Abstract
Remyelination therapies, which are currently under development, have a great potential to delay, prevent or even reverse disability in multiple sclerosis patients. Several models are available to study the effectiveness of novel compounds in vivo, among which is the cuprizone model. This model is characterized by toxin-induced demyelination, followed by endogenous remyelination after cessation of the intoxication. Due to its high reproducibility and ease of use, this model enjoys high popularity among various research and industrial groups. In this review article, we will summarize recent findings using this model and discuss the potential of some of the identified compounds to promote remyelination in multiple sclerosis patients.
1. Introduction
The nervous system is simultaneously complicated and fascinating. No other scientific field has recorded more advances recorded in the last few decades than neuroscience. We owe these great advances to the new technologies that have been recently developed, enabling us to visualize and manipulate central nervous system (CNS) cells, as well as the development of appropriate in vivo [1] and in vitro [2] models to study CNS health and disease at the molecular level. Using these models, we can now study both tissue destruction and tissue repair.
The cells of the nervous system can be divided into nerve cells (neurons) and glial cells. Neurons are responsible for signal transmission by generating action potentials and passing them on to communicating neurons. The place where nerve cells communicate with each other is called the synapse. It is textbook knowledge that the central and peripheral nervous system consist not only of neurons, but also of other cells that are morphologically and functionally different from neurons. The co-discoverer of these non-neuronal cells in the mid-19th century, Rudolf Virchow, suspected a support and holding function, therefore naming them glial cells, derived from the Greek word glia for “glue”. Utilizing different staining methods by Santiago Ramón y Cajal, Pío del Río Hortega, and Camillo Golgi, glial cells were further subclassified at the end of the 19th century. The first differentiation of glial cells was made based on their size. Accordingly, microglia were distinguished from macroglia. The central macroglial cells include astrocytes, oligodendrocytes, and ependymal cells. This subclassification was wise: As is known today, microglial and macroglial cells have nothing to do with each other in terms of evolutionary history. Macroglial cells, i.e., oligodendrocytes, astrocytes, and ependymal cells, all originate from the neuroectoderm. In contrast, microglial cells represent immigrated blood cells; therefore, they originate from the mesoderm. Although the number of neurons in the human brain exceeds our imagination (about 100 billion), the number of glial cells exceeds that of neurons by a multiple. This review article will focus on the myelin-producing cells of the CNS, the oligodendrocytes, and their precursors.
The main function of oligodendrocytes and Schwann cells is the synthesis and maintenance of the myelin sheath, which is a lipid-rich biomembrane that spirals around the axons of most vertebrate nerve cells electrically insulating them. Compared to other biomembranes, myelin, which was discovered in 1854 by the pathologist Rudolf Virchow (1821–1902), has an exceptionally high lipid content (approximately 70% of dry weight) and a relatively low protein content (30%), with proteolipid protein (PLP) and myelin basic protein (MBP) being the most abundant ones [3]. Since myelin appears macroscopically white, highly myelinated regions in the CNS are also referred to as “white matter” in contrast to the less myelinated “gray matter” areas. Myelin sheaths along the axons are regularly interrupted by the nodes of Ranvier. Action potentials arise only at the nodes of Ranvier but not in the myelinated areas of the axon (id est, the internodes). This configuration allows for saltatory conduction, which is significantly faster than the continuous action potential propagation of non-myelinated fibers. In addition, this type of conduction saves energy, since an action potential only has to be built up at the location of the nodes and not continuously along an axon.
2. Remyelination Biology
There are various CNS disorders that are characterized by either dysmyelination or the destruction of a previously intact myelin sheath. Dysmyelination refers to a malformed and defective myelin sheath as opposed to the destruction of previously normal myelin that is seen in demyelinating conditions. In addition to the various forms of leukodystrophies, other genetically determined disorders, such as infantile amaurotic idiocy, hematosidosis, Niemann–Pick’s disease, and several of the aminoacidopathies are examples of dysmyelinating disorders [4]. The most frequent neurological disease with the central hallmark of myelin pathology is, however, multiple sclerosis (MS). In contrast to the above mentioned disorders, MS is a demyelinating condition in which intact myelin sheaths are destroyed by peripheral and central inflammatory cells. Pathological hallmarks, besides demyelination, are a focal breakdown of the blood–brain barrier (BBB), peripheral immune cell recruitment, neuronal and axonal damage, as well as microglia and astrocyte activation. During the initial phase of the disease, inflammation, mediated via the adaptive immune system, clinically results in specific behavioral deficits from which the affected patients can recover either entirely or partially. This disease phase is named ‘relapsing-remitting MS (RRMS)’. As the disease progresses, the frequency of new clinically detectable relapses decreases. Instead, there is a progressive accumulation of behavioral deficits from which the patients usually do not recover. This secondary disease phase is named ‘secondary progressive MS (SPMS)‘. Pathologically, SPMS is driven by a diffuse and chronic inflammatory process inside and around the brain and spinal cord parenchyma [5,6]. Eventually, patients initially present with a progressive disease course, which is named ‘primary progressive MS (PPMS)’.
Although MS is the most frequent demyelinating disease, white matter degeneration, oligodendrocyte dysfunction, or myelin destruction have been observed in other CNS disorders as well, such as Alzheimer’s disease [7], stroke [8,9], spinal cord injury [10], schizophrenia [11] or amyotrophic lateral sclerosis [12]. Consequently, cells of the oligodendrocyte lineage have been shown to exert important roles beyond those related to myelination, including regulation of angiogenesis in the normal postnatal brain [13], or antigen presentation and phagocytosis in mouse models of MS [14,15,16]. Remyelination is a multistep, complicated process that is very effective in young adults but loses effectiveness as one ages [17,18].
Since myelin debris is a potent inhibitor of remyelination, clearance of myelin debris by microglia and/or recruited monocytes in the first step is pivotal [19,20,21,22]. In a second step, due to the expanding (neuro-) inflammatory milieu, oligodendrocyte progenitor cells (OPCs) are activated, eventually proliferate [23], and migrate into the demyelinated area [24]. In the next series of molecular events, the OPCs differentiate into premyelinating oligodendrocytes, which change their morphology from a bipolar to a highly-branched, multipolar cell type. Finally, the premyelinating oligodendrocytes extend a process to the denuded axon that generates a new, lipid-rich, multi-lamellar myelin sheath. Oligodendrocyte proliferation and differentiation represents a delicate and complicated balance, and a number of factors have been identified that regulate these cellular events, including the Sonic Hedgehog signaling pathways [25], the Wnt (Wingless-type MMTV integration site family) signaling pathway [26], fibroblast growth factor 2 [27], different GPR family members [28], the transcription factor SOX2 [29], AKT (AKT serine/threonine kinase 1) [30] or BDNF (brain-derived neurotrophic factor) [31].
Several observations suggest that remyelination ameliorates functional deficits: Firstly, following experimental demyelination of the optic nerve, recovery in visual conductance is tightly correlated with MBP re-expression [32]. Secondly, accelerated remyelination via muscarinic cholinergic receptor knockdown prevents axonal loss in the experimental autoimmune encephalomyelitis (EAE) model [33]. Thirdly, the inhibition of remyelination, that is, experimentally induced by X-irradiation, results in a significant increase in the extent of axonal degeneration and loss compared to non-irradiated mice [34]. There appear to be multiple sources of remyelinating oligodendrocytes, including parenchymal OPC distributed ubiquitously throughout the gray and white matter, neural stem cells (NSCs) located in the subventricular zone(s) [35], and Schwann cells migrating from the periphery into the CNS [36].
3. Remyelination In Vivo Models
To study the complex physiology of remyelination and the factors involved in its failure, animal models are unavoidable. Although various species have been applied in the past in the context of myelin research, including dogs [37], cats [38], and zebrafishes [39], small rodents, especially mice, are the most frequently used. To study myelin degeneration and repair, toxin models are enjoying great popularity. In principle, toxin-mediated demyelination with subsequent remyelination can be induced using either focal injection of lysolecithin (also called lysophosphatidylcholine (LPC)) or ethidium bromide [40] into myelin-rich white matter tracts, or the systemic administration of the copper-chelator cuprizone. All three experimental approaches show robust endogenous remyelination after the demyelinating insult [41,42,43]. In this article, we will focus on the cuprizone model. First, we will describe the characteristics and histopathological changes of the model and then present an overview of factors that were identified to regulate myelin repair.
Cuprizone, chemically known as bis-(cyclohexanone) oxaldehydrozone, is a synthetic chelating compound initially used to detect trace copper [44,45]. This compound became of interest in biomedical research when it was discovered to exert toxic effects in the CNS of laboratory mice [46]. The intoxication of young adult mice with cuprizone, mixed into standard rodent chow in a concentration of 0.2–0.5% (w/w), induces, within some days, oligodendrocyte stress, leading to oligodendrocyte degeneration [47]. This presumably primary oligodendrocyte insult leads to the activation of astrocytes and microglia, the latter phagocytosing the degenerating myelin sheaths. This results in the acute demyelination of distinct white and gray matter brain regions [48]. Since cuprizone chelates copper and copper is an essential trace element for a number of metalloenzymes involved in cellular respiration, it is widely presumed that CNS damage due to the cuprizone intoxication is a result of copper dyshomeostasis and, in consequence, mitochondrial dysfunction. In contrast to this theory, results of a recent study suggest that cuprizone’s toxicity is not due to copper depletion but instead due to a gain of toxicity induced by an unusual cuprizone:copper complex [44]. Whatever the precise underlying mechanism of the cuprizone-induced toxicity is, it triggers a highly reproducible demyelination of distinct white and gray matter regions. Of note, although demyelination is widespread, it occurs in a region-specific manner. For example, at the level of the rostral hippocampus, the medial part of the corpus callosum is almost entirely demyelinated. In contrast, neighboring white matter tracts such as the cingulum, the fornix, and the hippocampal fimbria are less severely affected. In addition to the white matter tracts, various gray matter areas are affected as well, such as the hippocampus [49], thalamus [50] or neocortex [51].
In the cuprizone model, demyelination is complete after an intoxication period of around 5 weeks (i.e., acute demyelination). In case the animals are provided standard chow, spontaneous, endogenous remyelination occurs, which is complete in a matter of weeks [52]. In contrast, prolonged cuprizone intoxication for 12–13 weeks induces chronic lesions which show a limited endogenous remyelination capacity [42]. Early remyelination is often monitored after a remyelination period of 2 weeks [52]. While the expression of different astrocyte marker proteins, among which are glial fibrillary acidic protein (GFAP), aldehyde dehydrogenase 1 family member L1 (ALDH1L1) or Vimentin, is highly increased in the demyelinated areas, expression levels decrease again after the cessation of cuprizone intoxication. Nevertheless, expression values remain elevated compared to those of control mice, indicating ongoing astrocyte activation during remyelination [53]. Consequently, some studies demonstrated that the modulation of astrocytes impacts myelin repair [54].
Conceptually, there are three sources of remyelinating cells in the cuprizone model. Firstly, the neural stem cells (NSCs) [35,55,56], which reside in the subventricular zone (SVZ) but can migrate into the corpus callosum, striatum, and fimbria. There, these cells can differentiate into NG2-positive non-myelinating and mature myelinating oligodendrocytes. Notably, the number of NSC-derived oligodendrocytes in vivo increased fourfold after a demyelinating lesion in the corpus callosum. This indicates that SVZ cells participate in myelin repair in the adult brain [55]. Of note, in the cuprizone model, SVZ-NSCs are recruited to the white matter tract corpus callosum during the remyelination phase and are capable of forming new oligodendrocytes. When these SVZ-derived NSCs were ablated, animals displayed reduced oligodendrocyte numbers within the lesioned corpus callosum [57]. The next cell type which can give rise to new myelinating oligodendrocytes are the OPCs, also known as NG2 glia. These OPCs are distributed ubiquitously throughout the CNS white and gray matter. In response to a demyelinating insult, OPCs proliferate rapidly and differentiate into re-myelinating oligodendrocytes, contributing to myelin repair [35,58]. Furthermore, the results of some studies suggest that adult oligodendrocytes, which survived the demyelinating insult, can participate in myelin repair [38,59]. No reports are available so far suggesting that Schwann cells participate in the remyelination process in the cuprizone model as well.
Various protocols have been applied to study the effectiveness of novel compounds to promote remyelination in the cuprizone model. After a 5-week cuprizone intoxication period, the corpus callosum is highly populated with OPCs. In case compound treatment is initiated at the time-point when animals are switched back to normal chow, one can easily study its effects on OPC differentiation and remyelination. However, potential OPC-generating effects might be missed if such a protocol is applied. Alternatively, one might initiate the compound treatment at the beginning of week 4, when OPC activation and proliferation starts (compare Figure 1). One should also be aware that after acute cuprizone-induced demyelination, endogenous remyelination occurs spontaneously. This does not allow the study of pro-myelinating effects in the non-supportive environment. Alternatively, one might either use aged animals [60,61] or apply prolonged, chronic cuprizone intoxication [62], where remyelination is significantly delayed.
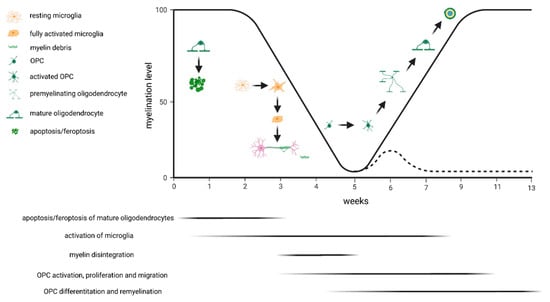
Figure 1.
Schematic presentation of the cuprizone-induced pathological changes. The black line indicates the percentage of myelinated fibers in the corpus callosum above the fornix during acute exposure to cuprizone. The dashed line indicates exposure to cuprizone for 13 weeks (i.e., chronic demyelination). The major event at week 1 is apoptosis/feroptosis of the mature oligodendrocytes. In a second step, this triggers the activation of microglia and astrocytes, both phagocytosing the degenerating myelin sheaths. Oligodendrocyte progenitor cells (OPCs) become activated and start to proliferate. If animals are provided normal chow after acute cuprizone-induced demyelination, endogenous remyelination occurs (solid black line) by OPC differentiation and remyelination. If, however, the cuprizone intoxication is continued, remyelination fails (dashed line).
4. Remyelination—A Clinical Perspective
In MS patients, independent of clinically detectable relapses or inflammation visualized by different imaging modalities, there is a continuous accumulation of clinical disability, a phenomenon which was recently termed by Bruce Cree and colleagues [63] “silent progression”. Since the observed disability progression was associated with accelerated brain atrophy, it is likely directly related to the accumulation of irreversible axonal and neuronal damage. There are several studies suggesting that the promotion of remyelination is one option to prevent this neurodegeneration. In the EAE model, an elegant study has shown that accelerated remyelination prevents axonal loss and improves functional recovery [33]. In post mortem MS tissues, it has been demonstrated that the density of acutely damaged axons is high during active and chronic demyelination but low in remyelinated shadow plaques. These observed patterns of axonal pathology in chronic active EAE were qualitatively and quantitatively similar to those found in MS tissues [64]. Furthermore, it has recently been demonstrated that failed remyelination of the nonhuman primate optic nerve leads to axon degeneration, retinal damage, and visual dysfunction [65]. Finally, the results of a recently published longitudinal combined positron emission tomography (PET)/magnetic resonance imaging (MRI) suggest that intralesional remyelination is associated with the microstructural preservation of surrounding tissues [66].
