Brassinosteroids (BRs) Role in Plant Development and Coping with Different Stresses
Abstract
:1. Introduction
2. Discovery of BRs in Different Plant Species
3. Occurrence of BRs in Plants
4. Chemical Structure of BRs
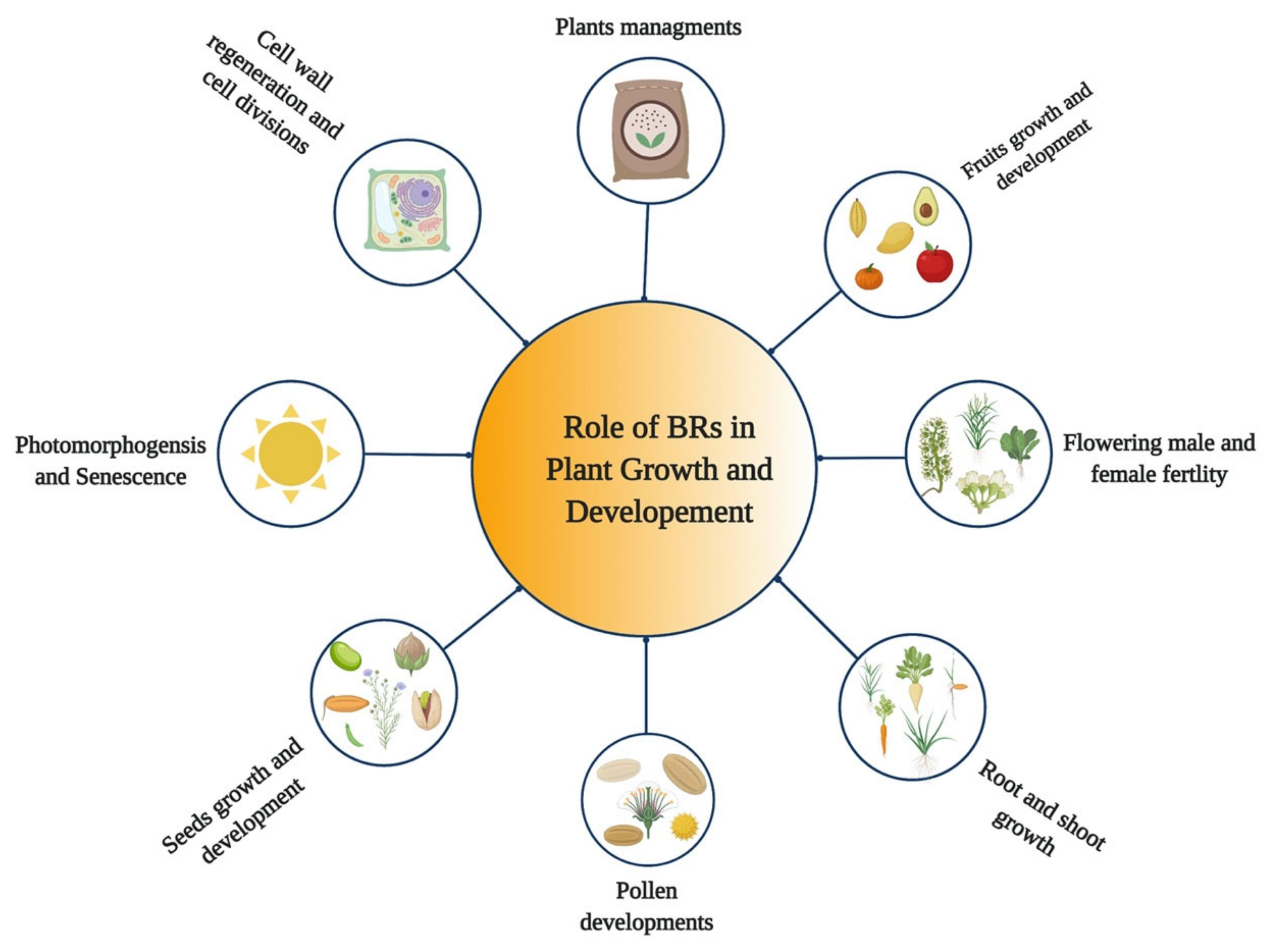
5. Role of BRs in Growth and Development of Plants
| Gene | Description of Gene | Crop/Plant | Role in Growth | Reference |
|---|---|---|---|---|
| CESA | The CESA gene superfamily, encoding the catalytic subunits of cellulose synthase | Arabidopsis (A. thaliana) | Plays a role in regulating the cellulose synthesis | [90] |
| CYCD3;1 | Cell division markers | Arabidopsis (A. thaliana) | Needed for normal cell cycle progression | [51] |
| Histone lysine methyltransferase SDG8 | In Arabidopsis, there are 43 SET Domain Groups (SDG), which contain proteins with conserved SET domains | Arabidopsis (A. thaliana) | Involved in BR-regulated gene expression | [62] |
| WRKY46, WRKY54, and WRKY70 | The WRKY family TFs are composed of over 70 members in Arabidopsis | Arabidopsis (A. thaliana) | Play positive roles in BR-regulated plant growth and drought stress | [61] |
| Brassinazole-resistant 1 (BZR1), and BES1-interacting MYC-like proteins (BIMs) | BZR1; BR-activated transcription factor (TF) and BIMs; bHLH TF | Arabidopsis (A. thaliana) | BR signaling promotes vegetative growth by inhibiting the floral transition | [91] |
| Transcripts of autophagy-related genes (ATGs) | Autophagy-related genes | Tomato (Solanum lycopersicum) | Enhanced level of BR triggers ATGs and formation of autophagosomes | [92] |
| VvHMGR | Plays a role in the mevalonate (MVA) pathway | Grape berries (Vitis vinifera) | Involved in increasing the anthocyanin content and promoting coloration. Accumulates the fruit sugar components, and decreases the tartaric acid content | [93] |
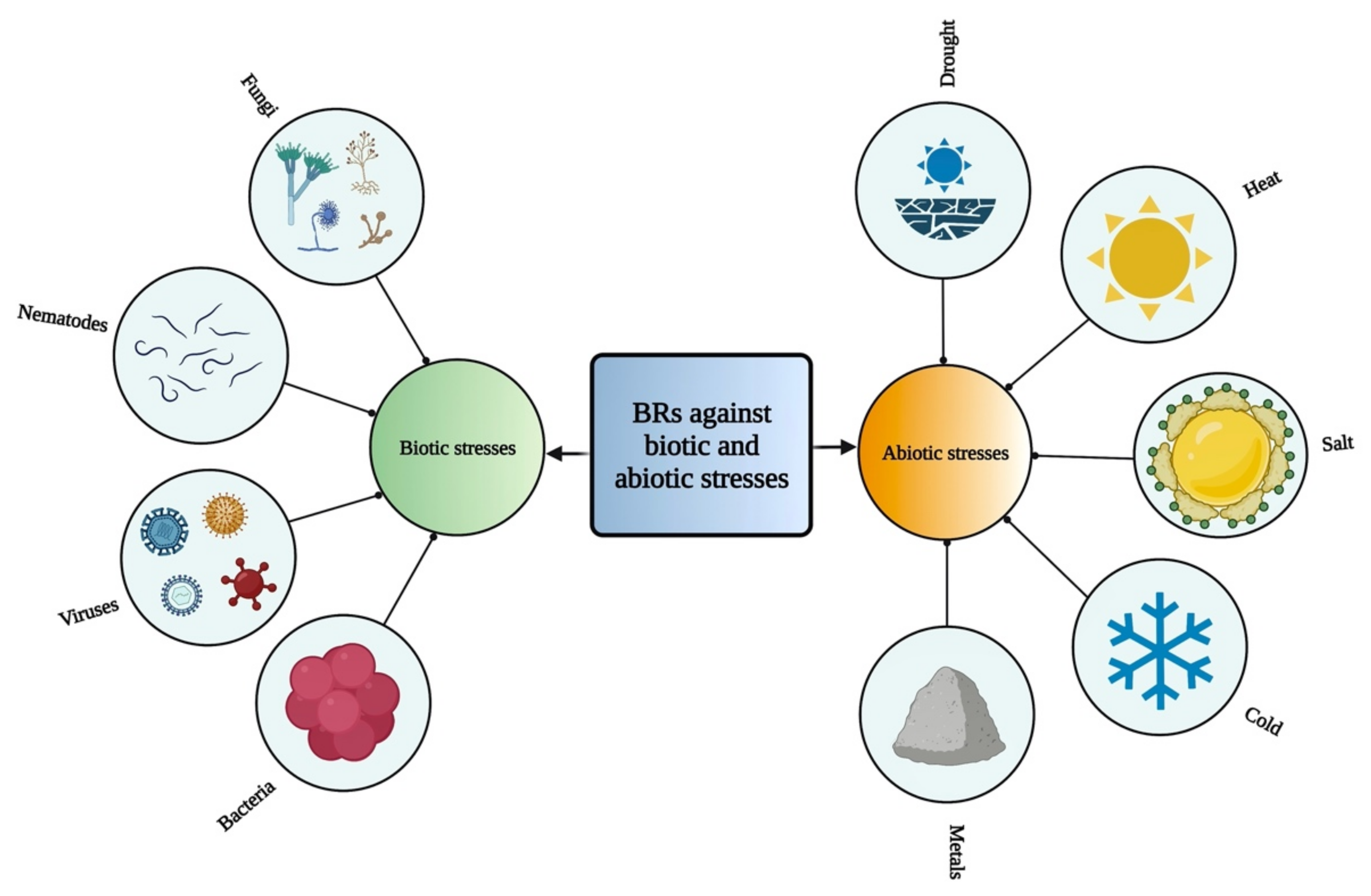
6. Role of BRs against Different Stresses in Plants
| Gene/BRs | Gene Function | Crop/Plant | Stress Type | Reference |
|---|---|---|---|---|
| Respiratory burst oxidase homolog (RBOH) | Involved in ROS generation | Cucumber (Cucumis sativus L.) | Cold and photo-oxidative stresses | [119] |
| DREB | Involved in regulating various cold stress-responsive genes | Rice (O. sativa L.) | Cold stress | [109,120] |
| Proline-5-caryboxylate synthetase 1 (P5CS1) | Involved in the proline biosynthesis | Arabidopsis (A. thaliana) | Salt stress | [121] |
| Abscisic acid stress ripening (ASR) | Involved in signal transduction | Mango (Mangifera indica L.) | Cold stress | [122] |
| YODA (YDA) | A TF involved in regulating stomatal conductance | Arabidopsis (A. thaliana) | Drought and salt stresses | [41] |
| CYP90b3, GSH1, and GST1 | Play a role in detoxification | Tomato (S. lycopersicum L.) | Phenanthrene stress | [123] |
| Remorin | Membrane skeleton protein | Mango (M. indica L.) | Drought stress | [122] |
| UBC32 | A stress-induced functional ubiquitin conjugation enzyme (E2) | Arabidopsis (A. thaliana) | Salt stress | [102] |
| Lipocalins | Involved in signal transduction | Mango (M. indica L.) | Cold stress | [122] |
| Submergence 1A (SUB1A) | An ethylene response factor (ERF), involved in conferring the submergence tolerance | Rice (O. sativa L.) | Submergence tolerance | [124] |
| Alternative oxidase (AOX) | Involved in protecting the plant photosystems | Tobacco (Nicotiana benthamiana) | Cold stress | [125] |
| Ferritin | Involved in iron storage | Rice (O. sativa L.) | Pesticide and salt stresses | [126] |
| Respiratory burst oxidase homolog 1 (RBOH1) | Involved in ROS generation | Tomato (S. lycopersicum) | Heat tolerance | [118] |
| Ascorbate peroxidase (APX) | Involved in the scavenging of ROS | Rice (O. sativa L.) | Pesticide and salt stresses | [127,128] |
| bes1-D | BRI1 EMS SUPRESSOR 1 | Arabidopsis (A. thaliana) | Tolerance to Cucumber mosaic virus (CMV) | [112] |
| Superoxide dismutase (SOD) | H2O2 biosynthesis | Rice (O. sativa L.) | Pesticide and salt stresses | [127,128] |
| Glutathione reductase (GR) | Involved in the scavenging of ROS | Rice (O. sativa L.) | Pesticide and salt stresses | [127,128] |
| Catalase (CAT) | Engaged in the scavenging of ROS | Rice (O. sativa L.) | Pesticide and salt stresses | [127,128] |
| No-expressor of pathogenesis-related genes1-1 (NPR1-1) | Involved in regulating various stress-responsive genes | Arabidopsis (A. thaliana) | Salt and hyper-thermal stresses | [129] |
| 1-aminocyclopropane-1-carboxylate synthase (ACS) | An ethylene synthesis enzyme | Tomato (S. lycopersicum) | Salt stress | [103] |
| Cesta (CES) | TFs that are involved in regulating several cold stress-responsive genes | Arabidopsis (A. thaliana) | Cold stress | [98] |
| BZR1 and BES1 | Basic helix-loop-helix TFs play a role in the BR-signaling pathway | Arabidopsis (A. thaliana) | Freezing tolerance | [99] |
| WRKY | Involved in regulating various stress-responsive genes | Arabidopsis (A. thaliana) | Drought stress | [61] |
