MicroRNAs: A Link between Mammary Gland Development and Breast Cancer
Abstract
1. Introduction
2. Mammary Gland Development
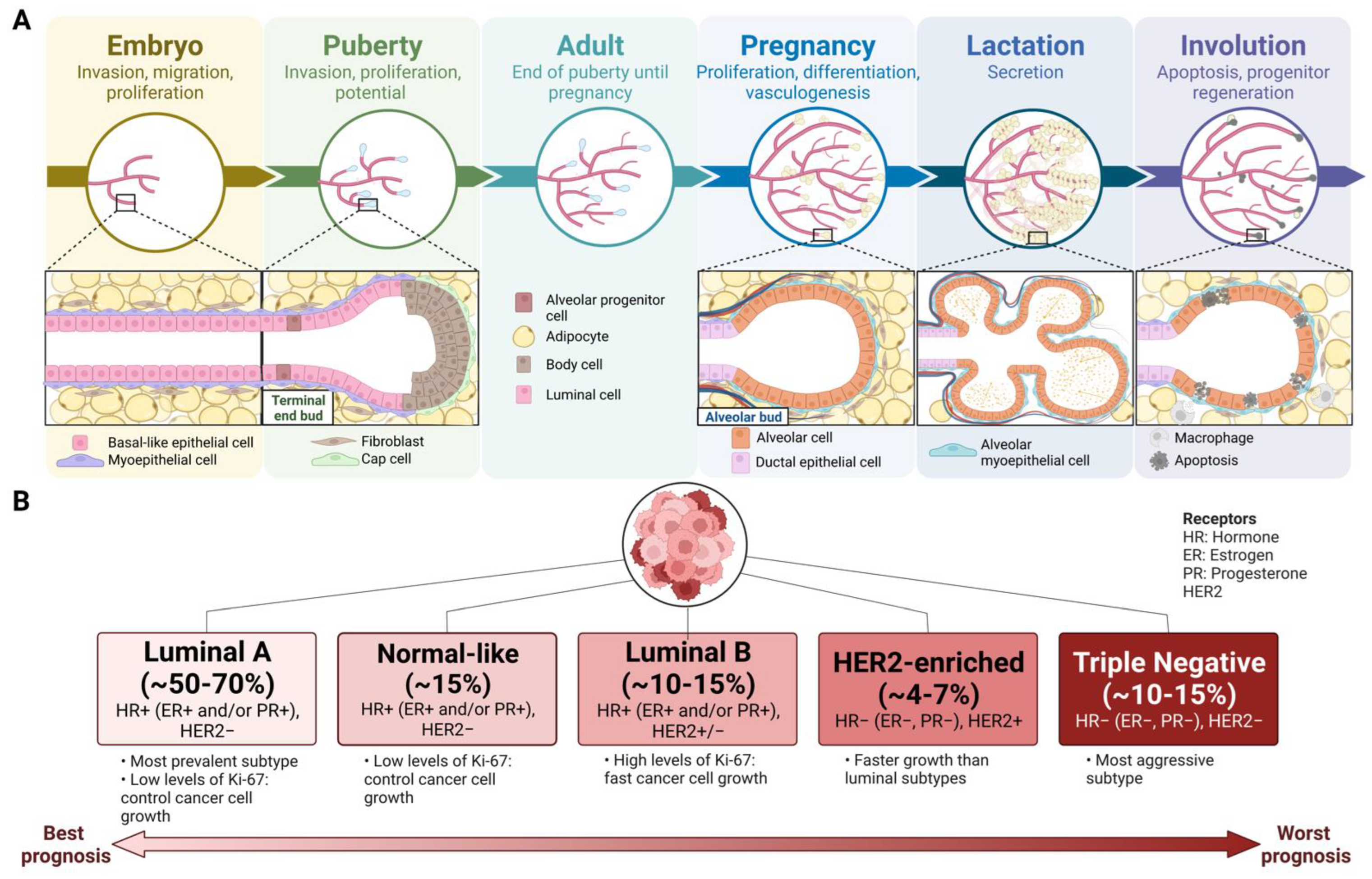
3. Breast Cancer
4. MicroRNAs
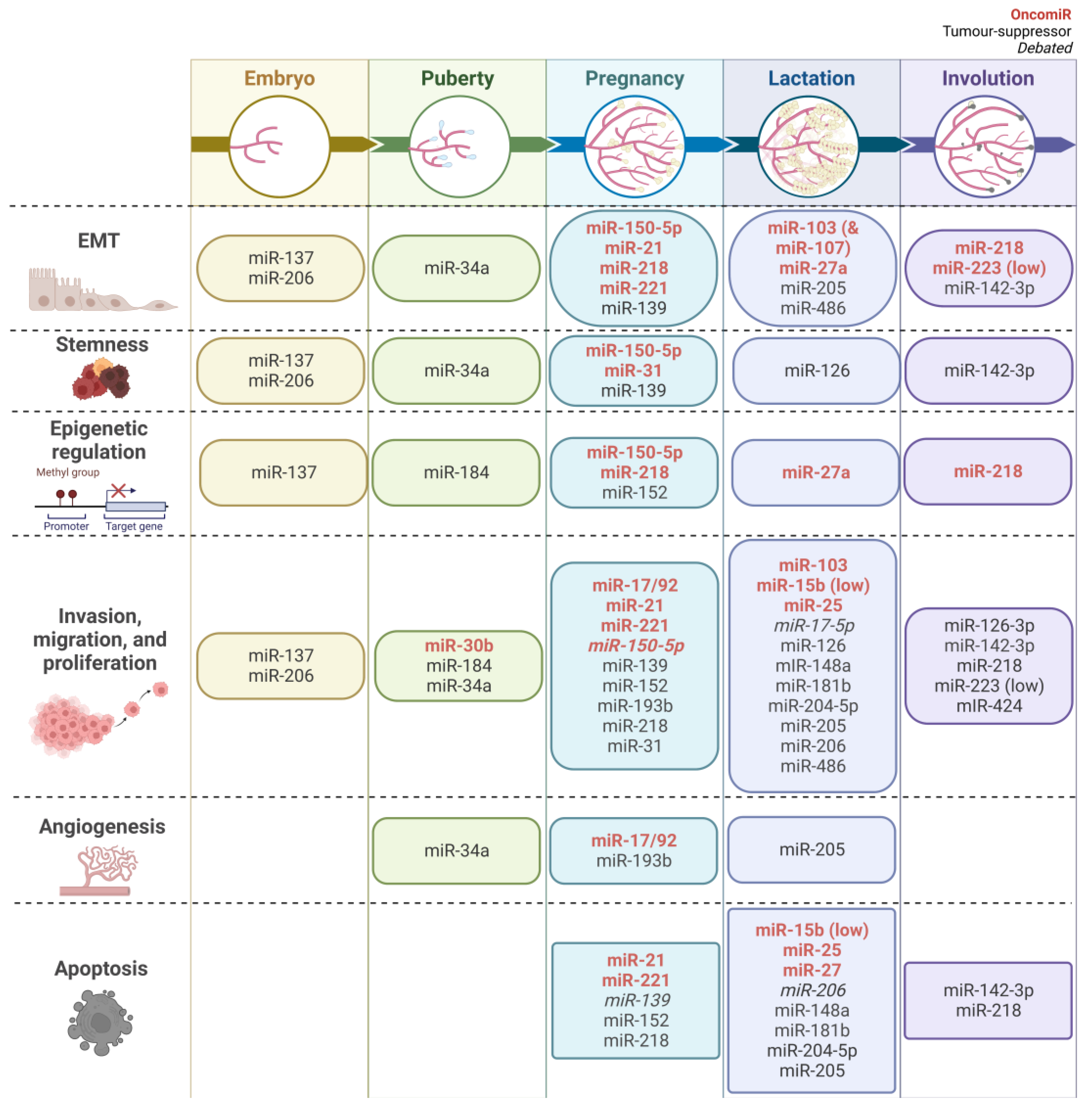
5. MiRNAs in Mammary Gland Development and Breast Cancer
5.1. EMT
5.2. Stemness Characteristics
5.3. Epigenetic Regulation
5.4. Invasion, Migration, and Proliferation
5.5. Angiogenesis
5.6. Apoptosis
| MiRNA | Development | Breast Cancer 1 | ||
|---|---|---|---|---|
| Population Characteristics | Outcomes | Population Characteristics | Outcomes | |
| Embryo stage | ||||
| miR-137 | Embryos from ICR (CD-1) time-mated pregnant mice [34] |
| Ex vivo tissue, breast cancer cell lines | Oncogenic ↑ EMT, invasion [76] Tumour suppressive ↓ tumour weight, volume, invasion, proliferation, migration, EMT, drug resistance, stemness [34,36,37,38,77,78,79,80] |
| miR-206 | Embryos from ICR time-mated pregnant mice [41] |
| Ex vivo tissue, breast cancer cell lines | Oncogenic ↑ migration, invasion, proliferation [44] Tumour suppressive ↓ proliferation, drug resistance, metastasis, stemness ↑ apoptosis [27,42,43,81,82] 2 |
| Puberty stage | ||||
| miR-184 | 5-week-old β-actin-GFP reporter FVB/n mice [51] |
| Ex vivo tissue, breast cancer cell lines, mouse tumour models | Tumour suppressive ↓ proliferation, invasion, methylation, metastasis ↑ cell cycle arrest [51,54,83] |
| miR-34a | miR-34-knockout C57BL/6J (Trp53 strain) mice [45] |
| Ex vivo tissue, breast cancer cell lines, mouse tumour models, review | Tumour suppressive ↓ stemness, invasion, migration, tumour volume and growth, EMT, proliferation, drug resistance ↑ apoptosis, cell cycle arrest [27,51,54,63,83,84,85,86,87,88,89,90,91,92,93,94,95] 2 |
| miR-489 | 6-week-old FVP mice [96] |
| Ex vivo tissue, breast cancer cell lines, mouse tumour models | Tumour suppressive ↓ proliferation, migration, invasion, drug resistance, stemness, tumour volume ↑ apoptosis, sensitivity to drugs [96,97,98,99,100,101,102,103,104,105] |
| 4- and 6-week-old MMTV-miR-489 mice (n = 9) |
| |||
| Virgin adult and pregnancy | ||||
| miR-17/92 cluster | miR-17-92bfl/fl;MMTV-Cre mice [55] |
