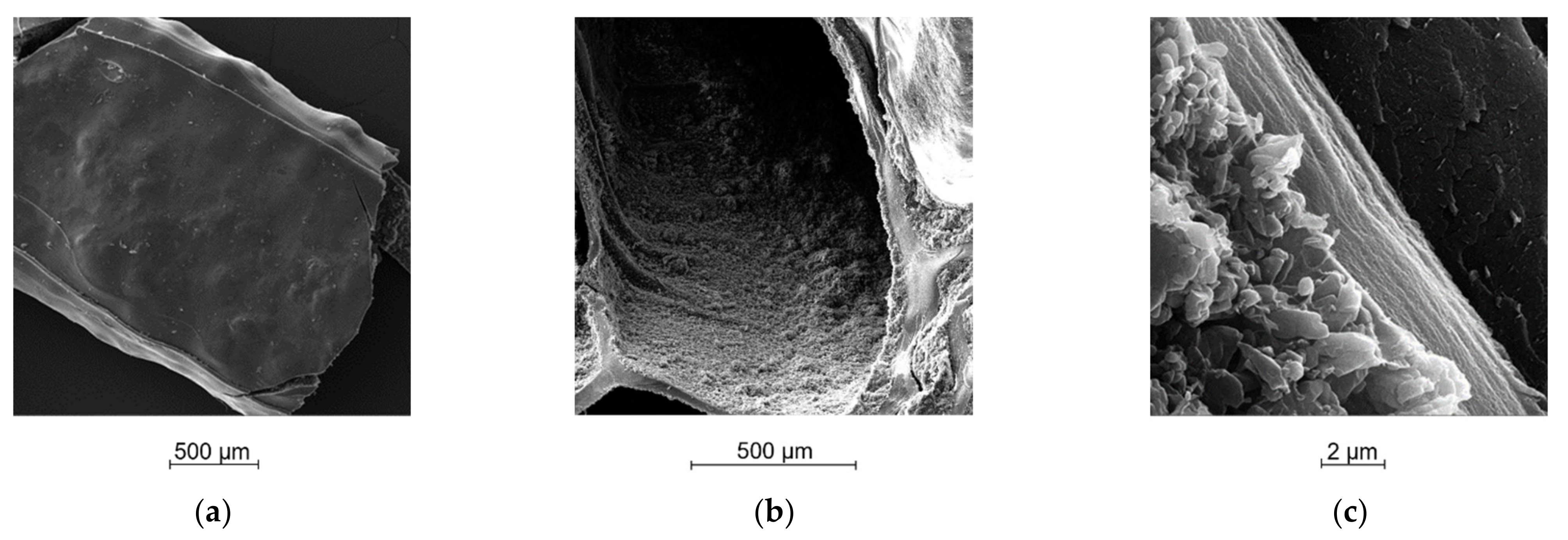
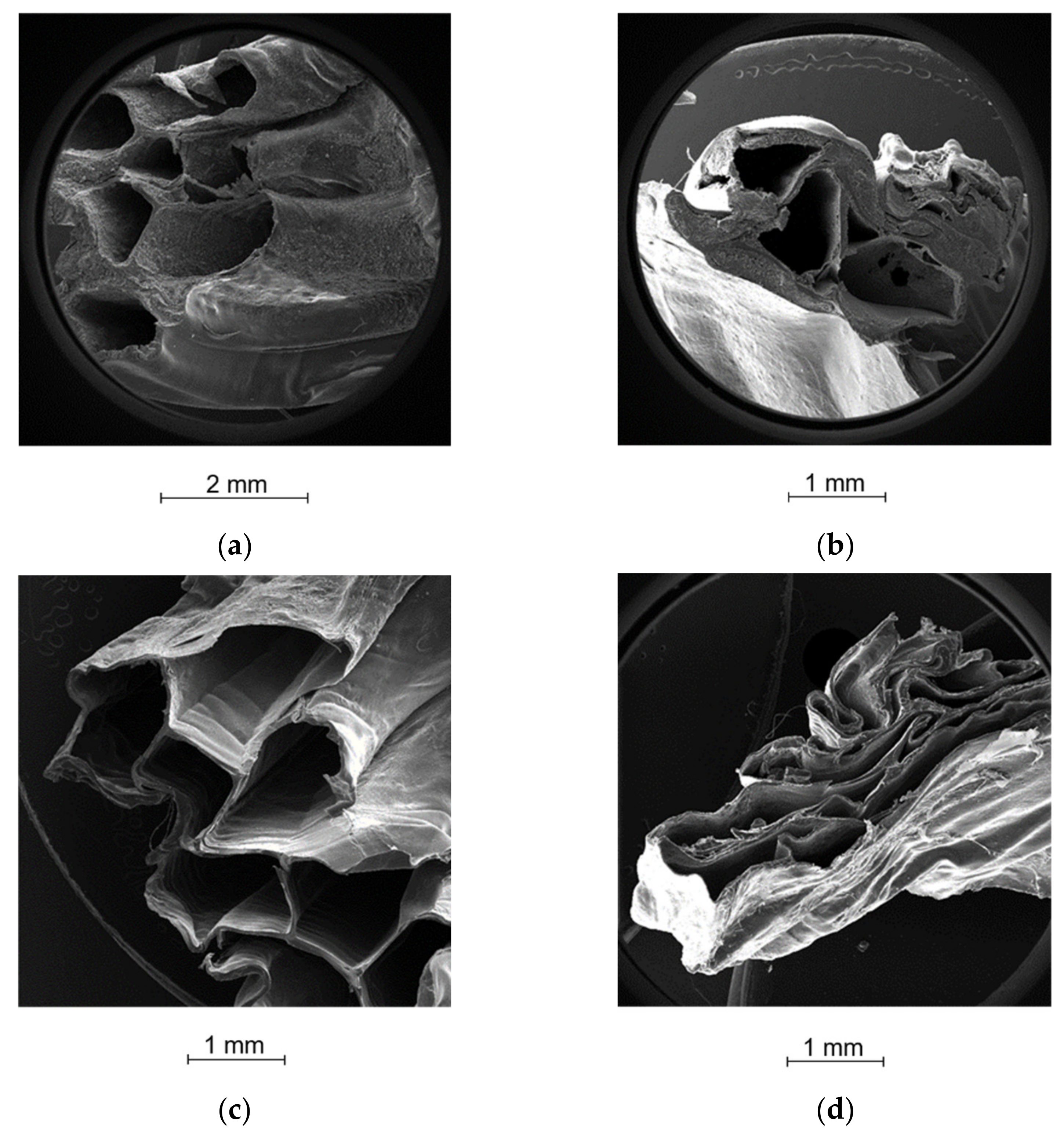
2.5. Morphological Analysis
2.6. Drug Content Analysis and Drug-Loading Efficiency
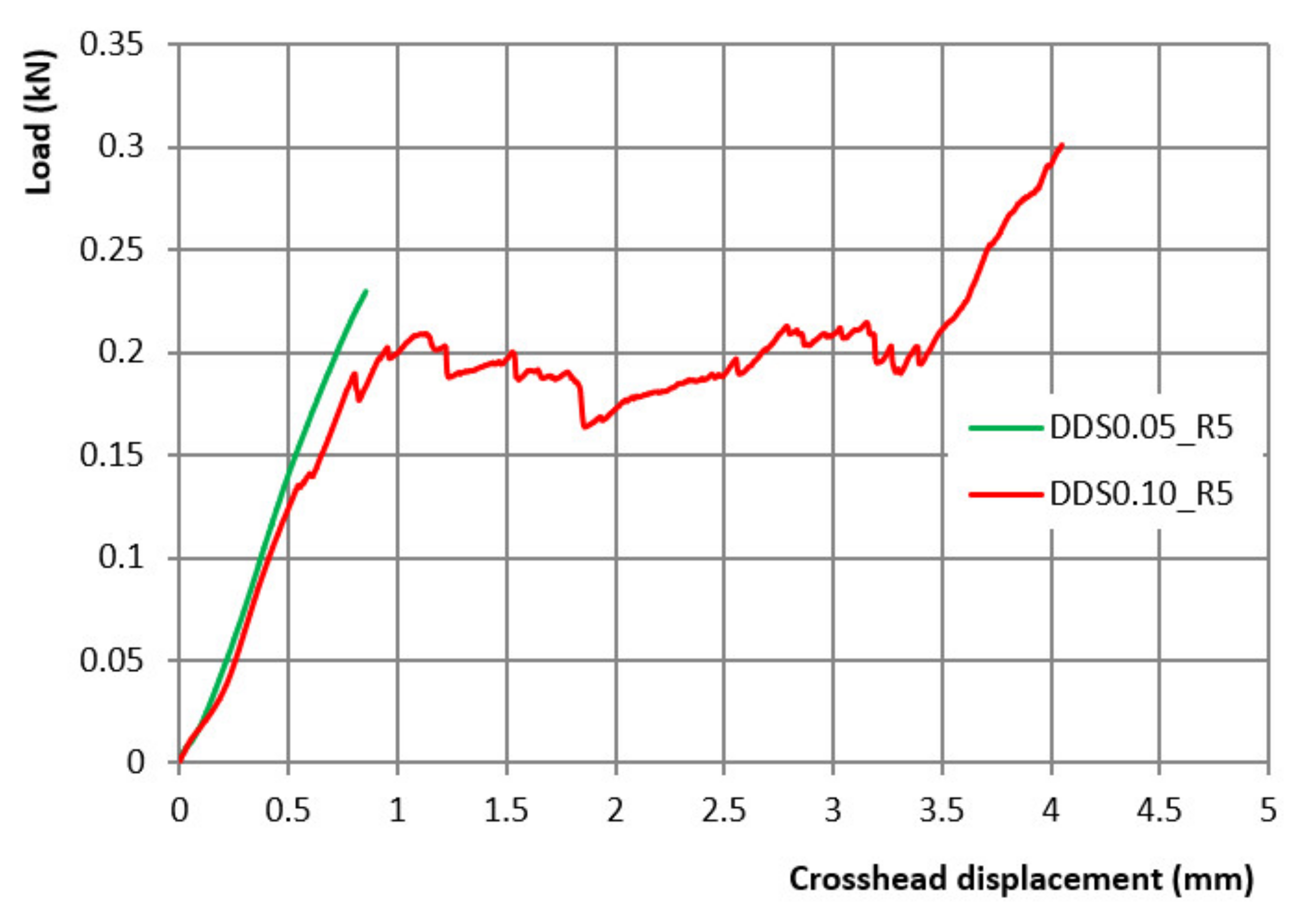
2.7. DDS Mechanical Strength
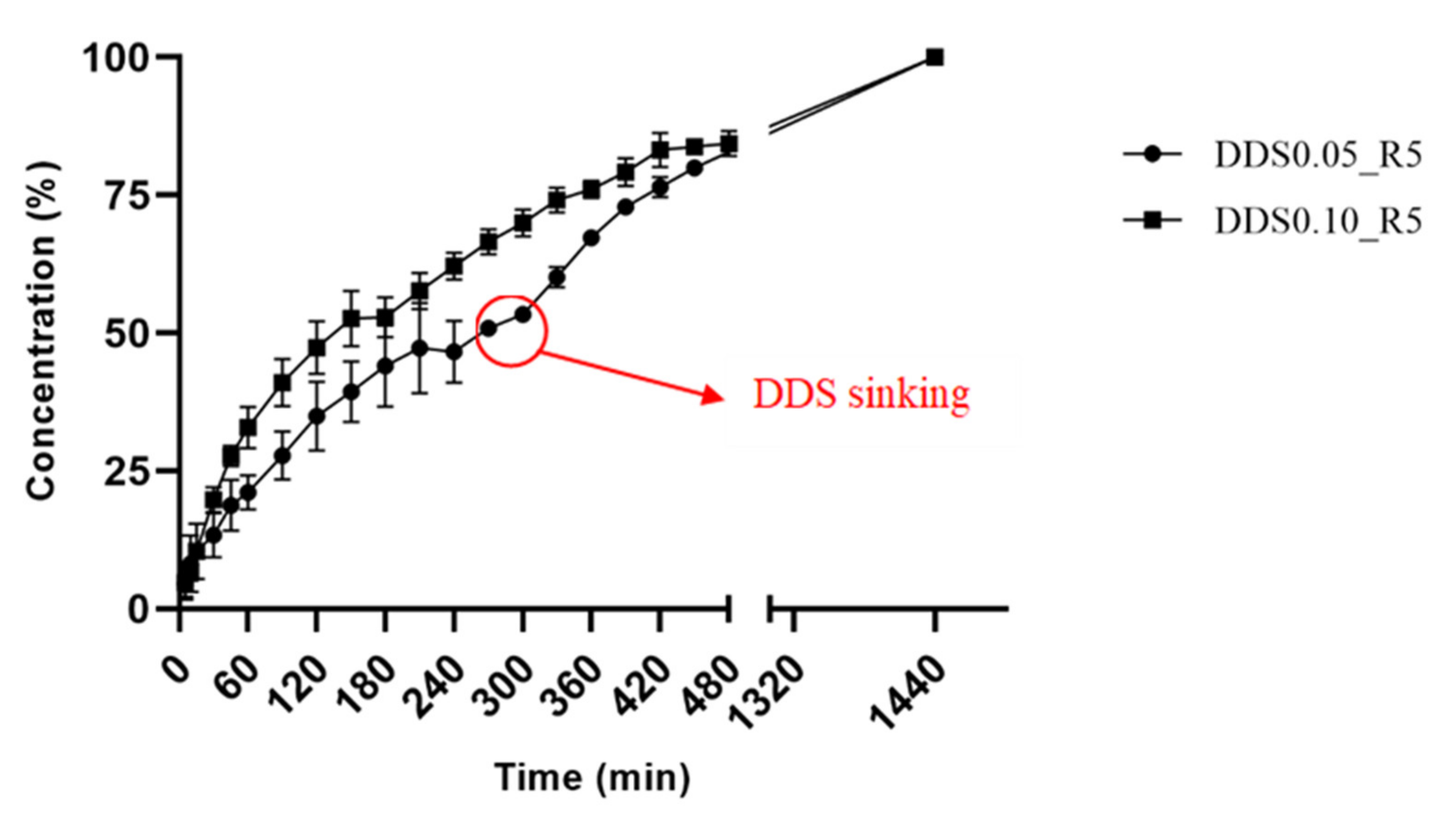
2.8. Buoyancy and In Vitro Drug Release
2.9. Release Kinetics Fitting Studies
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Alginate and Crosslinking Ink Preparation
4.2.2. Rheological Study
4.2.3. Design, Development, and Production of DDS
4.2.4. DSC Thermal Analysis
4.2.5. FT-IR Analysis
4.2.6. Morphological Analysis
4.2.7. DDS Printing Reproducibility: Mass and Dimensional Analysis
4.2.8. Drug Content Analysis and Drug-Loading Efficiency
4.2.9. DDS Mechanical Strength
4.2.10. Buoyancy and In Vitro Drug Release Evaluation
4.2.11. Release Kinetics Fitting Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McKellar, Q.; Scott, E. The benzimidazole anthelmintic agents-a review. J. Vet. Pharmacol. Ther. 1990, 13, 223–247. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.X.; Weller, P.F. Antiparasitic drugs. N. Engl. J. Med. 1996, 334, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Spasov, A.; Smirnova, L.; Iezhitsa, I.; Sergeeva, S.; Ozerov, A. Pharmacokinetics of benzimidazole derivatives. Vopr. Meditsinskoi Khimii 2002, 48, 233–258. [Google Scholar]
- Lopez-Garcia, M.L.; Torrado-Duran, S.; Torrado-Duran, J.; Martínez-Fernández, A.R.; Bolás-Fernández, F. Albendazole versus ricobendazole (albendazole-sulphoxide) against enteral and parenteral stages of Trichinella spiralis in mice. Int. J. Parasitol. 1997, 27, 781–785. [Google Scholar] [CrossRef]
- Lifschitz, A.; Lanusse, C.; Alvarez, L. Host pharmacokinetics and drug accumulation of anthelmintics within target helminth parasites of ruminants. N. Z. Vet. J. 2017, 65, 76–184. [Google Scholar] [CrossRef]