In principle, two different strategies can be followed to promote remyelination. Firstly, by transplanting cell populations promoting myelin repair, and secondly, by interfering with endogenous pathways orchestrating endogenous remyelination (or its failure). Concerning the first strategy, there is only one completed study so far, the “Neural Stem Cell Transplantation in Multiple Sclerosis Patients (STEMS)”, which has been coordinated by Gianvito Martino and colleagues (ClinicalTrials.gov Identifier: NCT03269071). In this phase 1 trial, human fetal-derived neural stem cells were administered intrathecally into progressive MS patients. While the safety of the approach appears to be good, no results have been presented so far concerning the efficacy of this treatment strategy.
A number of different studies are currently being conducted to investigate the potency of drugs to interfere with endogenous remyelination regulators. The results are, so far, heterogeneous. The randomized, double-blind, placebo-controlled, parallel group, phase 2a trial “CCMR One” that investigated the potency of the retinoid X receptor agonist bexarotene failed, mainly due to safety reasons [67]. All bexarotene-treated participants had at least one adverse event. The SYNERGY trial investigated the potency of opicinumab, a fully humanized anti-LINGO-1 antibody, to promote myelin repair. LINGO-1 expression is upregulated in MS lesions, and blockade using antagonistic antibodies or genetic deletion results in increased axonal myelination, both in vitro and in vivo, with amelioration of the disease in the EAE model [68]. Disappointingly, SYNERGY did not meet the primary endpoint, which was the percentage of participants achieving confirmed disability improvement over 72 weeks [69]. To our knowledge, the development of anti-LINGO1 for remyelination in MS has been stopped.
In contrast to these negative outcomes, the results of the ReBUILD trial suggest that clemastine fumarate might be a potential drug supporting endogenous remyelination in MS patients [70]. In this study, relapsing MS patients with chronic (>6 months) demyelinating optic neuropathy that were treated with clemastine, an anti-histamine and anticholinergic medication, demonstrated improved visual-evoked potential latencies. Of note, the potency of this drug to promote remyelination was discovered in vitro, using micropillar arrays as a high-throughput screening platform for novel therapeutics [2]. Other trials investigating the effectiveness of clemastine as a myelin repair therapy are currently ongoing (ClinicalTrials.gov Identifiers NCT05359653 (ReVIVE), NCT05338450 (RESTORE), NCT05131828 (CCMR Two); NCT05338450 (RESTORE), others).
5. Findings in the Cuprizone Model
Table 1 lists the studies published since 2016 using the cuprizone de-/remyelination model. A number of very interesting observations have been made using this model. As outlined above, different sources of remyelinating oligodendrocytes exist. NSCs are located in the subventricular zone, which is responsible for the lifelong generation of interneuron subtypes and oligodendrocytes. Using a novel in silico screening approach, the FDA-approved anti-inflammatory corticosteroid medrysone was identified as a potential regulator of NSC-driven remyelination [71]. After 9 weeks of cuprizone intoxication, medrysone promoted the recovery of myelin basic protein expression, the repopulation of the corpus callosum with oligodendrocytes, and the reformation of nodes of Ranvier numbers [54]. Subsequent studies showed that inhibition of the GLI family zinc finger 1 (Gli1) [72] is functionally involved in NSC-driven remyelination. Another study investigated pro-myelinating effects of the antipsychotic drugs haloperidol and clozapine [73]. In 1972, myelin and oligodendrocyte abnormalities had already been described on the ultrastructural level in the brains of schizophrenic patients [74], and these findings have been confirmed and expanded by others [75,76,77]. The preservation of oligodendrocytes and/or the restoration of damaged myelin sheaths might, thus, be an attractive therapeutic concept in schizophrenia. Patergnani and colleagues demonstrated, using the cuprizone model, that both antipsychotic drugs reversed cuprizone-induced MBP loss and myelin fragmentation in the cerebellum, paralleled by improved motor-performance and amelioration of catalepsy signs. Not surprisingly, clinical trials addressing this topic are ongoing.

Table 1.
Summary of publications using the cuprizone model to study which factors regulate remyelination. The terms “cuprizone” and “remyelination” were applied using the PubMed database. Manuscripts from 2016–2022 were included. HC = Histochemistry; IHC = Immunohistochemistry; IF = Immunofluorescence; RT-PCR = reverse transcription polymerase chain reaction; WB = western blotting; TEM = transmission electron microscopy; MRI = magnet resonance imaging; FACS = fluorescence aided cell sorting, (sc)RNA-seq = (single cell)RNA-sequencing, RNA-isH = RNA in situ hybridization. The “*” in column “Citation” indicates that a clinical trial/clinical trials are either ongoing or have already been completed (information retrieved from ClinicalTrials.gov in December 2022; search terms used were “multiple sclerosis” and “the respective compounds”; no pathways were included in this analysis).
In the following subchapters we will give a brief insight into recent findings. While it is out of the scope of this review article to discuss all studies conducted so far, we will briefly comment on some compounds and/or strategies identified as potential pro-myelinating agents to be tested in clinical trials. This selection is based on our own expertise working with this model in the past, and on compounds that are currently being evaluated by other groups as potential pro-myelinating agents.
5.1. Anti-LINGO-1 Therapy
A major obstacle for successful axon regeneration in the adult CNS arises from inhibitory molecules in CNS myelin debris, which signal through a common receptor complex of neurons consisting of the ligand-binding Nogo-66 receptor (NgR) and two transmembrane co-receptors, p75 and LINGO-1 (Leucine-rich repeat and Immunoglobin-like domain-containing protein 1) [214]. Activation of this receptor complex with, for example, oligodendrocyte myelin glycoprotein, myelin associated glycoprotein (MAG), or neurite outgrowth inhibitor (Nogo) inhibits axonal regeneration.
In 2005, Mi and colleagues reported that LINGO-1 is also expressed by oligodendrocytes [215]. Reduction in LINGO-1 expression via RNAi lentivirus infection or antagonism via the induced expression of a dominant-negative LINGO-1 variant both promoted the differentiation of cultured oligodendrocytes. In contrast, overexpression of full-length LINGO-1 had the opposite effect and inhibited oligodendrocyte differentiation, indicating that endogenous LINGO-1 expression may inhibit oligodendrocyte differentiation and remyelination. Functional experiments revealed that LINGO-1 antagonism promotes oligodendrocyte differentiation via the upregulation of FYN and, consequently, the downregulation of RhoA-GTP. Developmental myelination was accelerated in LINGO-1 knockout mice, compared to wildtypes. Notably, it has been shown that both, oligodendrocyte-derived and axonal-derived LINGO-1, suppresses oligodendrocyte differentiation [216]. Pro-myelinating activities of LINGO-1 antagonism have subsequently been demonstrated in MOG-induced EAE [68], brain slice cultures, LPC-induced focal demyelination and the cuprizone model [106,215]. After some promising results in the RENEW [217] and SYNERGY trial [69], Biogen announced that it is discontinuing the clinical development of opicinumab, an anti-LINGO1 antibody, based on data from the Phase 2 AFFINITY clinical trial.
5.2. Clemastine
Clemastine was identified in 2014 using so-called micropillar arrays as a high-throughput screening platform for potential remyelinating therapies [2]. This approach’s principle is quantifying OPC-derived versus mature oligodendrocyte-derived membranes wrapping around micropillar arrays of compressed silica. After performing a screen of 1000 bioactive molecules, the authors found clusters of compounds that promoted either the proliferation or differentiation of cultured OPCS, but not both. Among others, the authors identified eight FDA-approved antimuscarinic compounds that significantly enhanced oligodendrocyte differentiation and membrane wrapping, including clemastine. Clemastine is a widely available first-generation anti-histamine with a favorable safety profile. It is used primarily for the symptomatic treatment of allergies and also exhibits antimuscarinic properties. In subsequently performed in vivo experiments using the LPC-model, clemastine treatment enhanced the differentiation of oligodendrocytes and accelerated remyelination. Soon after that initial publication, pro-remyelinating properties of clemastine were also demonstrated in the cuprizone model [218]. In this study, the potency of clemastine was tested to accelerate remyelination after a 6-week cuprizone intoxication period. Clemastine augmented myelin recovery in the corpus callosum, cortex, and hippocampus, as determined by anti-MBP staining intensities, paralleled by higher numbers of CC1+ mature oligodendrocytes. As mentioned above, the results of the ReBUILD trial suggest that clemastine fumarate might be a potential drug supporting endogenous remyelination in MS patients [70]. Of note, results of additional preclinical trials suggest that clemastine might also be beneficial in other neurological disorders, including hypoxic brain injury [219], age-related memory deficits [220], or Alzheimer’s disease [221]. Currently, a phase 3 trial (RESTORE; Clemastine Fumarate as Remyelinating Treatment in Internuclear Ophthalmoparesis and multiple sclerosis; NCT05338450) is being conducted to assess the (long-term) efficacy of clemastine fumarate in a clinical model for MS (i.e., in patients with internuclear ophthalmoparesis and MS). The authors expect the final results of their studies in May 2024.
5.3. GPR17-Receptor Modulators
In a sentinel study, Maria Abbracchio’s lab from the University of Milan in Italy showed that the expression of GPR17, a receptor for uracil nucleotides and cysLTs (e.g., UDP-glucose and LTD(4)), is expressed in neurons and parenchymal OPCs, and that, upon induction of brain injury using an established focal ischemia model, the expression of GPR17 increases in neurons as well as proliferating OPCs. From a functional point of view, the in vitro exposure of isolated OPCs to the GPR17 endogenous ligands UDP-glucose and LTD(4) promoted the expression of myelin basic protein, suggesting pro-myelinating effects [222]. The same group demonstrated later that the in vivo knockdown of GPR17 by an antisense oligonucleotide strategy during experimental spinal cord injury induction ameliorated disease severity [223]. Finding that Gpr17 overexpression inhibited oligodendrocyte differentiation and maturation both in vivo and in vitro [224] paved the way for considering GPR17 antagonism as a potential remyelinating strategy in MS. Several years later, it was subsequently demonstrated that loss of GPR17, either globally or specifically in oligodendrocytes, led to an earlier onset of remyelination after LPC-induced myelin injury in mice. Similarly, pharmacological inhibition of GPR17 with pranlukast promoted remyelination [225,226]. In the cuprizone model, high GPR17 expression positively correlated with the intrinsic remyelination capacity [227]. The fact that neurons also express GPR17 and GPR17 antagonism ameliorates neuronal damage in different models [228,229] lets us speculate that GPR17 antagonism not just promotes remyelination but, at the same time, might exert neuroprotective properties.
5.4. Sphingosine-1-Phosphate Modulators
The sphingosine-1-phosphate receptors (S1PR) system, which consists of five receptor subtypes (from S1PR1 to 5), is involved in various functions, including cell migration, proliferation, and differentiation. S1PR1 receptors are found on the outside of lymphocytes, and their activation triggers lymphocytes to leave lymph nodes and enter the bloodstream, ultimately making their way into the target tissue. Consequently, antagonism of S1PR1 activity blocks the egress of lymphocytes from the lymph nodes and, in consequence, exerts anti-inflammatory activities [230]. In addition, S1PR1 and 5 are expressed by cells of the CNS, as demonstrated by several groups [231,232,233,234]. On a cellular level, S1PR1 is predominately expressed by astrocytes and microglia, whereas cells of the oligodendrocytic lineage are the major cell type expressing S1PR5 in the CNS. Among the available S1P receptor modulators, fingolimod (non-selective, S1PR1-3-4-5), siponimod, ponesimod, and ozanimod have already been approved by regulatory authorities for the treatment of MS [235].
Some reports claim that the modulation of S1PR activities might promote remyelination. Fingolimod, a non-selective S1PR modulator, is a prodrug that has to be activated by phosphorylation via the Sphingosine kinase 2. Consequently, Sphingosine kinase 2 is required for the modulation of lymphocyte traffic by fingolimod [236]. Following cuprizone withdrawal, spontaneous remyelination occurred in wildtype but not in Sphingosine kinase 2-/- mice, and myelin thickness in these mice was found to be reduced with aging [104]. These results suggest that the S1PR-signalling cascade is involved in regulating myelin repair. In line with this assumption, fingolimod showed protective effects in the LPC model [237,238] and EAE [239] but failed to enhance remyelination in the cuprizone model [240,241,242]. No studies have been published to date regarding the pro-remyelinating potency of ozanimod. However, the results of two studies suggest that ozanimod might protect cells of the oligodendrocyte lineage [243,244].
In 2020, the selective S1PR1 and 5 modulator siponimod (trade name Mayzent®) received EU approval for treating adults suffering from SPMS with disease activity demonstrated by clinical relapses or imaging of inflammatory activity. As treatment with siponimod has an overall stabilizing effect regarding clinical and radiological outcome measures [245], it is discussed whether some of these protective effects might be modulated by the induction of remyelination. Using a Xenopus tadpole screening approach, Mannioui and colleagues identified siponimod among the most efficient molecules favoring remyelination [246]. Furthermore, increased remyelination, determined by evaluations of magnetization transfer ratio and T2-weighted MRT imaging, was also observed in the cuprizone model [97]. In EAE, siponimod prevented the degeneration of synapses after intracerebroventricular infusion [247]. In slice cultures, where the CNS and the peripheral immune system are virtually uncoupled, siponimod attenuated lysophosphatidic choline-mediated demyelination [248], whereas in vivo siponimod increased myelin basic protein levels after lysophosphatidic choline-induced focal demyelination [249]. Finally, our group recently showed that siponimod protects mature oligodendrocytes in an S1PR5-dependent manner [250]. Further studies addressing the regenerative properties of siponimod are currently ongoing in our laboratory, and the results will hopefully be published soon.
5.5. Sex Hormones
The observation that males are less likely to develop MS and often have a more severe disease course than females, and the phenomenon that the relapse rate in female MS patients significantly decreases during pregnancy [251], lead to several projects investigating the potency of sex steroids, particularly estrogens, progesterone and testosterone, to ameliorate the MS disease course. In 2013, Hussain and colleagues demonstrated that in castrated male and female mice, testosterone promoted remyelination after chronic cuprizone intoxication. Testosterone also promoted remyelination of cerebellar slice cultures after LPC-induced demyelination [252,253]. Functional experiments further showed that this protective effect of testosterone involves androgen receptor signaling. In 2019, TestOsterone TreatmEnt on Neuroprotection and Myelin Repair in Relapsing Remitting Multiple Sclerosis (TOTEM-RRMS), a phase-2 randomized, placebo-controlled trial, was initiated to investigate potential protective effects of testosterone in RRMS patients (ClinicalTrials.gov Identifiers: NCT03910738). The authors expect the final results of their studies in May 2023.
6. Summary and Conclusions
Several pre-clinical and some clinical studies are currently being carried out to test the pro-remyelinating properties of novel compounds. Although stakeholders, investors and regulatory agencies frequently require that compound effectiveness be demonstrated in the EAE model of MS, direct remyelinating properties can be studied straightforwardly using the cuprizone model. There is good reason st believe that novel compounds will be approved in the next decade to prevent disease progression in MS patients.
