The Role of MicroRNAs in Myocarditis—What Can We Learn from Clinical Trials?
Abstract
1. Introduction
1.1. Basic Knowledge and Statistics about Myocarditis
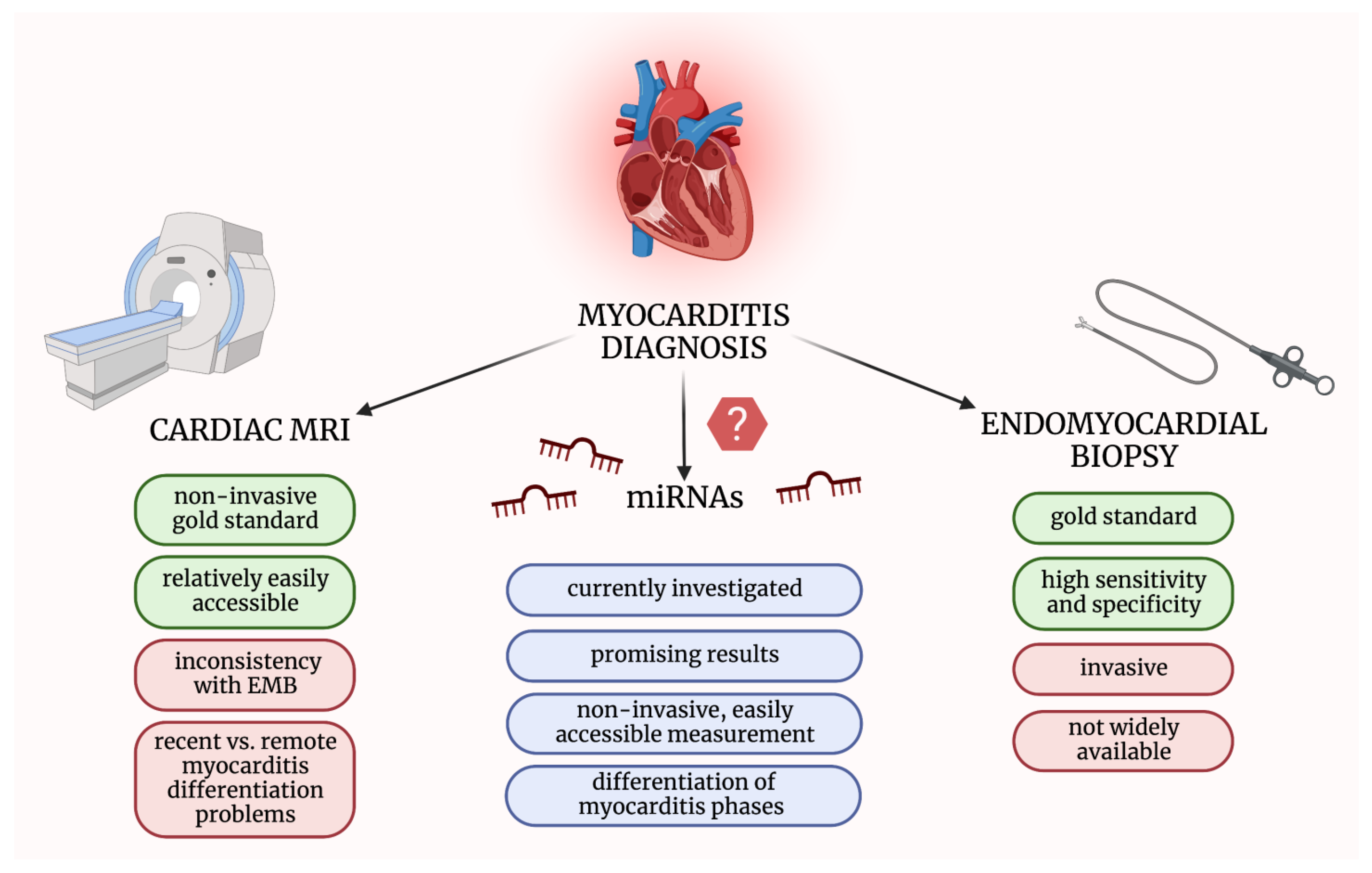
1.2. Available Diagnostic and Prognostic Tools in Myocarditis
1.3. Biosynthesis and Function of miRNAs
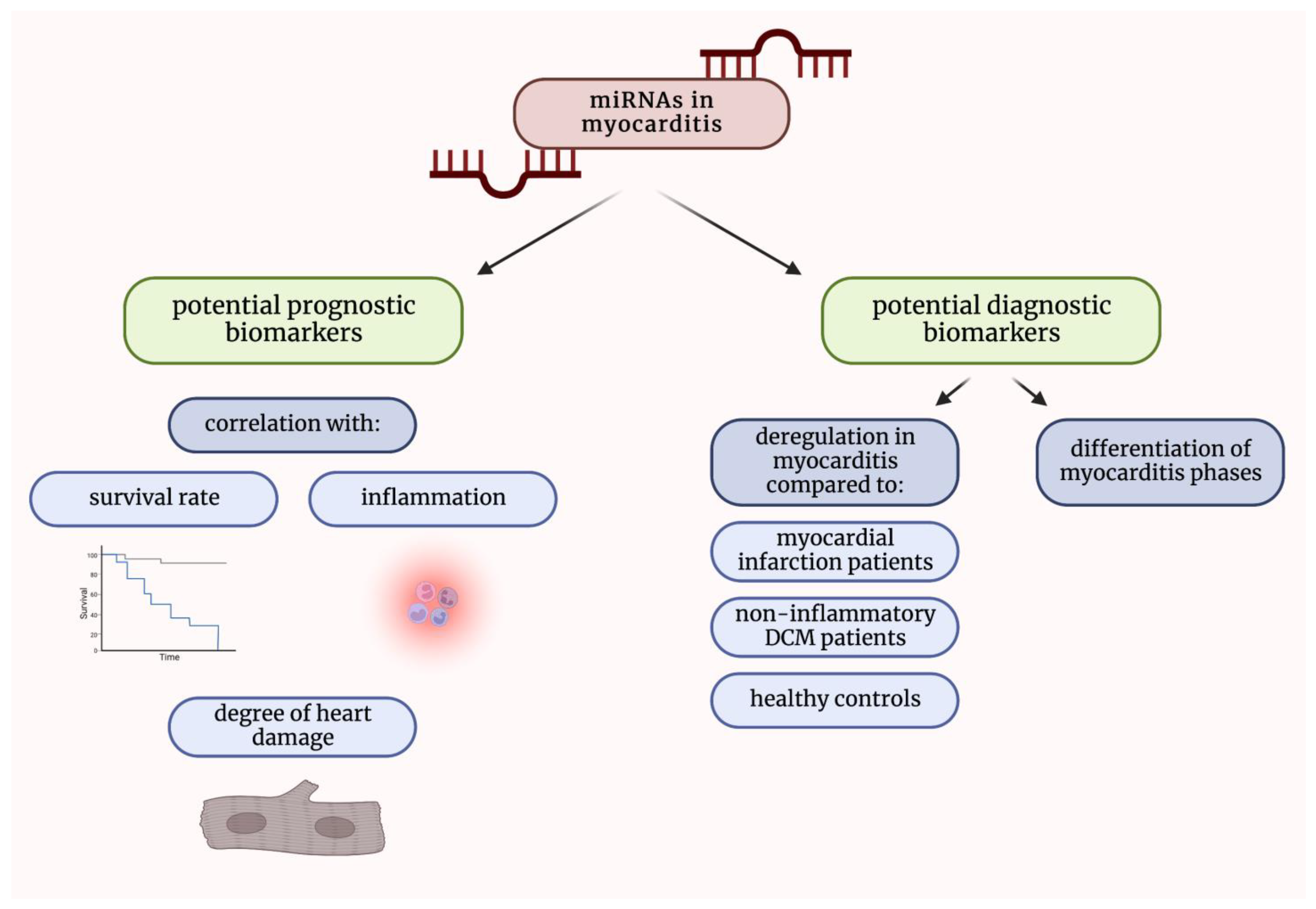
1.4. The Putative Role of miRNAs in Myocarditis
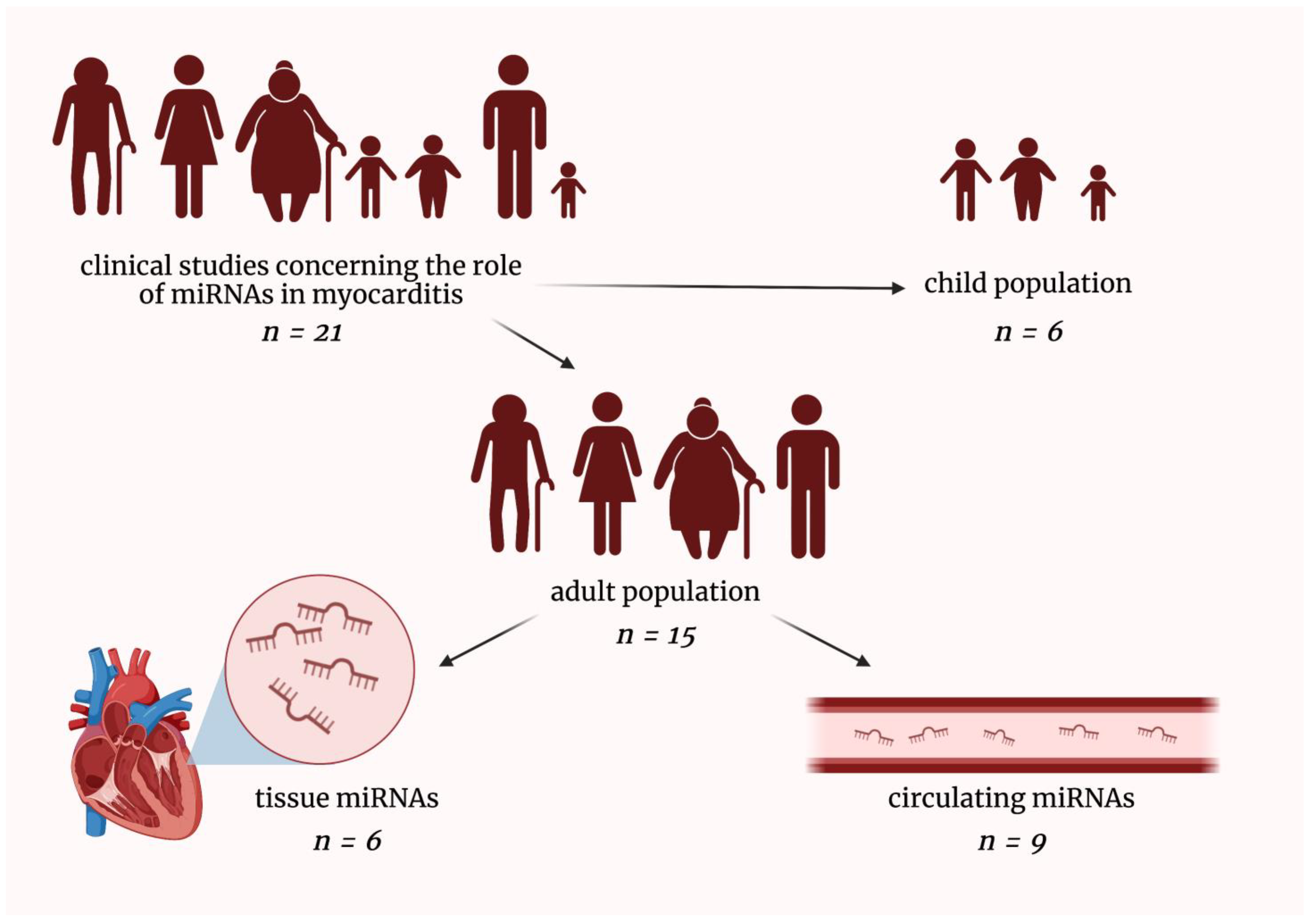
2. MicroRNAs in Patients Suffering from Myocarditis
2.1. MicroRNAs in Pediatric Myocarditis Patients
2.2. Tissue MicroRNAs in Adult Myocarditis Patients
2.3. Circulating MicroRNAs in Adult Myocarditis Patients
3. Conclusions
4. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richardson, P.; McKenna, W.; Bristow, M.; Maisch, B.; Mautner, B.; O’Connell, J.; Olsen, E.; Thiene, G.; Goodwin, J.; Gyarfas, I.; et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation 1996, 93, 841–842. [Google Scholar] [CrossRef] [PubMed]
- Caforio, A.L.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef] [PubMed]
- Tschöpe, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.P.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hübner, N.; et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2021, 18, 169–193. [Google Scholar] [CrossRef] [PubMed]
- Lampejo, T.; Durkin, S.M.; Bhatt, N.; Guttmann, O. Acute myocarditis: Aetiology, diagnosis and management. Clin. Med. 2021, 21, e505–e510. [Google Scholar] [CrossRef]
- Cooper, L.T., Jr. Myocarditis. N. Engl. J. Med. 2009, 360, 1526–1538. [Google Scholar] [CrossRef]
- Tymińska, A.; Ozierański, K.; Skwarek, A.; Kapłon-Cieślicka, A.; Baritussio, A.; Grabowski, M.; Marcolongo, R.; Caforio, A.L. Personalized Management of Myocarditis and Inflammatory Cardiomyopathy in Clinical Practice. J. Pers. Med. 2022, 12, 183. [Google Scholar] [CrossRef]
- Schultheiss, H.P.; Kühl, U.; Cooper, L.T. The management of myocarditis. Eur. Heart J. 2011, 32, 2616–2625. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013, 128, e240–e327. [Google Scholar] [CrossRef]
- Pilati, M.; Rebonato, M.; Formigari, R.; Butera, G. Endomyocardial Biopsy in Pediatric Myocarditis and Dilated Cardiomyopathy: A Tool in Search for a Role. J. Cardiovasc. Dev. Dis. 2022, 9, 24. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Zeiher, A.M.; Nagel, E. T1 and T2 mapping in myocarditis: Seeing beyond the horizon of Lake Louise criteria and histopathology. Expert Rev. Cardiovasc. Ther. 2018, 16, 319–330. [Google Scholar] [CrossRef]