- Shahare, M.B.; Kadam, V.J.; Jagdale, D.M.; Gandhi, P.S.; Gaikwad, P.L. Synthesis and evaluation of novel anthelmentic benzimidazole derivatives. Int. J. Res. Pharm. Chem. 2012, 2, 132–136. [Google Scholar]
- Lanusse, C.; Virkel, G.; Sanchez, S.; Alvarez, L.; Lifschitz, A.; Imperiale, F.; Monfrinotti, A. Ricobendazole kinetics and availability following subcutaneous administration of a novel injectable formulation to calves. Res. Vet. Sci. 1998, 65, 5–10. [Google Scholar] [CrossRef]
- Wu, Z.; Razzak, M.; Tucker, I.G.; Medlicott, N.J. Physicochemical characterization of ricobendazole: I. Solubility, lipophilicity, and ionization characteristics. J. Pharm. Sci. 2005, 94, 983–993. [Google Scholar] [CrossRef]
- Ibrahim, M.; Naguib, Y.W.; Sarhan, H.A.; Abdelkader, H. Gastro-retentive oral drug delivery systems: A promising approach for narrow absorption window drugs. J. Adv. Biomed. Pharm. Sci. 2019, 2, 98–110. [Google Scholar] [CrossRef]
- Dib, A.; Paredes, A.J.; Eliópulos, N.; Farias, C.E.; Suárez, G.; Aldrovandi, A.; Palma, S.D.; Allemandi, D.A.; Lanusse, C.E.; Sanchez Bruni, S.F. Pharmacokinetic Assessment of Novel Controlled Release Formulations of Ricobendazole Intended for Oral Administration in Dogs. J. Clin. Pharmacol. 2015, 5. [Google Scholar] [CrossRef] [Green Version]
- Bhosale, A.R.; Shinde, J.V.; Chavan, R.S. A Comprehensive Review on Floating Drug Delivery System (FDDS). J. Drug Deliv. Ther. 2020, 10, 174–182. [Google Scholar] [CrossRef]
- Auriemma, G.; Cerciello, A.; Sansone, F.; Pinto, A.; Morello, S.; Aquino, R.P. Polysaccharides based gastroretentive system to sustain piroxicam release: Development and in vivo prolonged anti-inflammatory effect. Int. J. Biol. Macromol. 2018, 120, 2303–2312. [Google Scholar] [CrossRef] [PubMed]