| Review | Oncogenic ↑ proliferation, migration, invasion, angiogenesis, metastasis, chemoresistance [28,58,106] 2 |
| miR-193b | C57BL/6 miR-193b−/− mice [64] |
| Ex vivo tissue, breast cancer cell lines | Tumour suppressive ↓ growth, metastasis, migration, invasion, stemness, chemoresistance ↑ apoptosis [66,107,108,109,110,111,112,113] |
| miR-21 | Stat5fl/fl;Cre mice, miR-21−/− mice [56] |
| Review | Oncogenic ↑ invasion, migration, proliferation, metastasis, radiotherapy and chemoresistance, tumour growth ↓ apoptosis [58,59,114,115,116,117] 2 |
| Pregnancy and lactation | ||||
| miR-27a | Three-year-old Xinong Saanen Dairy Goat (n = 3) [118] |
| Ex vivo tissue, breast cancer cell lines, review | Oncogenic ↑ cell growth, EMT, demethylation of tumour suppressor ↓ apoptosis [81,114,119] 2 |
| miR-139 | Holstein cows mid-pregnancy (n = 3), mid-lactation (n = 3, 90 days in milk) [46] |
| Ex vivo tissue, breast cancer cell lines, mouse tumour models | Oncogenic ↓ apoptosis [120] Tumour suppressive ↓ proliferation, migration, invasion, EMT, stemness ↑ apoptosis [121,122,123,124,125,126,127,128,129,130] |
| miR-150-5p | Stop-150fl/fl C57BL/6 mice [131] |
| Oncogenic ↑ cell proliferation, drug resistance, migration, EMT, stem-like characteristics [50,132,133] | |
| miR-204-5p | Pregnant and lactating C57BL/6J mice (n = 6 per group) [134] |
| Ex vivo tissue, breast cancer cell lines | [27,135,136,137,138] 2 |
| HC11 cells |
| |||
| miR-206 | Mammary gland from 2- month adult, pregnancy day 10, and lactation day 6 [42] |
| Ex vivo tissue, breast cancer cell lines | Oncogenic ↑ migration, invasion, proliferation [44] Tumour suppressive ↓ proliferation, drug resistance, metastasis, stemness ↑ apoptosis [27,42,43,81,82] 2 |
| miR-486 | Multiparous Holstein cows in high-quality lactation (n = 3), low-quality lactation (n = 3), and pregnancy (n = 3) [139] |
| Ex vivo tissue, breast cancer cell lines | Tumour suppressive ↓ invasion, migration, stemness, proliferation, EMT ↑ apoptosis, radiosensitivity, chemosensitivity, cell cycle arrest [140,141,142,143] |
| Bovine mammary epithelial cells |
| |||
| Pregnancy, lactation and involution | ||||