- Gannon, M.P.; Schaub, E.; Grines, C.L.; Saba, S.G. State of the art: Evaluation and prognostication of myocarditis using cardiac MRI. J. Magn. Reson. Imaging 2019, 49, e122–e131. [Google Scholar] [CrossRef] [PubMed]
- Liguori, C.; Farina, D.; Vaccher, F.; Ferrandino, G.; Bellini, D.; Carbone, I. Myocarditis: Imaging up to date. Radiol. Med. 2020, 125, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, E.; Veronese, G.; Bottiroli, M.; Wang, D.W.; Cipriani, M.; Garascia, A.; Pedrotti, P.; Adler, E.D.; Frigerio, M. Update on acute myocarditis. Trends Cardiovasc. Med. 2021, 31, 370–379. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, R.E., 3rd; Boehmer, J.P.; Hruban, R.H.; Hutchins, G.M.; Kasper, E.K.; Hare, J.M.; Baughman, K.L. Long-term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. N. Engl. J. Med. 2000, 342, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Procyk, G.; Bilicki, D.; Balsam, P.; Lodziński, P.; Grabowski, M.; Gąsecka, A. Extracellular Vesicles in Atrial Fibrillation-State of the Art. Int. J. Mol. Sci. 2022, 23, 7591. [Google Scholar] [CrossRef]
- Gartshteyn, Y.; Tamargo, M.; Fleischer, S.; Kapoor, T.; Li, J.; Askanase, A.; Winchester, R.; Geraldino-Pardilla, L. Endomyocardial biopsies in the diagnosis of myocardial involvement in systemic lupus erythematosus. Lupus 2020, 29, 199–204. [Google Scholar] [CrossRef]
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef]
- Lu, J.; Clark, A.G. Impact of microRNA regulation on variation in human gene expression. Genome Res. 2012, 22, 1243–1254. [Google Scholar] [CrossRef]
- Hoefig, K.P.; Heissmeyer, V. MicroRNAs grow up in the immune system. Curr. Opin. Immunol. 2008, 20, 281–287. [Google Scholar] [CrossRef]
- Barwari, T.; Joshi, A.; Mayr, M. MicroRNAs in Cardiovascular Disease. J. Am. Coll. Cardiol. 2016, 68, 2577–2584. [Google Scholar] [CrossRef]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell. Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef] [PubMed]
- Creugny, A.; Fender, A.; Pfeffer, S. Regulation of primary microRNA processing. FEBS Lett. 2018, 592, 1980–1996. [Google Scholar] [CrossRef]
