Effects of Phytochelatin-like Gene on the Resistance and Enrichment of Cd2+ in Tobacco
Abstract
1. Introduction
2. Results
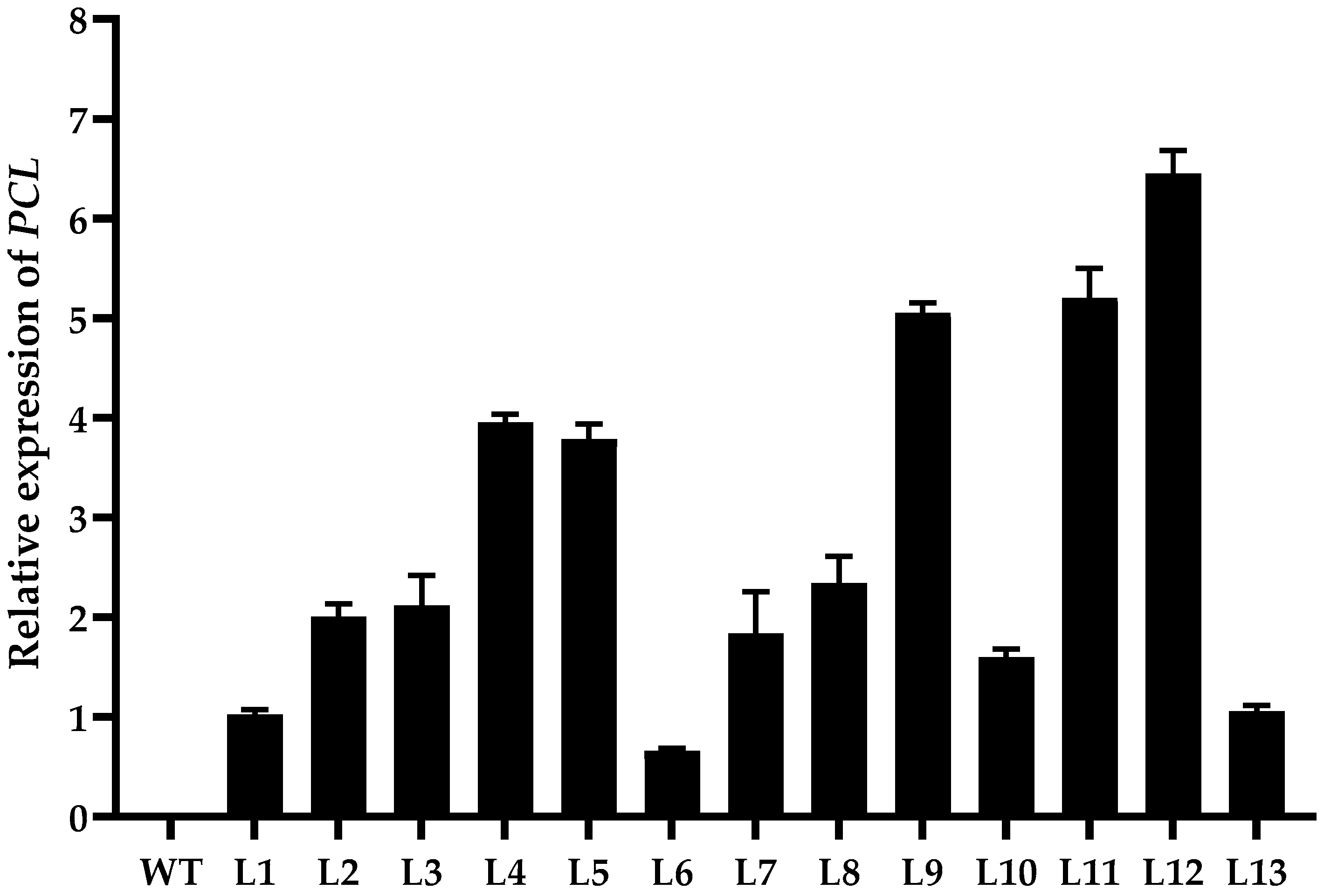
2.1. Transgenic Tobacco with PCL High Expression
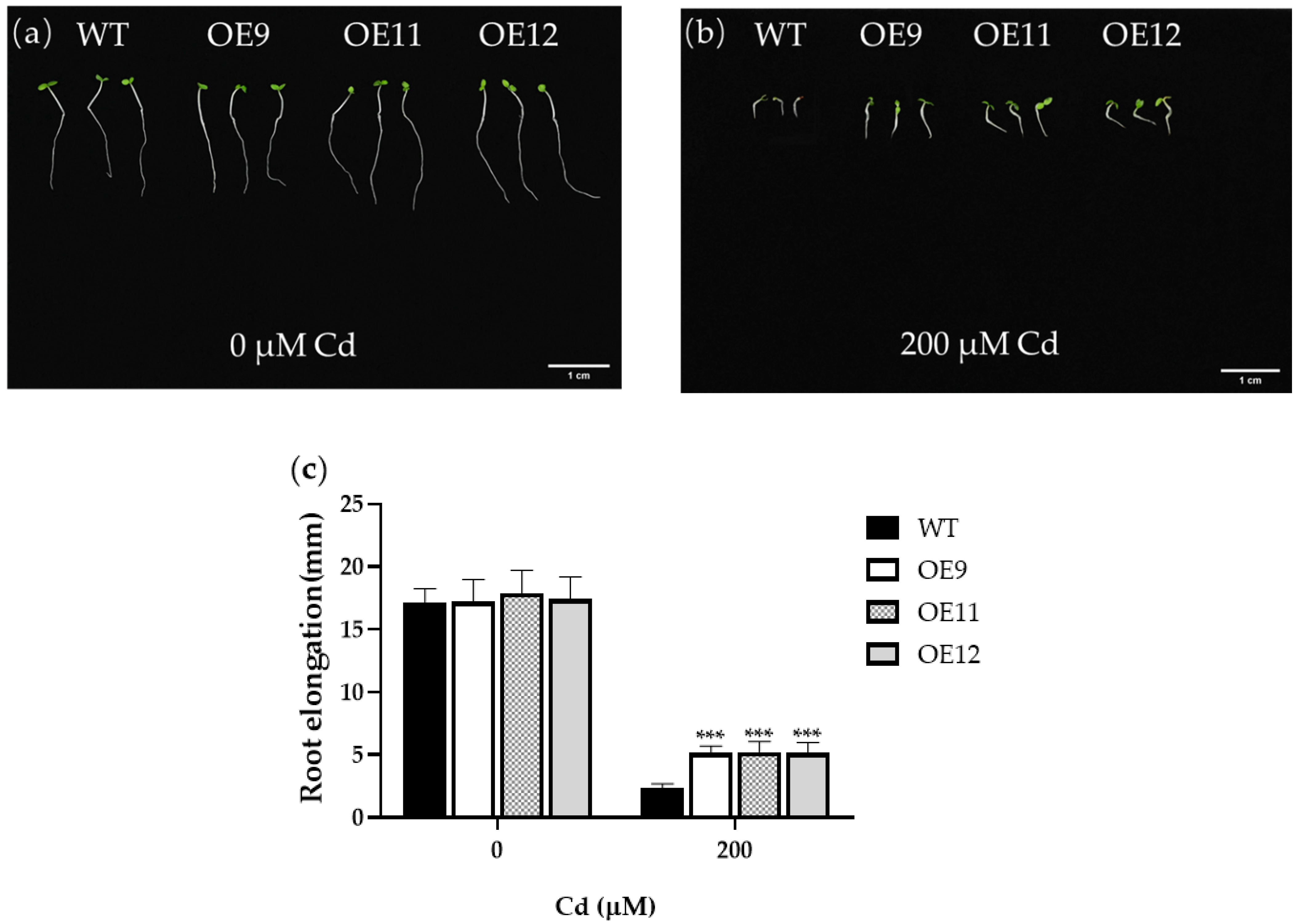
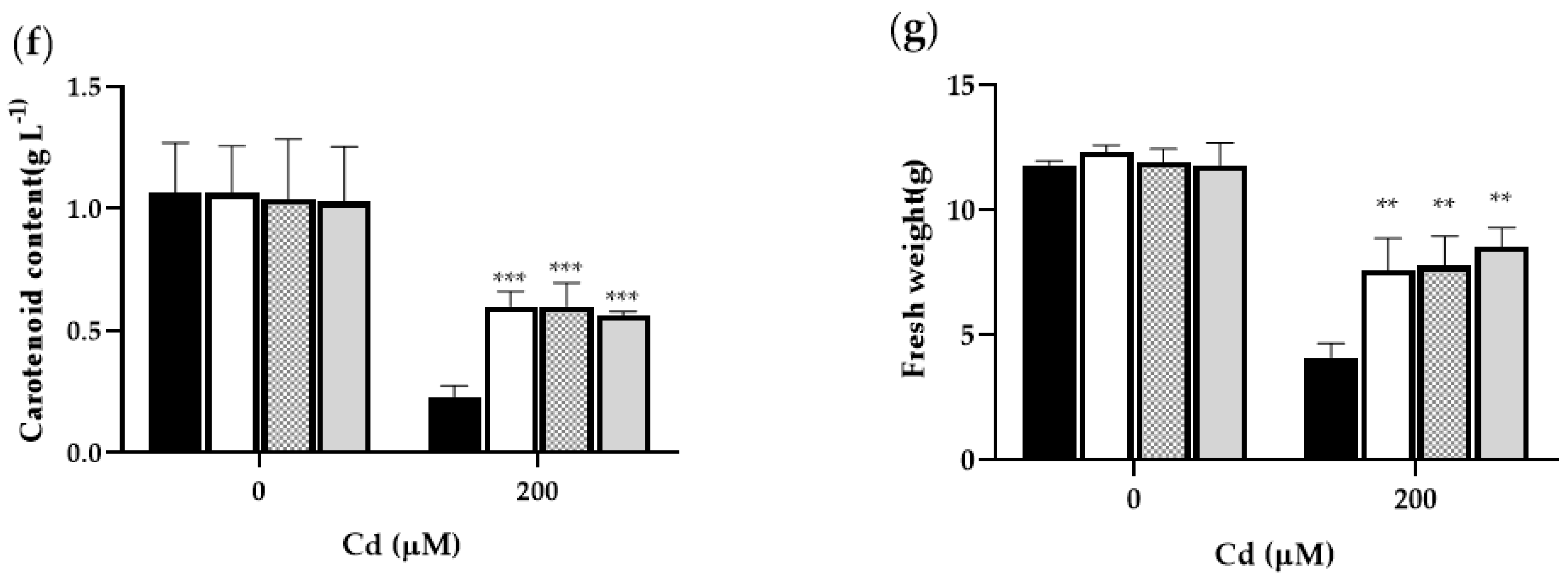
2.2. PCL Gene Improved the Endurance of Tobacco to Cd
2.3. PCL Gene Increases Cd Accumulation in Tobacco
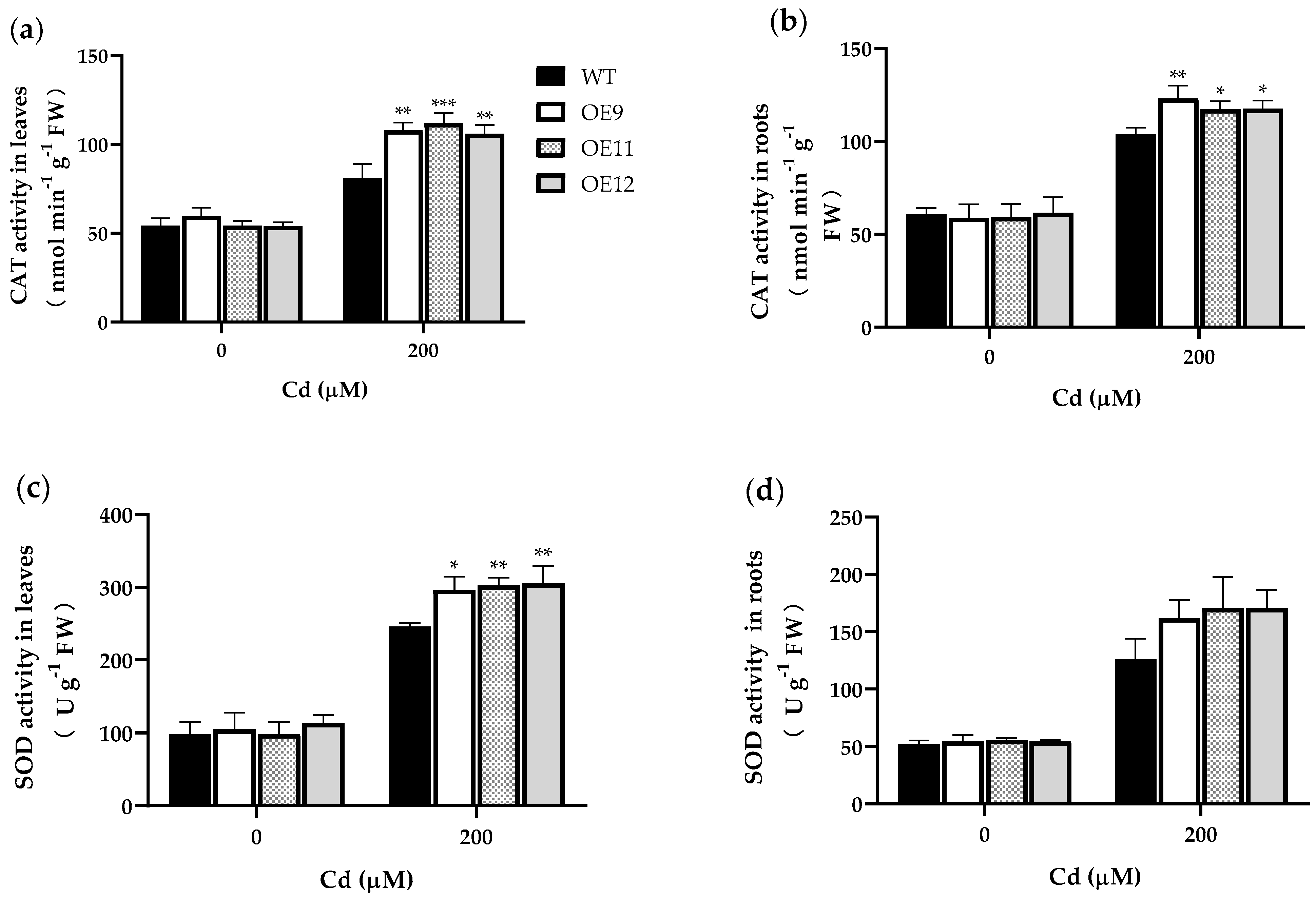
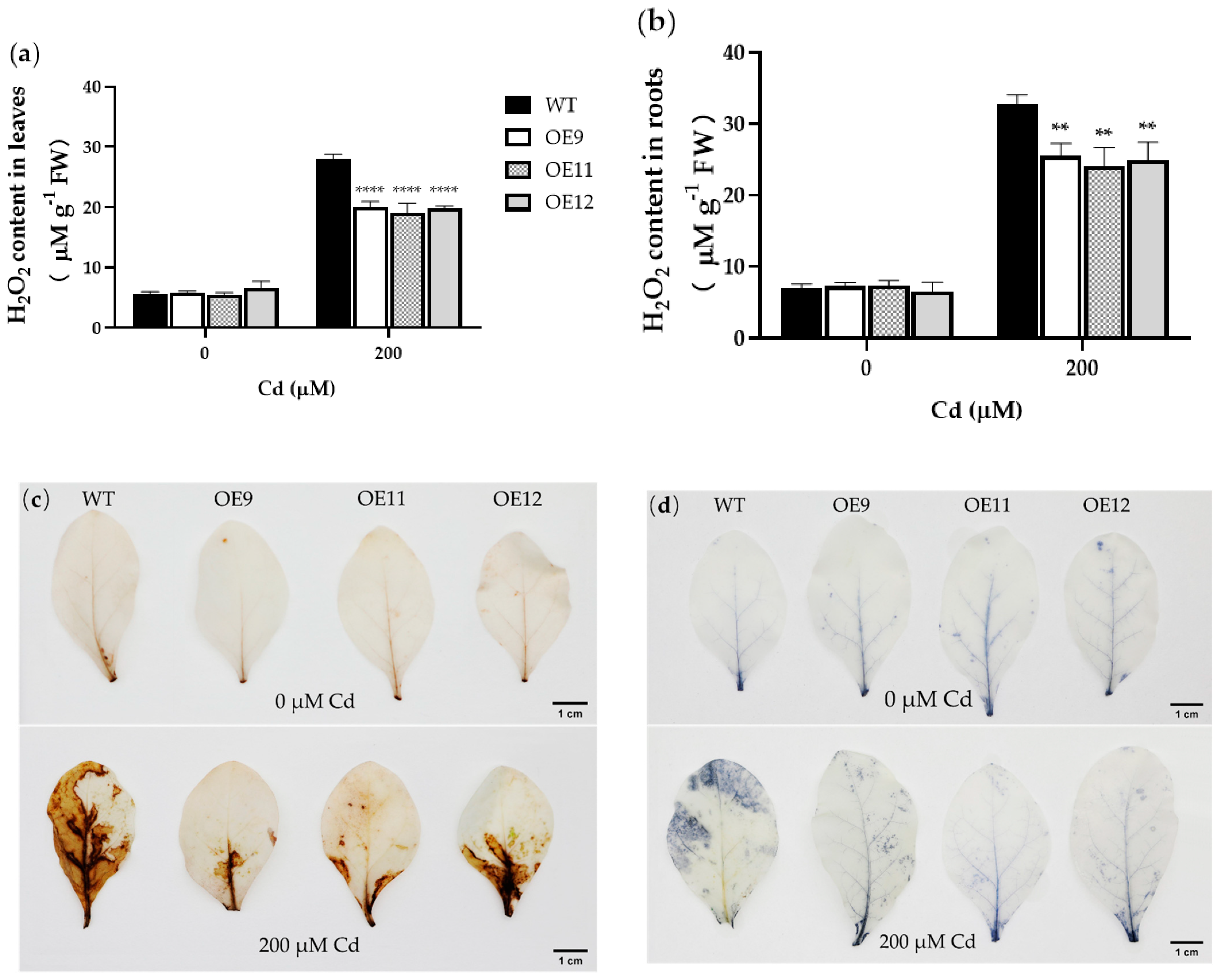
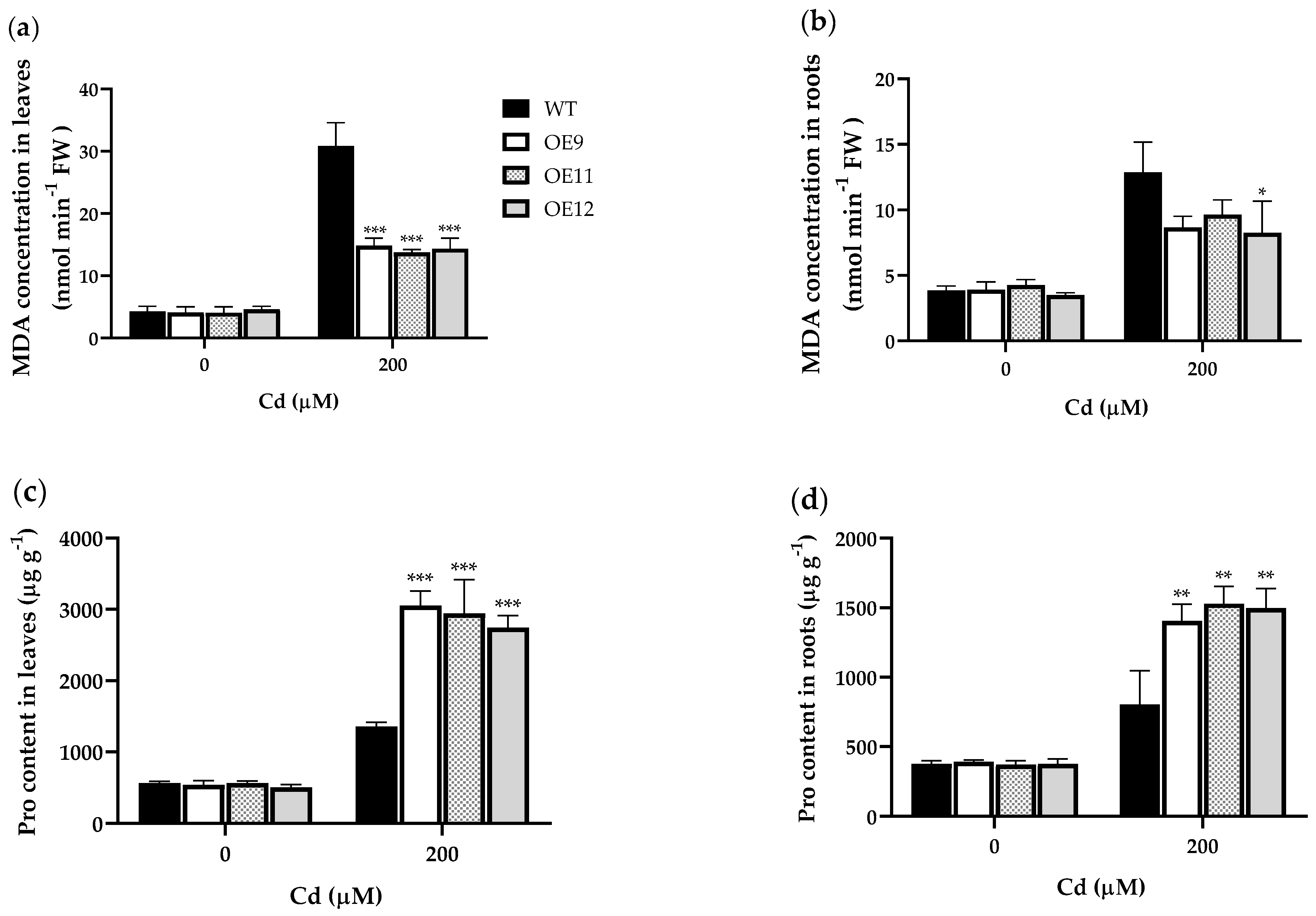
2.4. PCL Increases Antioxidant Enzyme Activities in Tobacco
2.5. PCL Transgenic Tobacco Was More Tolerant to Osmotic and Oxidative Stress
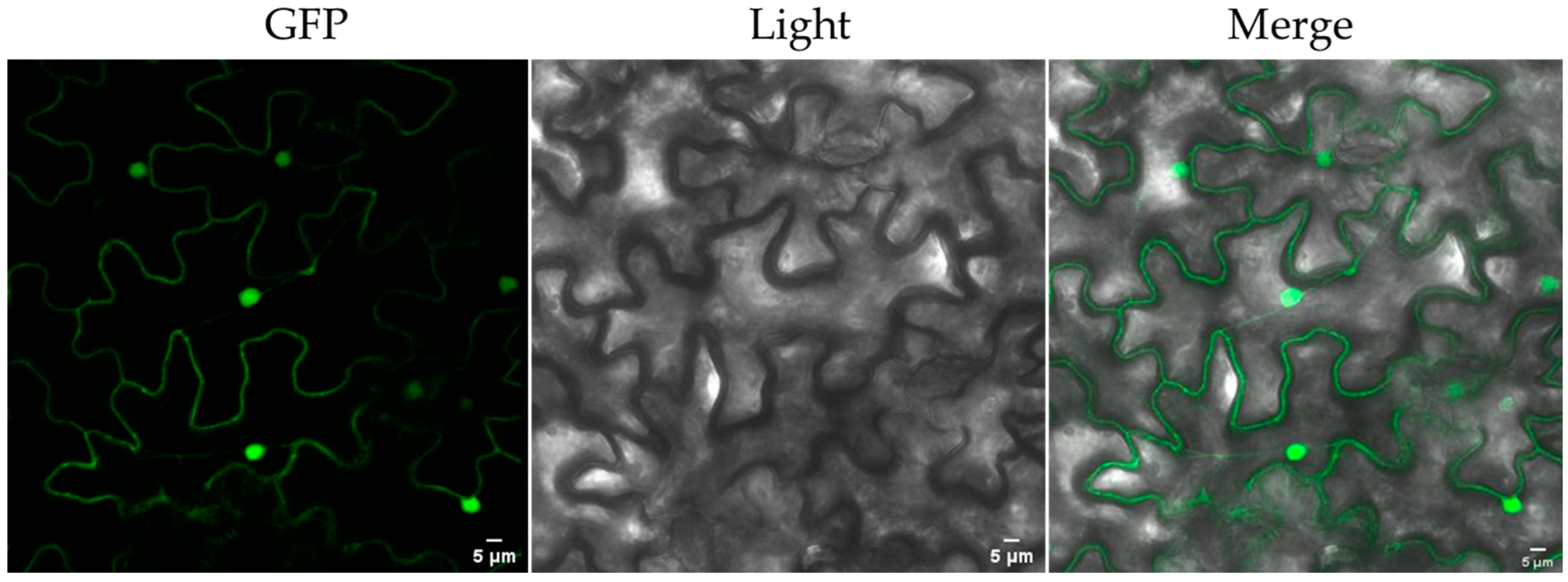
2.6. Subcellular Localization of PCL
3. Discussion
4. Materials and Methods
4.1. Experimental Materials and Reagents
4.2. Generation of Tobacco Plants Expressing PCL
4.3. Growth Conditions and Treatments
4.4. Determination of Cadmium in Plants
4.5. Determination of Chlorophyll Content in Plants
4.6. Measurement of Antioxidant Enzyme Activity
4.7. Determination of MDA and Pro Content in Plants
4.8. Determination of H2O2 Content
4.9. DAB and NBT Staining
4.10. Subcellular Localization of PCL Peptide
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [PubMed]
