Photodynamic Activity of Protoporphyrin IX-Immobilized Cellulose Monolith for Nerve Tissue Regeneration
Abstract
:1. Introduction
2. Results and Discussion
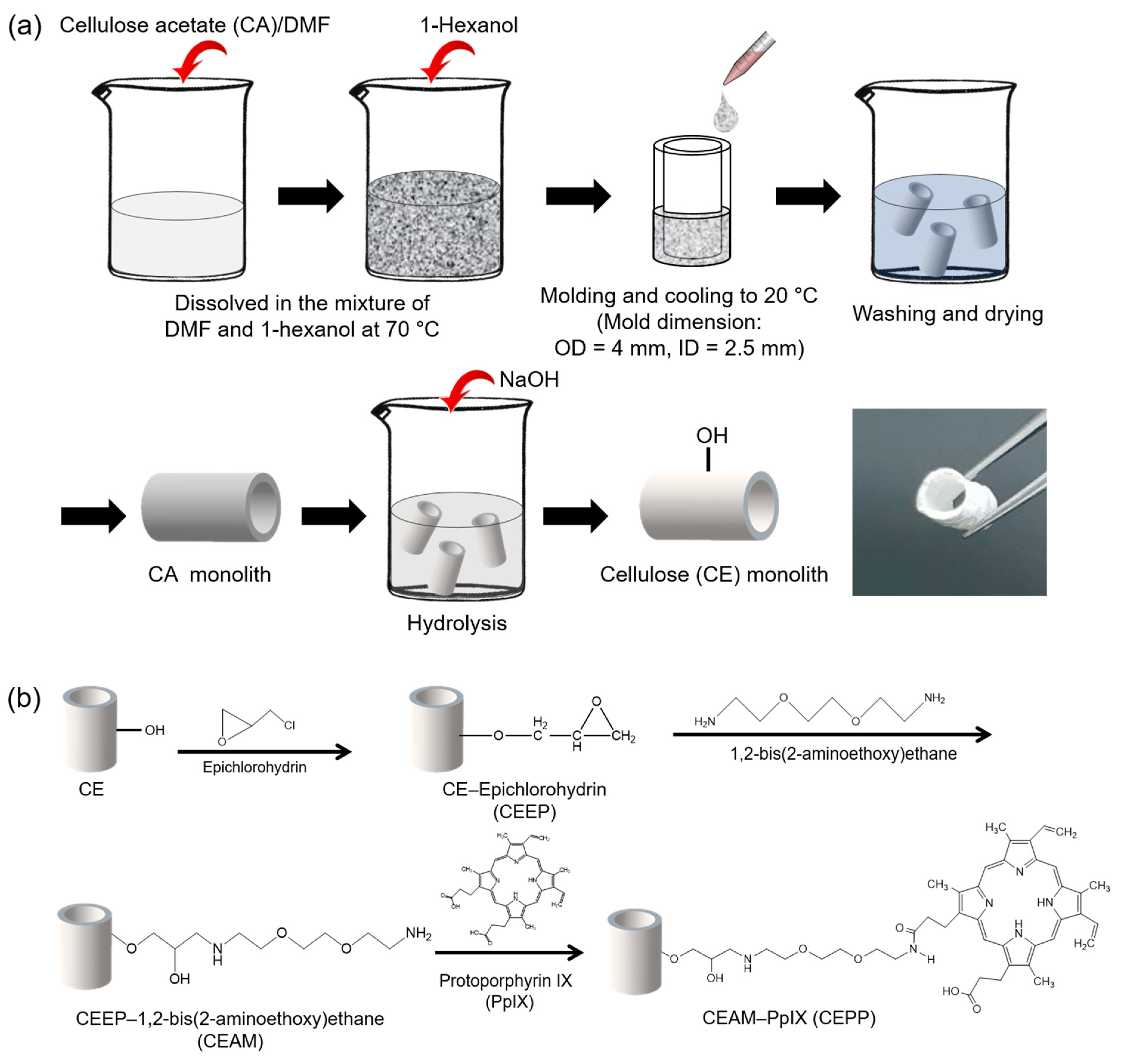
2.1. Fabrication of the CEPP Monolith
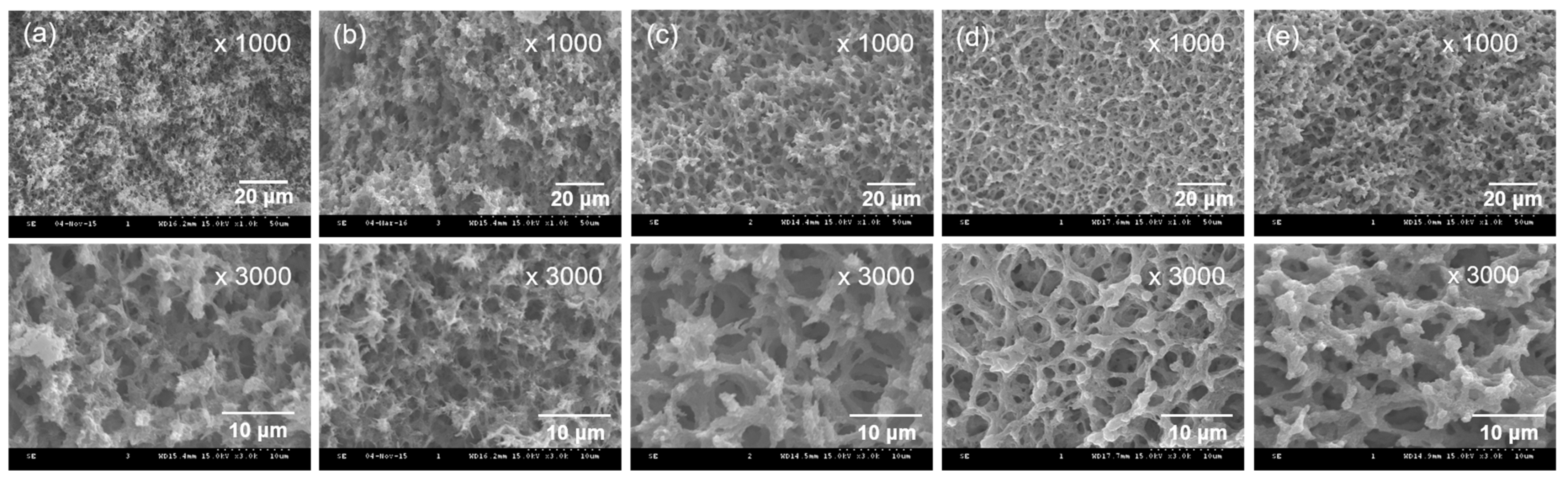
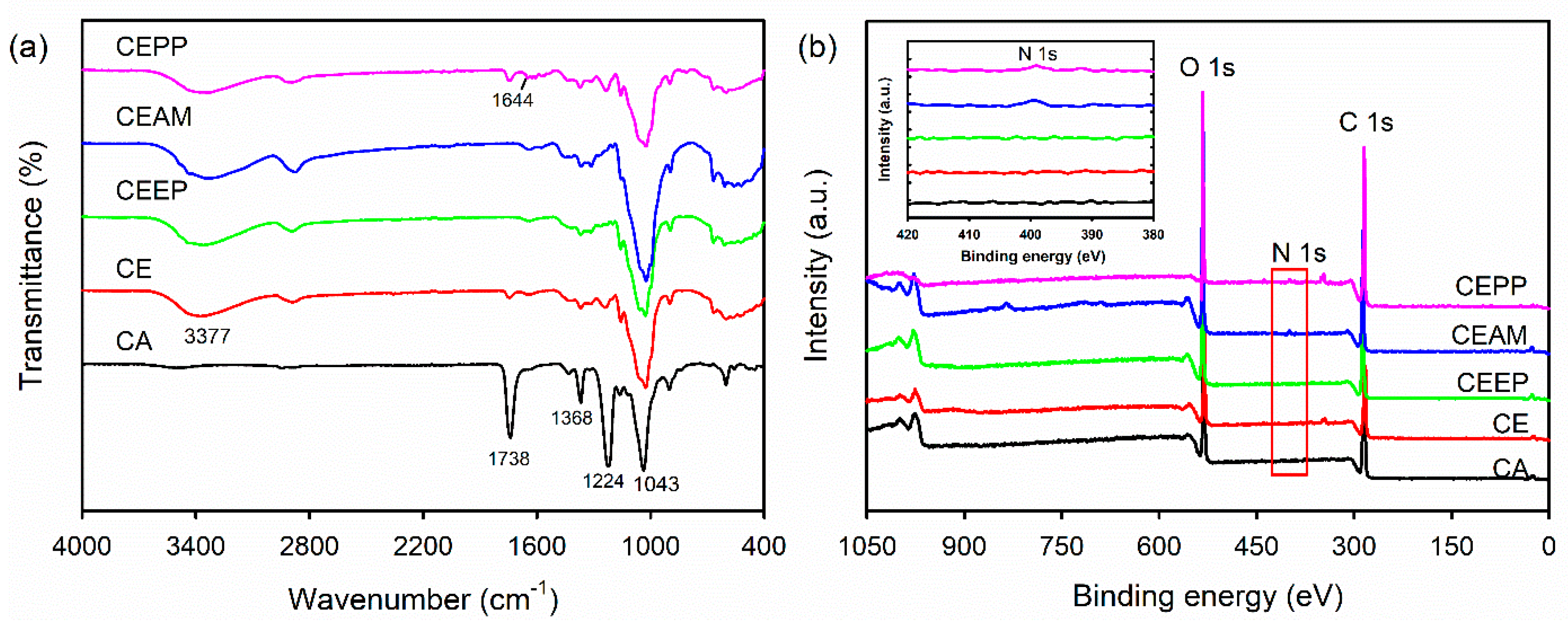
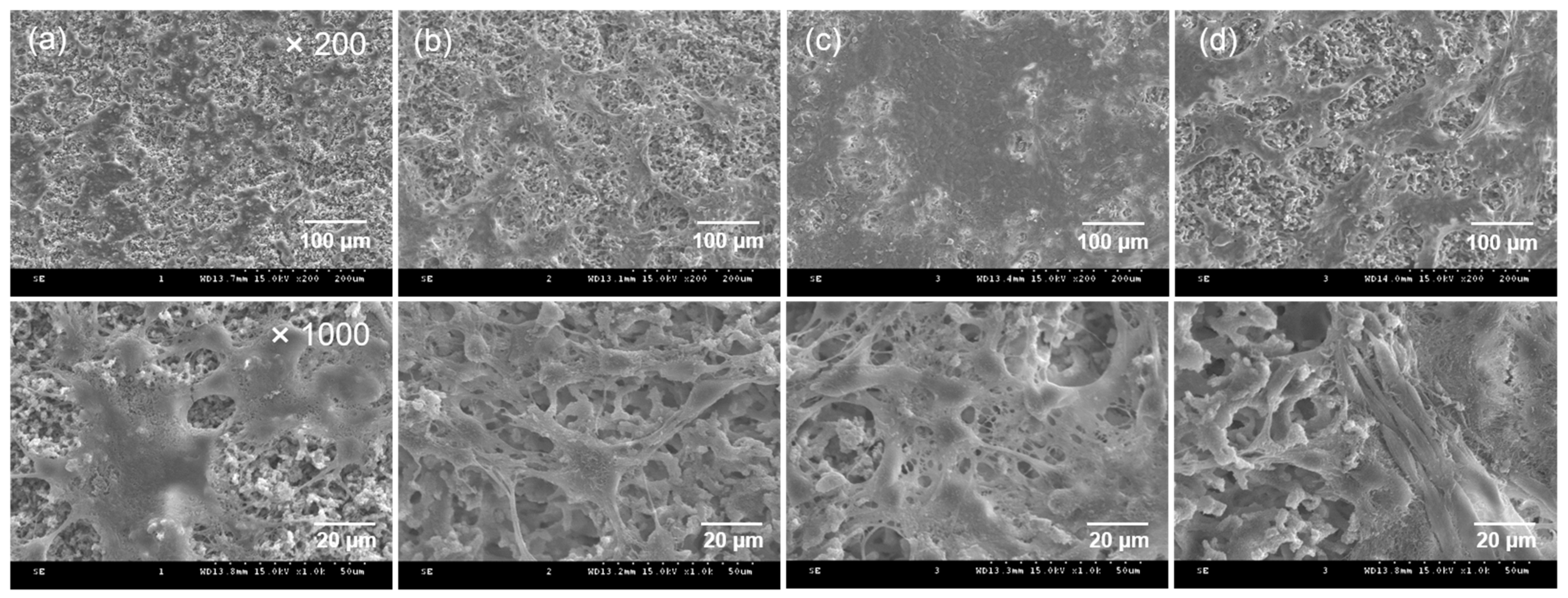
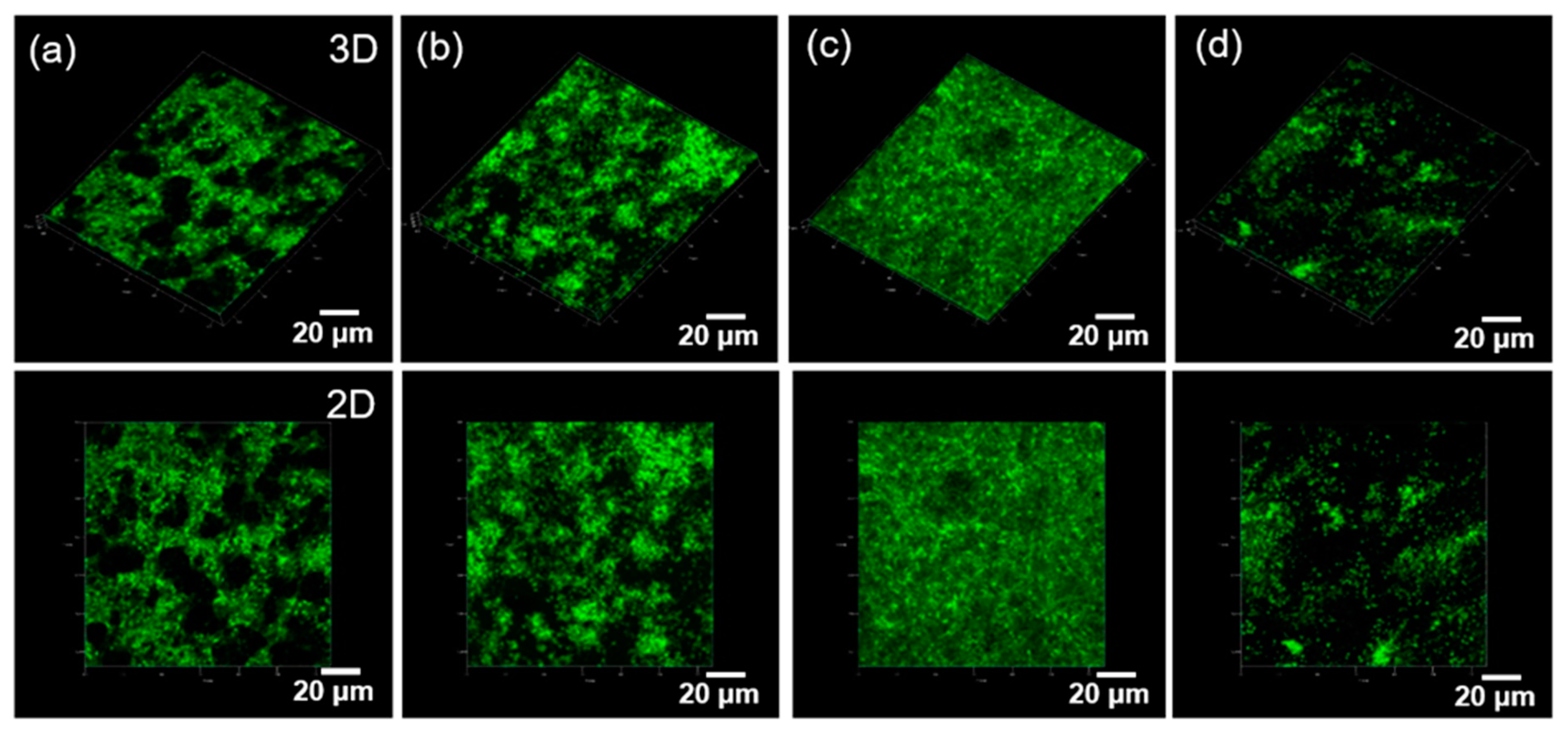
2.2. Physicochemical Properties of the CEPP Monolith
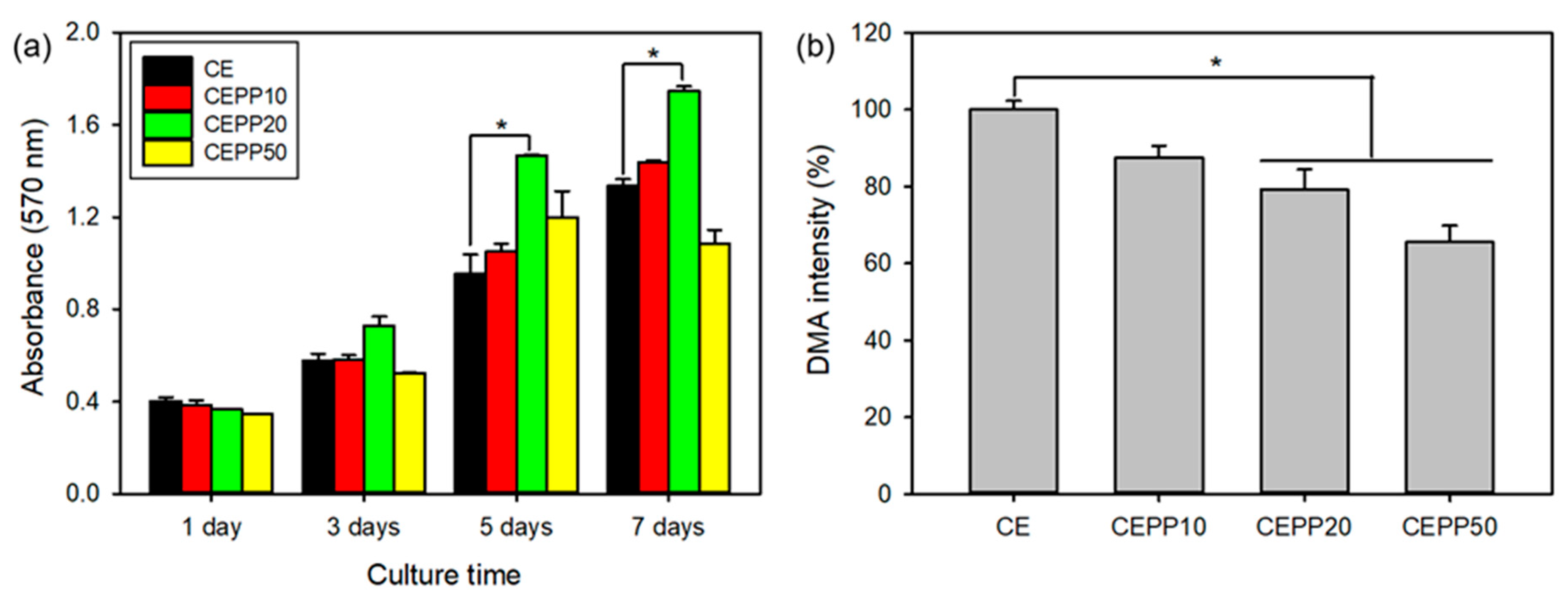
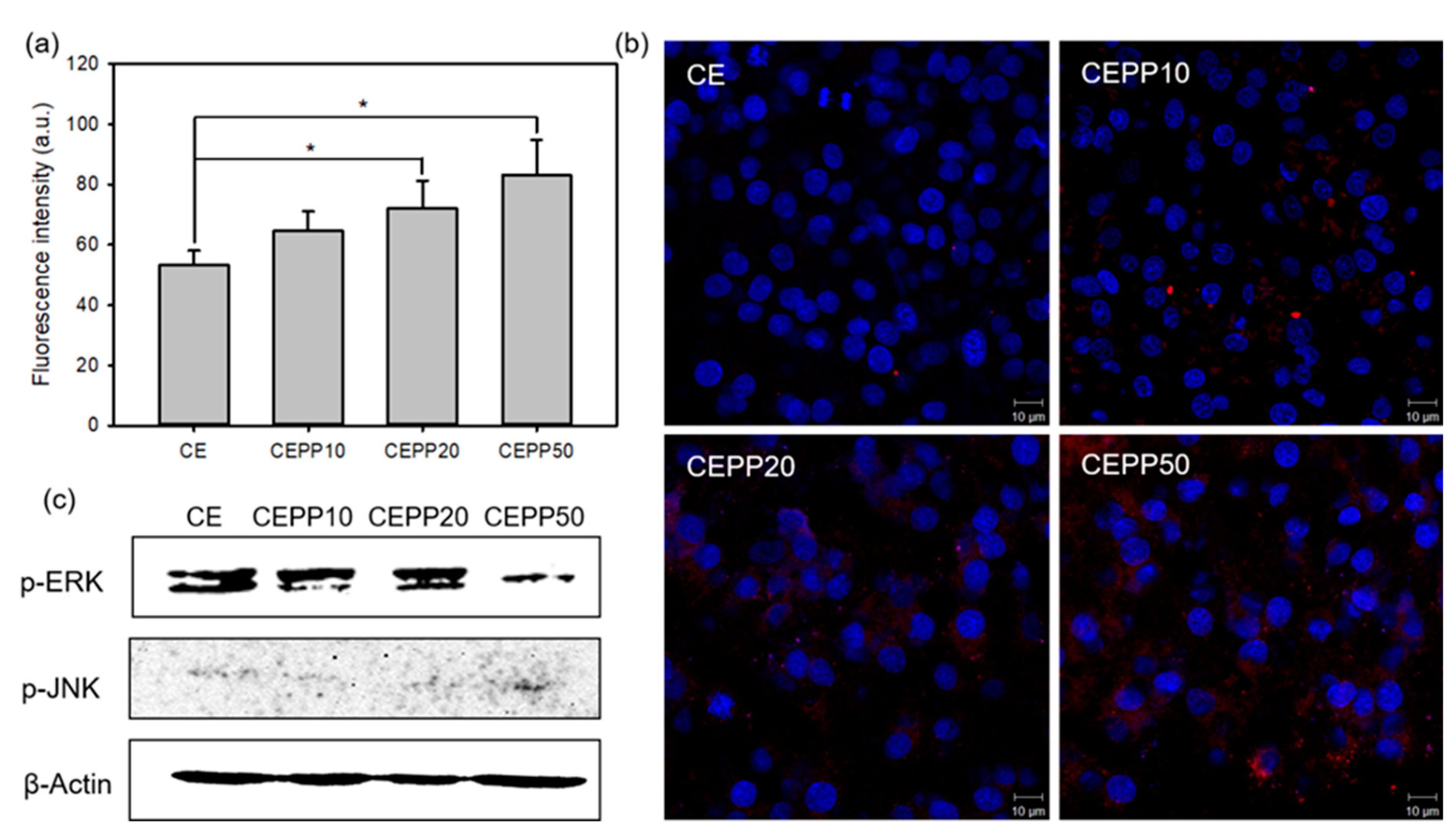
2.3. In Vitro Bioactivity of the CEPP Monolith
3. Materials and Methods
3.1. Materials
3.2. Fabrication of the CE Monolith
3.3. Preparation of the PpIX-Immobilized CE Monolith
3.4. Surface Morphology and Physicochemical Properties of the PpIX-Immobilized CE Monolith
3.5. Singlet Oxygen Detection
3.6. In Vitro Bioactivity of the PpIX-Immobilized CE Monolith
3.7. Intracellular ROS Generation Assays
3.8. Western Blot
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Si, J.; Yang, Y.; Xing, X.; Yang, F.; Shan, P. Controlled degradable chitosan/collagen composite scaffolds for application in nerve tissue regeneration. Polym. Degrad. Stab. 2019, 166, 73–85. [Google Scholar] [CrossRef]