- Michlewski, G.; Cáceres, J.F. Post-transcriptional control of miRNA biogenesis. RNA 2019, 25, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, B.C.; Ooi, J.Y.; Lin, R.C.; McMullen, J.R. miRNA therapeutics: A new class of drugs with potential therapeutic applications in the heart. Future Med. Chem. 2015, 7, 1771–1792. [Google Scholar] [CrossRef] [PubMed]
- Iwakawa, H.O.; Tomari, Y. Life of RISC: Formation, action, and degradation of RNA-induced silencing complex. Mol. Cell 2022, 82, 30–43. [Google Scholar] [CrossRef]
- McGeary, S.E.; Lin, K.S.; Shi, C.Y.; Pham, T.M.; Bisaria, N.; Kelley, G.M.; Bartel, D.P. The biochemical basis of microRNA targeting efficacy. Science 2019, 366, eaav1741. [Google Scholar] [CrossRef]
- Helwak, A.; Kudla, G.; Dudnakova, T.; Tollervey, D. Mapping the human miRNA interactome by CLASH reveals frequent noncanonical binding. Cell 2013, 153, 654–665. [Google Scholar] [CrossRef]
- Colpaert, R.M.W.; Calore, M. Epigenetics and microRNAs in cardiovascular diseases. Genomics 2021, 113, 540–551. [Google Scholar] [CrossRef]
- Inoue, J.; Inazawa, J. Cancer-associated miRNAs and their therapeutic potential. J. Hum. Genet. 2021, 66, 937–945. [Google Scholar] [CrossRef]
- Halushka, P.V.; Goodwin, A.J.; Halushka, M.K. Opportunities for microRNAs in the Crowded Field of Cardiovascular Biomarkers. Annu. Rev. Pathol. 2019, 14, 211–238. [Google Scholar] [CrossRef]
- Oh, J.H.; Kim, G.B.; Seok, H. Implication of microRNA as a potential biomarker of myocarditis. Clin. Exp. Pediatr. 2022, 65, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Procyk, G.; Klimczak-Tomaniak, D.; Sygitowicz, G.; Tomaniak, M. Circulating and Platelet MicroRNAs in Cardiovascular Risk Assessment and Antiplatelet Therapy Monitoring. J. Clin. Med. 2022, 11, 1763. [Google Scholar] [CrossRef] [PubMed]
- Neri, M.; Fabbri, M.; D’Errico, S.; Di Paolo, M.; Frati, P.; Gaudio, R.M.; La Russa, R.; Maiese, A.; Marti, M.; Pinchi, E.; et al. Regulation of miRNAs as new tool for cutaneous vitality lesions demonstration in ligature marks in deaths by hanging. Sci. Rep. 2019, 9, 20011. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, A.; Chiti, E.; Maiese, A.; Turillazzi, E.; Spinetti, I. MicroRNAs: An Update of Applications in Forensic Science. Diagnostics 2020, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Tan, R.; Wong, L.; Fekete, R.; Halsey, J. Quantitation of microRNAs by real-time RT-qPCR. Methods Mol. Biol. 2011, 687, 113–134. [Google Scholar] [CrossRef]