To date, magnetic resonance imaging for the treatment management of MS primarily relies on T1-weighted, T2-weighted, fluid-attenuated inversion recovery, and gadolinium-based contrast agent-enhanced sequences, which generate tissue and lesion contrast through differences in water content, proton relaxation, and BBB integrity related to inflammation, demyelination, and neurodegeneration. Notably, the visualization of remyelination is much more complex but advances in this field have been made. For example, diffusion-based modalities such as DTI (diffusion tensor imaging) or proton relaxation-based modalities, such as MTI (magnetization transfer imaging), have become widespread, and are arguably the most commonly used proxies for myelin content in clinical trial settings today [254]. An excellent review article addressing this topic has recently been published [255], highlighting the urgent need to further develop and validate these methods to visualize myelin degeneration and repair.
It will be interesting to see whether or not molecules identified as pro-myelinating agents in the cuprizone model demonstrate beneficial effects in future clinical trials. Several factors might play a role in this context. Although lymphocytes are recruited into the demyelinated areas in the cuprizone model [256], T-cell densities are higher in RRMS patients compared to those in the cuprizone model, which might be relevant due to the suppressive impact of Th17 cells on remyelination pathways [257]. Furthermore, B-cells and plasma cells play important roles in MS but not in the cuprizone model. Another important aspect is the time window to be defined to treat MS patients with pro-myelinating compounds. While, in the cuprizone model, the time window to initiate the treatment is well-defined (id est, during or after acute/chronic demyelination), several different lesions with different remyelination kinetics co-exist in MS patients, which makes the situation much more complex. Finally, species-specific differences in oligodendrocyte functions should be considered, as discussed recently in [258]. In fact, in comparison to myelination, non-myelinating functions of oligodendrocytes, such as metabolic support to axons, regulation of axonal and dendritic growth, the regulation of inflammation and angiogenesis, the synthesis of extracellular matrix to form perineuronal nets, or the regulatory impact on blood-brain barrier function are less well-characterized but should be considered in this context.
Funding
This research received no external funding.
Acknowledgments
The kind help of Concordia Lubrich for preparing the Table 1 is appreciated.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Scheld, M.; Rüther, B.J.; Große-Veldmann, R.; Ohl, K.; Tenbrock, K.; Dreymüller, D.; Fallier-Becker, P.; Zendedel, A.; Beyer, C.; Clarner, T.; et al. Neurodegeneration Triggers Peripheral Immune Cell Recruitment into the Forebrain. J. Neurosci. Off. J. Soc. Neurosci. 2016, 36, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Mei, F.; Fancy, S.P.J.; Shen, Y.A.; Niu, J.; Zhao, C.; Presley, B.; Miao, E.; Lee, S.; Mayoral, S.R.; Redmond, S.A.; et al. Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nat. Med. 2014, 20, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Hammel, G.; Zivkovic, S.; Ayazi, M.; Ren, Y. Consequences and mechanisms of myelin debris uptake and processing by cells in the central nervous system. Cell. Immunol. 2022, 380, 104591. [Google Scholar] [CrossRef]
- Poser, C.M. Dysmyelination revisited. Arch. Neurol. 1978, 35, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Tobin, W.O.; Kalinowska-Lyszczarz, A.; Weigand, S.D.; Guo, Y.; Tosakulwong, N.; Parisi, J.E.; Metz, I.; Frischer, J.M.; Lassmann, H.; Brück, W.; et al. Clinical Correlation of Multiple Sclerosis Immunopathologic Subtypes. Neurology 2021, 97, e1906–e1913. [Google Scholar] [CrossRef] [PubMed]
- Cree, B.A.C.; Arnold, D.L.; Chataway, J.; Chitnis, T.; Fox, R.J.; Pozo Ramajo, A.; Murphy, N.; Lassmann, H. Secondary Progressive Multiple Sclerosis: New Insights. Neurology 2021, 97, 378–388. [Google Scholar] [CrossRef]
- Caso, F.; Agosta, F.; Mattavelli, D.; Migliaccio, R.; Canu, E.; Magnani, G.; Marcone, A.; Copetti, M.; Falautano, M.; Comi, G.; et al. White Matter Degeneration in Atypical Alzheimer Disease. Radiology 2015, 277, 162–172. [Google Scholar] [CrossRef]
- Bonfanti, E.; Gelosa, P.; Fumagalli, M.; Dimou, L.; Viganò, F.; Tremoli, E.; Cimino, M.; Sironi, L.; Abbracchio, M.P. The role of oligodendrocyte precursor cells expressing the GPR17 receptor in brain remodeling after stroke. Cell Death Dis. 2017, 8, e2871. [Google Scholar] [CrossRef]
- Li, S.; Rao, J.H.; Lan, X.Y.; Li, X.; Chu, C.Y.; Liang, Y.; Janowski, M.; Zhang, H.T.; Walczak, P. White matter demyelination predates axonal injury after ischemic stroke in cynomolgus monkeys. Exp. Neurol. 2021, 340, 113655. [Google Scholar] [CrossRef]
- Waxman, S.G. Demyelination in spinal cord injury and multiple sclerosis: What can we do to enhance functional recovery? J. Neurotrauma 1992, 9 (Suppl. 1), S105–S117. [Google Scholar]
- Hakak, Y.; Walker, J.R.; Li, C.; Wong, W.H.; Davis, K.L.; Buxbaum, J.D.; Haroutunian, V.; Fienberg, A.A. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc. Natl. Acad. Sci. USA 2001, 98, 4746–4751. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Li, Y.; Fukaya, M.; Lorenzini, I.; Cleveland, D.W.; Ostrow, L.W.; Rothstein, J.D.; Bergles, D.E. Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat. Neurosci. 2013, 16, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Yuen, T.J.; Silbereis, J.C.; Griveau, A.; Chang, S.M.; Daneman, R.; Fancy, S.P.J.; Zahed, H.; Maltepe, E.; Rowitch, D.H. Oligodendrocyte-encoded HIF function couples postnatal myelination and white matter angiogenesis. Cell 2014, 158, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Falcão, A.M.; van Bruggen, D.; Marques, S.; Meijer, M.; Jäkel, S.; Agirre, E.; Floriddia, E.M.; Vanichkina, D.P.; Williams, A.; Guerreiro-Cacais, A.O.; et al. Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis. Nat. Med. 2018, 24, 1837–1844. [Google Scholar] [CrossRef]
- Kirby, L.; Jin, J.; Cardona, J.G.; Smith, M.D.; Martin, K.A.; Wang, J.; Strasburger, H.; Herbst, L.; Alexis, M.; Karnell, J.; et al. Oligodendrocyte precursor cells present antigen and are cytotoxic targets in inflammatory demyelination. Nat. Commun. 2019, 10, 3887. [Google Scholar] [CrossRef]
- Jäkel, S.; Agirre, E.; Mendanha Falcão, A.; van Bruggen, D.; Lee, K.W.; Knuesel, I.; Malhotra, D.; Ffrench-Constant, C.; Williams, A.; Castelo-Branco, G. Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature 2019, 566, 543–547. [Google Scholar] [CrossRef]
- Natrajan, M.S.; de la Fuente, A.G.; Crawford, A.H.; Linehan, E.; Nuñez, V.; Johnson, K.R.; Wu, T.; Fitzgerald, D.C.; Ricote, M.; Bielekova, B.; et al. Retinoid X receptor activation reverses age-related deficiencies in myelin debris phagocytosis and remyelination. Brain J. Neurol. 2015, 138 Pt 12, 3581–3597. [Google Scholar] [CrossRef]
- Gilson, J.; Blakemore, W.F. Failure of remyelination in areas of demyelination produced in the spinal cord of old rats. Neuropathol. Appl. Neurobiol. 1993, 19, 173–181. [Google Scholar] [CrossRef]
- Kotter, M.R.; Li, W.W.; Zhao, C.; Franklin, R.J. Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 328–332. [Google Scholar] [CrossRef]
- Standiford, M.M.; Grund, E.M.; Howe, C.L. Citrullinated myelin induces microglial TNFα and inhibits endogenous repair in the cuprizone model of demyelination. J. Neuroinflammation 2021, 18, 305. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, L.; Fitzner, D.; Bosch-Queralt, M.; Weil, M.T.; Su, M.; Sen, P.; Ruhwedel, T.; Mitkovski, M.; Trendelenburg, G.; Lütjohann, D.; et al. Defective cholesterol clearance limits remyelination in the aged central nervous system. Science 2018, 359, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Lampron, A.; Larochelle, A.; Laflamme, N.; Préfontaine, P.; Plante, M.M.; Sánchez, M.G.; Yong, V.W.; Stys, P.K.; Tremblay, M.; Rivest, S. Inefficient clearance of myelin debris by microglia impairs remyelinating processes. J. Exp. Med. 2015, 212, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.H.; Xu, Y.; Medynets, M.; Monaco, M.C.G.; Major, E.O.; Nath, A.; Wang, T. Activated T cells induce proliferation of oligodendrocyte progenitor cells via release of vascular endothelial cell growth factor-A. Glia 2018, 66, 2503–2513. [Google Scholar] [CrossRef]
- Levine, J.M.; Reynolds, R. Activation and proliferation of endogenous oligodendrocyte precursor cells during ethidium bromide-induced demyelination. Exp. Neurol. 1999, 160, 333–347. [Google Scholar] [CrossRef]
- Ferent, J.; Zimmer, C.; Durbec, P.; Ruat, M.; Traiffort, E. Sonic Hedgehog signaling is a positive oligodendrocyte regulator during demyelination. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 1759–1772. [Google Scholar] [CrossRef] [PubMed]
- Fancy, S.P.; Baranzini, S.E.; Zhao, C.; Yuk, D.I.; Irvine, K.A.; Kaing, S.; Sanai, N.; Franklin, R.J.; Rowitch, D.H. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 2009, 23, 1571–1585. [Google Scholar] [CrossRef]
- Magy, L.; Keita, M.; Richard, L.; Piaser, M.; Vallat, J.M. Transient exposure to FGF2 enhances myelination in embryonic brain cell cocultures. Exp. Neurol. 2003, 181, 17–24. [Google Scholar] [CrossRef]
- Chiou, B.; Gao, C.; Giera, S.; Folts, C.J.; Kishore, P.; Yu, D.; Oak, H.C.; Jiang, R.; Piao, X. Cell type-specific evaluation of ADGRG1/GPR56 function in developmental central nervous system myelination. Glia 2021, 69, 413–423. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, X.; Gui, X.; Croteau, C.; Song, L.; Xu, J.; Wang, A.; Bannerman, P.; Guo, F. Sox2 Is Essential for Oligodendroglial Proliferation and Differentiation during Postnatal Brain Myelination and CNS Remyelination. J. Neurosci. Off. J. Soc. Neurosci. 2018, 38, 1802–1820. [Google Scholar] [CrossRef]
- Narayanan, S.P.; Flores, A.I.; Wang, F.; Macklin, W.B. Akt signals through the mammalian target of rapamycin pathway to regulate CNS myelination. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 6860–6870. [Google Scholar] [CrossRef]
- Xiao, J.; Wong, A.W.; Willingham, M.M.; van den Buuse, M.; Kilpatrick, T.J.; Murray, S.S. Brain-derived neurotrophic factor promotes central nervous system myelination via a direct effect upon oligodendrocytes. Neuro-Signals 2010, 18, 186–202. [Google Scholar] [CrossRef]
- Mozafari, S.; Sherafat, M.A.; Javan, M.; Mirnajafi-Zadeh, J.; Tiraihi, T. Visual evoked potentials and MBP gene expression imply endogenous myelin repair in adult rat optic nerve and chiasm following local lysolecithin induced demyelination. Brain Res. 2010, 1351, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Mei, F.; Lehmann-Horn, K.; Shen, Y.A.; Rankin, K.A.; Stebbins, K.J.; Lorrain, D.S.; Pekarek, K.; Sagan, A.S.; Xiao, L.; Teuscher, C.; et al. Accelerated remyelination during inflammatory demyelination prevents axonal loss and improves functional recovery. eLife 2016, 5, e18246. [Google Scholar] [CrossRef] [PubMed]
- Irvine, K.A.; Blakemore, W.F. Remyelination protects axons from demyelination-associated axon degeneration. Brain J. Neurol. 2008, 131 Pt 6, 1464–1477. [Google Scholar] [CrossRef] [PubMed]
- Serwanski, D.R.; Rasmussen, A.L.; Brunquell, C.B.; Perkins, S.S.; Nishiyama, A. Sequential Contribution of Parenchymal and Neural Stem Cell-Derived Oligodendrocyte Precursor Cells toward Remyelination. Neuroglia 2018, 1, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Blight, A.R.; Young, W. Central axons in injured cat spinal cord recover electrophysiological function following remyelination by Schwann cells. J. Neurol. Sci. 1989, 91, 15–34. [Google Scholar] [CrossRef]
- Duncan, I.D.; Marik, R.L.; Broman, A.T.; Heidari, M. Thin myelin sheaths as the hallmark of remyelination persist over time and preserve axon function. Proc. Natl. Acad. Sci. USA 2017, 114, E9685–E9691. [Google Scholar] [CrossRef]
- Duncan, I.D.; Radcliff, A.B.; Heidari, M.; Kidd, G.; August, B.K.; Wierenga, L.A. The adult oligodendrocyte can participate in remyelination. Proc. Natl. Acad. Sci. USA 2018, 115, E11807–E11816. [Google Scholar] [CrossRef]
