The Reactions of N,N′-Diphenyldithiomalondiamide with Arylmethylidene Meldrum’s Acids
Abstract
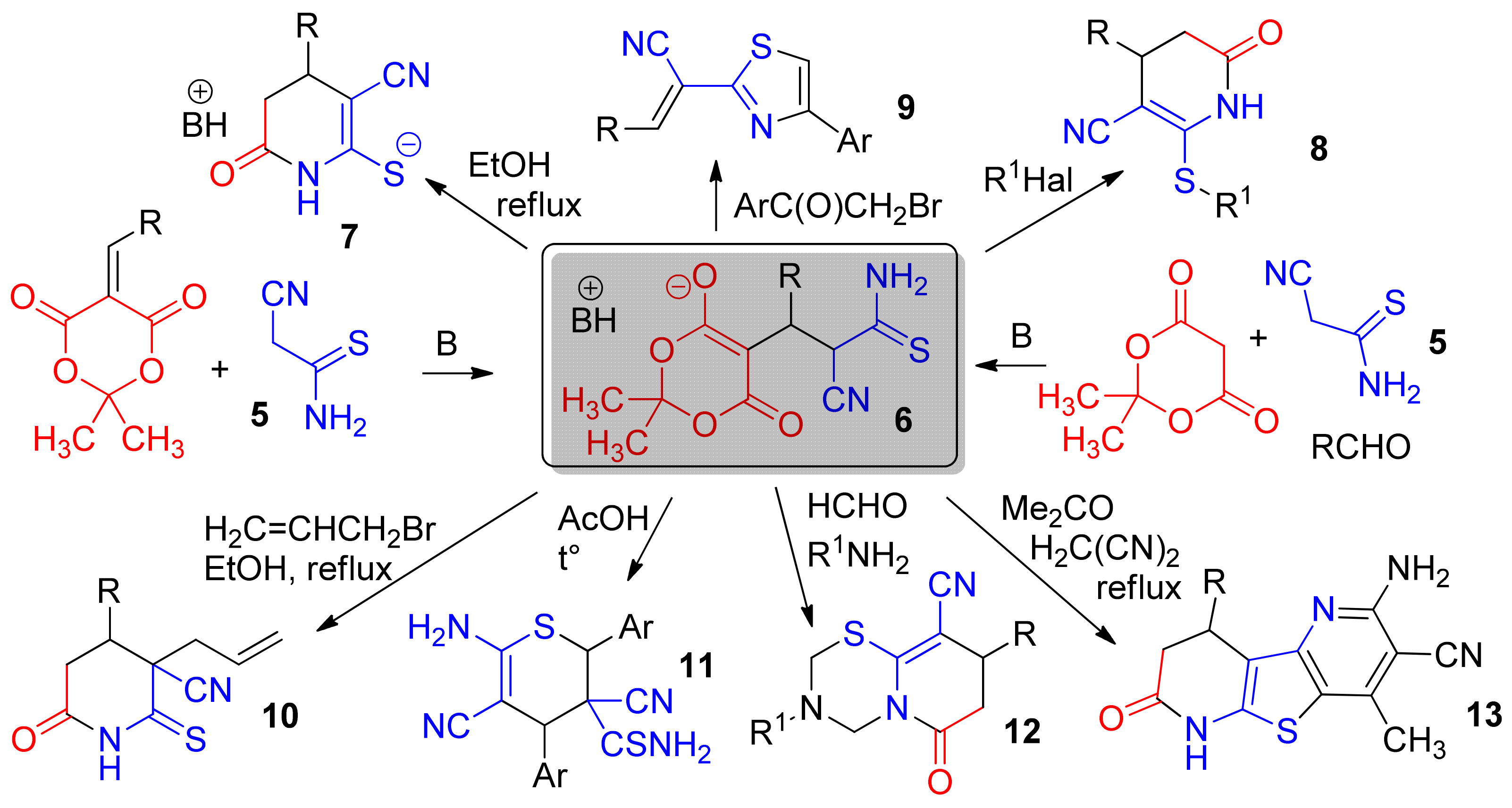
1. Introduction
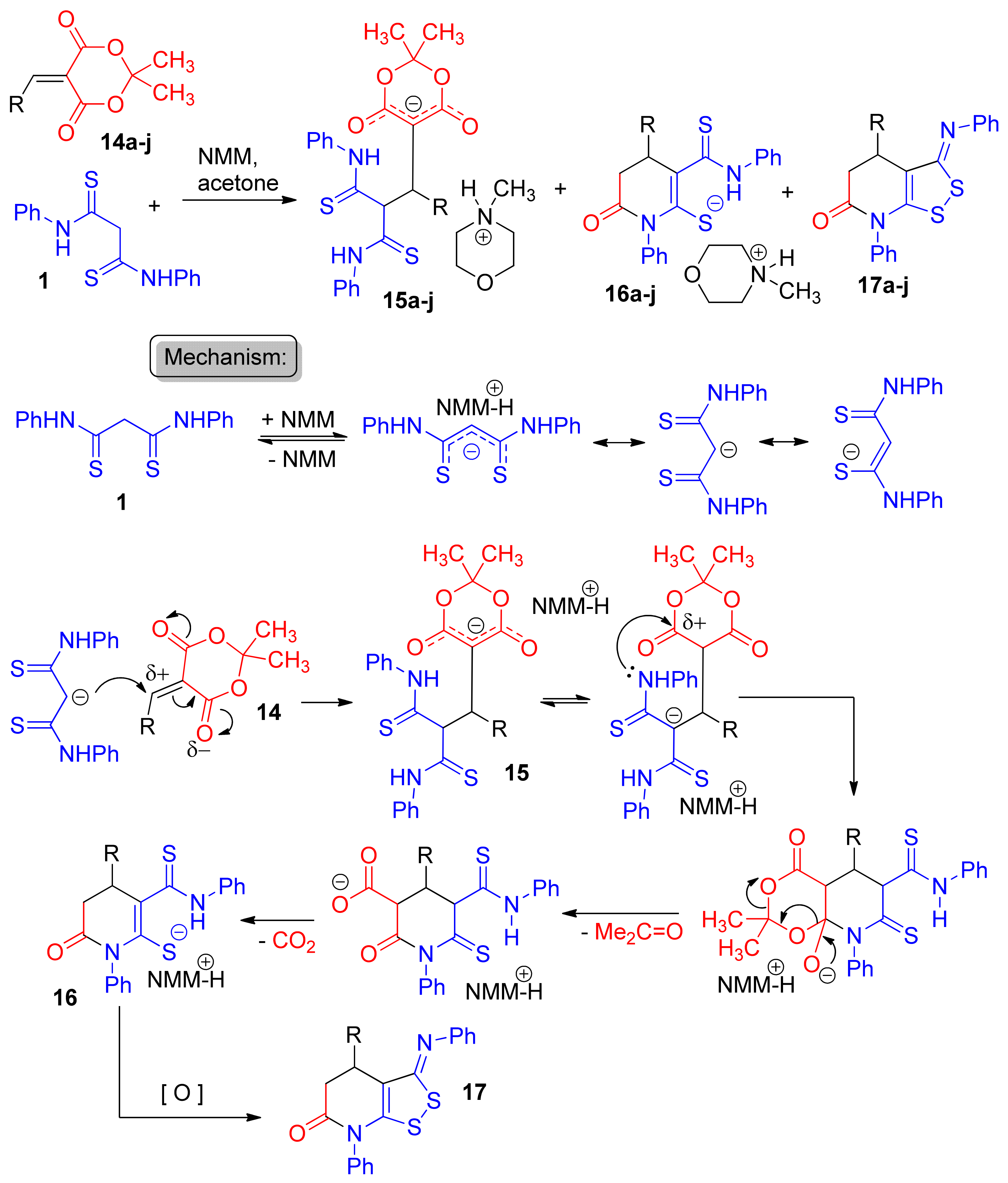
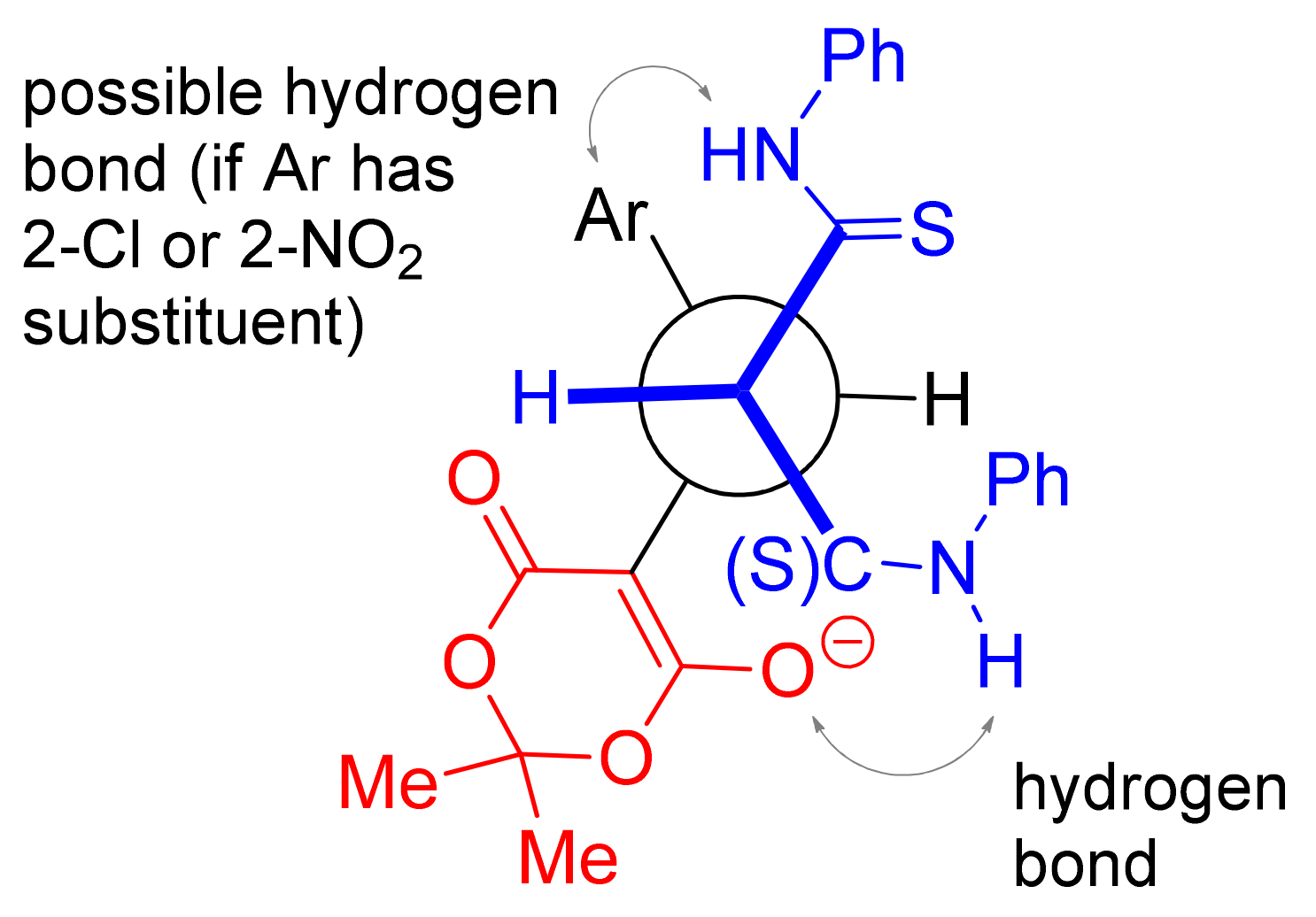
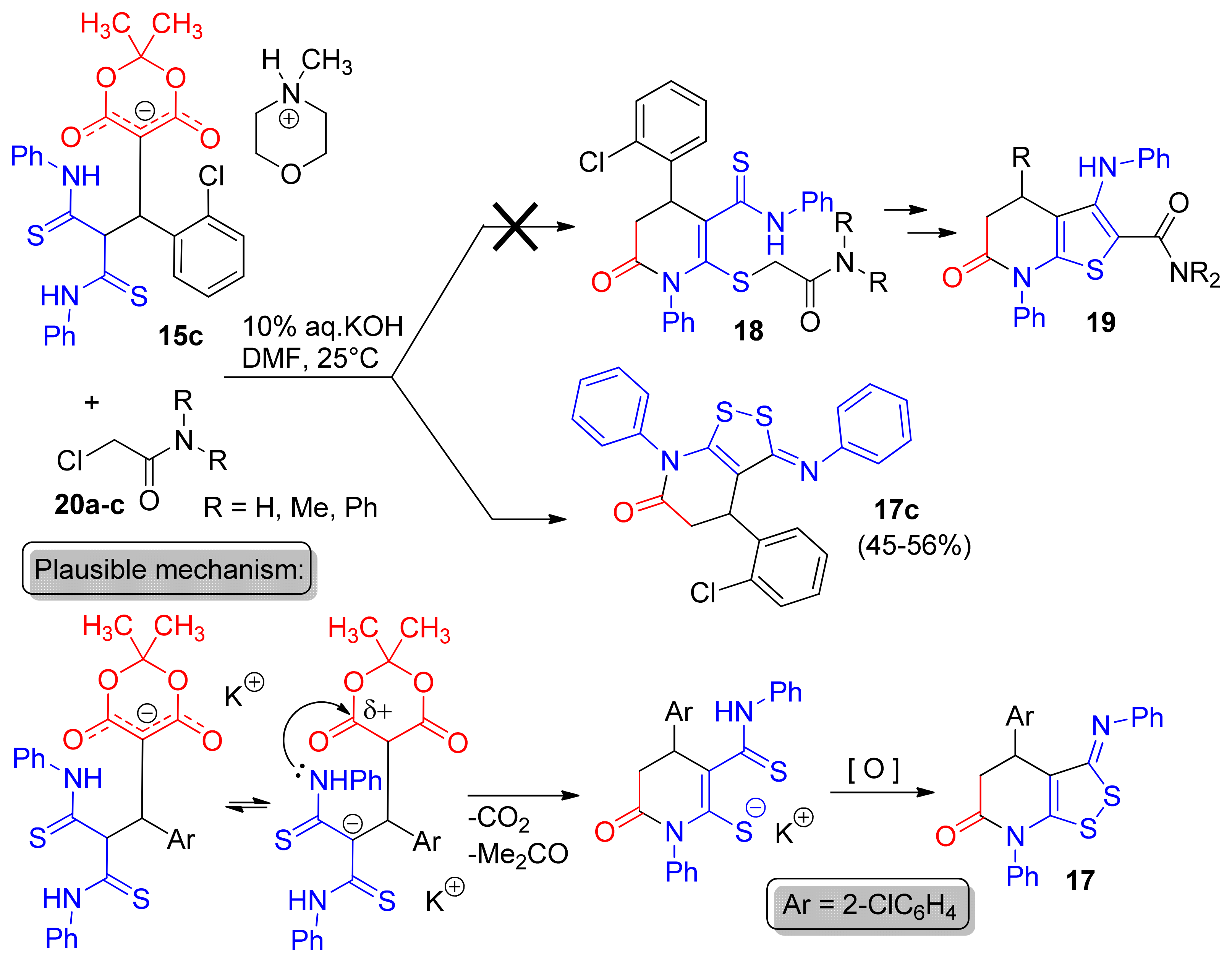
2. Results and Discussion
| Entries | Starting Reagents 14 | Reaction Conditions | Products (Yields) 1 | ||
|---|---|---|---|---|---|
| Michael Adducts 15 | Pyridine-2-Thiolates 16 | Dithiolo-Pyridines 17 | |||
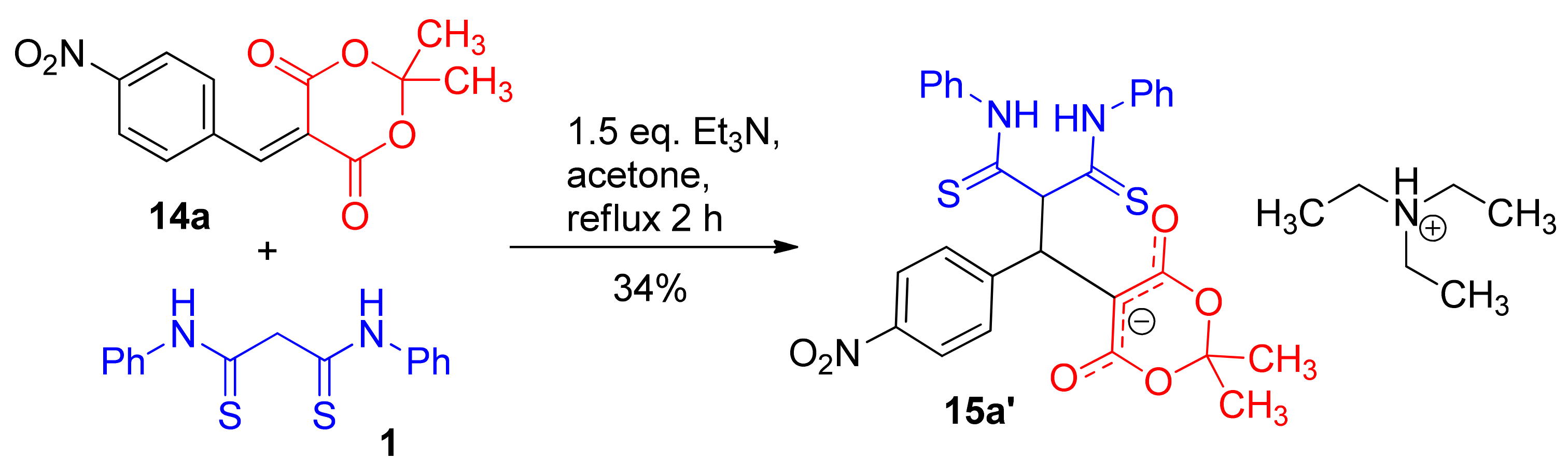
| entry 1 | 14a 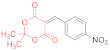 | 1.5 eq. Et3N, acetone, reflux 2 h | 15a′ (34%) | ND 2 | ND |
| entry 2 | 14a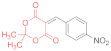 | 1.5 eq. NMM, EtOH, reflux 2 h | 15a (42%) | traces | ND |
| entry 3 | 14a | 1.5 eq. NMM, acetone, reflux 1 h | 15a (56%) | traces | ND |
| entry 4 | 14a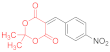 | 1.5 eq. NMM, acetone, 25 °C | 15a (9%) | ND | ND |
| entry 5 | 14a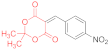 | 1.5 eq. NMM, acetone, reflux 2.5 h | 15a (24%) | ND | 17a (21%) |
| entry 6 | 14b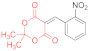 | 1.5 eq. NMM, acetone, reflux 1 h | 15b (57%) | traces | ND |
| entry 7 | 14b | 1.5 eq. NMM, acetone, reflux 2.5 h | 15b (36%) | 16b (13%) | ND |
| entry 8 | 14c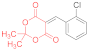 | 1.5 eq. NMM, acetone, reflux 20 min | 15c (43%) | ND | ND |
| entry 9 | 14c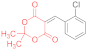 | 1.5 eq. NMM, acetone, reflux 40 min | 15c (60%) | traces | ND |
| entry 10 | 14c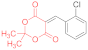 | 1.5 eq. NMM, acetone, reflux 3 h | 15c (58%) | 16b (<5%) | ND |
| entry 11 | 14d | 1.5 eq. NMM, acetone, reflux 40 min | 15d (19%) | 16d (19%) | ND |
| entry 12 | 14e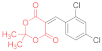 | 1.5 eq. NMM, acetone, reflux 35 min | 15e (44%) | 16e (22%) | ND |
| entry 13 | 14f | 1.5 eq. NMM, acetone, reflux 80 min | 15f (33%) | 16f (2%) | ND |
| entry 14 | 14f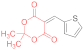 | 1.5 eq. NMM, acetone, reflux 3 h | 15f (35%) | 16f (20%) | 17f (8%) |
| entry 15 | 14g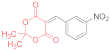 | 1.5 eq. NMM, acetone, reflux 80 min | 15g (18%) | 16g (14%) | 17g (12%) |
| entry 16 | 14h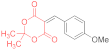 | 1.5 eq. NMM, acetone, reflux 1 h | 15h (27%) | 16h (7%) | traces |
| entry 17 | 14h | 1.5 eq. NMM, acetone, reflux 4 h | 15h (19%) | 16h (4%) | 17h (13%) |
| entry 18 | 14i | 1.5 eq. NMM, acetone, reflux 1.5 h | 15i (20%) | 16h (~2%) | ND |
| entry 19 | 14j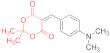 | 1.5 eq. NMM, acetone, reflux 80 min | ND | ND | 17j (29%) |
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Becker, J.; Stidsen, C.E. Recent developments in the synthesis and chemistry of 2(1H)-pyridinethiones and related compounds. Sulfur Rep. 1988, 8, 105–146. [Google Scholar] [CrossRef]
- Litvinov, V.P.; Rodinovskaya, L.A.; Sharanin, Y.A.; Shestopalov, A.M.; Senning, A. Advances in the chemistry of 3-cyanopyridin-2(1H)-ones, -thiones, and -selenones. J. Sulfur Chem. 1992, 13, 1–142. [Google Scholar] [CrossRef]
- Litvinov, V.P. Advances in the chemistry of hydrogenated 3-cyanopyridine-2(1H)-thiones and -selenones. Phosphorus Sulfur Silicon Relat. Elem. 1993, 74, 139–156. [Google Scholar] [CrossRef]