- Nie, X.; He, M.; Wang, J.; Chen, P.; Wang, F.; Lai, J.; Li, C.; Yu, T.; Zuo, H.; Cui, G.; et al. Circulating miR-4763-3p Is a Novel Potential Biomarker Candidate for Human Adult Fulminant Myocarditis. Mol. Ther. Methods Clin. Dev. 2020, 17, 1079–1087. [Google Scholar] [CrossRef]
- Corsten, M.F.; Papageorgiou, A.; Verhesen, W.; Carai, P.; Lindow, M.; Obad, S.; Summer, G.; Coort, S.L.; Hazebroek, M.; van Leeuwen, R.; et al. MicroRNA profiling identifies microRNA-155 as an adverse mediator of cardiac injury and dysfunction during acute viral myocarditis. Circ. Res. 2012, 111, 415–425. [Google Scholar] [CrossRef]
- Yan, M.; Wang, J.; Wang, S.; Zhang, Y.; Liu, L.; Zhao, H. Expression Levels of MicroRNA-146b and Anti-Cardiac Troponin I in Serum of Children with Viral Myocarditis and Their Clinical Significance. Iran. J. Public Health 2021, 50, 510–519. [Google Scholar] [CrossRef]
- Xia, K.; Zhang, Y.; Sun, D. miR-217 and miR-543 downregulation mitigates inflammatory response and myocardial injury in children with viral myocarditis by regulating the SIRT1/AMPK/NF-κB signaling pathway. Int. J. Mol. Med. 2020, 45, 634–646. [Google Scholar] [CrossRef]
- Goldberg, L.; Tirosh-Wagner, T.; Vardi, A.; Abbas, H.; Pillar, N.; Shomron, N.; Nevo-Caspi, Y.; Paret, G. Circulating MicroRNAs: A Potential Biomarker for Cardiac Damage, Inflammatory Response, and Left Ventricular Function Recovery in Pediatric Viral Myocarditis. J. Cardiovasc. Transl. Res. 2018, 11, 319–328. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, L.; Sun, H.; Yu, Z.; Liu, X.; Luo, X.; Li, C.; Sun, D.; Li, T. MicroRNA-381 protects myocardial cell function in children and mice with viral myocarditis via targeting cyclooxygenase-2 expression. Exp. Ther. Med. 2018, 15, 5510–5516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, L.; Sun, H.; Liu, X.; Luo, X.; Li, C.; Sun, D.; Li, T. Overexpression of microRNA-133b reduces myocardial injuries in children with viral myocarditis by targeting Rab27B gene. Cell. Mol. Biol. 2017, 63, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, T.; Cui, H.; Zhang, Y. Analysis of the Indicating Value of Cardiac Troponin I, Tumor Necrosis Factor-α, Interleukin-18, Mir-1 and Mir-146b for Viral Myocarditis among Children. Cell. Physiol. Biochem. 