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Health risk assessment of dietary cadmium intake: Do current guidelines indicate how much is safe? Environ. Health Perspect. 2017, 125, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Ihedioha, J.N.; Okoye, C.O. Dietary intake and health risk assessment of lead and cadmium via consumption of cow meat for an urban population in Enugu State, Nigeria. Ecotoxicol. Environ. Saf. 2013, 93, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Zheng, W.; Wang, W.; Dai, F.; Zhang, Z.; Yuan, Y.; Wang, Q. Health risk assessment of Chinese consumers to cadmium via dietary intake. J. Trace Elem. Med. Biol. 2017, 44, 137–145. [Google Scholar] [CrossRef]
- Clemens, S.; Ma, J.F. Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu. Rev. Plant Biol. 2016, 67, 489–512. [Google Scholar] [CrossRef] [PubMed]
- Huybrechts, M.; Cuypers, A.; Deckers, J.; Iven, V.; Vandionant, S.; Jozefczak, M.; Hendrix, S. Cadmium and plant development: An agony from seed to seed. Int. J. Mol. Sci. 2019, 20, 3971. [Google Scholar] [CrossRef]
- Mithofer, A.; Schulze, B.; Boland, W. Biotic and heavy metal stress response in plants: Evidence for common signals. FEBS Lett. 2004, 566, 1–5. [Google Scholar] [CrossRef]
- Kupper, H.; Parameswaran, A.; Leitenmaier, B.; Trtilek, M.; Setlik, I. Cadmium-induced inhibition of photosynthesis and long-term acclimation to cadmium stress in the hyperaccumulator Thlaspi caerulescens. New Phytol. 2007, 175, 655–674. [Google Scholar] [CrossRef]
- Usman, K.; Al-Ghouti, M.A.; Abu-Dieyeh, M.H. The assessment of cadmium, chromium, copper, and nickel tolerance and bioaccumulation by shrub plant Tetraena qataranse. Sci. Rep. 2019, 9, 5658–5668. [Google Scholar] [CrossRef]