- Häberlein, F.; Mingardo, E.; Merten, N.; Schulze Köhling, N.K.; Reinoß, P.; Simon, K.; Japp, A.; Nagarajan, B.; Schrage, R.; Pegurier, C.; et al. Humanized zebrafish as a tractable tool for in vivo evaluation of pro-myelinating drugs. Cell Chem. Biol. 2022, 29, 1541–1555.e7. [Google Scholar] [CrossRef]
- Blakemore, W.F.; Franklin, R.J. Remyelination in experimental models of toxin-induced demyelination. Curr. Top. Microbiol. Immunol. 2008, 318, 193–212. [Google Scholar]
- Huang, J.K.; Jarjour, A.A.; Nait Oumesmar, B.; Kerninon, C.; Williams, A.; Krezel, W.; Kagechika, H.; Bauer, J.; Zhao, C.; Baron-Van Evercooren, A.; et al. Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat. Neurosci. 2011, 14, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Kipp, M.; Gingele, S.; Pott, F.; Clarner, T.; van der Valk, P.; Denecke, B.; Gan, L.; Siffrin, V.; Zipp, F.; Dreher, W.; et al. BLBP-expression in astrocytes during experimental demyelination and in human multiple sclerosis lesions. Brain Behav. Immun. 2011, 25, 1554–1568. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, V.A.; Tardif, V.; Lyssiotis, C.A.; Green, C.C.; Kerman, B.; Kim, H.J.; Padmanabhan, K.; Swoboda, J.G.; Ahmad, I.; Kondo, T.; et al. A regenerative approach to the treatment of multiple sclerosis. Nature 2013, 502, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.L.; Teo, W.; Hernandez, Y.; Brideau, C.; Cummins, K.; Kuipers, H.F.; Stys, P.K. Cuprizone-induced Demyelination in Mouse Brain is not due to Depletion of Copper. ASN Neuro 2022, 14, 17590914221126367. [Google Scholar] [CrossRef]
- Peterson, R.E.; Bollier, M.E. Spectrophotometric Determination of Serum Copper with Biscyclohexanoneoxalyldihydrazone. Anal. Chem. 1955, 27, 1195–1197. [Google Scholar] [CrossRef]
- Carlton, W.W. Response of mice to the chelating agents sodium diethyldithiocarbamate, alpha-benzoinoxime, and biscyclohexanone oxaldihydrazone. Toxicol. Appl. Pharmacol. 1966, 8, 512–521. [Google Scholar] [CrossRef]
- Fischbach, F.; Nedelcu, J.; Leopold, P.; Zhan, J.; Clarner, T.; Nellessen, L.; Beißel, C.; van Heuvel, Y.; Goswami, A.; Weis, J.; et al. Cuprizone-induced graded oligodendrocyte vulnerability is regulated by the transcription factor DNA damage-inducible transcript 3. Glia 2019, 67, 263–276. [Google Scholar] [CrossRef]
- Goldberg, J.; Clarner, T.; Beyer, C.; Kipp, M. Anatomical Distribution of Cuprizone-Induced Lesions in C57BL6 Mice. J. Mol. Neurosci. MN 2015, 57, 166–175. [Google Scholar] [CrossRef]
- Norkute, A.; Hieble, A.; Braun, A.; Johann, S.; Clarner, T.; Baumgartner, W.; Beyer, C.; Kipp, M. Cuprizone treatment induces demyelination and astrocytosis in the mouse hippocampus. J. Neurosci. Res. 2009, 87, 1343–1355. [Google Scholar] [CrossRef]
- Wagenknecht, N.; Becker, B.; Scheld, M.; Beyer, C.; Clarner, T.; Hochstrasser, T.; Kipp, M. Thalamus Degeneration and Inflammation in Two Distinct Multiple Sclerosis Animal Models. J. Mol. Neurosci. MN 2016, 60, 102–114. [Google Scholar] [CrossRef]
- Skripuletz, T.; Lindner, M.; Kotsiari, A.; Garde, N.; Fokuhl, J.; Linsmeier, F.; Trebst, C.; Stangel, M. Cortical demyelination is prominent in the murine cuprizone model and is strain-dependent. Am. J. Pathol. 2008, 172, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Palavra, F.; Viana, S.D.; Henriques, S.; Dinis, J.; Martins, J.; Madeira, M.H.; Santiago, R.; Petrella, L.; Sereno, J.; Castelo-Branco, M.; et al. Defining milestones for the study of remyelination using the cuprizone mouse model: How early is early? Mult. Scler. Relat. Disord. 2022, 63, 103886. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Rodriguez, M.L.A.; Gingele, S.; Schröder, L.J.; Möllenkamp, T.; Stangel, M.; Skripuletz, T.; Gudi, V. Astroglial and oligodendroglial markers in the cuprizone animal model for de- and remyelination. Histochem. Cell Biol. 2022, 158, 15–38. [Google Scholar] [CrossRef] [PubMed]
- Silva Oliveira Junior, M.; Schira-Heinen, J.; Reiche, L.; Han, S.; de Amorim, V.C.M.; Lewen, I.; Gruchot, J.; Göttle, P.; Akkermann, R.; Azim, K.; et al. Myelin repair is fostered by the corticosteroid medrysone specifically acting on astroglial subpopulations. EBioMedicine 2022, 83, 104204. [Google Scholar] [CrossRef] [PubMed]
- Menn, B.; Garcia-Verdugo, J.M.; Yaschine, C.; Gonzalez-Perez, O.; Rowitch, D.; Alvarez-Buylla, A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 7907–7918. [Google Scholar] [CrossRef] [PubMed]
- Nait-Oumesmar, B.; Picard-Riera, N.; Kerninon, C.; Decker, L.; Seilhean, D.; Höglinger, G.U.; Hirsch, E.C.; Reynolds, R.; Baron-Van Evercooren, A. Activation of the subventricular zone in multiple sclerosis: Evidence for early glial progenitors. Proc. Natl. Acad. Sci. USA 2007, 104, 4694–4699. [Google Scholar] [CrossRef] [PubMed]
- Butti, E.; Bacigaluppi, M.; Chaabane, L.; Ruffini, F.; Brambilla, E.; Berera, G.; Montonati, C.; Quattrini, A.; Martino, G. Neural Stem Cells of the Subventricular Zone Contribute to Neuroprotection of the Corpus Callosum after Cuprizone-Induced Demyelination. J. Neurosci. Off. J. Soc. Neurosci. 2019, 39, 5481–5492. [Google Scholar] [CrossRef]
- Watanabe, M.; Toyama, Y.; Nishiyama, A. Differentiation of proliferated NG2-positive glial progenitor cells in a remyelinating lesion. J. Neurosci. Res. 2002, 69, 826–836. [Google Scholar] [CrossRef]
- Yeung, M.S.Y.; Djelloul, M.; Steiner, E.; Bernard, S.; Salehpour, M.; Possnert, G.; Brundin, L.; Frisén, J. Dynamics of oligodendrocyte generation in multiple sclerosis. Nature 2019, 566, 538–542. [Google Scholar] [CrossRef]
- Gingele, S.; Henkel, F.; Heckers, S.; Moellenkamp, T.M.; Hümmert, M.W.; Skripuletz, T.; Stangel, M.; Gudi, V. Delayed Demyelination and Impaired Remyelination in Aged Mice in the Cuprizone Model. Cells 2020, 9, 945. [Google Scholar] [CrossRef]
- Shields, S.A.; Gilson, J.M.; Blakemore, W.F.; Franklin, R.J. Remyelination occurs as extensively but more slowly in old rats compared to young rats following gliotoxin-induced CNS demyelination. Glia 1999, 28, 77–83. [Google Scholar] [CrossRef]
- Hibbits, N.; Yoshino, J.; Le, T.Q.; Armstrong, R.C. Astrogliosis during acute and chronic cuprizone demyelination and implications for remyelination. ASN Neuro 2012, 4, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Cree, B.A.C.; Hollenbach, J.A.; Bove, R.; Kirkish, G.; Sacco, S.; Caverzasi, E.; Bischof, A.; Gundel, T.; Zhu, A.H.; Papinutto, N.; et al. Silent progression in disease activity-free relapsing multiple sclerosis. Ann. Neurol. 2019, 85, 653–666. [Google Scholar] [PubMed]
- Kornek, B.; Storch, M.K.; Weissert, R.; Wallstroem, E.; Stefferl, A.; Olsson, T.; Linington, C.; Schmidbauer, M.; Lassmann, H. Multiple sclerosis and chronic autoimmune encephalomyelitis: A comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am. J. Pathol. 2000, 157, 267–276. [Google Scholar] [CrossRef]
- Sarrazin, N.; Chavret-Reculon, E.; Bachelin, C.; Felfli, M.; Arab, R.; Gilardeau, S.; Brazhnikova, E.; Dubus, E.; Yaha-Cherif, L.; Lorenceau, J.; et al. Failed remyelination of the nonhuman primate optic nerve leads to axon degeneration, retinal damages, and visual dysfunction. Proc. Natl. Acad. Sci. USA 2022, 119, e2115973119. [Google Scholar] [CrossRef]
- Ricigliano, V.A.G.; Tonietto, M.; Hamzaoui, M.; Poirion, É.; Lazzarotto, A.; Bottlaender, M.; Gervais, P.; Maillart, E.; Stankoff, B.; Bodini, B. Spontaneous remyelination in lesions protects the integrity of surrounding tissues over time in multiple sclerosis. Eur. J. Neurol. 2022, 29, 1719–1729. [Google Scholar] [CrossRef]
- Brown, J.W.L.; Cunniffe, N.G.; Prados, F.; Kanber, B.; Jones, J.L.; Needham, E.; Georgieva, Z.; Rog, D.; Pearson, O.R.; Overell, J.; et al. Safety and efficacy of bexarotene in patients with relapsing-remitting multiple sclerosis (CCMR One): A randomised, double-blind, placebo-controlled, parallel-group, phase 2a study. Lancet Neurol. 2021, 20, 709–720. [Google Scholar] [CrossRef]
- Mi, S.; Hu, B.; Hahm, K.; Luo, Y.; Kam Hui, E.S.; Yuan, Q.; Wong, W.M.; Wang, L.; Su, H.; Chu, T.H.; et al. LINGO-1 antagonist promotes spinal cord remyelination and axonal integrity in MOG-induced experimental autoimmune encephalomyelitis. Nat. Med. 2007, 13, 1228–1233. [Google Scholar] [CrossRef]
- Cadavid, D.; Mellion, M.; Hupperts, R.; Edwards, K.R.; Calabresi, P.A.; Drulović, J.; Giovannoni, G.; Hartung, H.P.; Arnold, D.L.; Fisher, E.; et al. Safety and efficacy of opicinumab in patients with relapsing multiple sclerosis (SYNERGY): A randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2019, 18, 845–856. [Google Scholar] [CrossRef]
- Green, A.J.; Gelfand, J.M.; Cree, B.A.; Bevan, C.; Boscardin, W.J.; Mei, F.; Inman, J.; Arnow, S.; Devereux, M.; Abounasr, A.; et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): A randomised, controlled, double-blind, crossover trial. Lancet 2017, 390, 2481–2489. [Google Scholar] [CrossRef] [PubMed]
- Azim, K.; Angonin, D.; Marcy, G.; Pieropan, F.; Rivera, A.; Donega, V.; Cantù, C.; Williams, G.; Berninger, B.; Butt, A.M.; et al. Pharmacogenomic identification of small molecules for lineage specific manipulation of subventricular zone germinal activity. PLoS Biol. 2017, 15, e2000698. [Google Scholar] [CrossRef]
- Samanta, J.; Grund, E.M.; Silva, H.M.; Lafaille, J.J.; Fishell, G.; Salzer, J.L. Inhibition of Gli1 mobilizes endogenous neural stem cells for remyelination. Nature 2015, 526, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Patergnani, S.; Bonora, M.; Ingusci, S.; Previati, M.; Marchi, S.; Zucchini, S.; Perrone, M.; Wieckowski, M.R.; Castellazzi, M.; Pugliatti, M.; et al. Antipsychotic drugs counteract autophagy and mitophagy in multiple sclerosis. Proc. Natl. Acad. Sci. USA 2021, 118, e2020078118. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, T.; Sumiyoshi, S.; Deshimaru, M.; Suzuki, T.; Tomonari, H. Electron microscopic study on schizophrenia. Mechanism of pathological changes. Acta Neuropathol. 1972, 20, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Uranova, N.; Orlovskaya, D.; Vikhreva, O.; Zimina, I.; Kolomeets, N.; Vostrikov, V.; Rachmanova, V. Electron microscopy of oligodendroglia in severe mental illness. Brain Res. Bull. 2001, 55, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Tkachev, D.; Mimmack, M.L.; Ryan, M.M.; Wayland, M.; Freeman, T.; Jones, P.B.; Starkey, M.; Webster, M.J.; Yolken, R.H.; Bahn, S. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet 2003, 362, 798–805. [Google Scholar] [CrossRef]
- Schmitt, A.; Tatsch, L.; Vollhardt, A.; Schneider-Axmann, T.; Raabe, F.J.; Roell, L.; Heinsen, H.; Hof, P.R.; Falkai, P.; Schmitz, C. Decreased Oligodendrocyte Number in Hippocampal Subfield CA4 in Schizophrenia: A Replication Study. Cells 2022, 11, 3242. [Google Scholar] [CrossRef]
- Yamanaka, K.; Nakamura, K.; Shibahara, T.; Takashima, M.; Takaki, H.; Hidaka, M.; Komori, M.; Yoshikawa, Y.; Wakisaka, Y.; Ago, T.; et al. Deletion of Nox4 enhances remyelination following cuprizone-induced demyelination by increasing phagocytic capacity of microglia and macrophages in mice. Glia 2022. [Google Scholar] [CrossRef]
- Sun, J.X.; Zhu, K.Y.; Wang, Y.M.; Wang, D.J.; Zhang, M.Z.; Sarlus, H.; Benito-Cuesta, I.; Zhao, X.Q.; Zou, Z.F.; Zhong, Q.Y.; et al. Activation of TRPV1 receptor facilitates myelin repair following demyelination via the regulation of microglial function. Acta Pharmacol. Sin. 2022. [Google Scholar] [CrossRef]
- Madadi, S.; Shiri, E.; Pasbakhsh, P.; Tahmasebi, F.; Kazemzadeh, S.; Zibara, K.; Kashani, I.R. Combination Therapy of Mesenchymal Stem Cell Transplantation and Astrocyte Ablation Improve Remyelination in a Cuprizone-Induced Demyelination Mouse Model. Mol. Neurobiol. 2022, 59, 7278–7292. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, M.; Zhang, Y.; Han, M. Stemazole Promotes Oligodendrocyte Precursor Cell Survival In Vitro and Remyelination In Vivo. Int. J. Mol. Sci. 2022, 23, 10756. [Google Scholar] [CrossRef] [PubMed]
- Loix, M.; Wouters, E.; Vanherle, S.; Dehairs, J.; McManaman, J.L.; Kemps, H.; Swinnen, J.V.; Haidar, M.; Bogie, J.F.J.; Hendriks, J.J.A. Perilipin-2 limits remyelination by preventing lipid droplet degradation. Cell. Mol. Life Sci. CMLS 2022, 79, 515. [Google Scholar] [CrossRef] [PubMed]