2016, 40, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Besler, C.; Urban, D.; Watzka, S.; Lang, D.; Rommel, K.P.; Kandolf, R.; Klingel, K.; Thiele, H.; Linke, A.; Schuler, G.; et al. Endomyocardial miR-133a levels correlate with myocardial inflammation, improved left ventricular function, and clinical outcome in patients with inflammatory cardiomyopathy. Eur. J. Heart Fail. 2016, 18, 1442–1451. [Google Scholar] [CrossRef]
- Ferreira, L.R.; Frade, A.F.; Santos, R.H.; Teixeira, P.C.; Baron, M.A.; Navarro, I.C.; Benvenuti, L.A.; Fiorelli, A.I.; Bocchi, E.A.; Stolf, N.A.; et al. MicroRNAs miR-1, miR-133a, miR-133b, miR-208a and miR-208b are dysregulated in Chronic Chagas disease Cardiomyopathy. Int. J. Cardiol. 2014, 175, 409–417. [Google Scholar] [CrossRef]
- Bao, J.L.; Lin, L. MiR-155 and miR-148a reduce cardiac injury by inhibiting NF-κB pathway during acute viral myocarditis. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2349–2356. [Google Scholar]
- Chen, Z.G.; Liu, H.; Zhang, J.B.; Zhang, S.L.; Zhao, L.H.; Liang, W.Q. Upregulated microRNA-214 enhances cardiac injury by targeting ITCH during coxsackievirus infection. Mol. Med. Rep. 2015, 12, 1258–1264. [Google Scholar] [CrossRef]
- Kühl, U.; Rohde, M.; Lassner, D.; Gross, U.M.; Escher, F.; Schultheiss, H.P. miRNA as activity markers in Parvo B19 associated heart disease. Herz 2012, 37, 637–643. [Google Scholar] [CrossRef]
- Blanco-Domínguez, R.; Sánchez-Díaz, R.; de la Fuente, H.; Jiménez-Borreguero, L.J.; Matesanz-Marín, A.; Relaño, M.; Jiménez-Alejandre, R.; Linillos-Pradillo, B.; Tsilingiri, K.; Martín-Mariscal, M.L.; et al. A Novel Circulating MicroRNA for the Detection of Acute Myocarditis. N. Engl. J. Med. 2021, 384, 2014–2027. [Google Scholar] [CrossRef]
- Aleshcheva, G.; Pietsch, H.; Escher, F.; Schultheiss, H.P. MicroRNA profiling as a novel diagnostic tool for identification of patients with inflammatory and/or virally induced cardiomyopathies. ESC Heart Fail. 2021, 8, 408–422. [Google Scholar] [CrossRef]