- Finger-Teixeira, A.; Ishii-Iwamoto, E.L.; Marchiosi, R.; Coelho, E.M.P.; Constantin, R.P.; Dos Santos, W.D.; Soares, A.R.; Ferrarese-Filho, O. Cadmium uncouples mitochondrial oxidative phosphorylation and induces oxidative cellular stress in soybean roots. Environ. Sci. Pollut. Res. Int. 2021, 28, 67711–67723. [Google Scholar] [CrossRef]
- Grill, E.; Winnacker, E.L.; Zenk, M.H. Phytochelatins: The principal heavy-metal complexing peptides of higher plants. Science 1985, 230, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Grill, E.; Loffler, S.; Winnacker, E.L.; Zenk, M.H. Phytochelatins, the heavy-metal-binding peptides of plants, are synthesized from glutathione by a specific gamma-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase). Proc. Natl. Acad. Sci. USA 1989, 86, 6838–6842. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Rai, J.P. Phytochelatins: Peptides involved in heavy metal detoxification. Appl. Biochem. Biotechnol. 2010, 160, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Vogeli-Lange, R.; Wagner, G.J. Subcellular localization of cadmium and cadmium-binding peptides in tobacco leaves: Implication of a transport function for cadmium-binding peptides. Plant Physiol. 1990, 92, 1086–1093. [Google Scholar] [CrossRef]
- Zhu, S.; Shi, W.; Jie, Y. Overexpression of BnPCS1, a novel phytochelatin synthase gene from ramie (Boehmeria nivea), enhanced Cd tolerance, accumulation, and translocation in Arabidopsis thaliana. Front. Plant Sci. 2021, 12, 639189. [Google Scholar] [CrossRef]