| BRL3 | A vascular-enriched member of the BR receptor family | Arabidopsis (A. thaliana) | Drought stress | [96] |
| BZR1 | The main regulator of BR response | Tomato (S. lycopersicum) and Arabidopsis (A. thaliana) | Thermotolerance | [100,101] |
7. BRs Signaling in Plants
8. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, S.; Cai, Z.; Wang, X. The primary signaling outputs of brassinosteroids are regulated by abscisic acid signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 4543–4548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vardhini, B.V.; Anjum, N.A. Brassinosteroids make plant life easier under abiotic stresses mainly by modulating major components of antioxidant defense system. Front. Environ. Sci. 2015, 2, 67. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Yuan, L.; Wang, J.; Xie, S.; Zheng, Y.; Nie, L.; Zhu, S.; Hou, J.; Chen, G.; Wang, C. Transcriptome analysis reveals a positive effect of brassinosteroids on the photosynthetic capacity of wucai under low temperature. BMC Genom. 2019, 20, 810. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ahammed, G.J.; Li, G.; Bai, P.; Jiang, Y.; Wang, S.; Chen, S. Ethylene is involved in red light-induced anthocyanin biosynthesis in cabbage (Brassica oleracea). Int. J. Agric. Biol. 2019, 21, 955–963. [Google Scholar]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [Green Version]
- Xiong, L.; Schumaker, K.S.; Zhu, J.-K. Cell signaling during cold, drought, and salt stress. Plant Cell 2002, 14, S165–S183. [Google Scholar] [CrossRef] [Green Version]
- Planas-Riverola, A.; Gupta, A.; Betegón-Putze, I.; Bosch, N.; Ibañes, M.; Caño-Delgado, A.I. Brassinosteroid signaling in plant development and adaptation to stress. Development 2019, 146, dev151894. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.-Y.; Bai, M.-Y.; Oh, E.; Zhu, J.-Y. Brassinosteroid signaling network and regulation of photomorphogenesis. Annu. Rev. Genet. 2012, 46, 701–724. [Google Scholar] [CrossRef]
- Tong, H.; Chu, C. Functional specificities of brassinosteroid and potential utilization for crop improvement. Trends Plant Sci. 2018, 23, 1016–1028. [Google Scholar] [CrossRef]
- Mitchell, J.; Mandava, N.; Worley, J.; Plimmer, J.; Smith, M. Brassins—A new family of plant hormones from rape pollen. Nature 1970, 225, 1065–1066. [Google Scholar] [CrossRef]
- Grove, M.D.; Spencer, G.F.; Rohwedder, W.K.; Mandava, N.; Worley, J.F.; Warthen, J.D.; Steffens, G.L.; Flippen-Anderson, J.L.; Cook, J.C. Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature 1979, 281, 216–217. [Google Scholar] [CrossRef]
- Li, J.; Chory, J. A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell 1997, 90, 929–938. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.-J.; Russinova, E. Brassinosteroid signalling. Curr. Biol. 2020, 30, R294–R298. [Google Scholar] [CrossRef] [PubMed]
- Bajguz, A.; Tretyn, A. The chemical characteristic and distribution of brassinosteroids in plants. Phytochemistry 2003, 62, 1027–1046. [Google Scholar] [CrossRef]
- Bajguz, A.; Tretyn, A. The chemical structures and occurrence of brassinosteroids in plants. In Brassinosteroids; Springer: Berlin/Heidelberg, Germany, 2003; pp. 1–44. [Google Scholar]
- Hussain, M.A.; Fahad, S.; Sharif, R.; Jan, M.F.; Mujtaba, M.; Ali, Q.; Ahmad, A.; Ahmad, H.; Amin, N.; Ajayo, B.S. Multifunctional role of brassinosteroid and its analogues in plants. Plant Growth Regul. 2020, 92, 141–156. [Google Scholar] [CrossRef]
- Bajguz, A. Brassinosteroids–occurence and chemical structures in plants. In Brassinosteroids: A Class of Plant Hormone; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1–27. [Google Scholar]
- Ohnishi, T. Recent advances in brassinosteroid biosynthetic pathway: Insight into novel brassinosteroid shortcut pathway. J. Pestic. Sci. 2018, 43, 159–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; He, Y. Roles of Brassinosteroids in Plant Reproduction. Int. J. Mol. Sci. 2020, 21, 872. [Google Scholar] [CrossRef] [Green Version]
- Hayat, S.; Ahmad, A.; Ahmad, A. Brassinosteroids: Bioactivity and Crop Productivity; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Fedina, E.; Yarin, A.; Mukhitova, F.; Blufard, A.; Chechetkin, I. Brassinosteroid-induced changes of lipid composition in leaves of Pisum sativum L. during senescence. Steroids 2017, 117, 25–28. [Google Scholar] [CrossRef]
- Yokota, T.; Ohnishi, T.; Shibata, K.; Asahina, M.; Nomura, T.; Fujita, T.; Ishizaki, K.; Kohchi, T. Occurrence of brassinosteroids in non-flowering land plants, liverwort, moss, lycophyte and fern. Phytochemistry 2017, 136, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, S.; Yokota, T. Biosynthesis and metabolism of brassinosteroids. Annu. Rev. Plant Biol. 2003, 54, 137–164. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Liang, H.; Chen, G.; Tang, B.; Tian, S.; Hu, Z. Isolation of the brassinosteroid receptor genes and recharacterization of dwarf plants by silencing of SlBRI1 in tomato. Plant Growth Regul. 2019, 89, 59–71. [Google Scholar] [CrossRef]