- Real, J.P.; Barberis, M.E.; Camacho, N.M.; Bruni, S.S.; Palma, S.D. Design of novel oral ricobendazole formulation applying melting solidification printing process (MESO-PP): An innovative solvent-free alternative method for 3D printing using a simplified concept and low temperature. Int. J. Pharm. 2020, 587, 119653. [Google Scholar] [CrossRef] [PubMed]
- Basit, A.W.; Gaisford, S. 3D Printing of Pharmaceuticals; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Yi, H.-G.; Kim, H.; Kwon, J.; Choi, Y.-J.; Jang, J.; Cho, D.-W. Application of 3D bioprinting in the prevention and the therapy for human diseases. Signal Transduct. Target. Ther. 2021, 6, 177. [Google Scholar] [CrossRef]
- Wallis, M.; Al-Dulimi, Z.; Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. 3D printing for enhanced drug delivery: Current state-of-the-art and challenges. Drug Dev. Ind. Pharm. 2020, 46, 1385–1401. [Google Scholar] [CrossRef]
- Choi, W.J.; Hwang, K.S.; Kwon, H.J.; Lee, C.; Kim, C.H.; Kim, T.H.; Heo, S.W.; Kim, J.-H.; Lee, J.-Y. Rapid development of dual porous poly (lactic acid) foam using fused deposition modeling (FDM) 3D printing for medical scaffold application. Mater. Sci. Eng. C 2020, 110, 110693. [Google Scholar] [CrossRef]
- Chen, G.; Xu, Y.; Kwok, P.C.L.; Kang, L. Pharmaceutical applications of 3D printing. Addit. Manuf. 2020, 34, 101209. [Google Scholar] [CrossRef]
- Vithani, K.; Goyanes, A.; Jannin, V.; Basit, A.W.; Gaisford, S.; Boyd, B.J. An overview of 3D printing technologies for soft materials and potential opportunities for lipid-based drug delivery systems. Pharm. Res. 2019, 36, 4. [Google Scholar] [CrossRef] [Green Version]
- Seoane-Viaño, I.; Januskaite, P.; Alvarez-Lorenzo, C.; Basit, A.W.; Goyanes, A. Semi-solid extrusion 3D printing in drug delivery and biomedicine: Personalised solutions for healthcare challenges. J. Control. Release 2021, 332, 367–389. [Google Scholar] [CrossRef]