- Fan, W.; Guo, Q.; Liu, C.; Liu, X.; Zhang, M.; Long, D.; Xiang, Z.; Zhao, A. Two mulberry phytochelatin synthase genes confer zinc/cadmium tolerance and accumulation in transgenic Arabidopsis and tobacco. Gene 2018, 645, 95–104. [Google Scholar] [CrossRef]
- Hall, J.L. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef]
- Li, Y.; Dhankher, O.; Carreira, L.; Lee, D.; Chen, A.; Schroeder, J.; Balish, R.; Meagher, R. Overexpression of phytochelatin synthase in Arabidopsis leads to enhanced arsenic tolerance and cadmium hypersensitivity. Plant Cell Physiol. 2004, 45, 1787–1797. [Google Scholar] [CrossRef]
- Wojas, S.; Clemens, S.; Hennig, J.; Sklodowska, A.; Kopera, E.; Schat, H.; Bal, W.; Antosiewicz, D. Overexpression of phytochelatin synthase in tobacco: Distinctive effects of AtPCS1 and CePCS genes on plant response to cadmium. J. Exp. Bot. 2008, 59, 2205–2219. [Google Scholar] [CrossRef]
- Khan, M.N.; Mobin, M.; Abbas, Z.K.; AlMutairi, K.A.; Siddiqui, Z.H. Role of nanomaterials in plants under challenging environments. Plant Physiol. Biochem. 2017, 110, 194–209. [Google Scholar] [CrossRef]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Alam, P.; Balawi, T.; Altalayan, F.; Ahanger, M.; Ashraf, M. Sodium nitroprusside (SNP) improves tolerance to arsenic (As) toxicity in Vicia faba through the modifications of biochemical attributes, antioxidants, ascorbate-glutathione cycle and glyoxalase cycle. Chemosphere 2020, 244, 125480. [Google Scholar] [CrossRef] [PubMed]