- Benraiss, A.; Mariani, J.N.; Tate, A.; Madsen, P.M.; Clark, K.M.; Welle, K.A.; Solly, R.; Capellano, L.; Bentley, K.; Chandler-Militello, D.; et al. A TCF7L2-responsive suppression of both homeostatic and compensatory remyelination in Huntington disease mice. Cell Rep. 2022, 40, 111291. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Du, X.; Yang, Q.; Li, M.; Ran, Q.; Liu, Q.; Yang, L.; Sun, L.; Guo, Y.; Li, Y.; et al. Ginsenoside Rg1 promotes remyelination and functional recovery in demyelinating disease by enhancing oligodendrocyte precursor cells-mediated myelin repair. Phytomedicine Int. J. Phytother. Phytopharm. 2022, 106, 154309. [Google Scholar] [CrossRef] [PubMed]
- Honarvar, F.; Hojati, V.; Zare, L.; Bakhtiari, N.; Javan, M. Ursolic Acid Enhances Myelin Repair in Adult Mice Brains and Stimulates Exhausted Oligodendrocyte Progenitors to Remyelinate. J. Mol. Neurosci. MN 2022, 72, 2081–2093. [Google Scholar] [CrossRef]
- Yuan, J.; Xu, N.; Tao, Y.; Han, X.; Yang, L.; Liang, J.; Jin, H.; Zhang, X.; Wu, H.; Shi, H.; et al. Total astragalosides promote oligodendrocyte precursor cell differentiation and enhance remyelination in cuprizone-induced mice through suppression of Wnt/β-catenin signaling pathway. J. Ethnopharmacol. 2022, 298, 115622. [Google Scholar] [CrossRef]
- Mojaverrostami, S.; Khadivi, F.; Zarini, D.; Mohammadi, A. Combination effects of mesenchymal stem cells transplantation and anodal transcranial direct current stimulation on a cuprizone-induced mouse model of multiple sclerosis. J. Mol. Histol. 2022, 53, 817–831. [Google Scholar] [CrossRef]
- Gharighnia, S.; Omidi, A.; Ragerdi Kashani, I.; Sepand, M.R.; Pour Beiranvand, S. Ameliorative effects of acetyl-L-carnitine on corpus callosum and functional recovery in demyelinated mouse model. Int. J. Neurosci. 2022, 1–11. [Google Scholar] [CrossRef]
- Titus, H.E.; Xu, H.; Robinson, A.P.; Patel, P.A.; Chen, Y.; Fantini, D.; Eaton, V.; Karl, M.; Garrison, E.D.; Rose, I.V.L.; et al. Repurposing the cardiac glycoside digoxin to stimulate myelin regeneration in chemically-induced and immune-mediated mouse models of multiple sclerosis. Glia 2022, 70, 1950–1970. [Google Scholar] [CrossRef]
- Dupree, J.L.; Paez, P.M.; Tiwari-Woodruff, S.K.; Denton, T.T.; Hensley, K.; Angeliu, C.G.; Boullerne, A.I.; Kalinin, S.; Egge, S.; Cheli, V.T.; et al. Lanthionine Ketimine Ethyl Ester Accelerates Remyelination in a Mouse Model of Multiple Sclerosis. ASN Neuro 2022, 14, 17590914221112352. [Google Scholar] [CrossRef]
- Suo, N.; He, B.; Cui, S.; Yang, Y.; Wang, M.; Yuan, Q.; Xie, X. The orphan G protein-coupled receptor GPR149 is a negative regulator of myelination and remyelination. Glia 2022, 70, 1992–2008. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Villarreal, O.D.; Wang, Y.C.; Ragoussis, J.; Rivest, S.; Gosselin, D.; Richard, S. PRMT1 is required for the generation of MHC-associated microglia and remyelination in the central nervous system. Life Sci. Alliance 2022, 5, e202201467. [Google Scholar] [CrossRef] [PubMed]
- Bauch, J.; Faissner, A. The Extracellular Matrix Proteins Tenascin-C and Tenascin-R Retard Oligodendrocyte Precursor Maturation and Myelin Regeneration in a Cuprizone-Induced Long-Term Demyelination Animal Model. Cells 2022, 11, 1773. [Google Scholar] [CrossRef] [PubMed]
- Begum, Z.; Subramanian, V.; Raghunath, G.; Gurusamy, K.; Vijayaraghavan, R.; Sivanesan, S. Efficacy of different intensity of aquatic exercise in enhancing remyelination and neuronal plasticity using cuprizone model in male Wistar rats. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2022, 31, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhu, X.; Yan, W.; Zhang, Z.; Cui, E.; Wu, Y.; Li, C.; Pan, J.; Yan, Q.; Chai, X.; et al. Dietary Supplementation With Acer truncatum Oil Promotes Remyelination in a Mouse Model of Multiple Sclerosis. Front. Neurosci. 2022, 16, 860280. [Google Scholar] [CrossRef]
- Ammar, R.A.; Mohamed, A.F.; Kamal, M.M.; Safar, M.M.; Abdelkader, N.F. Neuroprotective effect of liraglutide in an experimental mouse model of multiple sclerosis: Role of AMPK/SIRT1 signaling and NLRP3 inflammasome. Inflammopharmacology 2022, 30, 919–934. [Google Scholar] [CrossRef]
- Dietrich, M.; Hecker, C.; Martin, E.; Langui, D.; Gliem, M.; Stankoff, B.; Lubetzki, C.; Gruchot, J.; Göttle, P.; Issberner, A.; et al. Increased Remyelination and Proregenerative Microglia Under Siponimod Therapy in Mechanistic Models. Neurol. (R) Neuroimmunol. Neuroinflammation 2022, 9, e1161. [Google Scholar] [CrossRef]
- Ai, R.; Xing, K.; Deng, X.; Han, J.; Hao, D.; Qi, W.; Han, B.; Yang, Y.; Li, X.; Zhang, Y. Baicalin Promotes CNS Remyelination via PPARγ Signal Pathway. Neurol. (R) Neuroimmunol. Neuroinflammation 2022, 9, e1142. [Google Scholar] [CrossRef]
- Wang, X.; Chang, L.; Wan, X.; Tan, Y.; Qu, Y.; Shan, J.; Yang, Y.; Ma, L.; Hashimoto, K. (R)-ketamine ameliorates demyelination and facilitates remyelination in cuprizone-treated mice: A role of gut-microbiota-brain axis. Neurobiol. Dis. 2022, 165, 105635. [Google Scholar] [CrossRef]
- Barati, S.; Kashani, I.R.; Tahmasebi, F. The effects of mesenchymal stem cells transplantation on A1 neurotoxic reactive astrocyte and demyelination in the cuprizone model. J. Mol. Histol. 2022, 53, 333–346. [Google Scholar] [CrossRef]
- Paton, K.; Robichon, K.; Templeton, N.; Denny, L.; Al Abadey, A.; Luo, D.; Prisinzano, T.; La Flamme, A.; Kivell, B. The Salvinorin Analogue, Ethoxymethyl Ether Salvinorin B, Promotes Remyelination in Preclinical Models of Multiple Sclerosis. Front. Neurol. 2021, 12, 782190. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liao, B.; Xiao, D.; Wu, W.; Xiao, Y.; Alexander, T.; Song, S.; Zhao, Z.; Zhang, Y.; Wang, Z.; et al. Encapsulation of bryostatin-1 by targeted exosomes enhances remyelination and neuroprotection effects in the cuprizone-induced demyelinating animal model of multiple sclerosis. Biomater. Sci. 2022, 10, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Gudi, V.; Schäfer, N.; Gingele, S.; Stangel, M.; Skripuletz, T. Regenerative Effects of CDP-Choline: A Dose-Dependent Study in the Toxic Cuprizone Model of De- and Remyelination. Pharmaceuticals 2021, 14, 1156. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; McEwen, H.P.; Duncan, T.; Lee, J.Y.; Teo, J.D.; Don, A.S. Sphingosine kinase 2 is essential for remyelination following cuprizone intoxication. Glia 2021, 69, 2863–2881. [Google Scholar] [CrossRef]
- Sadeghirashed, S.; Kazemi, F.; Taheri, S.; Ebrahimi, M.; Arasteh, J. A novel probiotic strain exerts therapeutic effects on mouse model of multiple sclerosis by altering the expression of inflammasome and IDO genes and modulation of T helper cytokine profile. Metab. Brain Dis. 2022, 37, 197–207. [Google Scholar] [CrossRef]
- Moradbeygi, K.; Parviz, M.; Rezaeizadeh, H.; Zargaran, A.; Sahraian, M.A.; Mehrabadi, S.; Nikbakhtzadeh, M.; Zahedi, E. Anti-LINGO-1 improved remyelination and neurobehavioral deficit in cuprizone-induced demyelination. Iran. J. Basic Med. Sci. 2021, 24, 900–907. [Google Scholar]
- Wang, Y.; Zhang, Y.; Zhang, S.; Kim, B.; Hull, V.; Xu, J.; Prabhu, P.; Gregory, M.; Martinez-Cerdeno, V.; Zhan, X.; et al. PARP1-mediated PARylation activity is essential for oligodendroglial differentiation and CNS myelination. Cell Rep. 2021, 37, 109695. [Google Scholar] [CrossRef]
- Selkirk, J.; Dines, K.; Yan, Y.; Ching, N.; Dalvie, D.; Biswas, S.; Bortolato, A.; Schkeryantz, J.; Lopez, C.; Ruiz, I.; et al. Deconstructing the Pharmacological Contribution of Sphingosine-1 Phosphate Receptors to Mouse Models of Multiple Sclerosis Using the Species Selectivity of Ozanimod, a Dual Modulator of Human Sphingosine 1-Phosphate Receptor Subtypes 1 and 5. J. Pharmacol. Exp. Ther. 2021, 379, 386–399. [Google Scholar] [CrossRef]
- Safaei, H.; Eftekhari, S.; Aliomrani, M. Analysis of platelet-derived growth factor receptor A and oligodendrocyte transcription factor 2 markers following Hydroxychloroquine administration in animal induced multiple sclerosis model. Metab. Brain Dis. 2021, 36, 2101–2110. [Google Scholar] [CrossRef]
- Mausner-Fainberg, K.; Benhamou, M.; Golan, M.; Kimelman, N.; Danon, U.; Marom, E.; Karni, A. Specific Blockade of Bone Morphogenetic Protein-2/4 Induces Oligodendrogenesis and Remyelination in Demyelinating Disorders. Neurother. J. Am. Soc. Exp. NeuroTherapeutics 2021, 18, 1798–1814. [Google Scholar] [CrossRef]
- El Sharouny, S.; Shaaban, M.; Elsayed, R.; Tahef, A.; Abd ElWahed, M. N-acetylcysteine protects against cuprizone-induced demyelination: Histological and immunohistochemical study. Folia Morphol. 2021, 81, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Danesh-Seta, T.; Emami, F.; Nasr-Esfahani, M.; Ghaedi, K.; Aliomrani, M. Bee Venom-Derived BBB Shuttle and its Correlation with Oligodendrocyte Proliferation Markers in Mice Model of Multiple Sclerosis. Neurotox. Res. 2021, 39, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kunjamma, R.; Weiner, M.; Chan, J.; Popko, B. Prolonging the integrated stress response enhances CNS remyelination in an inflammatory environment. eLife 2021, 10, e65469. [Google Scholar] [CrossRef]
- Manousi, A.; Göttle, P.; Reiche, L.; Cui, Q.; Healy, L.; Akkermann, R.; Gruchot, J.; Schira-Heinen, J.; Antel, J.; Hartung, H.; et al. Identification of novel myelin repair drugs by modulation of oligodendroglial differentiation competence. EBioMedicine 2021, 65, 103276. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ji, J.; Zha, Z.; Zhao, H.; Xue, B.; Jin, L.; Wang, L. Effect and Mechanism of Catalpol on Remyelination via Regulation of the NOTCH1 Signaling Pathway. Front. Pharmacol. 2021, 12, 628209. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Reichelt, M.; Kyauk, R.; Ngu, H.; Shen, Y.; Foreman, O.; Modrusan, Z.; Friedman, B.; Sheng, M.; Yuen, T. Multiple sclerosis risk gene Mertk is required for microglial activation and subsequent remyelination. Cell Rep. 2021, 34, 108835. [Google Scholar] [CrossRef]
- Pouzol, L.; Baumlin, N.; Sassi, A.; Tunis, M.; Marrie, J.; Vezzali, E.; Farine, H.; Mentzel, U.; Martinic, M. ACT-1004-1239, a first-in-class CXCR7 antagonist with both immunomodulatory and promyelinating effects for the treatment of inflammatory demyelinating diseases. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2021, 35, e21431. [Google Scholar] [CrossRef]
- Denny, L.; Al Abadey, A.; Robichon, K.; Templeton, N.; Prisinzano, T.; Kivell, B.; La Flamme, A. Nalfurafine reduces neuroinflammation and drives remyelination in models of CNS demyelinating disease. Clin. Transl. Immunol. 2021, 10, e1234. [Google Scholar] [CrossRef]
- Sy, M.; Brandt, A.; Lee, S.; Newton, B.; Pawling, J.; Golzar, A.; Rahman, A.; Yu, Z.; Cooper, G.; Scheel, M.; et al. N-acetylglucosamine drives myelination by triggering oligodendrocyte precursor cell differentiation. J. Biol. Chem. 2020, 295, 17413–17424. [Google Scholar] [CrossRef]
- Lee, J.; Villarreal, O.; Chen, X.; Zandee, S.; Young, Y.; Torok, C.; Lamarche-Vane, N.; Prat, A.; Rivest, S.; Gosselin, D.; et al. QUAKING Regulates Microexon Alternative Splicing of the Rho GTPase Pathway and Controls Microglia Homeostasis. Cell Rep. 2020, 33, 108560. [Google Scholar] [CrossRef]
- El-Etr, M.; Akwa, Y.; Rame, M.; Schumacher, M.; Sitruk-Ware, R. Nestorone®, a 19nor-progesterone derivative boosts remyelination in an animal model of demyelination. CNS Neurosci. Ther. 2021, 27, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Khalilian, B.; Madadi, S.; Fattahi, N.; Abouhamzeh, B. Coenzyme Q10 enhances remyelination and regulate inflammation effects of cuprizone in corpus callosum of chronic model of multiple sclerosis. J. Mol. Histol. 2021, 52, 125–134. [Google Scholar] [CrossRef]
- Thompson, K.; Tsirka, S. Guanabenz modulates microglia and macrophages during demyelination. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Dong, F.; Liu, D.; Jiang, F.; Liu, Y.; Wu, X.; Qu, X.; Liu, J.; Chen, Y.; Fan, H.; Yao, R. Conditional Deletion of Foxg1 Alleviates Demyelination and Facilitates Remyelination via the Wnt Signaling Pathway in Cuprizone-Induced Demyelinated Mice. Neurosci. Bull. 2021, 37, 15–30. [Google Scholar] [CrossRef]
- Huang, Y.; Song, Y.; Isaac, M.; Miretzky, S.; Patel, A.; Geoffrey McAuliffe, W.; Dreyfus, C. Tropomyosin Receptor Kinase B Expressed in Oligodendrocyte Lineage Cells Functions to Promote Myelin Following a Demyelinating Lesion. ASN Neuro 2020, 12, 1759091420957464. [Google Scholar] [CrossRef] [PubMed]
