Parametric Drug Release Optimization of Anti-Inflammatory Drugs by Gold Nanoparticles for Topically Applied Ocular Therapy
Abstract
1. Introduction
2. Results and Discussion
2.1. Influence of Drug-Loading Parameters on Drug Release
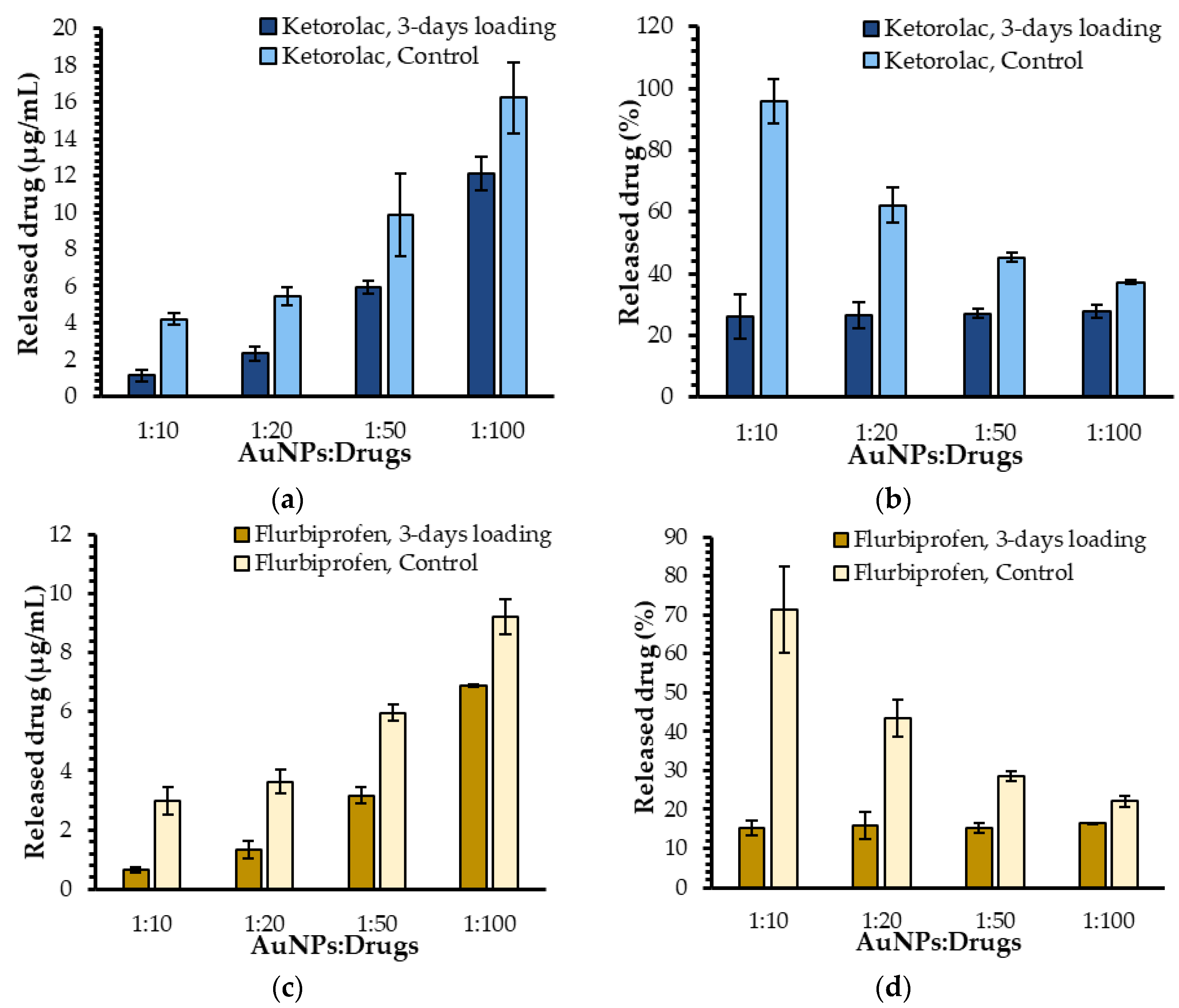
2.1.1. Influence of the Ratio of AuNPs to Drug Molecules on Drug Release
2.1.2. Influence of the Nature of the Loaded Drug on Drug Release
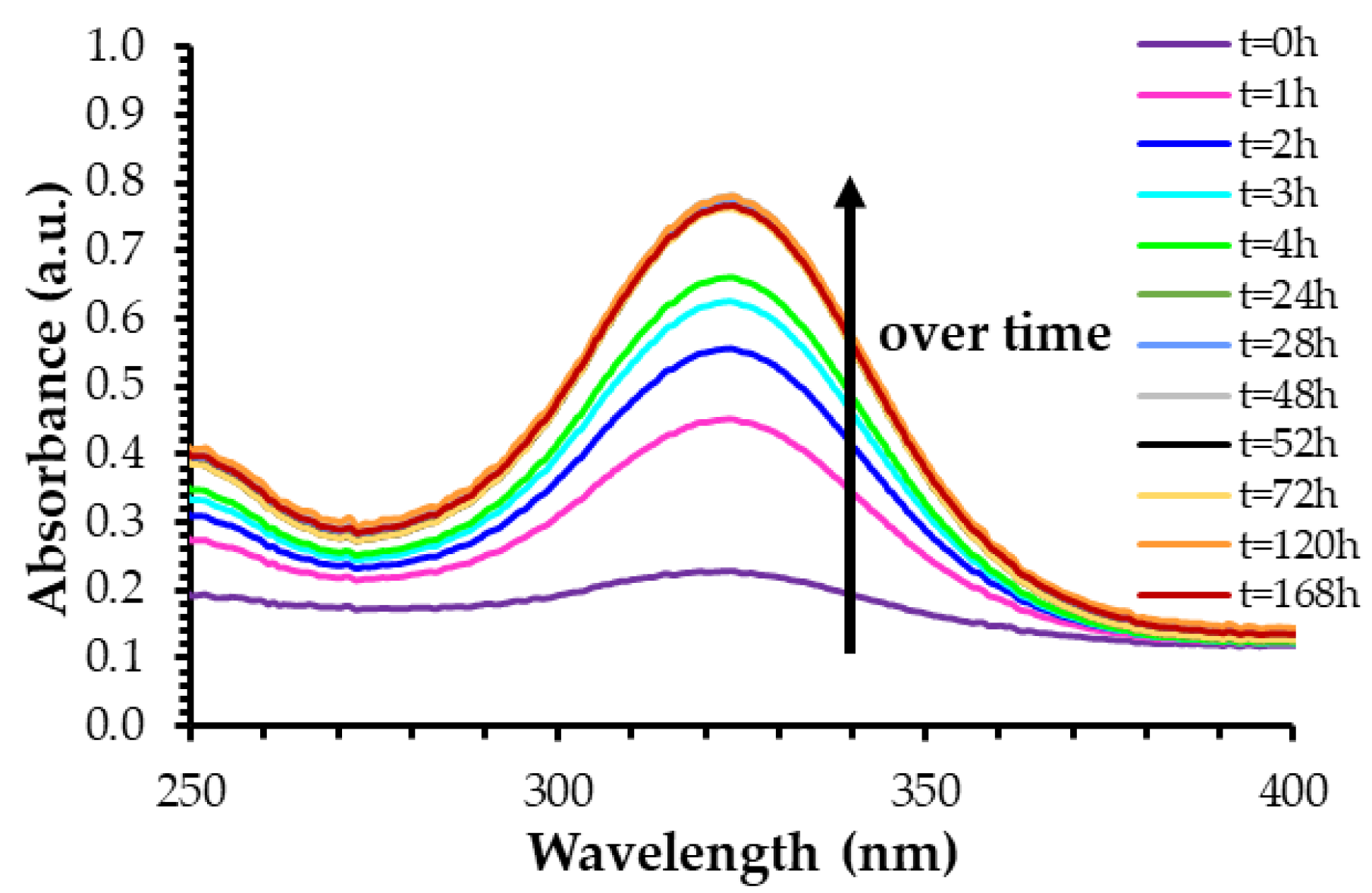
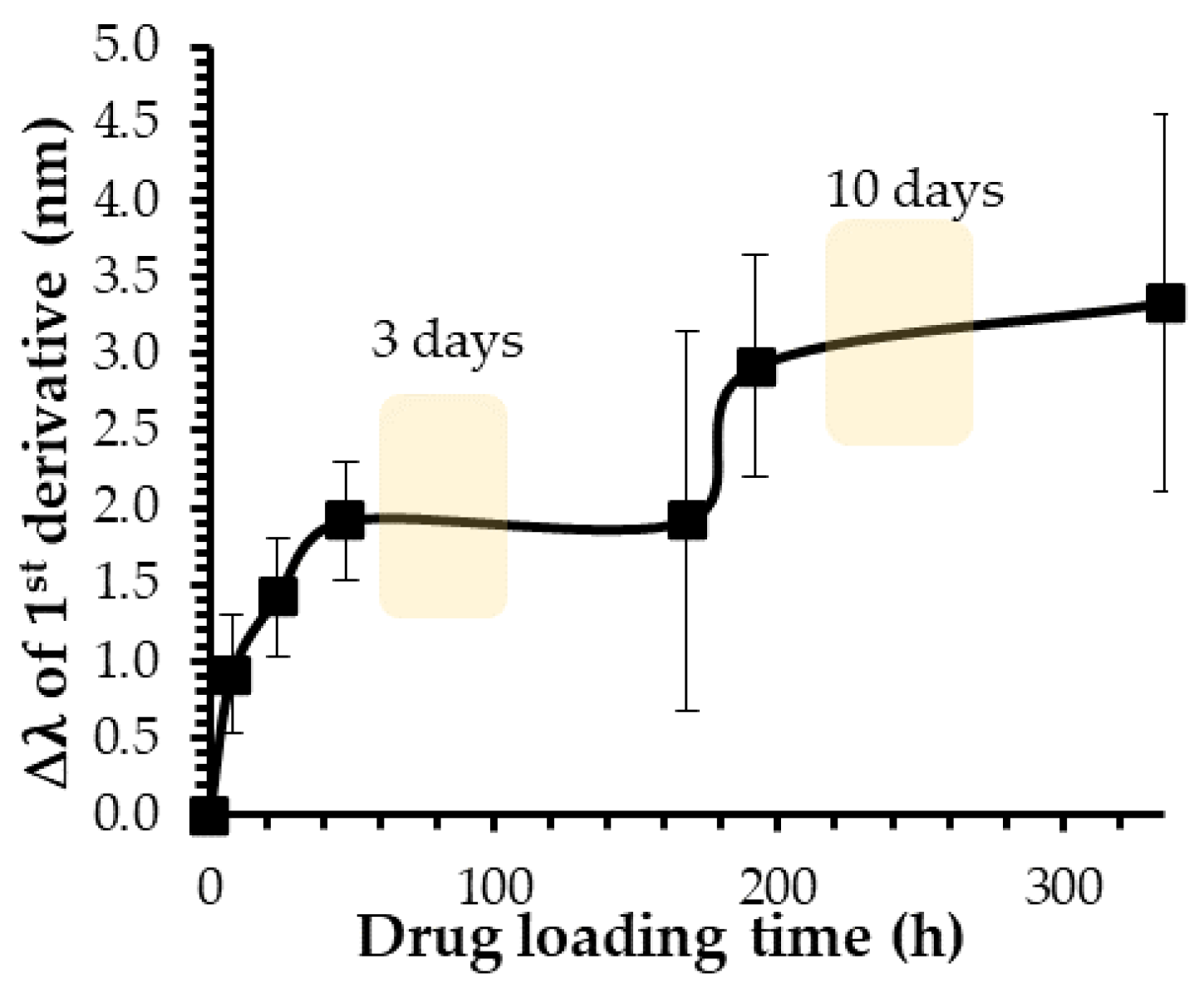
2.1.3. Influence of Loading Time on Drug Release
2.2. Influence of AuNPs-Related Parameters on the Drug Release
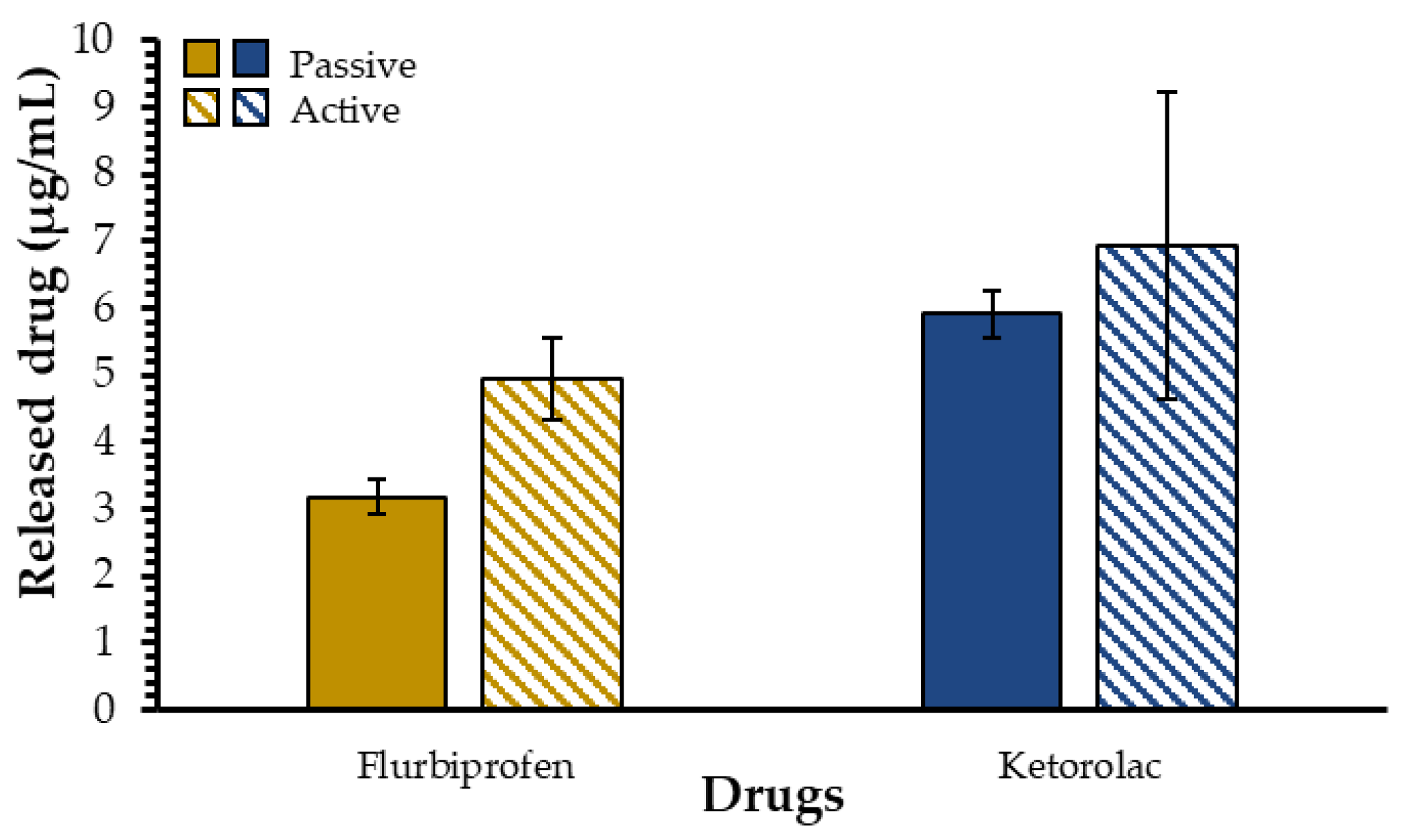
2.3. Influence of Experimental Protocol Parameters on Drug Release
3. Materials and Methods
3.1. Materials
3.2. Drug-Loading Kinetics
3.2.1. Preparation of AuNP Samples
3.2.2. UV–Visible Spectroscopy
3.2.3. First and Second Derivatives of Plasmon Band Spectra
3.3. Drug-Loading Quantification
3.4. Drug Release Experiments
3.4.1. Preparation of AuNPs Samples
3.4.2. Parameters and Dialysis Bags
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Etzioni, D.A.; Liu, J.H.; Maggard, M.A.; Ko, C.Y. The Aging Population and Its Impact on the Surgery Workforce. Ann. Surg. 2003, 238, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.; Wang, P.; Schein, O.D.; Srikumaran, D.; Makary, M.; Woreta, F.A. Prescribing Patterns and Costs Associated with Postoperative Eye Drop Use in Medicare Beneficiaries Undergoing Cataract Surgery. Ophthalmology 2020, 127, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Erichsen, J.H.; Forman, J.L.; Holm, L.M.; Kessel, L. Effect of