- Wawrzynski, A.; Kopera, E.; Wawrzynska, A.; Kaminska, J.; Bal, W.; Sirko, A. Effects of simultaneous expression of heterologous genes involved in phytochelatin biosynthesis on thiol content and cadmium accumulation in tobacco plants. J. Exp. Bot. 2006, 57, 2173–2182. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, M.; Dresler, S.; Plak, A.; Tukiendorf, A. Naturally evolved enhanced Cd tolerance of Dianthus carthusianorum L. is not related to accumulation of thiol peptides and organic acids. Environ. Sci. Pollut. Res. Int. 2015, 22, 7906–7917. [Google Scholar] [CrossRef]
- Lee, S.; Moon, J.S.; Ko, T.S.; Petros, D.; Goldsbrough, P.B.; Korban, S.S. Overexpression of Arabidopsis phytochelatin synthase paradoxically leads to hypersensitivity to cadmium stress. Plant Physiol. 2003, 131, 656–663. [Google Scholar] [CrossRef]
- Guo, J.; Dai, X.; Xu, W.; Ma, M. Overexpressing GSH1 and AsPCS1 simultaneously increases the tolerance and accumulation of cadmium and arsenic in Arabidopsis thaliana. Chemosphere 2008, 72, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Vatamaniuk, O.K.; Mari, S.; Lu, Y.P.; Rea, P.A. AtPCS1, a phytochelatin synthase from Arabidopsis: Isolation and in vitro reconstitution. Proc. Natl. Acad. Sci. USA 1999, 96, 7110–7115. [Google Scholar] [CrossRef]