| miR-103 | 30 healthy three-year-old Xinong Saanen dairy goats mid-lactation (120 days after parturition) and dry lactation (60 days before parturition) [144] |
| Ex vivo tissue, breast cancer cell lines | Oncogenic ↑ metastasis, EMT [145,146] |
| miR-152 | Mammary gland from Han ewes (n = 3) Day −8, −6, −4, −1 from parturition (involution), and 1 week after parturition [147] |
| Ex vivo tissue, breast cancer cell lines, mouse tumour models | Tumour suppressive ↓ proliferation, migration, invasion, cell survival, EMT, stemness, methylation, chemotherapy resistance, metastasis ↑ apoptosis, cell cycle arrest [148,149,150,151,152,153,154,155,156,157] |
| miR-218 | Mammary gland from Han ewes (n = 3) Day −8, −6, −4, −1 from parturition (involution), and 1 week after parturition [147] |
| Ex vivo tissue, breast cancer cell lines | Oncogenic ↑ metastasis, invasion, migration, EMT, methylation [158,159,160,161,162] Tumour suppressive ↓ proliferation, migration, chemoresistance, invasion ↑ apoptosis [163,164,165,166,167,168] |
| miR-223 | FVB MMTV-Δ16HER2 miR-223 knockout mice [169] |
| Breast cancer cell lines, review | Oncogenic ↑ EMT, metastasis, drug resistance [170] Tumour suppressive ↓ drug resistance, proliferation, migration, EMT ↑ apoptosis [169,171,172] |
| miR-31 | TRE-miR-31 transgenic mice from C57BL/6J background [173] |
| Breast cancer cell lines | Tumour suppressive ↓ invasion, migration, proliferation ↑ apoptosis, chemotherapy sensitivity [27,174,175,176] 2 |
| Lactation | ||||
| miR-148a | Three-year-old Xinong Saanen dairy goats non-pregnant, early-lactation, peak-lactation, late-lactation (15, 60, 150 days after parturition), and dry-lactation [177] |
| Breast cancer cell lines, mouse tumour models | Oncogenic Inhibition led to ↓ proliferation [178] Tumour suppressive ↓ proliferation, metastasis, chemoresistance, stemness ↑ apoptosis [27,179,180,181,182,183,184] 2 |
| miR-17-5p | Three-year-old Xinong Saanen dairy goats non-pregnant, early-lactation, peak-lactation, late-lactation (15, 60, 150 days after parturition), and dry-lactation [177] |
| Ex vivo tissue, breast cancer cell lines, mouse tumour models | Tumour suppressive ↓ cell proliferation ↑ apoptosis [185,186] Oncogenic ↑ migration, invasion, proliferation, cell growth, angiogenesis, metastasis [58,187,188] 2 |