Anti-Inflammatory Regimen on Early Postoperative Inflammation after Cataract Surgery. J. Cataract Refract. Surg. 2021, 47, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Masse, F.; Ouellette, M.; Lamoureux, G.; Boisselier, E. Gold Nanoparticles in Ophthalmology. Med. Res. Rev. 2019, 39, 302–327. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, B.; Bozdag, S.; Unlü, N. Dendrimeric Systems and Their Applications in Ocular Drug Delivery. Sci. World J. 2013, 2013, 732340. [Google Scholar] [CrossRef]
- Salter, A.; Chen, A.; Lee, D.; Greenberg, P. Compliance with Postoperative Cataract Surgery Care in an Urban Teaching Hospital. Rhode Island Med. J. 2014, 97, 48–49. [Google Scholar]
- Gaudana, R.; Jwala, J.; Boddu, S.H.S.; Mitra, A.K. Recent Perspectives in Ocular Drug Delivery. Pharm. Res. 2008, 26, 1197. [Google Scholar] [CrossRef]
- Bron, A.J.; Tiffany, J.M.; Gouveia, S.M.; Yokoi, N.; Voon, L.W. Functional Aspects of the Tear Film Lipid Layer. Exp. Eye Res. 2004, 78, 347–360. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Willcox, M.; Madigan, M.; Wasinger, V.; Schiller, B.; Walsh, B.; Graham, P.; Kearsley, J.; Li, Y. Tear Fluid Protein Biomarkers. Adv. Clin. Chem. 2013, 62, 151–196. [Google Scholar] [CrossRef]
- Dartt, D.A.; Willcox, M.D.P. Complexity of the Tear Film: Importance in Homeostasis and Dysfunction during Disease. Exp. Eye Res. 2013, 117, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Kopacz, D.; Niezgoda, Ł.; Fudalej, E.; Nowak, A.; Maciejewicz, P. Tear Film—Physiology and Disturbances in Various Diseases and Disorders. In Ocular Surface Diseases; Łucja, N., Ed.; IntechOpen: Rijeka, Croatia, 2020; Chapter 1; ISBN 978-1-83880-960-7. [Google Scholar]