- Zou, L.; Qu, M.; Zeng, L.; Xiong, G. The molecular basis of the interaction between Brassinosteroid induced and phosphorous deficiency induced leaf inclination in rice. Plant Growth Regul. 2020, 91, 263–276. [Google Scholar] [CrossRef]
- Wendeborn, S.; Lachia, M.; Jung, P.M.; Leipner, J.; Brocklehurst, D.; De Mesmaeker, A.; Gaus, K.; Mondière, R. Biological Activity of Brassinosteroids–Direct Comparison of Known and New Analogs in planta. Helv. Chim. Acta 2017, 100, 1–46. [Google Scholar] [CrossRef]
- Hayat, S.; Yusuf, M.; Bhardwaj, R.; Bajguz, A. Brassinosteroids: Plant Growth and Development; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Bajguz, A.; Chmur, M.; Gruszka, D. Comprehensive overview of the Brassinosteroid biosynthesis pathways: Substrates, products, inhibitors, and connections. Front. Plant Sci. 2020, 11, 1034. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K. Natural Occurrences of Brassinosteroids; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1991; pp. 26–35. [Google Scholar]
- Bishop, G.J.; Nomura, T.; Yokota, T.; Harrison, K.; Noguchi, T.; Fujioka, S.; Takatsuto, S.; Jones, J.D.; Kamiya, Y. The tomato DWARF enzyme catalyses C-6 oxidation in brassinosteroid biosynthesis. Proc. Natl. Acad. Sci. USA 1999, 96, 1761–1766. [Google Scholar] [CrossRef] [Green Version]
- Fujioka, S. Natural occurrence of brassinosteroids in the plant kingdom. In Brassinosteroid: Steroidal Plant Hormones; Springer: Amsterdam, The Netherlands, 1999; pp. 21–45. [Google Scholar]
- Fang, P.; Yan, M.; Chi, C.; Wang, M.; Zhou, Y.; Zhou, J.; Shi, K.; Xia, X.; Foyer, C.H.; Yu, J. Brassinosteroids act as a positive regulator of photoprotection in response to chilling stress. Plant Physiol. 2019, 180, 2061–2076. [Google Scholar] [CrossRef]
- Peres, A.L.G.; Soares, J.S.; Tavares, R.G.; Righetto, G.; Zullo, M.A.; Mandava, N.B.; Menossi, M. Brassinosteroids, the sixth class of phytohormones: A molecular view from the discovery to hormonal interactions in plant development and stress adaptation. Int. J. Mol. Sci. 2019, 20, 331. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.-H. Designed Manipulation of the Brassinosteroid Signal to Enhance Crop Yield. Front. Plant Sci. 2020, 11, 854. [Google Scholar] [CrossRef]
- Singh, A.P.; Savaldi-Goldstein, S. Growth control: Brassinosteroid activity gets context. J. Exp. Bot. 2015, 66, 1123–1132. [Google Scholar] [CrossRef] [Green Version]
- Fàbregas, N.; Caño-Delgado, A.I. Turning on the microscope turret: A new view for the study of brassinosteroid signaling in plant development. Physiol. Plant. 2014, 151, 172–183. [Google Scholar] [CrossRef]
- Lv, B.; Tian, H.; Zhang, F.; Liu, J.; Lu, S.; Bai, M.; Li, C.; Ding, Z. Brassinosteroids regulate root growth by controlling reactive oxygen species homeostasis and dual effect on ethylene synthesis in Arabidopsis. PLoS Genet. 2018, 14, e1007144. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yu, F.; Xie, Q. Balancing growth and adaptation to stress: Crosstalk between brassinosteroid and abscisic acid signaling. Plant Cell Environ. 2020, 43, 2325–2335. [Google Scholar] [CrossRef]
- Oh, E.; Zhu, J.-Y.; Bai, M.-Y.; Arenhart, R.A.; Sun, Y.; Wang, Z.-Y. Cell elongation is regulated through a central circuit of interacting transcription factors in the Arabidopsis hypocotyl. eLife 2014, 3, e03031. [Google Scholar] [CrossRef] [PubMed]
- Hacham, Y.; Holland, N.; Butterfield, C.; Ubeda-Tomas, S.; Bennett, M.J.; Chory, J.; Savaldi-Goldstein, S. Brassinosteroid perception in the epidermis controls root meristem size. Development 2011, 138, 839–848. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.-W.; Michniewicz, M.; Bergmann, D.C.; Wang, Z.-Y. Brassinosteroid regulates stomatal development by GSK3-mediated inhibition of a MAPK pathway. Nature 2012, 482, 419–422. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.; Rozhon, W.; Bigeard, J.; Pflieger, D.; Husar, S.; Pitzschke, A.; Teige, M.; Jonak, C.; Hirt, H.; Poppenberger, B. Brassinosteroid-regulated GSK3/Shaggy-like kinases phosphorylate mitogen-activated protein (MAP) kinase kinases, which control stomata development in Arabidopsis thaliana. J. Biol. Chem. 2013, 288, 7519–7527. [Google Scholar] [CrossRef] [Green Version]
- Vogler, F.; Schmalzl, C.; Englhart, M.; Bircheneder, M.; Sprunck, S. Brassinosteroids promote Arabidopsis pollen germination and growth. Plant Reprod. 2014, 27, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, R.; Fujioka, S.; Demura, T.; Takatsuto, S.; Yoshida, S.; Fukuda, H. Brassinosteroid levels increase drastically prior to morphogenesis of tracheary elements. Plant Physiol. 2001, 125, 556–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, M.; Kondo, Y.; Fukuda, H. BES1 and BZR1 redundantly promote phloem and xylem differentiation. Plant Cell Physiol. 2018, 59, 590–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Han, S.; Lee, H.-Y.; Jeong, B.; Heo, T.-Y.; Hyun, T.K.; Kim, K.; Je, B.I.; Lee, H.; Shim, D. Brassinosteroids facilitate xylem differentiation and wood formation in tomato. Planta 2019, 249, 1391–1403. [Google Scholar] [CrossRef]