| miR-181b | Three-year-old Xinong Saanen dairy goats non-pregnant, early-lactation, peak-lactation, late-lactation (15, 60, 150 days after parturition), and dry-lactation [189] |
| Breast cancer cell lines, review | Tumour suppressive ↓ cell proliferation, migration, invasion ↑ apoptosis [190] Oncogenic ↑ migration, proliferation, chemoresistance, cell cycle, EMT [58] 2 |
| miR-25 | Three-year-old Xinong Saanen dairy goats non-pregnant, early-lactation, peak-lactation, late-lactation (15, 60, 120 days after parturition), and dry-lactation [191] |
| Ex vivo tissue, breast cancer cell lines, tumour mouse models | Oncogenic ↓ apoptosis ↑ migration, invasion, proliferation, chemoresistance, EMT, tumour volume [192,193,194,195,196,197] |
| Involution | ||||
| miR-424(322)/503 | miR-424(322) and miR-503 knockout mice [73] |
| Ex vivo tissue, breast cancer cell lines, tumour mouse models | Tumour suppressive ↓ migration, drug resistance, invasion, tumorigenesis, EMT, stemness, invasion, tumour growth ↑ apoptosis, cell cycle arrest [27,74,75,198,199,200,201,202,203,204,205] 2 |
| Virgin adult, pregnancy, lactation and involution | ||||
| miR-126 | Mouse (strain not specified) at virgin, pregnancy day 5, lactation day 0, lactation day 5, lactation day 10, involution day 10 [206] |
| Ex vivo tissue, breast cancer cell lines, tumour mouse models | Tumour suppressive ↓ metastasis, angiogenesis, cell growth, proliferation, EMT markers, migration, drug resistance ↑ cell cycle arrest [207,208,209,210,211,212,213,214,215,216,217,218,219] |
| miR-126-3p | Female BALB/C mice mammary tissue from virgin, pregnancy, lactation, and involution at 3 time points within each (n = 1/time point) [220] |
| Breast cancer cell lines | Tumour suppressive ↓ invasion, migration [210,221] |
| miR-142-3p | Female BALB/c mice mammary tissue from virgin 4, 5, 7 weeks, pregnancy 5, 13, 18 days, lactation 3, 7, 13 days, involution 2, 5, 10 days [69] |
| Ex vivo tissue, breast cancer cell lines, tumour mouse models | Tumour suppressive ↓ invasion, migration, proliferation, chemoresistance, cell size, cell volume, EMT, metastasis ↑ apoptosis, cell cycle arrest [222,223,224] [70,71,72,225,226,227,228,229] Oncogenic ↑ metastasis [230] |