- Chen, J.H.; He, J.; Zhou, R.; Zheng, N. Expression and Significance of Circulating microRNA-29b in Adult Fulminant Myocarditis. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2022, 44, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, D.; Rommel, K.P.; Blazek, S.; Klingel, K.; Gutberlet, M.; Lücke, C.; Büttner, P.; Thiele, H.; Adams, V.; Lurz, P.; et al. The potential role of plasma miR-155 and miR-206 as circulatory biomarkers in inflammatory cardiomyopathy. ESC Heart Fail. 2021, 8, 1850–1860. [Google Scholar] [CrossRef] [PubMed]
- Marketou, M.; Kontaraki, J.; Patrianakos, A.; Kochiadakis, G.; Anastasiou, I.; Fragkiadakis, K.; Plevritaki, A.; Papadaki, S.T.; Chlouverakis, G.; Parthenakis, F. Peripheral Blood MicroRNAs as Potential Biomarkers of Myocardial Damage in Acute Viral Myocarditis. Genes 2021, 12, 420. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Wang, D.; Jiang, X.; Zhang, M.; Lv, K. Serum exosome microRNA panel as a noninvasive biomarker for molecular diagnosis of fulminant myocarditis. Mol. Ther. Methods Clin. Dev. 2021, 20, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.L.; Li, M.F.; Cui, F.; Feng, F.; Kong, L.; Zhang, F.H.; Hao, H.; Yin, M.X.; Liu, Y. Altered exosomal miR-181d and miR-30a related to the pathogenesis of CVB3 induced myocarditis by targeting SOCS3. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2208–2215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.Y.; Zhao, Z.; Jin, Z. Expression of miR-98 in myocarditis and its influence on transcription of the FAS/FASL gene pair. Genet. Mol. Res. 2016, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
| Ref. | Year | Population | Comparison | miRNA | Outcome | Methodology |
|---|---|---|---|---|---|---|
| Yan et al. [38] | 2021 | 48 VMC pediatric pts | 40 HCs | miR-146b | ↑ miR-146b in VMC pts compared to HCs | miRs in serum by qPCR |
| Xia et al. [39] | 2020 | 30 VMC pediatric pts | HCs | miR-217 miR-543 | ↑ miR-217 and miR-543 in study groups compared to controls | miRs in blood by qPCR |
| Goldberg et al. [40] | 2018 | 8 VMC pediatric pts | comparison of acute, subacute and resolution/chronic phase of VMC | miR-21 miR-208a | ↑ miR-208a in acute phase compared to other phases ↓ miR-21 in resolution/chronic phase compared to other phases | miRs in serum by qPCR |