- Litvinov, V.P. Partially hydrogenated pyridinechalcogenones. Russ. Chem. Bull. 1998, 47, 2053–2073. [Google Scholar] [CrossRef]
- Litvinov, V.P.; Krivokolysko, S.G.; Dyachenko, V.D. Synthesis and properties of 3-cyanopyridine-2(1H)-chalcogenones. Chem. Heterocycl. Compd. 1999, 35, 509–540. [Google Scholar] [CrossRef]
- Litvinov, V.P. The chemistry of 3-cyanopyridine-2(1H)-chalcogenones. Russ. Chem. Rev. 2006, 75, 577–599. [Google Scholar] [CrossRef]
- Kuthan, J.; Šcebek, P.; Böuhm, S. Developments in the chemistry of thiopyrans, selenopyrans, and teluropyrans. Adv. Heterocycl. Chem. 1994, 59, 179–244. [Google Scholar]
- Elnagdi, M.H.; Moustafa, M.S.; Al-Mousawi, S.M.; Mekheimer, R.A.; Sadek, K.U. Recent developments in utility of green multi-component reactions for the efficient synthesis of polysubstituted pyrans, thiopyrans, pyridines, and pyrazoles. Mol. Divers. 2015, 19, 625–651. [Google Scholar] [CrossRef]
- Sosnovskikh, V.Y. Synthesis and properties of 2,3-heteroannulated thiochromones-hetero analogs of thioxanthone. Chem. Heterocycl. Compd. 2019, 55, 103–125. [Google Scholar] [CrossRef]
- Litvinchuk, M.B.; Bentya, A.V.; Slyvka, N.Y.; Vovk, M.V. 2-Ylidene-1,3-thiazolidines and their nonhydrogenated analogs: Methods of synthesis and chemical properties. Chem. Heterocycl. Compd. 2020, 56, 1130–1145. [Google Scholar] [CrossRef]
- Taubert, K.; Kraus, S.; Schulze, B. Isothiazol-3(2H)-Ones, Part I: Synthesis, reactions and biological activity. J. Sulfur Chem. 2002, 23, 79–121. [Google Scholar] [CrossRef]
- Kletskov, A.V.; Bumagin, N.A.; Zubkov, F.I.; Grudinin, D.G.; Potkin, V.I. Isothiazoles in the design and synthesis of biologically active substances and ligands for metal complexes. Synthesis 2020, 52, 159–188. [Google Scholar] [CrossRef]
- Metwally, M.A.; Abdel-Latif, E.; Bondock, S. Thiocarbamoyl derivatives as synthons in heterocyclic synthesis. J. Sulfur Chem. 2007, 28, 431–466. [Google Scholar] [CrossRef]
- Shafran, Y.; Glukhareva, T.; Dehaen, W.; Bakulev, V. Recent developments in the chemistry of 1,2,3-thiadiazoles. Adv. Heterocycl. Chem. 2018, 126, 109–172. [Google Scholar]
- Bakulev, V.; Shafran, Y.; Dehaen, W. Progress in intermolecular and intramolecular reactions of thioamides with diazo compounds and azides. Tetrahedron Lett. 2019, 60, 513–523. [Google Scholar] [CrossRef]
- Bakhite, E.A.-G. Recent trends in the chemistry of thienopyridines. Phosphorus Sulfur Silicon Relat. Elem. 2003, 178, 929–992. [Google Scholar] [CrossRef]
- Litvinov, V.P.; Dotsenko, V.V.; Krivokolysko, S.G. Thienopyridines: Synthesis, properties, and biological activity. Russ. Hcem. Bull. Int. Ed. 2005, 54, 864–904. [Google Scholar] [CrossRef]
- Litvinov, V.P.; Dotsenko, V.V.; Krivokolysko, S.G. The chemistry of thienopyridines. Adv. Heterocycl. Chem. 2007, 93, 117–178. [Google Scholar]
- Sajadikhah, S.S.; Marandi, G. Recent approaches to the synthesis of thieno[2,3-b]pyridines (microreview). Chem. Heterocycl. Compd. 2019, 55, 1171–1173. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Buryi, D.S.; Lukina, D.Y.; Krivokolysko, S.G. Recent advances in the chemistry of thieno[2,3-b]pyridines 1. Methods of synthesis of thieno[2,3-b]pyridines. Russ. Chem. Bull. Int. Ed. 2020, 69, 1829–1858. [Google Scholar] [CrossRef]