- Dong, X.; Liu, S.; Yang, Y.; Gao, S.; Li, W.; Cao, J.; Wan, Y.; Huang, Z.; Fan, G.; Chen, Q.; et al. Aligned microfiber-induced macrophage polarization to guide Schwann-cell-enabled peripheral nerve regeneration. Biomaterials 2021, 272, 120767. [Google Scholar] [CrossRef]
- de Siqueira-Santos, R.; Sardella-Silva, G.; Nascimento, M.A.; Teixeira de Oliveira, L.; Coelho-Sampaio, T.; Ribeiro-Resende, V.T. Biological activity of laminin/polylaminin-coated poly-ε-caprolactone filaments on the regeneration and tissue replacement of the rat sciatic nerve. Mater. Today Bio 2019, 3, 100026. [Google Scholar] [CrossRef]
- Daly, W.T.; Knight, A.M.; Wang, H.; de Boer, R.; Giusti, G.; Dadsetan, M.; Spinner, R.J.; Yaszemski, M.J.; Windebank, A.J. Comparison and characterization of multiple biomaterial conduits for peripheral nerve repair. Biomaterials 2013, 31, 5789–5797. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Shah, M.B.; Lee, P.; Yu, X. Tissue-engineered spiral nerve conduit for peripheral nerve regeneration. Acta Biomater. 2018, 73, 302–311. [Google Scholar] [CrossRef]