- Roth, L.; Zidane, B.; Festa, L.; Putatunda, R.; Romer, M.; Monnerie, H.; Jordan-Sciutto, K.; Grinspan, J. Differential effects of integrase strand transfer inhibitors, elvitegravir and raltegravir, on oligodendrocyte maturation: A role for the integrated stress response. Glia 2021, 69, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Wan, R.; Cheli, V.; Santiago-González, D.; Rosenblum, S.; Wan, Q.; Paez, P. Impaired Postnatal Myelination in a Conditional Knockout Mouse for the Ferritin Heavy Chain in Oligodendroglial Cells. J. Neurosci. Off. J. Soc. Neurosci. 2020, 40, 7609–7624. [Google Scholar] [CrossRef] [PubMed]
- Mazloumfard, F.; Mirian, M.; Eftekhari, S.; Aliomrani, M. Hydroxychloroquine effects on miR-155-3p and miR-219 expression changes in animal model of multiple sclerosis. Metab. Brain Dis. 2020, 35, 1–9. [Google Scholar] [CrossRef]
- Ljunggren-Rose, Å.; Natarajan, C.; Matta, P.; Pandey, A.; Upender, I.; Sriram, S. Anacardic acid induces IL-33 and promotes remyelination in CNS. Proc. Natl. Acad. Sci. USA 2020, 117, 21527–21535. [Google Scholar] [CrossRef]
- Cignarella, F.; Filipello, F.; Bollman, B.; Cantoni, C.; Locca, A.; Mikesell, R.; Manis, M.; Ibrahim, A.; Deng, L.; Benitez, B.; et al. TREM2 activation on microglia promotes myelin debris clearance and remyelination in a model of multiple sclerosis. Acta Neuropathol. 2020, 140, 513–534. [Google Scholar] [CrossRef]
- de la Vega Gallardo, N.; Penalva, R.; Dittmer, M.; Naughton, M.; Falconer, J.; Moffat, J.; de la Fuente, A.; Hombrebueno, J.; Lin, Z.; Perbal, B.; et al. Dynamic CCN3 expression in the murine CNS does not confer essential roles in myelination or remyelination. Proc. Natl. Acad. Sci. USA 2020, 117, 18018–18028. [Google Scholar] [CrossRef] [PubMed]
- Mojaverrostami, S.; Pasbakhsh, P.; Madadi, S.; Nekoonam, S.; Zarini, D.; Noori, L.; Shiri, E.; Salama, M.; Zibara, K.; Kashani, I. Calorie restriction promotes remyelination in a Cuprizone-Induced demyelination mouse model of multiple sclerosis. Metab. Brain Dis. 2020, 35, 1211–1224. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, C.; García-Martin, A.; Garrido-Rodríguez, M.; Mestre, L.; Feliú, A.; Guaza, C.; Calzado, M.; Muñoz, E. Effects of EHP-101 on inflammation and remyelination in murine models of Multiple sclerosis. Neurobiol. Dis. 2020, 143, 104994. [Google Scholar] [CrossRef]
- Sullivan, G.; Knutsen, A.; Peruzzotti-Jametti, L.; Korotcov, A.; Bosomtwi, A.; Dardzinski, B.; Bernstock, J.; Rizzi, S.; Edenhofer, F.; Pluchino, S.; et al. Transplantation of induced neural stem cells (iNSCs) into chronically demyelinated corpus callosum ameliorates motor deficits. Acta Neuropathol. Commun. 2020, 8, 1–23. [Google Scholar] [CrossRef]
- Zhou, X.; Nicholson, A.; Ren, Y.; Brooks, M.; Jiang, P.; Zuberi, A.; Phuoc, H.; Perkerson, R.; Matchett, B.; Parsons, T.; et al. Loss of TMEM106B leads to myelination deficits: Implications for frontotemporal dementia treatment strategies. Brain J. Neurol. 2020, 143, 1905–1919. [Google Scholar] [CrossRef]
- Windrem, M.; Schanz, S.; Zou, L.; Chandler-Militello, D.; Kuypers, N.; Nedergaard, M.; Lu, Y.; Mariani, J.; Goldman, S. Human Glial Progenitor Cells Effectively Remyelinate the Demyelinated Adult Brain. Cell Rep. 2020, 31, 107658. [Google Scholar] [CrossRef]
- Bacmeister, C.M.; Barr, H.J.; McClain, C.R.; Thornton, M.A.; Nettles, D.; Welle, C.G.; Hughes, E.G. Motor learning promotes remyelination via new and surviving oligodendrocytes. Nat. Neurosci. 2020, 23, 819–831. [Google Scholar] [CrossRef]
- Shao, Y.; Ding, J.; He, Q.; Ma, Q.; Liu, Q.; Zhang, C.; Lv, H.; Liu, J. Effect of Sox10 on remyelination of the hippocampus in cuprizone-induced demyelinated mice. Brain Behav. 2020, 10, e01623. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Ciric, B.; Curtis, M.; Chen, W.; Rostami, A.; Zhang, G. A dual effect of ursolic acid to the treatment of multiple sclerosis through both immunomodulation and direct remyelination. Proc. Natl. Acad. Sci. USA 2020, 117, 9082–9093. [Google Scholar] [CrossRef]
- Bernal-Chico, A.; Manterola, A.; Cipriani, R.; Katona, I.; Matute, C.; Mato, S. P2x7 receptors control demyelination and inflammation in the cuprizone model. Brain Behav. Immun.-Health 2020, 4, 100062. [Google Scholar] [CrossRef]
- Kuhbandner, K.; Hoffmann, A.; González Alvarado, M.; Seyler, L.; Bäuerle, T.; Winkler, J.; Linker, R. alpha-Synuclein: A Modulator During Inflammatory CNS Demyelination. J. Mol. Neurosci. MN 2020, 70, 1038–1049. [Google Scholar] [CrossRef] [PubMed]
- Zamora, N.; Cheli, V.; Santiago González, D.; Wan, R.; Paez, P. Deletion of Voltage-Gated Calcium Channels in Astrocytes during Demyelination Reduces Brain Inflammation and Promotes Myelin Regeneration in Mice. J. Neurosci. Off. J. Soc. Neurosci. 2020, 40, 3332–3347. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.; Zhang, J.; Titus, H.; Karl, M.; Merzliakov, M.; Dorfman, A.; Karlik, S.; Stewart, M.; Watt, R.; Facer, B.; et al. Nanocatalytic activity of clean-surfaced, faceted nanocrystalline gold enhances remyelination in animal models of multiple sclerosis. Sci. Rep. 2020, 10, 1936. [Google Scholar] [CrossRef]
- Yin, J.; He, Y.; An, J.; Miao, Q.; Sui, R.; Wang, Q.; Yu, J.; Xiao, B.; Ma, C. Dynamic Balance of Microglia and Astrocytes Involved in the Remyelinating Effect of Ginkgolide B. Front. Cell. Neurosci. 2020, 13, 572. [Google Scholar] [CrossRef]
- Tomas-Roig, J.; Agbemenyah, H.; Celarain, N.; Quintana, E.; Ramió-Torrentà, L.; Havemann-Reinecke, U. Dose-dependent effect of cannabinoid WIN-55,212-2 on myelin repair following a demyelinating insult. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Yoon, H.; Choi, C.; Triplet, E.; Langley, M.; Kleppe, L.; Kim, H.; Simon, W.; Scarisbrick, I. Blocking the Thrombin Receptor Promotes Repair of Demyelinated Lesions in the Adult Brain. J. Neurosci. Off. J. Soc. Neurosci. 2020, 40, 1483–1500. [Google Scholar] [CrossRef]
- Tian, J.; Li, X.; Zhao, L.; Shen, P.; Wang, Z.; Zhu, L.; Li, C.; Su, C.; Zhang, Y. Glycyrrhizic acid promotes neural repair by directly driving functional remyelination. Food Funct. 2020, 11, 992–1005. [Google Scholar] [CrossRef]
- Mohammadi-Rad, M.; Ghasemi, N.; Aliomrani, M. Evaluation of apamin effects on myelination process in C57BL/6 mice model of multiple sclerosis. Res. Pharm. Sci. 2019, 14, 424–431. [Google Scholar]
- Nystad, A.; Lereim, R.; Wergeland, S.; Oveland, E.; Myhr, K.; Bø, L.; Torkildsen, Ø. Fingolimod downregulates brain sphingosine-1-phosphate receptor 1 levels but does not promote remyelination or neuroprotection in the cuprizone model. J. Neuroimmunol. 2020, 339, 577091. [Google Scholar] [CrossRef]
- Abo Taleb, H.; Alghamdi, B. Neuroprotective Effects of Melatonin during Demyelination and Remyelination Stages in a Mouse Model of Multiple Sclerosis. J. Mol. Neurosci. MN 2020, 70, 386–402. [Google Scholar] [CrossRef]
- Nyamoya, S.; Steinle, J.; Chrzanowski, U.; Kaye, J.; Schmitz, C.; Beyer, C.; Kipp, M. Laquinimod Supports Remyelination in Non-Supportive Environments. Cells 2019, 8, 1363. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, H.; Zang, C.; Dong, Y.; Shang, J.; Chen, J.; Wang, Y.; Liu, H.; Zhang, Z.; Xu, H.; et al. CXCR2 antagonism promotes oligodendrocyte precursor cell differentiation and enhances remyelination in a mouse model of multiple sclerosis. Neurobiol. Dis. 2020, 134, 104630. [Google Scholar] [CrossRef]
- Zhao, Z.; Bao, X.; Zhang, Z.; Liu, H.; Zhang, D. Phloroglucinol derivative compound 21 attenuates cuprizone-induced multiple sclerosis mice through promoting remyelination and inhibiting neuroinflammation. Sci. China Life Sci. 2020, 63, 905–914. [Google Scholar] [CrossRef]
- Houshmand, F.; Barati, M.; Golab, F.; Ramezani-Sefidar, S.; Tanbakooie, S.; Tabatabaei, M.; Amiri, M.; Sanadgol, N. Metformin-induced AMPK activation stimulates remyelination through induction of neurotrophic factors, downregulation of NogoA and recruitment of Olig2+ precursor cells in the cuprizone murine model of multiple sclerosis. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2019, 27, 583–592. [Google Scholar] [CrossRef]
- Binamé, F.; Pham-Van, L.; Spenlé, C.; Jolivel, V.; Birmpili, D.; Meyer, L.; Jacob, L.; Meyer, L.; Mensah-Nyagan, A.; Po, C.; et al. Disruption of Sema3A/Plexin-A1 inhibitory signalling in oligodendrocytes as a therapeutic strategy to promote remyelination. EMBO Mol. Med. 2019, 11, e10378. [Google Scholar] [CrossRef]
- Nguyen, H.; Wood, R.; Prawdiuk, A.; Furness, S.; Xiao, J.; Murray, S.; Fletcher, J. TrkB Agonist LM22A-4 Increases Oligodendroglial Populations During Myelin Repair in the Corpus Callosum. Front. Mol. Neurosci. 2019, 12, 205. [Google Scholar] [CrossRef]
- Wasko, N.; Kulak, M.; Paul, D.; Nicaise, A.; Yeung, S.; Nichols, F.; Khanna, K.; Crocker, S.; Pachter, J.; Clark, R. Systemic TLR2 tolerance enhances central nervous system remyelination. J. Neuroinflammation 2019, 16, 1–15. [Google Scholar] [CrossRef]
- Templeton, N.; Kivell, B.; McCaughey-Chapman, A.; Connor, B.; La Flamme, A. Clozapine administration enhanced functional recovery after cuprizone demyelination. PLoS ONE 2019, 14, e0216113. [Google Scholar] [CrossRef]
- Voskuhl, R.; Itoh, N.; Tassoni, A.; Matsukawa, M.; Ren, E.; Tse, V.; Jang, E.; Suen, T.; Itoh, Y. Gene expression in oligodendrocytes during remyelination reveals cholesterol homeostasis as a therapeutic target in multiple sclerosis. Proc. Natl. Acad. Sci. USA 2019, 116, 10130–10139. [Google Scholar] [CrossRef]
- Govier-Cole, A.; Wood, R.; Fletcher, J.; Gonsalvez, D.; Merlo, D.; Cate, H.; Murray, S.; Xiao, J. Inhibiting Bone Morphogenetic Protein 4 Type I Receptor Signaling Promotes Remyelination by Potentiating Oligodendrocyte Differentiation. eNeuro 2019, 6. [Google Scholar] [CrossRef]
- Cui, X.; Guo, Y.; Fang, J.; Shi, C.; Suo, N.; Zhang, R.; Xie, X. Donepezil, a drug for Alzheimer’s disease, promotes oligodendrocyte generation and remyelination. Acta Pharmacol. Sin. 2019, 40, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Suo, N.; Guo, Y.; He, B.; Gu, H.; Xie, X. Inhibition of MAPK/ERK pathway promotes oligodendrocytes generation and recovery of demyelinating diseases. Glia 2019, 67, 1320–1332. [Google Scholar] [CrossRef] [PubMed]
- Madadi, S.; Pasbakhsh, P.; Tahmasebi, F.; Mortezaee, K.; Khanehzad, M.; Boroujeni, F.; Noorzehi, G.; Kashani, I. Astrocyte ablation induced by La-aminoadipate (L-AAA) potentiates remyelination in a cuprizone demyelinating mouse model. Metab. Brain Dis. 2019, 34, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Sharma, G.; Gupta, V.; Kaur, R.; Thakur, K.; Malik, R.; Kumar, A.; Kaushal, N.; Katare, O.; Raza, K. Oral Delivery of Methylthioadenosine to the Brain Employing Solid Lipid Nanoparticles: Pharmacokinetic, Behavioral, and Histopathological Evidences. AAPS PharmSciTech 2019, 20, 74. [Google Scholar] [CrossRef]
- de Rosa, V.; Secondo, A.; Pannaccione, A.; Ciccone, R.; Formisano, L.; Guida, N.; Crispino, R.; Fico, A.; Polishchuk, R.; Annunziato, L.; et al. D-Aspartate treatment attenuates myelin damage and stimulates myelin repair. EMBO Mol. Med. 2019, 11, e9278. [Google Scholar]
- Jin, M.; Li, Q.; Gu, Y.; Wan, B.; Huang, J.; Xu, X.; Huang, R.; Zhang, Y. Leonurine suppresses neuroinflammation through promoting oligodendrocyte maturation. J. Cell. Mol. Med. 2019, 23, 1470–1485. [Google Scholar] [CrossRef]
- Thompson, K.; Nissen, J.; Pretory, A.; Tsirka, S. Tuftsin Combines With Remyelinating Therapy and Improves Outcomes in Models of CNS Demyelinating Disease. Front. Immunol. 2018, 9, 2784. [Google Scholar] [CrossRef]
- Yu, X.; Cheng, G.; Zhang, L.; Zhang, Y.; Wang, Q.; Zhao, M.; Zeng, L.; Hu, Y.; Feng, L. N-Phenylquinazolin-2-amine Yhhu4952 as a novel promotor for oligodendrocyte differentiation and myelination. Sci. Rep. 2018, 8, 14040. [Google Scholar] [CrossRef]
- Popescu, D.; Huang, H.; Singhal, N.; Shriver, L.; McDonough, J.; Clements, R.; Freeman, E. Vitamin K enhances the production of brain sulfatides during remyelination. PLoS ONE 2018, 13, e0203057. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, L.; Fang, Z.; Kong, J.; Huang, Q.; Xu, H. Adenosine Promotes the Recovery of Mice from the Cuprizone-Induced Behavioral and Morphological Changes while Effecting on Microglia and Inflammatory Cytokines in the Brain. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2018, 13, 412–425. [Google Scholar] [CrossRef]