- Larionova, N.A.; Shestopalov, A.M.; Rodinovskaya, L.A.; Zubarev, A.A. Synthesis of biologically active heterocycles via a domino sequence involving an SN2/Thorpe–Ziegler Reaction Step. Synthesis 2022, 54, 217–245. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Frolov, K.A.; Krivokolysko, S.G. Synthesis of partially hydrogenated 1,3,5-thiadiazines by Mannich reaction. Chem. Heterocycl. Compd. 2015, 51, 109–127. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Frolov, K.A.; Chigorina, E.A.; Khrustaleva, A.N.; Bibik, E.Y.; Krivokolysko, S.G. New possibilities of the Mannich reaction in the synthesis of N-, S,N-, and Se,N-heterocycles. Russ. Chem. Bull. Int. Ed. 2019, 68, 691–707. [Google Scholar] [CrossRef]
- Abdel-Galil, F.M.; Sherif, S.M.; Elnagdi, M.H. Utility of cyanoacetamide and cyanothioacetamide in heterocyclic synthesis. Heterocycles 1986, 24, 2023–2048. [Google Scholar]
- Litvinov, V.P. Cyanoacetamides and their thio- and selenocarbonyl analogues as promising reagents for fine organic synthesis. Russ. Chem. Rev. 1999, 68, 737–763. [Google Scholar] [CrossRef]
- Dyachenko, V.D.; Dyachenko, I.V.; Nenajdenko, V.G. Cyanothioacetamide: A polyfunctional reagent with broad synthetic utility. Russ. Chem. Rev. 2018, 87, 1–27. [Google Scholar] [CrossRef]
- Britsun, V.N.; Esipenko, A.N.; Lozinskii, M.O. Heterocyclization of thioamides containing an active methylene group (review). Chem. Heterocycl. Compd. 2008, 44, 1429–1459. [Google Scholar] [CrossRef]
- Lozynskii, M.O.; Britsun, V.M.; Esipenko, A.M.; Borysevych, A.M. Thio- and dithioamides of malonic acid: Synthesis, structure and heterocyclizations. Ukr. Khim. Zhurn. 2008, 74, 3–21. [Google Scholar]
- Barnikow, G.; Kath, V.; Richter, D. Isothiocyanate. II. N,N′-Aryl-substituierte Dithiomalonsäurediamide. J. Prakt. Chem. 1965, 30, 63–66. [Google Scholar] [CrossRef]
- Sinotsko, A.E.; Bespalov, A.V.; Pashchevskaya, N.V.; Dotsenko, V.V.; Aksenov, N.A.; Aksenova, I.V. N,N′-Diphenyldithiomalonodiamide: Structural Features, Acidic Properties, and In Silico Estimation of Biological Activity. Russ. J. Gen. Chem. 2021, 91, 2136–2150. [Google Scholar] [CrossRef]
- Barnikow, G.; Kunzek, H. Nickel-Chelate von N,N′-Diaryl-dithiomalonsäure-diamiden. Z. Chem. 1966, 6, 343. [Google Scholar] [CrossRef]
- Peyronel, G.; Pellacani, G.C.; Benetti, G.; Pollacci, G. Nickel(II) complexes with dithiomalonamide and NN′-diphenyldithiomalonamide. J. Chem. Soc. Dalton Trans. 1973, 879–882. [Google Scholar] [CrossRef]
- Pellacani, G.C. Palladium(II) complexes with dithiomalonamide and N,N′-diphenyldithiomalonamide. Can. J. Chem. 1974, 52, 3454–3458. [Google Scholar] [CrossRef]
- Pellacani, G.C.; Peyronel, G.; Pollacci, G.; Coronati, R. Zinc(II) complexes of dithiomalonamide, N,N′-dimethyl- and N,N′-diphenyl-dithiomalonamide: ZnLX2 (X = Cl, Br, I) and ZnL2(ClO4)2. J. Inorg. Nucl. Chem. 1976, 38, 1619–1621. [Google Scholar] [CrossRef]
- Pellacani, G.C.; Peyronel, G.; Malavasi, W.; Menabue, L. Antimony and bismuth trihalide complexes of dithiomalonamide, N,N′-dimethyl- and N,N′-diphenyl-dithiomalonamide. J. Inorg. Nucl. Chem. 1977, 39, 1855–1857. [Google Scholar] [CrossRef]