- Lee, B.D.; Hwang, S. Tobacco phytochelatin synthase (NtPCS1) plays important roles in cadmium and arsenic tolerance and in early plant development in tobacco. Plant Biotechnol. Rep. 2015, 9, 107–114. [Google Scholar] [CrossRef]
- Shukla, D.; Kesari, R.; Mishra, S.; Dwivedi, S.; Tripathi, R.D.; Nath, P.; Trivedi, P.K. Expression of phytochelatin synthase from aquatic macrophyte Ceratophyllum demersum L. enhances cadmium and arsenic accumulation in tobacco. Plant Cell Rep. 2012, 31, 1687–1699. [Google Scholar] [CrossRef]
- Blum, R.; Meyer, K.C.; Wunschmann, J.; Lendzian, K.J.; Grill, E. Cytosolic action of phytochelatin synthase. Plant Physiol. 2010, 153, 159–169. [Google Scholar] [CrossRef]
- Zhang, X.; Rui, H.; Zhang, F.; Hu, Z.; Xia, Y.; Shen, Z. Overexpression of a functional Vicia sativa PCS1 homolog increases cadmium tolerance and phytochelatins synthesis in Arabidopsis. Front. Plant Sci. 2018, 9, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Peng, J.; Zhang, G.; Yi, H.; Fu, Y.; Gong, J. Regulation of the phytochelatin synthase gene AtPCS2 in Arabidopsis thaliana. Sci. Sin. Vitae 2013, 43, 1112. [Google Scholar]
- Lazo, G.R.; Stein, P.A.; Ludwig, R.A. A DNA transformation-competent Arabidopsis genomic library in Agrobacterium. Biotechnology 1991, 9, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Svab, Z.; Maliga, P. High-frequency plastid transformation in tobacco by selection for a chimeric aadA gene. Proc. Natl. Acad. Sci. USA 1993, 90, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Peng, Y.B.; Pelleschi-Travier, S.; Fan, Y.; Lu, Y.F.; Lu, Y.M.; Gao, X.P.; Shen, Y.Y.; Delrot, S.; Zhang, D.P. Evidence for apoplasmic phloem unloading in developing apple fruit. Plant Physiol. 