- Stahl, U.; Willcox, M.; Stapleton, F. Osmolality and Tear Film Dynamics. Clin. Exp. Optom. 2012, 95, 3–11. [Google Scholar] [CrossRef]
- Arita, R.; Fukuoka, S.; Morishige, N. New Insights into the Morphology and Function of Meibomian Glands. Exp. Eye Res. 2017, 163, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, M.; Masse, F.; Lefebvre-Demers, M.; Maestracci, Q.; Grenier, P.; Millar, R.; Bertrand, N.; Prieto, M.; Boisselier, É. Insights into Gold Nanoparticles as a Mucoadhesive System. Sci. Rep. 2018, 8, 14357. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Guerrero, V.; Bravo-Osuna, I.; Pastoriza, P.; Molina-Martinez, I.T.; Herrero-Vanrell, R. Novel Technologies for the Delivery of Ocular Therapeutics in Glaucoma. J. Drug Deliv. Sci. Technol. 2017, 42, 181–192. [Google Scholar] [CrossRef]
- Hornof, M.D.; Bernkop-Schnürch, A. In Vitro Evaluation of the Permeation Enhancing Effect of Polycarbophil–Cysteine Conjugates on the Cornea of Rabbits. J. Pharm. Sci. 2002, 91, 2588–2592. [Google Scholar] [CrossRef]
- Suri, R.; Beg, S.; Kohli, K. Target Strategies for Drug Delivery Bypassing Ocular Barriers. J. Drug Deliv. Sci. Technol. 2020, 55, 101389. [Google Scholar] [CrossRef]
- Das Neves, J.; Bahia, M.F.; Amiji, M.M.; Sarmento, B. Mucoadhesive Nanomedicines: Characterization and Modulation of Mucoadhesion at the Nanoscale. Expert Opin. Drug Deliv. 2011, 8, 1085–1104. [Google Scholar] [CrossRef]