- Nolan, T.M.; Vukašinović, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional regulators of plant growth, development, and stress responses. Plant Cell 2020, 32, 295–318. [Google Scholar] [CrossRef] [Green Version]
- Ahammed, G.J.; Li, X.; Liu, A.; Chen, S. Brassinosteroids in Plant Tolerance to Abiotic Stress. J. Plant Growth Regul. 2020, 39, 1451–1464. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, Z.-Y.; Mora-Garcia, S.; Li, J.; Yoshida, S.; Asami, T.; Chory, J. BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell 2002, 109, 181–191. [Google Scholar] [CrossRef] [Green Version]
- Zadvornova, Y.; Alekseichuk, G.; Laman, N.; Khripach, V.; Grut, S. Effect of brassinosteroids on activation of the cell cycle during germination of Brassica oleracea L. seeds. Doklady Natsional’noi Akademii Nauk Belarusi 2005, 49, 70–73. [Google Scholar]
- González-García, M.-P.; Vilarrasa-Blasi, J.; Zhiponova, M.; Divol, F.; Mora-García, S.; Russinova, E.; Caño-Delgado, A.I. Brassinosteroids control meristem size by promoting cell cycle progression in Arabidopsis roots. Development 2011, 138, 849–859. [Google Scholar] [CrossRef] [Green Version]
- Nakaya, M.; Tsukaya, H.; Murakami, N.; Kato, M. Brassinosteroids control the proliferation of leaf cells of Arabidopsis thaliana. Plant Cell Physiol. 2002, 43, 239–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arteca, J.M.; Arteca, R.N. Brassinosteroid-induced exaggerated growth in hydroponically grown Arabidopsis plants. Physiol. Plant. 2001, 112, 104–112. [Google Scholar] [CrossRef]
- Ackerman-Lavert, M.; Savaldi-Goldstein, S. Growth models from a brassinosteroid perspective. Curr. Opin. Plant Biol. 2020, 53, 90–97. [Google Scholar] [CrossRef]
- Makarevitch, I.; Thompson, A.; Muehlbauer, G.J.; Springer, N.M. Brd1 gene in maize encodes a brassinosteroid C-6 oxidase. PLoS ONE 2012, 7, e30798. [Google Scholar]
- Hartwig, T.; Chuck, G.S.; Fujioka, S.; Klempien, A.; Weizbauer, R.; Potluri, D.P.V.; Choe, S.; Johal, G.S.; Schulz, B. Brassinosteroid control of sex determination in maize. Proc. Natl. Acad. Sci. USA 2011, 108, 19814–19819. [Google Scholar] [CrossRef] [Green Version]
- Jaillais, Y.; Vert, G. Brassinosteroids, gibberellins and light-mediated signalling are the three-way controls of plant sprouting. Nat. Cell Biol. 2012, 14, 788–790. [Google Scholar] [CrossRef]
- Zhu, X.; Liang, W.; Cui, X.; Chen, M.; Yin, C.; Luo, Z.; Zhu, J.; Lucas, W.J.; Wang, Z.; Zhang, D. Brassinosteroids promote development of rice pollen grains and seeds by triggering expression of Carbon Starved Anther, a MYB domain protein. Plant J. 2015, 82, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Båga, M.; Chibbar, R.N. Brassinosteroid receptor mutation influences starch granule size distribution in barley grains. Plant Physiol. Biochem. 2020, 154, 369–378. [Google Scholar] [CrossRef]
- Divi, U.K.; Krishna, P. Brassinosteroid: A biotechnological target for enhancing crop yield and stress tolerance. New Biotechnol. 2009, 26, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Nolan, T.M.; Ye, H.; Zhang, M.; Tong, H.; Xin, P.; Chu, J.; Chu, C.; Li, Z.; Yin, Y. Arabidopsis WRKY46, WRKY54, and WRKY70 transcription factors are involved in brassinosteroid-regulated plant growth and drought responses. Plant Cell 2017, 29, 1425–1439. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, J.; Xie, Z.; Liu, S.; Nolan, T.; Ye, H.; Zhang, M.; Guo, H.; Schnable, P.S.; Li, Z. Histone lysine methyltransferase SDG8 is involved in brassinosteroid-regulated gene expression in Arabidopsis thaliana. Mol. Plant 2014, 7, 1303–1315. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.-Y.; Jiang, W.-B.; Hu, Y.-W.; Wu, P.; Zhu, J.-Y.; Liang, W.-Q.; Wang, Z.-Y.; Lin, W.-H. BR signal influences Arabidopsis ovule and seed number through regulating related genes expression by BZR1. Mol. Plant 2013, 6, 456–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, W.-B.; Huang, H.-Y.; Hu, Y.-W.; Zhu, S.-W.; Wang, Z.-Y.; Lin, W.-H. Brassinosteroid regulates seed size and shape in Arabidopsis. Plant Physiol. 2013, 162, 1965–1977. [Google Scholar] [CrossRef]
- Jia, Z.; Giehl, R.F.; Meyer, R.C.; Altmann, T.; von Wirén, N. Natural variation of BSK3 tunes brassinosteroid signaling to regulate root foraging under low nitrogen. Nat. Commun. 2019, 10, 2378. [Google Scholar] [CrossRef] [Green Version]
- Tanabe, S.; Ashikari, M.; Fujioka, S.; Takatsuto, S.; Yoshida, S.; Yano, M.; Yoshimura, A.; Kitano, H.; Matsuoka, M.; Fujisawa, Y. A novel cytochrome P450 is implicated in brassinosteroid biosynthesis via the characterization of a rice dwarf mutant, dwarf11, with reduced seed length. Plant Cell 2005, 17, 776–790. [Google Scholar] [CrossRef] [Green Version]