| Zhang et al. [41] | 2018 | 26 VMC pediatric pts | 33 pediatric pts recovered from VMC | miR-381 | ↓ miR-381 in VMC pts compared to recovered pts | miRs in serum by qPCR |
| Zhang et al. [42] | 2017 | 36 VMC pediatric pts | HCs | miR-133b | ↓ miR-133b in VMC pts | miRs in blood by qPCR |
| Wang et al. [43] | 2016 | 119 VMC pediatric pts | 120 HCs | miR-1 miR-146b | ↓ miR-1 and ↑ miR-146b in VMC pts | miRs in serum by qPCR |
| Ref. | Year | Population | Comparison | miRNA | Outcome | Methodology |
|---|---|---|---|---|---|---|
| Besler et al. [44] | 2016 | 76 VMC pts | 22 DCM pts | miR-133a miR-155 | ↑ miR-133a, miR-155 in MC pts ↑ miR-133a associated with ↓ fibrosis and ↑ LV function miR-133a and miR-155 levels correlated with inflammatory cells count in MC pts | miRs in heart tissue by qPCR fibrosis, inflammatory cells by HP LV function in ECHO |
| Ferreira et al. [45] | 2014 | 10 CCC pts | 6 DCM pts 4 HCs | miR-1 miR-133a miR-133b miR-208a miR-208b | ↓ miR-1, miR-133a and miR-208b in CCC pts compared to others ↓ miR-133b and miR-208a in CCC pts compared to HCs | miRs in heart tissue by qPCR |
| Bao et al. [46] | 2014 | 6 acute MC pts | 6 HCs | miR-155 miR-148a | ↑ miR-155 and miR-148a in MC pts | miRs in heart biopsy by qPCR |
| Chen et al. [47] | 2015 | 8 acute VMC pts | 8 HCs | miR-214 miR-146b | ↓ miR-214 and miR-146b in VMC pts | miRs in heart tissue by qPCR |
| Kühl et al. [48] | 2012 | 15 active B19V infection pts | 45 inactive B19V infection pts | miRNAome | 29 differently-expressed miRs predicted miR-Ts involved in immune response and energy metabolism | miRs in heart tissue by qPCR |
| Corsten et al. [37] | 2012 | 4 acute MC pts | 6 HCs | miRNAome | 107 deregulated miRs in MC pts compared to HCs | miRs in heart biopsy by microarray analysis |
| Ref. | Year | Population | Comparison | miRNA | Outcome | Methodology |
|---|---|---|---|---|---|---|
| Blanco-Domínguez et al. [49] | 2021 | 39 acute MC pts | 39 STEMI pts 38 NSTEMI pts31 HCs | miR-Chr8:96 | ↑ miR-Chr8:96 in MC pts compared to other groups | miRs in plasma by qPCR |
| Nie et al. [36] | 2020 | 20 fulminant MC pts | 5 MI pts 10 HCs | miR-4763-3p miR-4281 | ↑ miR-4763-3p in fulminant MC pts compared to other groups↑ miR-4281 in fulminant MC pts compared to HCs | miRs in plasma by microarray and qPCR |