- Masse, F.; Desjardins, P.; Ouellette, M.; Couture, C.; Omar, M.M.; Pernet, V.; Guérin, S.; Boisselier, E. Synthesis of Ultrastable Gold Nanoparticles as a New Drug Delivery System. Molecules 2019, 24, 2929. [Google Scholar] [CrossRef] [PubMed]
- Boisselier, E.; Salmon, L.; Ruiz, J.; Astruc, D. How to Very Efficiently Functionalize Gold Nanoparticles by “Click” Chemistry. Chem. Commun. 2008, 44, 5788–5790. [Google Scholar] [CrossRef]
- Rippel, R.; Seifalian, A. Gold Revolution-Gold Nanoparticles for Modern Medicine and Surgery. J. Nanosci. Nanotechnol. 2011, 11, 3740–3748. [Google Scholar] [CrossRef]
- Cabuzu, D.; Cirja, A.; Puiu, R.; Mihai Grumezescu, A. Biomedical Applications of Gold Nanoparticles. Curr. Top. Med. Chem. 2015, 15, 1605–1613. [Google Scholar] [CrossRef]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent Biomedical Applications of Gold Nanoparticles: A Review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef]
- Jeong, H.-H.; Choi, E.; Ellis, E.; Lee, T.-C. Recent Advances in Gold Nanoparticles for Biomedical Applications: From Hybrid Structures to Multi-Functionality. J. Mater. Chem. B 2019, 7, 3480–3496. [Google Scholar] [CrossRef]
- Schein, O.D.; Cassard, S.D.; Tielsch, J.M.; Gower, E.W. Cataract Surgery among Medicare Beneficiaries. Ophthalmic Epidemiol. 2012, 19, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Molokhia, S.A.; Thomas, S.C.; Garff, K.J.; Mandell, K.J.; Wirostko, B.M. Anterior Eye Segment Drug Delivery Systems: Current Treatments and Future Challenges. J. Ocul. Pharmacol. Ther. 2013, 29, 92–105. [Google Scholar] [CrossRef]
- Bansal, S.A.; Kumar, V.; Karimi, J.; Singh, A.P.; Kumar, S. Role of Gold Nanoparticles in Advanced Biomedical Applications. Nanoscale Adv. 2020, 2, 3764–3787. [Google Scholar] [CrossRef]