- Fletcher, J.; Wood, R.; Nguyen, J.; Norman, E.; Jun, C.; Prawdiuk, A.; Biemond, M.; Nguyen, H.; Northfield, S.; Hughes, R.; et al. Targeting TrkB with a Brain-Derived Neurotrophic Factor Mimetic Promotes Myelin Repair in the Brain. J. Neurosci. Off. J. Soc. Neurosci. 2018, 38, 7088–7099. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Wang, J.; Mullin, A.; Caggiano, A.; Parry, T.; Colburn, R.; Pavlopoulos, E. The antibody rHIgM22 facilitates hippocampal remyelination and ameliorates memory deficits in the cuprizone mouse model of demyelination. Brain Res. 2018, 1694, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Cerina, M.; Narayanan, V.; Delank, A.; Meuth, P.; Graebenitz, S.; Göbel, K.; Herrmann, A.; Albrecht, S.; Daldrup, T.; Seidenbecher, T.; et al. Protective potential of dimethyl fumarate in a mouse model of thalamocortical demyelination. Brain Struct. Funct. 2018, 223, 3091–3106. [Google Scholar] [CrossRef] [PubMed]
- Wolf, Y.; Shemer, A.; Levy-Efrati, L.; Gross, M.; Kim, J.; Engel, A.; David, E.; Chappell-Maor, L.; Grozovski, J.; Rotkopf, R.; et al. Microglial MHC class II is dispensable for experimental autoimmune encephalomyelitis and cuprizone-induced demyelination. Eur. J. Immunol. 2018, 48, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.; Sullivan, G.; Armstrong, R. Genetic detection of Sonic hedgehog (Shh) expression and cellular response in the progression of acute through chronic demyelination and remyelination. Neurobiol. Dis. 2018, 115, 145–156. [Google Scholar] [CrossRef] [PubMed]
- El Waly, B.; Cayre, M.; Durbec, P. Promoting Myelin Repair through In Vivo Neuroblast Reprogramming. Stem Cell Rep. 2018, 10, 1492–1504. [Google Scholar] [CrossRef]
- Martin, N.; Molnar, V.; Szilagyi, G.; Elkjaer, M.; Nawrocki, A.; Okarmus, J.; Wlodarczyk, A.; Thygesen, E.; Palkovits, M.; Gallyas, F.; et al. Experimental Demyelination and Axonal Loss Are Reduced in MicroRNA-146a Deficient Mice. Front. Immunol. 2018, 9, 490. [Google Scholar] [CrossRef]
- Beckmann, N.; Giorgetti, E.; Neuhaus, A.; Zurbruegg, S.; Accart, N.; Smith, P.; Perdoux, J.; Perrot, L.; Nash, M.; Desrayaud, S.; et al. Brain region-specific enhancement of remyelination and prevention of demyelination by the CSF1R kinase inhibitor BLZ945. Acta Neuropathol. Commun. 2018, 6, 1–17. [Google Scholar] [CrossRef]
- Guo, Y.; Suo, N.; Cui, X.; Yuan, Q.; Xie, X. Vitamin C promotes oligodendrocytes generation and remyelination. Glia 2018, 66, 1302–1316. [Google Scholar] [CrossRef]
- Chen, Y.; Zhen, W.; Guo, T.; Zhao, Y.; Liu, A.; Rubio, J.; Krull, D.; Richardson, J.; Lu, H.; Wang, R. Histamine Receptor 3 negatively regulates oligodendrocyte differentiation and remyelination. PLoS ONE 2017, 12, e0189380. [Google Scholar] [CrossRef]
- Kuboyama, K.; Tanga, N.; Suzuki, R.; Fujikawa, A.; Noda, M. Protamine neutralizes chondroitin sulfate proteoglycan-mediated inhibition of oligodendrocyte differentiation. PLoS ONE 2017, 12, e0189164. [Google Scholar] [CrossRef] [PubMed]
- Zoupi, L.; Savvaki, M.; Kalemaki, K.; Kalafatakis, I.; Sidiropoulou, K.; Karagogeos, D. The function of contactin-2/TAG-1 in oligodendrocytes in health and demyelinating pathology. Glia 2018, 66, 576–591. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, R.; Baba, H.; Yamaguchi, Y. Unconventional Myosin ID is Involved in Remyelination After Cuprizone-Induced Demyelination. Neurochem. Res. 2018, 43, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Semnani, M.; Mashayekhi, F.; Azarnia, M.; Salehi, Z. Effects of green tea epigallocatechin-3-gallate on the proteolipid protein and oligodendrocyte transcription factor 1 messenger RNA gene expression in a mouse model of multiple sclerosis. Folia Neuropathol. 2017, 3, 199–205. [Google Scholar] [CrossRef]
- Ray, A.; DuBois, J.; Gruber, R.; Guzik, H.; Gulinello, M.; Perumal, G.; Raine, C.; Kozakiewicz, L.; Williamson, J.; Shafit-Zagardo, B. Loss of Gas6 and Axl signaling results in extensive axonal damage, motor deficits, prolonged neuroinflammation, and less remyelination following cuprizone exposure. Glia 2017, 65, 2051–2069. [Google Scholar] [CrossRef]
- Qin, J.; Sikkema, A.; van der Bij, K.; de Jonge, J.; Klappe, K.; Nies, V.; Jonker, J.; Kok, J.; Hoekstra, D.; Baron, W. GD1a Overcomes Inhibition of Myelination by Fibronectin via Activation of Protein Kinase A: Implications for Multiple Sclerosis. J. Neurosci. Off. J. Soc. Neurosci. 2017, 37, 9925–9938. [Google Scholar] [CrossRef]
- Santiago González, D.; Cheli, V.; Zamora, N.; Lama, T.; Spreuer, V.; Murphy, G.; Paez, P. Conditional Deletion of the L-Type Calcium Channel Cav1.2 in NG2-Positive Cells Impairs Remyelination in Mice. J. Neurosci. Off. J. Soc. Neurosci. 2017, 37, 10038–10051. [Google Scholar] [CrossRef]
- Stone, S.; Jamison, S.; Yue, Y.; Durose, W.; Schmidt-Ullrich, R.; Lin, W. NF-κB Activation Protects Oligodendrocytes against Inflammation. J. Neurosci. Off. J. Soc. Neurosci. 2017, 37, 9332–9344. [Google Scholar] [CrossRef]
- Werneburg, S.; Fuchs, H.; Albers, I.; Burkhardt, H.; Gudi, V.; Skripuletz, T.; Stangel, M.; Gerardy-Schahn, R.; Hildebrandt, H. Polysialylation at Early Stages of Oligodendrocyte Differentiation Promotes Myelin Repair. J. Neurosci. Off. J. Soc. Neurosci. 2017, 37, 8131–8141. [Google Scholar] [CrossRef]
- Yamamoto, S.; Yamashina, K.; Ishikawa, M.; Gotoh, M.; Yagishita, S.; Iwasa, K.; Maruyama, K.; Murakami-Murofushi, K.; Yoshikawa, K. Protective and therapeutic role of 2-carba-cyclic phosphatidic acid in demyelinating disease. J. Neuroinflammation 2017, 14, 1–14. [Google Scholar] [CrossRef]
- Chami, M.; Halmer, R.; Schnoeder, L.; Anne Becker, K.; Meier, C.; Fassbender, K.; Gulbins, E.; Walter, S. Acid sphingomyelinase deficiency enhances myelin repair after acute and chronic demyelination. PLoS ONE 2017, 12, e0178622. [Google Scholar] [CrossRef] [PubMed]
- Mullin, A.; Cui, C.; Wang, Y.; Wang, J.; Troy, E.; Caggiano, A.; Parry, T.; Colburn, R.; Pavlopoulos, E. rHIgM22 enhances remyelination in the brain of the cuprizone mouse model of demyelination. Neurobiol. Dis. 2017, 105, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Davidson, N.; Yu, F.; Kijpaisalratana, N.; Le, T.; Beer, L.; Radomski, K.; Armstrong, R. Leukemia/lymphoma-related factor (LRF) exhibits stage- and context-dependent transcriptional controls in the oligodendrocyte lineage and modulates remyelination. J. Neurosci. Res. 2017, 95, 2391–2408. [Google Scholar] [CrossRef] [PubMed]
- Karamita, M.; Barnum, C.; Möbius, W.; Tansey, M.; Szymkowski, D.; Lassmann, H.; Probert, L. Therapeutic inhibition of soluble brain TNF promotes remyelination by increasing myelin phagocytosis by microglia. JCI Insight 2017, 2, e87455. [Google Scholar] [CrossRef]
- Komegae, E.; Souza, T.; Grund, L.; Lima, C.; Lopes-Ferreira, M. Multiple functional therapeutic effects of TnP: A small stable synthetic peptide derived from fish venom in a mouse model of multiple sclerosis. PLoS ONE 2017, 12, e0171796. [Google Scholar] [CrossRef]
- Fan, H.; Chen, L.; Qu, X.; Ren, C.; Wu, X.; Dong, F.; Zhang, B.; Gao, D.; Yao, R. Transplanted miR-219-overexpressing oligodendrocyte precursor cells promoted remyelination and improved functional recovery in a chronic demyelinated model. Sci. Rep. 2017, 7, 41407. [Google Scholar] [CrossRef] [PubMed]
- Tejedor, L.; Wostradowski, T.; Gingele, S.; Skripuletz, T.; Gudi, V.; Stangel, M. The Effect of Stereotactic Injections on Demyelination and Remyelination: A Study in the Cuprizone Model. J. Mol. Neurosci. MN 2017, 61, 479–488. [Google Scholar] [CrossRef]
- Zhu, K.; Sun, J.; Kang, Z.; Zou, Z.; Wu, G.; Wang, J. Electroacupuncture Promotes Remyelination after Cuprizone Treatment by Enhancing Myelin Debris Clearance. Front. Neurosci. 2017, 10, 613. [Google Scholar] [CrossRef]
- Berghoff, S.; Gerndt, N.; Winchenbach, J.; Stumpf, S.; Hosang, L.; Odoardi, F.; Ruhwedel, T.; Böhler, C.; Barrette, B.; Stassart, R.; et al. Dietary cholesterol promotes repair of demyelinated lesions in the adult brain. Nat. Commun. 2017, 8, 14241. [Google Scholar] [CrossRef]
- Siegert, E.; Paul, F.; Rothe, M.; Weylandt, K. The effect of omega-3 fatty acids on central nervous system remyelination in fat-1 mice. BMC neuroscience 2017, 18, 19. [Google Scholar] [CrossRef]
- Ichihara, Y.; Doi, T.; Ryu, Y.; Nagao, M.; Sawada, Y.; Ogata, T. Oligodendrocyte Progenitor Cells Directly Utilize Lactate for Promoting Cell Cycling and Differentiation. J. Cell. Physiol. 2017, 232, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Hoyos, H.; Marder, M.; Ulrich, R.; Gudi, V.; Stangel, M.; Rabinovich, G.; Pasquini, L.; Pasquini, J. The Role of Galectin-3: From Oligodendroglial Differentiation and Myelination to Demyelination and Remyelination Processes in a Cuprizone-Induced Demyelination Model. Adv. Exp. Med. Biol. 2016, 949, 311–332. [Google Scholar] [PubMed]
- Saha, A.; Buntz, S.; Scotland, P.; Xu, L.; Noeldner, P.; Patel, S.; Wollish, A.; Gunaratne, A.; Gentry, T.; Troy, J.; et al. A cord blood monocyte-derived cell therapy product accelerates brain remyelination. JCI Insight 2016, 1, e86667. [Google Scholar] [CrossRef]
- Makinodan, M.; Ikawa, D.; Miyamoto, Y.; Yamauchi, J.; Yamamuro, K.; Yamashita, Y.; Toritsuka, M.; Kimoto, S.; Okumura, K.; Yamauchi, T.; et al. Social isolation impairs remyelination in mice through modulation of IL-6. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2016, 30, 4267–4274. [Google Scholar] [CrossRef] [PubMed]
- Mi, G.; Gao, Y.; Liu, S.; Ye, E.; Li, Y.; Jin, X.; Yang, H.; Yang, Z. Cyclin-dependent kinase inhibitor flavopiridol promotes remyelination in a cuprizone induced demyelination model. Cell Cycle (Georget. Tex.) 2016, 15, 2780–2791. [Google Scholar] [CrossRef]
- Choi, I.; Piccio, L.; Childress, P.; Bollman, B.; Ghosh, A.; Brandhorst, S.; Suarez, J.; Michalsen, A.; Cross, A.; Morgan, T.; et al. A Diet Mimicking Fasting Promotes Regeneration and Reduces Autoimmunity and Multiple Sclerosis Symptoms. Cell Rep. 2016, 15, 2136–2146. [Google Scholar] [CrossRef]
- Cruz-Martinez, P.; González-Granero, S.; Molina-Navarro, M.; Pacheco-Torres, J.; García-Verdugo, J.; Geijo-Barrientos, E.; Jones, J.; Martinez, S. Intraventricular injections of mesenchymal stem cells activate endogenous functional remyelination in a chronic demyelinating murine model. Cell Death Dis. 2016, 7, e2223. [Google Scholar] [CrossRef]
- Xu, H.; Lu, H.; Xu, Z.; Luan, L.; Li, C.; Xu, Y.; Dong, K.; Zhang, J.; Li, X.; Li, Y.; et al. Discovery of CNS Penetrant CXCR2 Antagonists for the Potential Treatment of CNS Demyelinating Disorders. ACS Med. Chem. Lett. 2016, 7, 397–402. [Google Scholar] [CrossRef]
- Ghaiad, H.; Nooh, M.; El-Sawalhi, M.; Shaheen, A. Resveratrol Promotes Remyelination in Cuprizone Model of Multiple Sclerosis: Biochemical and Histological Study. Mol. Neurobiol. 2017, 54, 3219–3229. [Google Scholar] [CrossRef]
- El-Tahry, H.; Marei, H.; Shams, A.; El-Shahat, M.; Abdelaziz, H.; Abd El-Kader, M. The effect of triiodothyronine on maturation and differentiation of oligodendrocyte progenitor cells during remyelination following induced demyelination in male albino rat. Tissue Cell 2016, 48, 242–251. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z.; Li, Y.; Lu, M.; Zhang, Y.; Elias, S.; Chopp, M. Thymosin beta4 promotes oligodendrogenesis in the demyelinating central nervous system. Neurobiol. Dis. 2016, 88, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, S.; Tian, Y.; Wu, X.; He, Y.; Li, C.; Namaka, M.; Kong, J.; Li, H.; Xiao, L. Quetiapine Inhibits Microglial Activation by Neutralizing Abnormal STIM1-Mediated Intercellular Calcium Homeostasis and Promotes Myelin Repair in a Cuprizone-Induced Mouse Model of Demyelination. Front. Cell. Neurosci. 2015, 9, 492. [Google Scholar] [CrossRef] [PubMed]
- Zendedel, A.; Kashani, I.; Azimzadeh, M.; Pasbakhsh, P.; Omidi, N.; Golestani, A.; Beyer, C.; Clarner, T. Regulatory effect of triiodothyronine on brain myelination and astrogliosis after cuprizone-induced demyelination in mice. Metab. Brain Dis. 2016, 31, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Mosyak, L.; Wood, A.; Dwyer, B.; Buddha, M.; Johnson, M.; Aulabaugh, A.; Zhong, X.; Presman, E.; Benard, S.; Kelleher, K.; et al. The structure of the Lingo-1 ectodomain, a module implicated in central nervous system repair inhibition. J. Biol. Chem. 2006, 281, 36378–36390. [Google Scholar] [CrossRef]