- Azad, M.A.; Olawuni, D.; Kimbell, G.; Badruddoza, A.Z.M.; Hossain, M.; Sultana, T. Polymers for extrusion-based 3D printing of pharmaceuticals: A holistic materials–process perspective. Pharmaceutics 2020, 12, 124. [Google Scholar] [CrossRef] [Green Version]
- Reddy Dumpa, N.; Bandari, S.; Repka, M.A. Novel gastroretentive floating pulsatile drug delivery system produced via hot-melt extrusion and fused deposition modeling 3D printing. Pharmaceutics 2020, 12, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, H.-G.; Choi, Y.-J.; Kang, K.S.; Hong, J.M.; Pati, R.G.; Park, M.N.; Shim, I.K.; Lee, C.M.; Kim, S.C.; Cho, D.-W. A 3D-printed local drug delivery patch for pancreatic cancer growth suppression. J. Control. Release 2016, 238, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.; Chai, H.; Wang, X.; Yang, J.; Li, J.; Zhao, Y.; Cai, W.; Tao, T.; Xiang, X. Fused deposition modeling (FDM) 3D printed tablets for intragastric floating delivery of domperidone. Sci. Rep. 2017, 7, 2829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Zhang, S.; Sun, W.; Cui, M.; Wen, H.; Li, Q.; Pan, W.; Yang, X. Flexibility of 3D extruded printing for a novel controlled-release puerarin gastric floating tablet: Design of internal structure. AAPS Pharmscitech 2019, 20, 236. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guan, X.; Cui, M.; Zhu, Z.; Chen, K.; Wen, H.; Jia, D.; Hou, J.; Xu, W.; Yang, X. Preparation and investigation of novel gastro-floating tablets with 3D extrusion-based printing. Int. J. Pharm. 2018, 535, 325–332. [Google Scholar] [CrossRef]
- Wen, H.; He, B.; Wang, H.; Chen, F.; Li, P.; Cui, M.; Li, Q.; Pan, W.; Yang, X. Structure-based gastro-retentive and controlled-release drug delivery with novel 3D printing. AAPS Pharmscitech 2019, 20, 68. [Google Scholar] [CrossRef]