- Hong, Z.; Ueguchi-Tanaka, M.; Fujioka, S.; Takatsuto, S.; Yoshida, S.; Hasegawa, Y.; Ashikari, M.; Kitano, H.; Matsuoka, M. The rice brassinosteroid-deficient dwarf2 mutant, defective in the rice homolog of Arabidopsis DIMINUTO/DWARF1, is rescued by the endogenously accumulated alternative bioactive brassinosteroid, dolichosterone. Plant Cell 2005, 17, 2243–2254. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zhu, K.; Wang, Z.; Zhang, H.; Gu, J.; Liu, L.; Yang, J.; Zhang, J. Brassinosteroids function in spikelet differentiation and degeneration in rice. J. Integr. Plant Biol. 2019, 61, 943–963. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Veerabomma, S.; Abdel-Mageed, H.A.; Fokar, M.; Asami, T.; Yoshida, S.; Allen, R.D. Brassinosteroid regulates fiber development on cultured cotton ovules. Plant Cell Physiol. 2005, 46, 1384–1391. [Google Scholar] [CrossRef]
- Luo, M.; Xiao, Y.; Li, X.; Lu, X.; Deng, W.; Li, D.; Hou, L.; Hu, M.; Li, Y.; Pei, Y. GhDET2, a steroid 5α-reductase, plays an important role in cotton fiber cell initiation and elongation. Plant J. 2007, 51, 419–430. [Google Scholar] [CrossRef]
- Janeczko, A.; Oklestkova, J.; Novak, O.; Śniegowska-Świerk, K.; Snaczke, Z.; Pociecha, E. Disturbances in production of progesterone and their implications in plant studies. Steroids 2015, 96, 153–163. [Google Scholar] [CrossRef]
- Wan, L.; Zhang, F.; Zhang, L.; Liu, L.; Chen, C.; Ma, N.; Zhang, C. Brassinosteroids promote seed development and physiological maturity of oilseed rape (Brassica napus L.). Oil Crop Sci. 2017, 1, 122–130. [Google Scholar]
- Zhang, S.; Hu, J.; Zhang, Y.; Xie, X.; Knapp, A. Seed priming with brassinolide improves lucerne (Medicago sativa L.) seed germination and seedling growth in relation to physiological changes under salinity stress. Aust. J. Agric. Res. 2007, 58, 811–815. [Google Scholar] [CrossRef]
- Symons, G.M.; Davies, C.; Shavrukov, Y.; Dry, I.B.; Reid, J.B.; Thomas, M.R. Grapes on steroids. Brassinosteroids are involved in grape berry ripening. Plant Physiol. 2006, 140, 150–158. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Xi, Z.-m.; Zhang, H.; Zhang, C.-j.; Zhang, Z.-w. Brassinosteroids are involved in controlling sugar unloading in Vitis vinifera ‘Cabernet Sauvignon’ berries during véraison. Plant Physiol. Biochem. 2015, 94, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, Z.; Qin, G.; Tian, S. Effects of brassinosteroids on postharvest disease and senescence of jujube fruit in storage. Postharvest Biol. Technol. 2010, 56, 50–55. [Google Scholar] [CrossRef]
- Meudt, W.; Thompson, M.; Bennett, H. Investigations on the Mechanism of the Brassinosteroid Response. III. Techniques for Potential Enhancement of Crop Production [Barley, Bean]. In Proceedings Annual Meeting; Plant Growth Regulator Society of America: Madison, WI, USA, 1983; pp. 312–318. [Google Scholar]
- Oh, M.-H. Brassinosteroids accelerate the rate of cell division in isolated petal protoplasts of Petunia hybrida. J. Plant Biotechnol. 2003, 5, 69–77. [Google Scholar]
- Kęsy, J.; Trzaskalska, A.; Galoch, E.; Kopcewicz, J. Inhibitory effect of brassinosteroids on the flowering of the short-day plant Pharbitis nil. Biol. Plant. 2003, 47, 597–600. [Google Scholar] [CrossRef]
- Trevisan, S.; Forestan, C.; Brojanigo, S.; Quaggiotti, S.; Varotto, S. Brassinosteroid application affects the growth and gravitropic response of maize by regulating gene expression in the roots, shoots and leaves. Plant Growth Regul. 2020, 92, 117–130. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, X.; Xia, X.-J.; Shi, K.; Zhou, Y.-H.; Yu, J.-Q. Enhanced photosynthetic capacity and antioxidant potential mediate brassinosteriod-induced phenanthrene stress tolerance in tomato. Environ. Pollut. 2015, 201, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Ye, Q.; Zhu, W.; Li, L.; Zhang, S.; Yin, Y.; Ma, H.; Wang, X. Brassinosteroids control male fertility by regulating the expression of key genes involved in Arabidopsis anther and pollen development. Proc. Natl. Acad. Sci. USA 2010, 107, 6100–6105. [Google Scholar] [CrossRef] [Green Version]
- Domagalska, M.A.; Sarnowska, E.; Nagy, F.; Davis, S.J. Genetic analyses of interactions among gibberellin, abscisic acid, and brassinosteroids in the control of flowering time in Arabidopsis thaliana. PLoS ONE 2010, 5, e14012. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Nagpal, P.; Vitart, V.; McMorris, T.C.; Chory, J. A role for brassinosteroids in light-dependent development of Arabidopsis. Science 1996, 272, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Gudesblat, G.E.; Schneider-Pizoń, J.; Betti, C.; Mayerhofer, J.; Vanhoutte, I.; Van Dongen, W.; Boeren, S.; Zhiponova, M.; De Vries, S.; Jonak, C. SPEECHLESS integrates brassinosteroid and stomata signalling pathways. Nat. Cell Biol. 2012, 14, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-Y.; Sae-Seaw, J.; Wang, Z.-Y. Brassinosteroid signalling. Development 2013, 140, 1615–1620. [Google Scholar] [CrossRef] [Green Version]