- Pal, T.; Ganguly, A.; Maity, D.S.; Livingstone, S.E. N,N′-diphenyldithiomalonamide as a gravimetric reagent for nickel and cobalt. Talanta 1986, 33, 973–977. [Google Scholar] [CrossRef]
- Pal, T.; Ganguly, A.; Pal, A. Spectrophotometric determination of cobalt and nickel with N,N′-diphenyldithiomalonamide and elucidation of structures of the compounds. J. Ind. Chem. Soc. 1988, 65, 655–657. [Google Scholar]
- Battaglia, L.P.; Bonamartini Corradi, A.; Marzotto, A.; Menabue, L.; Pellacani, G.C. Nickel(II) and palladium(II) complexes of dithiomalonamides: Ligands which favor the formation of π-conjugation systems in the coordination. J. Crystallogr. Spectrosc. Res. 1988, 18, 101–112. [Google Scholar] [CrossRef]
- Battaglia, L.P.; Bonamartini Corradi, A.; Marzotto, A.; Menabue, L.; Pellacani, G.C. Co-ordinative abilities of ligands which favour S,S chelation: Copper(I) halide complexes of N,N′-diphenyldithiomalonamide. The crystal and molecular structure of bis(N,N′-diphenyldithiomalonamide)copper(I) iodide–methanol (2/1). J. Chem. Soc. Dalton Trans. 1988, 1713–1718. [Google Scholar] [CrossRef]
- Singh, T.; Singh, R.; Verma, V.K. Evaluation of 2,4-dithiomalonamides as extreme pressure additives in the four-ball test. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 1990, 204, 103–107. [Google Scholar] [CrossRef]
- Singh, T. Tribochemistry and EP activity assessment of Mo-S complexes in lithium-base greases. Adv. Tribol. 2008, 2008, 947543. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, M.M. Substituted dithiomalonamides as inhibitor for the corrosion of AISI 304SS in phosphoric acid—Hydrochloric acid mixture. Anti-Corros. Methods Mater. 1993, 40, 4–7. [Google Scholar] [CrossRef]
- Schmidt, U. Synthesen mit den Thioamiden der Malonsäure, I. 3,5-Diamino-dithiyliumsalze. Ein neuer pseudoaromatischer Fünfring mit 1,2-Stellung der S-Atome. Chem. Ber. 1959, 92, 1171–1176. [Google Scholar] [CrossRef]
- Barnikow, G. Isothiocyanate, XIV. 3.5-Bis-arylamino-1.2-dithioliumsalze aus N.N′-Diaryl-dithiomalonsäure-diamiden. Chem. Ber. 1967, 100, 1389–1393. [Google Scholar] [CrossRef]
- Nizovtseva, T.V.; Komarova, T.N.; Nakhmanovich, A.S.; Larina, L.I.; Lopyrev, V.A.; Kalistratova, E.F. Reaction of Dithiomalonamide and Dianilide with α-Acetylene Ketones. Russ. J. Org. Chem. 2002, 38, 1205–1207. [Google Scholar] [CrossRef]
- Nizovtseva, T.V.; Komarova, T.N.; Nakhmanovich, A.S.; Larina, L.I.; Lopyrev, V.A. Synthesis of 1,3-dithiinium salts. Arkivoc 2003, 13, 191–195. [Google Scholar] [CrossRef]
- Elokhina, V.N.; Yaroshenko, T.I.; Nakhmanovich, A.S.; Larina, L.I.; Amosova, S.V. Reaction of dithiomalonic acid dianilide with substituted acetylenic ketones. Russ. J. Gen. Chem. 2006, 76, 1916–1918. [Google Scholar] [CrossRef]
- Volkova, K.A.; Nakhmanovich, A.S.; Elokhina, V.N.; Yaroshenko, T.I.; Larina, L.I.; Shulunova, A.M.; Amosova, S.V. Reaction of dithiomalonic acid dianilide with methyl propiolate. Russ. J. Org. Chem. 2007, 43, 768–770. [Google Scholar] [CrossRef]
- Obydennov, K.L.; Golovko, N.A.; Kosterina, M.F.; Pospelova, T.A.; Slepukhin, P.A.; Morzherin, Y.Y. Synthesis of 4-oxothiazolidine-2,5-diylidenes containing thioamide group based on dithiomalonamides. Russ. Chem. Bull. Int. Ed. 2014, 63, 1330–1336. [Google Scholar] [CrossRef]