- Gianturco, S.L.; Pavlech, L.L.; Storm, K.D.; Yoon, S.; Yuen, M.V.; Mattingly, A.N. Flurbiprofen: Summary Report. Univ. Md. Baltim. 2020. [Google Scholar]
- Solomon, K.D.; Cheetham, J.K.; DeGryse, R.; Brint, S.F.; Rosenthal, A. Topical Ketorolac Tromethamine 0.5% Ophthalmic Solution in Ocular Inflammation after Cataract Surgery. Ophthalmology 2001, 108, 331–337. [Google Scholar] [CrossRef]
- Amul, B.; Muthu, S.; Raja, M.; Sevvanthi, S. Molecular Structure, Spectroscopic (FT-IR, FT-Raman, NMR, UV-VIS), Chemical Reactivity and Biological Examinations of Ketorolac. J. Mol. Struct. 2020, 1210, 128040. [Google Scholar] [CrossRef]
- Wittpenn, J.R.; Silverstein, S.; Heier, J.; Kenyon, K.R.; Hunkeler, J.D.; Earl, M. A Randomized, Masked Comparison of Topical Ketorolac 0.4% Plus Steroid vs Steroid Alone in Low-Risk Cataract Surgery Patients. Am. J. Ophthalmol. 2008, 146, 554–560.e1. [Google Scholar] [CrossRef]
- Grzybowski, A.; Kanclerz, P. Do We Need Day-1 Postoperative Follow-up after Cataract Surgery? Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 855–861. [Google Scholar] [CrossRef]
- Mahmud, I.; Kelley, T.; Stowell, C.; Haripriya, A.; Boman, A.; Kossler, I.; Morlet, N.; Pershing, S.; Pesudovs, K.; Goh, P.P.; et al. A Proposed Minimum Standard Set of Outcome Measures for Cataract Surgery. JAMA Ophthalmol. 2015, 133, 1247–1252. [Google Scholar] [CrossRef]
- Westborg, I.; Mönestam, E. Optimizing Number of Postoperative Visits after Cataract Surgery: Safety Perspective. J. Cataract Refract. Surg. 2017, 43, 1184–1189. [Google Scholar] [CrossRef]