- Clouse, S.D. The molecular intersection of brassinosteroid-regulated growth and flowering in Arabidopsis. Proc. Natl. Acad. Sci. USA 2008, 105, 7345–7346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, B.; Li, J. Regulation of brassinosteroid biosynthesis and inactivation F. J. Integr. Plant Biol. 2012, 54, 746–759. [Google Scholar] [CrossRef]
- Rozhon, W.; Akter, S.; Fernandez, A.; Poppenberger, B. Inhibitors of brassinosteroid biosynthesis and signal transduction. Molecules 2019, 24, 4372. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.; Yang, C.; Wang, X. Brassinosteroids can regulate cellulose biosynthesis by controlling the expression of CESA genes in Arabidopsis. J. Exp. Bot. 2011, 62, 4495–4506. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Ou, Y.; Zhang, Z.; Li, J.; He, Y. Brassinosteroid signaling recruits histone 3 lysine-27 demethylation activity to FLOWERING LOCUS C chromatin to inhibit the floral transition in Arabidopsis. Mol. Plant 2018, 11, 1135–1146. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Cao, J.-J.; Wang, K.-X.; Xia, X.-J.; Shi, K.; Zhou, Y.-H.; Yu, J.-Q.; Zhou, J. BZR1 mediates brassinosteroid-induced autophagy and nitrogen starvation in tomato. Plant Physiol. 2019, 179, 671–685. [Google Scholar] [CrossRef] [Green Version]
- Zheng, T.; Dong, T.; Haider, M.S.; Jin, H.; Jia, H.; Fang, J. Brassinosteroid Regulates 3-Hydroxy-3-methylglutaryl CoA Reductase to Promote Grape Fruit Development. J. Agric. Food Chem. 2020, 68, 11987–11996. [Google Scholar] [CrossRef]
- Anjum, S.; Wang, L.; Farooq, M.; Hussain, M.; Xue, L.; Zou, C. Brassinolide application improves the drought tolerance in maize through modulation of enzymatic antioxidants and leaf gas exchange. J. Agron. Crop Sci. 2011, 197, 177–185. [Google Scholar] [CrossRef]
- Divi, U.K.; Rahman, T.; Krishna, P. Gene expression and functional analyses in brassinosteroid-mediated stress tolerance. Plant Biotechnol. J. 2016, 14, 419–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fàbregas, N.; Lozano-Elena, F.; Blasco-Escámez, D.; Tohge, T.; Martínez-Andújar, C.; Albacete, A.; Osorio, S.; Bustamante, M.; Riechmann, J.L.; Nomura, T. Overexpression of the vascular brassinosteroid receptor BRL3 confers drought resistance without penalizing plant growth. Nat. Commun. 2018, 9, 4680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, J.; Lobato, A. Brassinosteroids improve photosystem II efficiency, gas exchange, antioxidant enzymes and growth of cowpea plants exposed to water deficit. Physiol. Mol. Biol. Plants 2017, 23, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Eremina, M.; Unterholzner, S.J.; Rathnayake, A.I.; Castellanos, M.; Khan, M.; Kugler, K.G.; May, S.T.; Mayer, K.F.; Rozhon, W.; Poppenberger, B. Brassinosteroids participate in the control of basal and acquired freezing tolerance of plants. Proc. Natl. Acad. Sci. USA 2016, 113, E5982–E5991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Ye, K.; Shi, Y.; Cheng, J.; Zhang, X.; Yang, S. BZR1 positively regulates freezing tolerance via CBF-dependent and CBF-independent pathways in Arabidopsis. Mol. Plant 2017, 10, 545–559. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; Qin, K.; Song, X.; Zhang, Q.; Zhou, Y.; Xia, X.; Yu, J. BZR1 transcription factor regulates heat stress tolerance through FERONIA receptor-like kinase-mediated reactive oxygen species signaling in tomato. Plant Cell Physiol. 2018, 59, 2239–2254. [Google Scholar] [CrossRef]
- Ibañez, C.; Delker, C.; Martinez, C.; Bürstenbinder, K.; Janitza, P.; Lippmann, R.; Ludwig, W.; Sun, H.; James, G.V.; Klecker, M. Brassinosteroids dominate hormonal regulation of plant thermomorphogenesis via BZR1. Curr. Biol. 2018, 28, 303–310.e3. [Google Scholar] [CrossRef]
- Cui, F.; Liu, L.; Zhao, Q.; Zhang, Z.; Li, Q.; Lin, B.; Wu, Y.; Tang, S.; Xie, Q. Arabidopsis ubiquitin conjugase UBC32 is an ERAD component that functions in brassinosteroid-mediated salt stress tolerance. Plant Cell 2012, 24, 233–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, T.; Deng, X.; Zhou, X.; Zhu, L.; Zou, L.; Li, P.; Zhang, D.; Lin, H. Ethylene and hydrogen peroxide are involved in brassinosteroid-induced salt tolerance in tomato. Sci. Rep. 2016, 6, 35392. [Google Scholar] [CrossRef] [Green Version]
- Rajewska, I.; Talarek, M.; Bajguz, A. Brassinosteroids and response of plants to heavy metals action. Front. Plant Sci. 2016, 7, 629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahzad, B.; Tanveer, M.; Che, Z.; Rehman, A.; Cheema, S.A.; Sharma, A.; Song, H.; ur Rehman, S.; Zhaorong, D. Role of 24-epibrassinolide (EBL) in mediating heavy metal and pesticide induced oxidative stress in plants: A review. Ecotoxicol. Environ. Saf. 2018, 147, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Yin, Y.; Fei, S.-z. Brassinosteroid signaling network: Implications on yield and stress tolerance. Plant Cell Rep. 2013, 32, 1017–1030. [Google Scholar] [CrossRef]