- Beckert, R.; Gruner, M. Regioselektive Cyclisierung von Thiocarbonsäureamiden mit Bisimidoylchloriden—Synthese von Thiazolidinen mit einer Ketenacetal-Substruktur. Z. Naturforsch. B Chem. Sci. 1997, 52, 1245–1250. [Google Scholar] [CrossRef]
- Barnikow, G. Isothiocyanate, X. Pyrazole und Thiophene aus N.N′-Diaryl-dithiomalonsäure-diamiden. Liebigs Ann. Chem. 1966, 700, 46–49. [Google Scholar] [CrossRef]
- Degorce, S.; Jung, F.H.; Harris, C.S.; Koza, P.; Lecoq, J.; Stevenin, A. Diversity-orientated synthesis of 3,5-bis(arylamino)pyrazoles. Tetrahedron Lett. 2011, 52, 6719–6722. [Google Scholar] [CrossRef]
- Bakulev, V.A.; Lebedev, A.T.; Dankova, E.F.; Mokrushin, V.S.; Petrosyan, V.S. Two directions of cyclization of α-diazo-β-dithioamides. New rearrangements of 1,2,3-triazole-4-carbothiamides. Tetrahedron 1989, 45, 7329–7340. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Krivokolysko, S.G.; Frolov, K.A.; Chigorina, E.A.; Polovinko, V.V.; Dmitrienko, A.O.; Bushmarinov, I.S. Synthesis of [1,2]dithiolo[3,4-b]pyridines via the reaction of dithiomalondianilide with arylmethylidenemalononitriles. Chem. Heterocycl. Compd. 2015, 51, 389–392. [Google Scholar] [CrossRef]
- Goncharenko, M.P.; Sharanin, Y.A.; Turov, A.V. Meldrum’s acid in reactions with arylmethylenecyanothioacetamides. Russ. J. Org. Chem. 1993, 29, 1341–1347. [Google Scholar]
- Nesterov, V.N.; Krivokolysko, S.G.; Dyachenko, V.D.; Dotsenko, V.V.; Litvinov, V.P. Synthesis, properties, and structures of ammonium 4-aryl-5-cyano-2-oxo-1,2,3,4-tetrahydropyridine-6-thiolates. Russ. Chem. Bull. 1997, 46, 990–996. [Google Scholar] [CrossRef]
- Dyachenko, V.D.; Krivokolysko, S.G.; Litvinov, V.P. A new method for the synthesis of N-methylmorpholinium 4-aryl-5-cyano-2-oxo-1,2,3,4-tetrahydropyridine-6-thiolates and their properties. Russ. Chem. Bull. 1997, 46, 1758–1762. [Google Scholar] [CrossRef]
- Dyachenko, V.D.; Krivokolysko, S.G.; Litvinov, V.P. Synthesis and some properties of 4-alkyl-5-cyano-6-mercapto-3,4-dihydropyridin-2(1H)-ones. Russ. Chem. Bull. 1997, 46, 1912–1915. [Google Scholar] [CrossRef]
- Krivokolysko, S.G.; Dyachenko, V.D.; Litvinov, V.P. Synthesis and alkylation of N-methylmorpholinium 5-cyano-4-(3- and 4-hydroxyphenyl)-2-oxo-1,2,3,4-tetrahydropyridine-6-thiolates. Russ. Chem. Bull. 1999, 48, 2308–2311. [Google Scholar] [CrossRef]
- Krivokolysko, S.G.; Chernega, A.N.; Litvinov, V.P. Synthesis, structure, and alkylation of N-methylmorpholinium 5-[2-cyanoethyl-1-(4-hydroxy-3-methoxyphenyl)-2-thiocarbamoyl]-2,2-dimethyl-6-oxo-1,3-dioxa-4-cyclohexen-4-olate. Chem. Heterocycl. Compd. 2002, 38, 1269–1275. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Krivokolysko, S.G.; Chernega, A.N.; Litvinov, V.P. Fused sulfurcontaining pyridine systems 1. Synthesis and structures of tetrahydropyridothienopyridinone and tetrahydropyridothiopyranopyridinone derivatives. Russ. Chem. Bull. Int. Ed. 2003, 52, 969–977. [Google Scholar] [CrossRef]