- Solomon, D.; Gupta, N.; Mulla, N.S.; Shukla, S.; Guerrero, Y.A.; Gupta, V. Role of In Vitro Release Methods in Liposomal Formulation Development: Challenges and Regulatory Perspective. AAPS J. 2017, 19, 1669–1681. [Google Scholar] [CrossRef]
- Yu, M.; Yuan, W.; Li, D.; Schwendeman, A.; Schwendeman, S.P. Predicting Drug Release Kinetics from Nanocarriers inside Dialysis Bags. J. Control. Release 2019, 315, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Jalodia, K.; Kumar, P.; Gautam, H.K. Recent Advances in Nanoparticle-Mediated Drug Delivery. J. Drug Deliv. Sci. Technol. 2017, 41, 260–268. [Google Scholar] [CrossRef]
- Shetab Boushehri, M.A.; Lamprecht, A. Nanoparticles as Drug Carriers: Current Issues with in Vitro Testing. Nanomedicine 2015, 10, 3213–3230. [Google Scholar] [CrossRef]
- Kim, Y.; Fluckiger, L.; Hoffman, M.; Lartaud-Idjouadiene, I.; Atkinson, J.; Maincent, P. The Antihypertensive Effect of Orally Administered Nifedipine-Loaded Nanoparticles in Spontaneously Hypertensive Rats. Br. J. Pharmacol. 1997, 120, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Anderson, B.D. Determination of Drug Release Kinetics from Nanoparticles: Overcoming Pitfalls of the Dynamic Dialysis Method. Mol. Pharm. 2013, 10, 3076–3089. [Google Scholar] [CrossRef] [PubMed]
- Schwarzl, R.; Du, F.; Haag, R.; Netz, R.R. General Method for the Quantification of Drug Loading and Release Kinetics of Nanocarriers. Eur. J. Pharm. Biopharm. 2017, 116, 131–137. [Google Scholar] [CrossRef]
- Jain, S.K.; Chourasia, M.K.; Masuriha, R.; Soni, V.; Jain, A.; Jain, N.K.; Gupta, Y. Solid Lipid Nanoparticles Bearing Flurbiprofen for Transdermal Delivery. Drug Deliv. 2005, 12, 207–215. [Google Scholar] [CrossRef][Green Version]