- Daly, W.; Yao, L.; Zeugolis, D.; Windebank, A.; Pandit, A. A biomaterials approach to peripheral nerve regeneration: Bridging the peripheral nerve gap and enhancing functional recovery. J. R. Soc. Interface 2012, 9, 202–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signaling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Hong, S.H.; Koo, M.-A.; Lee, M.H.; Seon, G.M.; Park, Y.J.; Jeong, H.; Kim, D.; Park, J.-C. An effective method to generate controllable levels of ROS for the enhancement of HUVEC proliferation using a chlorin e6-immobilized PET film as a photo-functional biomaterial. Regen. Biomater. 2021, 8, rbab005. [Google Scholar] [CrossRef]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Covarrubias, L.; Hernandez-Garcia, D.; Schnabel, D.; Salas-Vidal, E.; Castro-Obregon, S. Function of reactive oxygen species during animal development: Passive or active? Dev. Biol. 2008, 320, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-mediated cellular signaling. Oxidative Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [Green Version]
- Sart, S.; Song, L.; Li, Y. Controlling redox status for stem cell survival, expansion, and differentiation. Oxidative Med. Cell. Longev. 2015, 2015, 105135. [Google Scholar] [CrossRef] [Green Version]
- Yue, Z.; Wen, F.; Gao, S.; Ang, M.Y.; Pallatjadka, P.K.; Liu, L.; Yu, H. Preparation of three-dimensional interconnected macroporous cellulosic hydrogels for soft tissue engineering. Biomaterials 2010, 31, 8141–8152. [Google Scholar] [CrossRef]
- Parajuli, P.; Acharya, S.; Hu, Y.; Abidi, N. Cellulose-based monoliths with enhanced surface area and porosity. J. Appl. Polym. Sci. 2020, 137, e48975. [Google Scholar] [CrossRef]
- Lin, W.-H.; Jana, S.C. Analysis of porous structures of cellulose aerogel monoliths and microparticles. Microporous Mesoporous Mater. 2021, 310, 110625. [Google Scholar] [CrossRef]
- Moon, J.Y.; Lee, J.; Hwang, T.I.; Park, C.H.; Kim, C.S. A multifunctional, one-step das foaming strategy for antimicrobial silver nanoparticle-decorated 3D cellulose nanofiber scaffolds. Carbohydr. Polym. 2021, 273, 118603. [Google Scholar] [CrossRef] [PubMed]
- Gateholm, P.; Klemm, D. Bacterial nanocellulose as a renewable material for biomedical applications. MRS Bull. 2010, 35, 208–213. [Google Scholar] [CrossRef]