- Dyachenko, V.D. A simple and efficient route to substituted 2-alkylsulfanyl-6-oxo-1,4,5,6-tetrahydropyridine-3-carbonitriles. Russ. J. Org. Chem. 2006, 42, 1877–1879. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Lebedeva, I.A.; Krivokolysko, S.G.; Povstyanoi, M.V.; Povstyanoi, V.M.; Kostyrko, E.O. Reaction of ethyl 4-aryl-6-bromomethyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylates with N-methylmorpholinium 3-cyano-1,4-dihydro- and 3-cyano-1,4,5,6-tetrahydropyridine-2-thiolates. Chem. Heterocycl. Compd. 2012, 48, 462–469. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Krivokolysko, S.G.; Litvinov, V.P. The Mannich reaction in the synthesis of N,S-containing heterocycles. 11. Synthesis of 3,3′-(1,4-phenylene)-bis(8-aryl-6-oxo-3,4,7,8-tetrahydro-2H,6H-pyrido[2,1-b][1,3,5]thiadiazine-9-carbonitriles). Russ. Chem. Bull. Int. Ed. 2012, 61, 131–135. [Google Scholar] [CrossRef]
- Osolodkin, D.I.; Kozlovskaya, L.I.; Dueva, E.V.; Dotsenko, V.V.; Rogova, Y.V.; Frolov, K.A.; Krivokolysko, S.G.; Romanova, E.G.; Morozov, A.S.; Karganova, G.G.; et al. Inhibitors of Tick-Borne Flavivirus Reproduction from Structure-Based Virtual Screening. ACS Med. Chem. Lett. 2013, 4, 869–874. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Frolov, K.A.; Pekhtereva, T.M.; Papaianina, O.S.; Suykov, S.Y.; Krivokolysko, S.G. Design and synthesis of pyrido[2,1-b][1,3,5]thiadiazine library via uncatalyzed Mannich-type reaction. ACS Comb. Sci. 2014, 16, 543–550. [Google Scholar] [CrossRef]
- Bibik, I.V.; Bibik, E.Y.; Dotsenko, V.V.; Frolov, K.A.; Krivokolysko, S.G.; Aksenov, N.A.; Aksenova, I.V.; Shcherbakov, S.V.; Ovcharov, S.N. Synthesis and analgesic activity of new heterocyclic cyanothioacetamide derivatives. Russ. J. Gen. Chem. 2021, 91, 154–166. [Google Scholar] [CrossRef]
- Krivokolysko, D.S.; Dotsenko, V.V.; Bibik, E.Y.; Samokish, A.A.; Venidiktova, Y.S.; Frolov, K.A.; Krivokolysko, S.G.; Vasilin, V.K.; Pankov, A.A.; Aksenov, N.A.; et al. New 4-(2-furyl)-1,4-dihydronicotinonitriles and 1,4,5,6-tetrahydronicotinonitriles: Synthesis, structure, and analgesic activity. Russ. J. Gen. Chem. 2021, 91, 1646–1660. [Google Scholar] [CrossRef]
- Krivokolysko, S.G.; Dyachenko, V.D.; Nesterov, V.N.; Litvinov, V.P. Novel stereoselective synthesis and molecular and crystalline structure of 3-allyl-4-(4-bromophenyl)-3-cyano-6-oxopiperidine-2-thione. Chem. Heterocycl. Compd. 2001, 37, 315–319. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Krivokolysko, S.G.; Chernega, A.N.; Litvinov, V.P. Synthesis and structure of pyrido[2,1-b][1,3,5]thiadiazine derivatives. Doklady Chem. 2003, 389, 92–96. [Google Scholar] [CrossRef]
- Bibik, E.Y.; Yaroshevskaya, O.G.; Devdera, A.V.; Demenko, A.V.; Zakharov, V.V.; Frolov, K.A.; Dotsenko, V.V.; Krivokolysko, S.G. Search for anti-inflammatory agents in the tetrahydropyrido[2,1-b][1,3,5]thiadiazine series. Pharm. Chem. J. 2017, 51, 648–651. [Google Scholar] [CrossRef]
- Bybik, E.Y.; Yaroshevskaya, O.G.; Demenko, A.V.; Frolov, K.A.; Dotsenko, V.V.; Kryvokolysko, S.G. The effect of derivatives of tetrahydropyrido[2,1-b][1,3,5]thiadiazine on hematologic indices of rats with subacute parotitis. Res. Results Pharmacol. 2018, 4, 77–84. [Google Scholar] [CrossRef]
- Bibik, E.Y.; Saphonova, A.A.; Yeryomin, A.V.; Frolov, K.A.; Dotsenko, V.V.; Krivokolysko, S.G. Study of analeptic activity of tetrahydropyrido[2,1-b][1,3,5]thiadiazine derivatives. Res. Result Pharmacol. Clin. Pharmacol. 2017, 3, 20–25. [Google Scholar]