| Aleshcheva et al. [50] | 2020 | 343 AMC or VMC pts | 71 DCM pts 85 HCs | Let-7f miR-197 miR-223 miR-93 miR-379 | deregulation of these miRNAs only in MC pts: ↑ miR-93, miR-197, miR-379 compared to other groups ↓ let7f, miR-223 compared to HCs | miRs in serum by qPCR |
| Chen et al. [51] | 2022 | fulminant MC pts | HCs | miR-29b miR-125b | ↑ miR-29b and miR-125b in fulminant MC patients compared to HCs | miRs in plasma by microarray analyses and qPCR |
| Obradovic et al. [52] | 2021 | 60 MC pts | 29 DCM pts | miR-155 miR-206 | ↑ miR-155 and miR-206 in MC pts compared to control group | miRs in plasma by qPCR |
| Marketou et al. [53] | 2021 | 40 acute VMC pts | 29 HCs | miR-21-5p miR-1-3p | ↑ miR-21-5p and miR-1-3p compared to control group | miRs in blood by qPCR |
| Zhang et al. [54] | 2021 | 99 fulminant MC pts | 32 non-fulminant MC pts 105 HCs | miR-30a miR-192 miR-146a miR-155 miR-320a | ↑ miR-30a, miR-192, miR-146a, miR-155 and miR-320a in fulminant MC pts compared to other groups | exosomal miRs by NGS and qPCR |
| Fan et al. [55] | 2019 | 23 acute MC pts | 12 HCs | miR-181d miR-30a miR-125a miR-155 miR-21 | ↑ miR-181d, miR-30a, miR-125a and ↓ miR-125a, miR-21 in MC pts compared to HCs | miRs in serum exosomes by qPCR |
| Zhang et al. [56] | 2016 | 50 MC pts | 50 HCs | miR-98 | ↓ miR-98 in MC pts | miRs in plasma by qPCR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grodzka, O.; Procyk, G.; Gąsecka, A. The Role of MicroRNAs in Myocarditis—What Can We Learn from Clinical Trials? Int. J. Mol. Sci. 2022, 23, 16022. https://doi.org/10.3390/ijms232416022
Grodzka O, Procyk G, Gąsecka A. The Role of MicroRNAs in Myocarditis—What Can We Learn from Clinical Trials? International Journal of Molecular Sciences. 2022; 23(24):16022. https://doi.org/10.3390/ijms232416022
Chicago/Turabian StyleGrodzka, Olga, Grzegorz Procyk, and Aleksandra Gąsecka. 2022. "The Role of MicroRNAs in Myocarditis—What Can We Learn from Clinical Trials?" International Journal of Molecular Sciences 23, no. 24: 16022. https://doi.org/10.3390/ijms232416022
APA StyleGrodzka, O., Procyk, G., & Gąsecka, A. (2022). The Role of MicroRNAs in Myocarditis—What Can We Learn from Clinical Trials? International Journal of Molecular Sciences, 23(24), 16022. https://doi.org/10.3390/ijms232416022