- Pan, X.; Jinka, S.; Singh, V.K.; Ramkumar, S.; Karim, M.N. Cellulose monolith with tunable functionality for immobilization of influenza virus. J. Bioeng. Biomed. Sci. 2018, 8, 1000246. [Google Scholar] [CrossRef]
- Yang, Z.; Asoh, T.-A.; Uyama, H. Cationic functionalization of cellulose monoliths using a urea-choline based deep eutectic solvent and their applications. Polym. Degrad. Stab. 2019, 160, 126–135. [Google Scholar] [CrossRef]
- Kanno, T.; Uyama, H. Unique leafy morphology of poly(lactic acid) monoliths controlled via novel phase separation technology. RSC Adv. 2017, 7, 33726–33732. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Xue, X.; Fan, K.; Liu, Q.; Zhang, S.; Peng, M.; Zhou, J.; Cao, Z. Moderate hypoxia modulates ABCG2 to promote the proliferation of mouse spermatogonial stem cells by maintaining mild ROS levels. Theriogenology 2020, 145, 149–157. [Google Scholar] [CrossRef]
- Kantonis, G.; Trikeriotis, M.; Ghanotakis, D.F. Biocompatible protoporphyrin IX-containing nanohybrids with potential applications in photodynamictherapy. J. Photochem. Photobiol. A Chem. 2007, 85, 62–66. [Google Scholar] [CrossRef]