- Bibik, E.Y.; Nekrasa, I.A.; Demenko, A.V.; Frolov, K.A.; Dotsenko, V.V.; Krivokolysko, S.G. Studying the adaptogenic activity of a series of tetrahydropyrido[2,1-b][1,3,5]thiadiazine derivatives. Bull. Siber. Med. 2019, 18, 21–28. (In Russian) [Google Scholar] [CrossRef]
- Wang, Y.; Duraiswami, C.; Madauss, K.P.; Tran, T.B.; Williams, S.P.; Deng, S.J.; Graybill, T.L.; Hammond, M.; Jones, D.G.; Grygielko, E.T.; et al. 2-Amino-9-aryl-3-cyano-4-methyl-7-oxo-6,7,8,9-tetrahydropyrido[2′,3′: 4,5]thieno[2,3-b]pyridine derivatives as selective progesterone receptor agonists. Bioorg. Med. Chem. Lett. 2009, 19, 4916–4919. [Google Scholar] [CrossRef] [PubMed]
- Frolov, K.A.; Dotsenko, V.V.; Krivokolysko, S.G.; Litvinov, V.P. Three-component condensation in the synthesis of substituted tetrahydropyridinethiolates. Russ. Chem. Bull. Int. Ed. 2005, 54, 1335–1336. [Google Scholar] [CrossRef]
- Frolov, K.A.; Dotsenko, V.V.; Krivokolysko, S.G. Synthesis and reactions of 1,2-bis [3-cyano-4-(2-fluorophenyl)-6-oxo-1,4,5,6-tetrahydropyridin-2-yl]diselane. Chem. Heterocycl. Compd. 2012, 48, 1006–1010. [Google Scholar] [CrossRef]
- Baggaley, K.H.; Jennings, L.J.A.; Tyrrell, A.W.R. Synthesis of 2-substituted isothiazolopyridin-3-ones. J. Heterocycl. Chem. 1982, 19, 1393–1396. [Google Scholar] [CrossRef]
- Borgna, P.; Pregnolato, M.; Invernizzi, A.G.; Mellerio, G. On the reaction between 3H-1,2-dithiolo[3,4-b]pyridine-3-thione and primary alkyl and arylalkylamines. J. Heterocycl. Chem. 1993, 30, 1079–1084. [Google Scholar] [CrossRef]
- Pregnolato, M.; Terreni, M.; Ubiali, D.; Pagani, G.; Borgna, P.; Pastoni, F.; Zampollo, F. 3H-[1,2]Dithiolo[3,4-b]pyridine-3-thione and its derivatives. Synthesis and antimicrobial activity. Il Farmaco 2000, 55, 669–679. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Krivokolysko, S.G. Oxidation of thioamides with the DMSO–HCl system: A convenient and efficient method for the synthesis of 1,2,4-thiadiazoles, isothiazolo[5,4-b]pyridines, and heterocyclic disulfides. Chem. Heterocycl. Compd. 2013, 49, 636–644. [Google Scholar] [CrossRef]
- Furdas, S.D.; Shekfeh, S.; Bissinger, E.M.; Wagner, J.M.; Schlimme, S.; Valkov, V.; Hendzel, M.; Jung, M.; Sippl, W. Synthesis and biological testing of novel pyridoisothiazolones as histone acetyltransferase inhibitors. Bioorg. Med. Chem. 2011, 19, 3678–3689. [Google Scholar] [CrossRef] [PubMed]
- Pagani, G.; Pregnolato, M.; Ubiali, D.; Terreni, M.; Piersimoni, C.; Scaglione, F.; Fraschini, F.; Rodríguez Gascón, A.; Pedraz Muñoz, J.L. Synthesis and in vitro anti-mycobacterium activity of N-alkyl-1,2-dihydro-2-thioxo-3-pyridinecarbothioamides. Preliminary toxicity and pharmacokinetic evaluation. J. Med. Chem. 2000, 43, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Deeb, A.; Essawy, A.; El-Gendy, A.M.; Shaban, A. Heterocyclic synthesis with 3-cyano-2(1H)-pyridinethione: Synthesis of 3-oxo-2,3-dihydroisothiazolo[5,4-b]pyridine and related compounds. Monatsh. Chem. 1990, 121, 281–287. [Google Scholar] [CrossRef]
- Hussain, S.M.; Sherif, S.M.; Youssef, M.M. New Synthesis of Polyfunctionally Substituted 2-Mercaptopyridines and Fused Pyridines. Gazz. Chim. Ital. 1994, 124, 97–102. [Google Scholar]