2004, 135, 574–586. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, H.; Zou, C.; Li, Y.; Chen, Y.; Wang, Z.; Jiang, Y.; Liu, A.; Zhao, P.; Wang, M.; et al. Combined inoculation with multiple arbuscular mycorrhizal fungi improves growth, nutrient uptake and photosynthesis in cucumber seedlings. Front. Microbiol. 2017, 8, 2516–2526. [Google Scholar] [CrossRef]
- Biehler, E.; Mayer, F.; Hoffmann, L.; Krause, E.; Bohn, T. Comparison of 3 spectrophotometric methods for carotenoid determination in frequently consumed fruits and vegetables. J. Food Sci. 2010, 75, 55–61. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. Biochem. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Mo, Y.; Wang, Y.; Yang, R.; Zheng, J.; Liu, C.; Li, H.; Ma, J.; Zhang, Y.; Wei, C.; Zhang, X. Regulation of plant growth, photosynthesis, antioxidation and osmosis by an Arbuscular mycorrhizal fungus in watermelon seedlings under well-watered and drought conditions. Front. Plant Sci. 2016, 7, 644–658. [Google Scholar] [CrossRef]
- Bai, T.; Li, C.; Ma, F.; Feng, F.; Shu, H.J.P. Responses of growth and antioxidant system to root-zone hypoxia stress in two Malus species. Plant Soil 2010, 327, 95–105. [Google Scholar] [CrossRef]