- Mi, S.; Miller, R.H.; Tang, W.; Lee, X.; Hu, B.; Wu, W.; Zhang, Y.; Shields, C.B.; Zhang, Y.; Miklasz, S.; et al. Promotion of central nervous system remyelination by induced differentiation of oligodendrocyte precursor cells. Ann. Neurol. 2009, 65, 304–315. [Google Scholar] [CrossRef]
- Lee, X.; Yang, Z.; Shao, Z.; Rosenberg, S.S.; Levesque, M.; Pepinsky, R.B.; Qiu, M.; Miller, R.H.; Chan, J.R.; Mi, S. NGF regulates the expression of axonal LINGO-1 to inhibit oligodendrocyte differentiation and myelination. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 220–225. [Google Scholar] [CrossRef]
- Cadavid, D.; Balcer, L.; Galetta, S.; Aktas, O.; Ziemssen, T.; Vanopdenbosch, L.; Frederiksen, J.; Skeen, M.; Jaffe, G.J.; Butzkueven, H.; et al. Safety and efficacy of opicinumab in acute optic neuritis (RENEW): A randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2017, 16, 189–199. [Google Scholar] [CrossRef]
- Li, Z.; He, Y.; Fan, S.; Sun, B. Clemastine rescues behavioral changes and enhances remyelination in the cuprizone mouse model of demyelination. Neurosci. Bull. 2015, 31, 617–625. [Google Scholar] [CrossRef]
- Cree, B.A.C.; Niu, J.; Hoi, K.K.; Zhao, C.; Caganap, S.D.; Henry, R.G.; Dao, D.Q.; Zollinger, D.R.; Mei, F.; Shen, Y.A.; et al. Clemastine rescues myelination defects and promotes functional recovery in hypoxic brain injury. Brain J. Neurol. 2018, 141, 85–98. [Google Scholar] [CrossRef]
- Wang, F.; Ren, S.Y.; Chen, J.F.; Liu, K.; Li, R.X.; Li, Z.F.; Hu, B.; Niu, J.Q.; Xiao, L.; Chan, J.R.; et al. Myelin degeneration and diminished myelin renewal contribute to age-related deficits in memory. Nat. Neurosci. 2020, 23, 481–486. [Google Scholar] [CrossRef]
- Chen, J.F.; Liu, K.; Hu, B.; Li, R.R.; Xin, W.; Chen, H.; Wang, F.; Chen, L.; Li, R.X.; Ren, S.Y.; et al. Enhancing myelin renewal reverses cognitive dysfunction in a murine model of Alzheimer’s disease. Neuron 2021, 109, 2292–2307.e5. [Google Scholar] [CrossRef] [PubMed]
- Lecca, D.; Trincavelli, M.L.; Gelosa, P.; Sironi, L.; Ciana, P.; Fumagalli, M.; Villa, G.; Verderio, C.; Grumelli, C.; Guerrini, U.; et al. The recently identified P2Y-like receptor GPR17 is a sensor of brain damage and a new target for brain repair. PLoS ONE 2008, 3, e3579. [Google Scholar] [CrossRef] [PubMed]
- Ceruti, S.; Villa, G.; Genovese, T.; Mazzon, E.; Longhi, R.; Rosa, P.; Bramanti, P.; Cuzzocrea, S.; Abbracchio, M.P. The P2Y-like receptor GPR17 as a sensor of damage and a new potential target in spinal cord injury. Brain J. Neurol. 2009, 132 Pt 8, 2206–2218. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, H.; Wang, S.; Koito, H.; Li, J.; Ye, F.; Hoang, J.; Escobar, S.S.; Gow, A.; Arnett, H.A.; et al. The oligodendrocyte-specific G protein-coupled receptor GPR17 is a cell-intrinsic timer of myelination. Nat. Neurosci. 2009, 12, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Ou, Z.; Sun, Y.; Lin, L.; You, N.; Liu, X.; Li, H.; Ma, Y.; Cao, L.; Han, Y.; Liu, M.; et al. Olig2-Targeted G-Protein-Coupled Receptor Gpr17 Regulates Oligodendrocyte Survival in Response to Lysolecithin-Induced Demyelination. J. Neurosci. Off. J. Soc. Neurosci. 2016, 36, 10560–10573. [Google Scholar] [CrossRef]
- Lu, C.; Dong, L.; Zhou, H.; Li, Q.; Huang, G.; Bai, S.J.; Liao, L. G-Protein-Coupled Receptor Gpr17 Regulates Oligodendrocyte Differentiation in Response to Lysolecithin-Induced Demyelination. Sci. Rep. 2018, 8, 4502. [Google Scholar] [CrossRef]
- Nyamoya, S.; Leopold, P.; Becker, B.; Beyer, C.; Hustadt, F.; Schmitz, C.; Michel, A.; Kipp, M. G-Protein-Coupled Receptor Gpr17 Expression in Two Multiple Sclerosis Remyelination Models. Mol. Neurobiol. 2019, 56, 1109–1123. [Google Scholar] [CrossRef]
- Bonfanti, E.; Bonifacino, T.; Raffaele, S.; Milanese, M.; Morgante, E.; Bonanno, G.; Abbracchio, M.P.; Fumagalli, M. Abnormal Upregulation of GPR17 Receptor Contributes to Oligodendrocyte Dysfunction in SOD1 G93A Mice. Int. J. Mol. Sci. 2020, 21, 2395. [Google Scholar] [CrossRef]
- Franke, H.; Parravicini, C.; Lecca, D.; Zanier, E.R.; Heine, C.; Bremicker, K.; Fumagalli, M.; Rosa, P.; Longhi, L.; Stocchetti, N.; et al. Changes of the GPR17 receptor, a new target for neurorepair, in neurons and glial cells in patients with traumatic brain injury. Purinergic Signal. 2013, 9, 451–462. [Google Scholar] [CrossRef]
- McGinley, M.P.; Cohen, J.A. Sphingosine 1-phosphate receptor modulators in multiple sclerosis and other conditions. Lancet 2021, 398, 1184–1194. [Google Scholar] [CrossRef]
- Novgorodov, A.S.; El-Alwani, M.; Bielawski, J.; Obeid, L.M.; Gudz, T.I. Activation of sphingosine-1-phosphate receptor S1P5 inhibits oligodendrocyte progenitor migration. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2007, 21, 1503–1514. [Google Scholar] [CrossRef] [PubMed]
- Van Doorn, R.; Van Horssen, J.; Verzijl, D.; Witte, M.; Ronken, E.; Van Het Hof, B.; Lakeman, K.; Dijkstra, C.D.; Van Der Valk, P.; Reijerkerk, A.; et al. Sphingosine 1-phosphate receptor 1 and 3 are upregulated in multiple sclerosis lesions. Glia 2010, 58, 1465–1476. [Google Scholar] [CrossRef] [PubMed]
- Janssen, S.; Schlegel, C.; Gudi, V.; Prajeeth, C.K.; Skripuletz, T.; Trebst, C.; Stangel, M. Effect of FTY720-phosphate on the expression of inflammation-associated molecules in astrocytes in vitro. Mol. Med. Rep. 2015, 12, 6171–6177. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Akiyama, T.; Irei, I.; Hamazaki, S.; Sadahira, Y. Cellular localization of sphingosine-1-phosphate receptor 1 expression in the human central nervous system. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2010, 58, 847–856. [Google Scholar] [CrossRef]
- Dal Buono, A.; Gabbiadini, R.; Alfarone, L.; Solitano, V.; Repici, A.; Vetrano, S.; Spinelli, A.; Armuzzi, A. Sphingosine 1-Phosphate Modulation in Inflammatory Bowel Diseases: Keeping Lymphocytes Out of the Intestine. Biomedicines 2022, 10, 1735. [Google Scholar] [CrossRef]
- Kharel, Y.; Lee, S.; Snyder, A.H.; Sheasley-O’neill, S.L.; Morris, M.A.; Setiady, Y.; Zhu, R.; Zigler, M.A.; Burcin, T.L.; Ley, K.; et al. Sphingosine kinase 2 is required for modulation of lymphocyte traffic by FTY720. J. Biol. Chem. 2005, 280, 36865–36872. [Google Scholar] [CrossRef]
- Hashemian, M.; Ghasemi-Kasman, M.; Parsian, H.; Sadeghi, F. Fingolimod (FTY720) improves the functional recovery and myelin preservation of the optic pathway in focal demyelination model of rat optic chiasm. Brain Res. Bull. 2019, 153, 109–121. [Google Scholar] [CrossRef]
- Yazdi, A.; Baharvand, H.; Javan, M. Enhanced remyelination following lysolecithin-induced demyelination in mice under treatment with fingolimod (FTY720). Neuroscience 2015, 311, 34–44. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z.G.; Li, Y.; Ding, X.; Shang, X.; Lu, M.; Elias, S.B.; Chopp, M. Fingolimod treatment promotes proliferation and differentiation of oligodendrocyte progenitor cells in mice with experimental autoimmune encephalomyelitis. Neurobiol. Dis. 2015, 76, 57–66. [Google Scholar] [CrossRef]
- Alme, M.N.; Nystad, A.E.; Bo, L.; Myhr, K.M.; Vedeler, C.A.; Wergeland, S.; Torkildsen, O. Fingolimod does not enhance cerebellar remyelination in the cuprizone model. J. Neuroimmunol. 2015, 285, 180–186. [Google Scholar] [CrossRef]
- Hu, Y.; Lee, X.; Ji, B.; Guckian, K.; Apicco, D.; Pepinsky, R.B.; Miller, R.H.; Mi, S. Sphingosine 1-phosphate receptor modulator fingolimod (FTY720) does not promote remyelination in vivo. Mol. Cell. Neurosci. 2011, 48, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Miron, V.E.; Dukala, D.; Proia, R.L.; Ludwin, S.K.; Traka, M.; Antel, J.P.; Soliven, B. Neurobiological effects of sphingosine 1-phosphate receptor modulation in the cuprizone model. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2011, 25, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Safaeinejad, F.; Asadi, S.; Ghafghazi, S.; Niknejad, H. The Synergistic Anti-Apoptosis Effects of Amniotic Epithelial Stem Cell Conditioned Medium and Ponesimod on the Oligodendrocyte Cells. Front. Pharmacol. 2021, 12, 691099. [Google Scholar] [CrossRef] [PubMed]
- Kihara, Y.; Jonnalagadda, D.; Zhu, Y.; Ray, M.; Ngo, T.; Palmer, C.; Rivera, R.; Chun, J. Ponesimod inhibits astrocyte-mediated neuroinflammation and protects against cingulum demyelination via S1P(1)-selective modulation. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2022, 36, e22132. [Google Scholar] [CrossRef] [PubMed]
- Regner-Nelke, L.; Pawlitzki, M.; Willison, A.; Rolfes, L.; Oezalp, S.H.; Nelke, C.; Kölsche, T.; Korsen, M.; Grothe, M.; Groppa, S.; et al. Real-world evidence on siponimod treatment in patients with secondary progressive multiple sclerosis. Neurol. Res. Pract. 2022, 4, 55. [Google Scholar] [CrossRef]
- Mannioui, A.; Vauzanges, Q.; Fini, J.B.; Henriet, E.; Sekizar, S.; Azoyan, L.; Thomas, J.L.; Pasquier, D.D.; Giovannangeli, C.; Demeneix, B.; et al. The Xenopus tadpole: An in vivo model to screen drugs favoring remyelination. Mult. Scler. (Houndmills Basingstoke Engl.) 2018, 24, 1421–1432. [Google Scholar] [CrossRef]
- Gentile, A.; Musella, A.; Bullitta, S.; Fresegna, D.; De Vito, F.; Fantozzi, R.; Piras, E.; Gargano, F.; Borsellino, G.; Battistini, L.; et al. Siponimod (BAF312) prevents synaptic neurodegeneration in experimental multiple sclerosis. J. Neuroinflammation 2016, 13, 207. [Google Scholar] [CrossRef]
- O’Sullivan, C.; Schubart, A.; Mir, A.K.; Dev, K.K. The dual S1PR1/S1PR5 drug BAF312 (Siponimod) attenuates demyelination in organotypic slice cultures. J. Neuroinflammation 2016, 13, 31. [Google Scholar] [CrossRef]
- Jackson, S.J.; Giovannoni, G.; Baker, D. Fingolimod modulates microglial activation to augment markers of remyelination. J. Neuroinflammation 2011, 8, 76. [Google Scholar] [CrossRef]
- Behrangi, N.; Heinig, L.; Frintrop, L.; Santrau, E.; Kurth, J.; Krause, B.; Atanasova, D.; Clarner, T.; Fragoulis, A.; Joksch, M.; et al. Siponimod ameliorates metabolic oligodendrocyte injury via the sphingosine-1 phosphate receptor 5. Proc. Natl. Acad. Sci. USA 2022, 119, e2204509119. [Google Scholar] [CrossRef]
- Kipp, M.; Hochstrasser, T.; Schmitz, C.; Beyer, C. Female sex steroids and glia cells: Impact on multiple sclerosis lesion formation and fine tuning of the local neurodegenerative cellular network. Neurosci. Biobehav. Rev. 2016, 67, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.; Ghoumari, A.M.; Bielecki, B.; Steibel, J.; Boehm, N.; Liere, P.; Macklin, W.B.; Kumar, N.; Habert, R.; Mhaouty-Kodja, S.; et al. The neural androgen receptor: A therapeutic target for myelin repair in chronic demyelination. Brain J. Neurol. 2013, 136 Pt 1, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Bielecki, B.; Mattern, C.; Ghoumari, A.M.; Javaid, S.; Smietanka, K.; Abi Ghanem, C.; Mhaouty-Kodja, S.; Ghandour, M.S.; Baulieu, E.E.; Franklin, R.J.; et al. Unexpected central role of the androgen receptor in the spontaneous regeneration of myelin. Proc. Natl. Acad. Sci. USA 2016, 113, 14829–14834. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Karakuzu, A.; Cohen-Adad, J.; Cercignani, M.; Nichols, T.E.; Stikov, N. An interactive meta-analysis of MRI biomarkers of myelin. eLife 2020, 9, e61523. [Google Scholar] [CrossRef] [PubMed]
- van der Weijden, C.W.J.; Biondetti, E.; Gutmann, I.W.; Dijkstra, H.; McKerchar, R.; de Paula Faria, D.; de Vries, E.F.J.; Meilof, J.F.; Dierckx, R.; Prevost, V.H.; et al. Quantitative myelin imaging with MRI and PET: An overview of techniques and their validation status. Brain J. Neurol. 2022, awac436. [Google Scholar] [CrossRef]
- Kaddatz, H.; Joost, S.; Nedelcu, J.; Chrzanowski, U.; Schmitz, C.; Gingele, S.; Gudi, V.; Stangel, M.; Zhan, J.; Santrau, E.; et al. Cuprizone-induced demyelination triggers a CD8-pronounced T cell recruitment. Glia 2021, 69, 925–942. [Google Scholar] [CrossRef]
- Baxi, E.G.; DeBruin, J.; Tosi, D.M.; Grishkan, I.V.; Smith, M.D.; Kirby, L.A.; Strasburger, H.J.; Fairchild, A.N.; Calabresi, P.A.; Gocke, A.R. Transfer of myelin-reactive th17 cells impairs endogenous remyelination in the central nervous system of cuprizone-fed mice. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 8626–8639. [Google Scholar] [CrossRef]
- Hines, J.H. Evolutionary Origins of the Oligodendrocyte Cell Type and Adaptive Myelination. Front. Neurosci. 2021, 15, 757360. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).