- Falcone, G.; Saviano, M.; Aquino, R.P.; Del Gaudio, P.; Russo, P. Coaxial semi-solid extrusion and ionotropic alginate gelation: A successful duo for personalized floating formulations via 3D printing. Carbohydr. Polym. 2021, 260, 117791. [Google Scholar] [CrossRef]
- Hölzl, K.; Lin, S.; Tytgat, L.; Van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink properties before, during and after 3D bioprinting. Biofabrication 2016, 8, 032002. [Google Scholar] [CrossRef]
- Paxton, N.; Smolan, W.; Böck, T.; Melchels, F.; Groll, J.; Jungst, T. Proposal to assess printability of bioinks for extrusion-based bioprinting and evaluation of rheological properties governing bioprintability. Biofabrication 2017, 9, 044107. [Google Scholar] [CrossRef]
- Mezger, T. The Rheology Handbook; Vincentz Network: Hannover, Germany, 2020. [Google Scholar]
- Xiong, S.; Chen, Y.; Jia, W.; Yang, X.; Liu, K.; Li, C.; Ling, G. Optimization of the co-axial dispensing nozzle of a 3D bioprinter for the fabrication of tubular structures with micro-channel encapsulation. J. Micromech. Microeng. 2021, 31, 045009. [Google Scholar] [CrossRef]
- Chaudhary, M.K.; Prajapati, P.; Srivastava, K.; Silva, K.F.; Joshi, B.D.; Tandon, P.; Ayala, A.P. Molecular interactions and vibrational properties of ricobendazole: Insights from quantum chemical calculation and spectroscopic methods. J. Mol. Struct. 2021, 1230, 129889. [Google Scholar] [CrossRef]
- Castillo, J.; Palomo-Canales, J.; Garcia, J.; Lastres, J.; Bolas, F.; Torrado, J. Preparation and characterization of albendazole β-cyclodextrin complexes. Drug Dev. Ind. Pharm. 1999, 25, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Falcone, G.; Mazzei, P.; Piccolo, A.; Esposito, T.; Mencherini, T.; Aquino, R.P.; Del Gaudio, P.; Russo, P. Advanced printable hydrogels from pre-crosslinked alginate as a new tool in semi solid extrusion 3D printing process. Carbohydr. Polym. 2021, 276, 118746. [Google Scholar] [CrossRef]