- Hoffman, R.S.; Braga-Mele, R.; Donaldson, K.; Emerick, G.; Henderson, B.; Kahook, M.; Mamalis, N.; Miller, K.M.; Realini, T.; Shorstein, N.H.; et al. Cataract Surgery and Nonsteroidal Antiinflammatory Drugs. J. Cataract Refract. Surg. 2016, 42, 1368–1379. [Google Scholar] [CrossRef]
- Xavier, J.; Vincent, S.; Meder, F.; Vollmer, F. Advances in Optoplasmonic Sensors—Combining Optical Nano/Microcavities and Photonic Crystals with Plasmonic Nanostructures and Nanoparticles. Nanophotonics 2018, 7, 1–38. [Google Scholar] [CrossRef]
- Martinsson, E.; Otte, M.A.; Shahjamali, M.M.; Sepulveda, B.; Aili, D. Substrate Effect on the Refractive Index Sensitivity of Silver Nanoparticles. J. Phys. Chem. C 2014, 118, 24680–24687. [Google Scholar] [CrossRef]
- Arcas, A.S.; Jaramillo, L.; Costa, N.S.; Allil, R.C.S.B.; Werneck, M.M. Localized Surface Plasmon Resonance-Based Biosensor on Gold Nanoparticles for Taenia Solium Detection. Appl. Opt. 2021, 60, 8137–8144. [Google Scholar] [CrossRef]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface Plasmon Resonance in Gold Nanoparticles: A Review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef]
- Daniel, M.-C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Loiseau, A.; Zhang, L.; Hu, D.; Salmain, M.; Mazouzi, Y.; Flack, R.; Liedberg, B.; Boujday, S. Core–Shell Gold/Silver Nanoparticles for Localized Surface Plasmon Resonance-Based Naked-Eye Toxin Biosensing. ACS Appl. Mater. Interfaces 2019, 11, 46462–46471. [Google Scholar] [CrossRef]