- Rezabeigi, E.; Wood-Adams, P.M.; Drew, R.A.L. Production of porous polylactic acid monoliths via nonsolvent induced phase separation. Polymer 2014, 55, 6743–6753. [Google Scholar] [CrossRef]
- Sofi, H.S.; Akram, T.; Shabir, N.; Vasita, R.; Jadhav, A.H.; Sheikh, F.A. Regenerated cellulose nanofibers from cellulose acetate: Incorporating hydroxyapatite (HAp) and silver (Ag) nanoparticles (NPs), as a scaffold for tissue engineering applications. Mater. Sci. Eng. C 2021, 118, 111547. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-R.; Kim, Y.-J. Hydrophilic chlorin e6-poly(amidoamine) dendrimer nanoconjugates for enhanced photodynamic therapy. Nanomaterials 2018, 8, 445. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.J.; Koo, H.; Lee, D.-E.; Min, S.; Lee, S.; Chen, X.; Choi, Y.; Leary, J.F.; Park, K.; Jeong, S.Y.; et al. Tumor-homing photosensitizer-conjugated glycol chitosan nanoparticles for synchronous photodynamic imaging and therapy based on cellular on/off system. Biomaterials 2011, 32, 4021–4029. [Google Scholar] [CrossRef]
- Chudal, L.; Pandey, N.K.; Phan, J.; Johnson, O.; Li, X.; Chen, W. Investigation of PPIX-Lipo-MnO2 to enhance photodynamic therapy by improving tumor hypoxia. Mater. Sci. Eng. C 2019, 104, 109979. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Müller, A.H.E. Architecture, self-assembly and properties of well-defined hybrid polymers based on polyhedral oligomeric silsequioxane (POSS). Prog. Polym. Sci. 2013, 38, 1121–1162. [Google Scholar] [CrossRef]
- Seo, S.R.; Chong, S.A.; Lee, S.-I.; Sung, J.Y.; Ahn, Y.S.; Chung, K.C.; Seo, J.T. Zn2+-induced ERK activation mediated by reactive oxygen species cause cell death in differentiated PC12 cells. J. Neurochem. 2001, 78, 600–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.P.; Kim, J.; Syed, N.; Tung, Y.-J.; Bhaskaran, A.; Mindos, T.; Mirsky, R.; Jessen, K.R.; Maurel, P.; Parkinson, D.B.; et al. p38 MAPK activation promotes denervated Schwann cell phenotype and function as a negative regulator of Schwann cell proliferation and myelination. J. Neurosci. 2012, 32, 7158–7168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.H.; Im, B.N.; Hwang, H.S.; Na, K. Gemcitabine loaded DSPE-PEG-PheoA liposome as a photomediated immune modulator for cholangiocarcinoma treatment. Biomaterials 2018, 183, 139–150. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.H.; Kim, K.H.; Kwon, O.H.; Kwon, O.K.; Uyama, H.; Kim, Y.-J. Photodynamic Activity of Protoporphyrin IX-Immobilized Cellulose Monolith for Nerve Tissue Regeneration. Int. J. Mol. Sci. 2022, 23, 1035. https://doi.org/10.3390/ijms23031035
Lee JH, Kim KH, Kwon OH, Kwon OK, Uyama H, Kim Y-J. Photodynamic Activity of Protoporphyrin IX-Immobilized Cellulose Monolith for Nerve Tissue Regeneration. International Journal of Molecular Sciences. 2022; 23(3):1035. https://doi.org/10.3390/ijms23031035
Chicago/Turabian StyleLee, Ji Hye, Ki Hong Kim, Oh Hyeong Kwon, Oh Kyoung Kwon, Hiroshi Uyama, and Young-Jin Kim. 2022. "Photodynamic Activity of Protoporphyrin IX-Immobilized Cellulose Monolith for Nerve Tissue Regeneration" International Journal of Molecular Sciences 23, no. 3: 1035. https://doi.org/10.3390/ijms23031035
APA StyleLee, J. H., Kim, K. H., Kwon, O. H., Kwon, O. K., Uyama, H., & Kim, Y.-J. (2022). Photodynamic Activity of Protoporphyrin IX-Immobilized Cellulose Monolith for Nerve Tissue Regeneration. International Journal of Molecular Sciences, 23(3), 1035. https://doi.org/10.3390/ijms23031035