- Wu, C.; Li, F.; Xu, H.; Zeng, W.; Yu, R.; Wu, X.; Shen, L.; Liu, Y.; Li, J. The potential role of brassinosteroids (BRs) in alleviating antimony (Sb) stress in Arabidopsis thaliana. Plant Physiol. Biochem. 2019, 141, 51–59. [Google Scholar] [CrossRef]
- Fridman, Y.; Savaldi-Goldstein, S. Brassinosteroids in growth control: How, when and where. Plant Sci. 2013, 209, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Kagale, S.; Divi, U.K.; Krochko, J.E.; Keller, W.A.; Krishna, P. Brassinosteroid confers tolerance in Arabidopsis thaliana and Brassica napus to a range of abiotic stresses. Planta 2007, 225, 353–364. [Google Scholar] [CrossRef]
- Sahni, S.; Prasad, B.D.; Liu, Q.; Grbic, V.; Sharpe, A.; Singh, S.P.; Krishna, P. Overexpression of the brassinosteroid biosynthetic gene DWF4 in Brassica napus simultaneously increases seed yield and stress tolerance. Sci. Rep. 2016, 6, 28298. [Google Scholar] [CrossRef] [PubMed]
- Nakashita, H.; Yasuda, M.; Nitta, T.; Asami, T.; Fujioka, S.; Arai, Y.; Sekimata, K.; Takatsuto, S.; Yamaguchi, I.; Yoshida, S. Brassinosteroid functions in a broad range of disease resistance in tobacco and rice. Plant J. 2003, 33, 887–898. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.-W.; Deng, X.-G.; Fu, F.-Q.; Lin, H.-H. Induction of plant virus defense response by brassinosteroids and brassinosteroid signaling in Arabidopsis thaliana. Planta 2015, 241, 875–885. [Google Scholar] [CrossRef]
- Chi, C.; Li, X.; Fang, P.; Xia, X.; Shi, K.; Zhou, Y.; Zhou, J.; Yu, J. Brassinosteroids act as a positive regulator of NBR1-dependent selective autophagy in response to chilling stress in tomato. J. Exp. Bot. 2020, 71, 1092–1106. [Google Scholar] [CrossRef]
- Soares, C.; de Sousa, A.; Pinto, A.; Azenha, M.; Teixeira, J.; Azevedo, R.A.; Fidalgo, F. Effect of 24-epibrassinolide on ROS content, antioxidant system, lipid peroxidation and Ni uptake in Solanum nigrum L. under Ni stress. Environ. Exp. Bot. 2016, 122, 115–125. [Google Scholar] [CrossRef]
- Zhou, Y.-l.; Huo, S.-f.; Wang, L.-t.; Meng, J.-f.; Zhang, Z.-w.; Xi, Z.-m. Exogenous 24-Epibrassinolide alleviates oxidative damage from copper stress in grape (Vitis vinifera L.) cuttings. Plant Physiol. Biochem. 2018, 130, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, L.; Kong, Q.; Cheng, F.; Niu, M.; Xie, J.; Nawaz, M.A.; Bie, Z. Comprehensive mineral nutrition analysis of watermelon grafted onto two different rootstocks. Hortic. Plant J. 2016, 2, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Hasan, S.A.; Hayat, S.; Ahmad, A. Brassinosteroids protect photosynthetic machinery against the cadmium induced oxidative stress in two tomato cultivars. Chemosphere 2011, 84, 1446–1451. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, J.; Li, X.; Xia, X.-J.; Zhou, Y.-H.; Shi, K.; Chen, Z.; Yu, J.-Q. H2O2 mediates the crosstalk of brassinosteroid and abscisic acid in tomato responses to heat and oxidative stresses. J. Exp. Bot. 2014, 65, 4371–4383. [Google Scholar] [CrossRef]
- Xia, X.-J.; Wang, Y.-J.; Zhou, Y.-H.; Tao, Y.; Mao, W.-H.; Shi, K.; Asami, T.; Chen, Z.; Yu, J.-Q. Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Plant Physiol. 2009, 150, 801–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, B.-Z.; Chen, X.; Xiang, C.-B.; Tang, N.; Zhang, Q.-F.; Xiong, L.-Z. Evaluation of seven function-known candidate genes for their effects on improving drought resistance of transgenic rice under field conditions. Mol. Plant 2009, 2, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Zeng, H.; Tang, Q.; Hua, X. Arabidopsis brassinosteroid mutants det2-1 and bin2-1 display altered salt tolerance. J. Plant Growth Regul. 2010, 29, 44–52. [Google Scholar] [CrossRef]
- Li, B.; Zhang, C.; Cao, B.; Qin, G.; Wang, W.; Tian, S. Brassinolide enhances cold stress tolerance of fruit by regulating plasma membrane proteins and lipids. Amino Acids 2012, 43, 2469–2480. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Gao, C.-J.; Ogweno, J.O.; Zhou, Y.-H.; Xia, X.-J.; Mao, W.-H.; Shi, K.; Yu, J.-Q. Brassinosteroids induce plant tolerance against phenanthrene by enhancing degradation and detoxification in Solanum lycopersicum L. Ecotoxicol. Environ. Saf. 2012, 80, 28–36. [Google Scholar] [CrossRef]
- Schmitz, A.J.; Folsom, J.J.; Jikamaru, Y.; Ronald, P.; Walia, H. SUB 1 A-mediated submergence tolerance response in rice involves differential regulation of the brassinosteroid pathway. New Phytol. 2013, 198, 1060–1070. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.-G.; Zhu, T.; Zhang, D.-W.; Lin, H.-H. The alternative respiratory pathway is involved in brassinosteroid-induced environmental stress tolerance in Nicotiana benthamiana. J. Exp. Bot. 2015, 66, 6219–6232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, I. Studies on Brassinosteroid Mediated Responses in Oryza sativa L. under Pesticide and Salt Stress Employing Molecular and Biochemical Approaches. Ph.D. Thesis, Guru Nanak Dev University, Amritsar, India, 2014. [Google Scholar]