- Chen, P.; Tran, N.T.; Wen, X.; Xiong, Q.; Liedberg, B. Inflection Point of the Localized Surface Plasmon Resonance Peak: A General Method to Improve the Sensitivity. ACS Sens. 2017, 2, 235–242. [Google Scholar] [CrossRef]
- Chen, P.; Liedberg, B. Curvature of the Localized Surface Plasmon Resonance Peak. Anal. Chem. 2014, 86, 7399–7405. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raiche-Marcoux, G.; Loiseau, A.; Maranda, C.; Poliquin, A.; Boisselier, E. Parametric Drug Release Optimization of Anti-Inflammatory Drugs by Gold Nanoparticles for Topically Applied Ocular Therapy. Int. J. Mol. Sci. 2022, 23, 16191. https://doi.org/10.3390/ijms232416191
Raiche-Marcoux G, Loiseau A, Maranda C, Poliquin A, Boisselier E. Parametric Drug Release Optimization of Anti-Inflammatory Drugs by Gold Nanoparticles for Topically Applied Ocular Therapy. International Journal of Molecular Sciences. 2022; 23(24):16191. https://doi.org/10.3390/ijms232416191
Chicago/Turabian StyleRaiche-Marcoux, Gabrielle, Alexis Loiseau, Cloé Maranda, Audrée Poliquin, and Elodie Boisselier. 2022. "Parametric Drug Release Optimization of Anti-Inflammatory Drugs by Gold Nanoparticles for Topically Applied Ocular Therapy" International Journal of Molecular Sciences 23, no. 24: 16191. https://doi.org/10.3390/ijms232416191
APA StyleRaiche-Marcoux, G., Loiseau, A., Maranda, C., Poliquin, A., & Boisselier, E. (2022). Parametric Drug Release Optimization of Anti-Inflammatory Drugs by Gold Nanoparticles for Topically Applied Ocular Therapy. International Journal of Molecular Sciences, 23(24), 16191. https://doi.org/10.3390/ijms232416191