- Formentini, E.; Mestorino, O.; Marino, E.; Errecalde, J. Pharmacokinetics of ricobendazole in calves. J. Vet. Pharmacol. Ther. 2001, 24, 199–202. [Google Scholar] [CrossRef]
- Russo, P.; Zacco, R.; Rekkas, D.M.; Politis, S.; Garofalo, E.; Del Gaudio, P.; Aquino, R.P. Application of experimental design for the development of soft-capsules through a prilling, inverse gelation process. J. Drug Deliv. Sci. Technol. 2019, 49, 577–585. [Google Scholar] [CrossRef]
- Higuchi, T. Rate of release of medicaments from ointment bases containing drugs in suspension. J. Pharm. Sci. 1961, 50, 874–875. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Del Gaudio, P.; De Cicco, F.; Sansone, F.; Aquino, R.P.; Adami, R.; Ricci, M.; Giovagnoli, S. Alginate beads as a carrier for omeprazole/SBA-15 inclusion compound: A step towards the development of personalized paediatric dosage forms. Carbohydr. Polym. 2015, 133, 464–472. [Google Scholar] [CrossRef]
| Code | DDS0.05_R5 | DDS0.10_R5 |
|---|---|---|
| After Printing | ||
| Mp (g) | 3.2 ± 0.2 | 3.2 ± 0.1 |
| Dp (%) | 110.2 ± 1.3 | 97.0 ± 2.7 |
| Hp (%) | 88.3 ± 2.8 | 104.7 ± 4.0 |
| After Drying | ||
| Md (%) | 7.9 ± 0.4 | 8.7 ± 0.2 |
| Dd (%) | 78.7 ± 7.6 | 81.0 ± 2.2 |
| Hd (%) | 33.3 ± 10.7 | 55.0 ± 6.6 |
| Drug Content (%) | 34.8 ± 1.3 | 39.0 ± 2.1 |
| Drug LoadingEfficiency (%) | 81.9 ± 4.1 | 99.2 ± 2.4 |
| Code | Higuchi | Korsmeyer–Peppas | |||
|---|---|---|---|---|---|
| r2adj * | Reduced χ2 † | r2adj * | Reduced χ2 † | n (S.E.) | |
| DDS0.05_R5 | 0.8615 | 7.83 | 0.9258 | 2.08 | 0.71 ± 0.08 |
| DDS0.10_R5 | 0.8813 | 6.42 | 0.9135 | 2.52 | 0.69 ± 0.05 |
| Code | Inks | |||
|---|---|---|---|---|