- Cao, K.; Yu, J.; Xu, D.; Ai, K.; Bao, E.; Zou, Z. Exposure to lower red to far-red light ratios improve tomato tolerance to salt stress. BMC Plant Biol. 2018, 18, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wu, Q.; Sprague, S.A.; Park, J.; Oh, M.; Rajashekar, C.B.; Koiwa, H.; Nakata, P.A.; Cheng, N.; Hirschi, K.D.; et al. Tomato expressing Arabidopsis glutaredoxin gene AtGRXS17 confers tolerance to chilling stress via modulating cold responsive components. Hortic. Res. 2015, 2, 15051–15061. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Li, M.; Liu, B.; Qin, Y.; Li, J.; Pan, Y.; Zhang, X. Effects of Phytochelatin-like Gene on the Resistance and Enrichment of Cd2+ in Tobacco. Int. J. Mol. Sci. 2022, 23, 16167. https://doi.org/10.3390/ijms232416167
Zheng Y, Li M, Liu B, Qin Y, Li J, Pan Y, Zhang X. Effects of Phytochelatin-like Gene on the Resistance and Enrichment of Cd2+ in Tobacco. International Journal of Molecular Sciences. 2022; 23(24):16167. https://doi.org/10.3390/ijms232416167
Chicago/Turabian StyleZheng, Yilin, Mengyu Li, Binman Liu, Yafei Qin, Jinhua Li, Yu Pan, and Xingguo Zhang. 2022. "Effects of Phytochelatin-like Gene on the Resistance and Enrichment of Cd2+ in Tobacco" International Journal of Molecular Sciences 23, no. 24: 16167. https://doi.org/10.3390/ijms232416167
APA StyleZheng, Y., Li, M., Liu, B., Qin, Y., Li, J., Pan, Y., & Zhang, X. (2022). Effects of Phytochelatin-like Gene on the Resistance and Enrichment of Cd2+ in Tobacco. International Journal of Molecular Sciences, 23(24), 16167. https://doi.org/10.3390/ijms232416167