- Gompper, R.; Elser, W. Stabile 1,4-Dipole aus Ketenacetalen und Schwefelkohlenstoff und ihre Verwendung zur Synthese von Heterocyclen. Angew. Chem. 1967, 79, 382–383. [Google Scholar] [CrossRef]
- Jian, F.; Zheng, J.; Li, Y.; Wang, J. Novel ((3Z,5Z)-3,5-bis(phenylimino)-1,2-dithiolan-4-yl) and 3H-[1,2]dithiolo[3,4-b]quinolin-4 (9H)-one heterocycles: An effective and facile green route. Green Chem. 2009, 11, 215–222. [Google Scholar] [CrossRef]
- Haasnoot, C.A.G.; de Leeuw, F.A.A.M.; Altona, C. The relationship between proton-proton NMR coupling constants and substituent electronegativities—I: An empirical generalization of the Karplus equation. Tetrahedron 1980, 36, 2783–2792. [Google Scholar] [CrossRef]
- Donders, L.A.; de Leeuw, F.A.A.M.; Altona, C. Relationship between proton-proton NMR coupling constants and substituent electronegativities. IV—An extended Karplus equation accounting for interactions between substituents and its application to coupling constant data calculated by the Extended Hückel method. Magn. Reson. Chem. 1989, 27, 556–563. [Google Scholar]
- Mohite, A.R.; Bhat, R.G. A practical and convenient protocol for the synthesis of (E)-α,β-unsaturated acids. Org. Lett. 2013, 15, 4564–4567. [Google Scholar] [CrossRef]
- Kaupp, G.; Naimi-Jamal, M.R.; Schmeyers, J. Solvent-free Knoevenagel condensations and Michael additions in the solid state and in the melt with quantitative yield. Tetrahedron 2003, 59, 3753–3760. [Google Scholar] [CrossRef]
- Krapivin, G.D.; Kul’nevich, V.G.; Val’ter, N.I. 2,2-Dimethyl-5-(5-R-2-furfurylidene)-1,3-dioxane-4,6-diones. 4. Synthesis, stereostructures, and properties of thiophene analogs. Chem. Heterocycl. Compd. 1989, 25, 1118–1121. [Google Scholar] [CrossRef]
- Sinotsko, A.E.; Dotsenko, V.V.; Aksenov, N.A. The Reactions of N,N′-Diphenyldithiomalonamide with Michael Acceptors. Chem. Proc. 2021, 3, 26. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinementand analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dotsenko, V.V.; Aksenov, A.V.; Sinotsko, A.E.; Varzieva, E.A.; Russkikh, A.A.; Levchenko, A.G.; Aksenov, N.A.; Aksenova, I.V. The Reactions of N,N′-Diphenyldithiomalondiamide with Arylmethylidene Meldrum’s Acids. Int. J. Mol. Sci. 2022, 23, 15997. https://doi.org/10.3390/ijms232415997
Dotsenko VV, Aksenov AV, Sinotsko AE, Varzieva EA, Russkikh AA, Levchenko AG, Aksenov NA, Aksenova IV. The Reactions of N,N′-Diphenyldithiomalondiamide with Arylmethylidene Meldrum’s Acids. International Journal of Molecular Sciences. 2022; 23(24):15997. https://doi.org/10.3390/ijms232415997
Chicago/Turabian StyleDotsenko, Victor V., Alexander V. Aksenov, Anna E. Sinotsko, Ekaterina A. Varzieva, Alena A. Russkikh, Arina G. Levchenko, Nicolai A. Aksenov, and Inna V. Aksenova. 2022. "The Reactions of N,N′-Diphenyldithiomalondiamide with Arylmethylidene Meldrum’s Acids" International Journal of Molecular Sciences 23, no. 24: 15997. https://doi.org/10.3390/ijms232415997
APA StyleDotsenko, V. V., Aksenov, A. V., Sinotsko, A. E., Varzieva, E. A., Russkikh, A. A., Levchenko, A. G., Aksenov, N. A., & Aksenova, I. V. (2022). The Reactions of N,N′-Diphenyldithiomalondiamide with Arylmethylidene Meldrum’s Acids. International Journal of Molecular Sciences, 23(24), 15997. https://doi.org/10.3390/ijms232415997