- Sharma, I.; Bhardwaj, R.; Pati, P.K. Mitigation of adverse effects of chlorpyrifos by 24-epibrassinolide and analysis of stress markers in a rice variety Pusa Basmati-1. Ecotoxicol. Environ. Saf. 2012, 85, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Sharma, I.; Bhardwaj, R.; Pati, P.K. Exogenous application of 28-homobrassinolide modulates the dynamics of salt and pesticides induced stress responses in an elite rice variety Pusa Basmati-1. J. Plant Growth Regul. 2015, 34, 509–518. [Google Scholar] [CrossRef]
- Wei, L.; Deng, X.-G.; Zhu, T.; Zheng, T.; Li, P.-X.; Wu, J.-Q.; Zhang, D.-W.; Lin, H.-H. Ethylene is involved in brassinosteroids induced alternative respiratory pathway in cucumber (Cucumis sativus L.) seedlings response to abiotic stress. Front. Plant Sci. 2015, 6, 982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clouse, S.D. Brassinosteroid signal transduction: From receptor kinase activation to transcriptional networks regulating plant development. Plant Cell 2011, 23, 1219–1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.-W.; Wang, Z.-Y. Brassinosteroid signal transduction from receptor kinases to transcription factors. Annu. Rev. Plant Biol. 2010, 61, 681–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.; Yuan, M.; Wang, R.; Yang, Y.; Wang, C.; Oses-Prieto, J.A.; Kim, T.-W.; Zhou, H.-W.; Deng, Z.; Gampala, S.S. PP2A activates brassinosteroid-responsive gene expression and plant growth by dephosphorylating BZR1. Nat. Cell Biol. 2011, 13, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Northey, J.G.; Liang, S.; Jamshed, M.; Deb, S.; Foo, E.; Reid, J.B.; McCourt, P.; Samuel, M.A. Farnesylation mediates brassinosteroid biosynthesis to regulate abscisic acid responses. Nat. Plants 2016, 2, 16114. [Google Scholar] [CrossRef]
- Praveena, J.; Dash, S.; Behera, L.; Rout, G.R. Brassinosteroids: A Multifunctional Phytohormone of Plant Development and Stress Responses. Curr. J. Appl. Sci. Technol. 2020, 39, 174–196. [Google Scholar] [CrossRef]
- Aldukhi, F.; Deb, A.; Zhao, C.; Moffett, A.S.; Shukla, D. Molecular Mechanism of Brassinosteroid Perception by the Plant Growth Receptor BRI1. J. Phys. Chem. B 2019, 124, 355–365. [Google Scholar] [CrossRef]
- Sun, Y.; Han, Z.; Tang, J.; Hu, Z.; Chai, C.; Zhou, B.; Chai, J. Structure reveals that BAK1 as a co-receptor recognizes the BRI1-bound brassinolide. Cell Res. 2013, 23, 1326–1329. [Google Scholar] [CrossRef]
- Lee, H.G.; Won, J.H.; Choi, Y.-R.; Lee, K.; Seo, P.J. Brassinosteroids Regulate Circadian Oscillation via the BES1/TPL-CCA1/LHY Module in Arabidopsis thaliana. Iscience 2020, 23, 101528. [Google Scholar] [CrossRef]
- Liu, X.; Yang, H.; Wang, Y.; Zhu, Z.; Zhang, W.; Li, J. Comparative transcriptomic analysis to identify brassinosteroid response genes. Plant Physiol. 2020, 184, 1072–1082. [Google Scholar] [CrossRef]
- Vukašinović, N.; Russinova, E. BRexit: Possible brassinosteroid export and transport routes. Trends Plant Sci. 2018, 23, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Vafeados, D.; Tao, Y.; Yoshida, S.; Asami, T.; Chory, J. A new class of transcription factors mediates brassinosteroid-regulated gene expression in Arabidopsis. Cell 2005, 120, 249–259. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Yu, X.; Thompson, A.; Guo, M.; Yoshida, S.; Asami, T.; Chory, J.; Yin, Y. Arabidopsis MYB30 is a direct target of BES1 and cooperates with BES1 to regulate brassinosteroid-induced gene expression. Plant J. 2009, 58, 275–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, H.; Li, L.; Guo, H.; Yin, Y. MYBL2 is a substrate of GSK3-like kinase BIN2 and acts as a corepressor of BES1 in brassinosteroid signaling pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 20142–20147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Ye, H.; Guo, H.; Johnson, A.; Zhang, M.; Lin, H.; Yin, Y. Transcription factor HAT 1 is phosphorylated by BIN 2 kinase and mediates brassinosteroid repressed gene expression in Arabidopsis. Plant J. 2014, 77, 59–70. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manghwar, H.; Hussain, A.; Ali, Q.; Liu, F. Brassinosteroids (BRs) Role in Plant Development and Coping with Different Stresses. Int. J. Mol. Sci. 2022, 23, 1012. https://doi.org/10.3390/ijms23031012
Manghwar H, Hussain A, Ali Q, Liu F. Brassinosteroids (BRs) Role in Plant Development and Coping with Different Stresses. International Journal of Molecular Sciences. 2022; 23(3):1012. https://doi.org/10.3390/ijms23031012
Chicago/Turabian StyleManghwar, Hakim, Amjad Hussain, Qurban Ali, and Fen Liu. 2022. "Brassinosteroids (BRs) Role in Plant Development and Coping with Different Stresses" International Journal of Molecular Sciences 23, no. 3: 1012. https://doi.org/10.3390/ijms23031012
APA StyleManghwar, H., Hussain, A., Ali, Q., & Liu, F. (2022). Brassinosteroids (BRs) Role in Plant Development and Coping with Different Stresses. International Journal of Molecular Sciences, 23(3), 1012. https://doi.org/10.3390/ijms23031012