| DDS0.05_R5 | Alginate | Crosslinking | ||
| Solvent | Solvent | |||
| H2O | H2O/EtOH 80/20 v/v | |||
| Ingredients | Ingredients | |||
| Sodium Alginate | 6% w/v | CaCl2 | 0.05 M | |
| RBZ | 5% w/v | |||
| HEC | 2% w/v | |||
| Tw | 0.1% v/v | |||
| Code | Inks | |||
| DDS0.10_R5 | Alginate | Crosslinking | ||
| Solvent | Solvent | |||
| H20 | H2O/EtOH 80/20 v/v | |||
| Ingredients | Ingredients | |||
| Sodium Alginate | 6% w/v | CaCl2 | 0.10 M | |
| RBZ | 5% w/v | |||
| HEC | 2% w/v | |||
| Tw | 0.1% v/v | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falcone, G.; Real, J.P.; Palma, S.D.; Aquino, R.P.; Del Gaudio, P.; Garofalo, E.; Russo, P. Floating Ricobendazole Delivery Systems: A 3D Printing Method by Co-Extrusion of Sodium Alginate and Calcium Chloride. Int. J. Mol. Sci. 2022, 23, 1280. https://doi.org/10.3390/ijms23031280
Falcone G, Real JP, Palma SD, Aquino RP, Del Gaudio P, Garofalo E, Russo P. Floating Ricobendazole Delivery Systems: A 3D Printing Method by Co-Extrusion of Sodium Alginate and Calcium Chloride. International Journal of Molecular Sciences. 2022; 23(3):1280. https://doi.org/10.3390/ijms23031280
Chicago/Turabian StyleFalcone, Giovanni, Juan P. Real, Santiago D. Palma, Rita P. Aquino, Pasquale Del Gaudio, Emilia Garofalo, and Paola Russo. 2022. "Floating Ricobendazole Delivery Systems: A 3D Printing Method by Co-Extrusion of Sodium Alginate and Calcium Chloride" International Journal of Molecular Sciences 23, no. 3: 1280. https://doi.org/10.3390/ijms23031280
APA StyleFalcone, G., Real, J. P., Palma, S. D., Aquino, R. P., Del Gaudio, P., Garofalo, E., & Russo, P. (2022). Floating Ricobendazole Delivery Systems: A 3D Printing Method by Co-Extrusion of Sodium Alginate and Calcium Chloride. International Journal of Molecular Sciences, 23(3), 1280. https://doi.org/10.3390/ijms23031280