| miR-15b | Mice 2 mammary gland from mature virgin (8 weeks), pregnancy day 5, lactation day 0, lactation day 5, lactation day 10 [231] |
| Ex vivo tissue, breast cancer cell lines | Oncogenic ↓ apoptosis ↑ migration, invasion, cell size, cell volume, proliferation [232,233,234] |
| miR-205 | miR-205-lacZ and miR-205fl/fl mice from C57BL6/129s mixed background [47] |
| Breast cancer cell lines, tumour mouse models | Tumour suppressive ↓ proliferation, migration, invasion, EMT, angiogenesis, radio/chemotherapy resistance ↑ apoptosis [27,48,81,114,235,236,237,238,239] 2 |
| SCID/beige mice transplanted with miR-205fl/fl mammary cells |
| |||
| miR-206 | MMTV-Cre Brca1Co/Co mice [42] |
| Ex vivo tissue, breast cancer cell lines | Oncogenic ↑ migration, invasion, proliferation [44] Tumour suppressive ↓ proliferation, drug resistance, metastasis, stemness ↑ apoptosis [27,42,43,81,82] 2 |
| MMTV miR-206 mice of FVB/NJ background [240] |
| |||
| miR-221 | Mice 3 mammary gland from mature virgin (8 weeks), pregnancy day 5, lactation day 0, lactation day 5, lactation day 10, involution day 10 [241] |
| Review | Oncogenic ↓ apoptosis ↑ drug resistance, EMT, proliferation, metastasis, invasion [58,114,242] 2 |
| miR-30b | MMTV-LTR miR-30b transgenic mice of FVB/N background [243] |
| Ex vivo tissue, breast cancer cell lines, review | Tumour suppressive ↑ chemotherapy sensitivity, cell cycle arrest [27] 2 Oncogenic ↑ proliferation, migration, invasion [244] |
6. Conclusions and Future Directions
7. Methods
7.1. Mammary Gland Development miRNA Search Strategy
7.2. Breast Cancer miRNA Search Strategy
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, CA. Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Prim. 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.P.; Chen, J.; Ward, W.E.; Thompson, L.U. Mammary gland morphogenesis is enhanced by exposure to flaxseed or its major lignan during suckling in rats. Exp. Biol. Med. 2004, 229, 147–157. [Google Scholar] [CrossRef]
- Avril-Sassen, S.; Goldstein, L.D.; Stingl, J.; Blenkiron, C.; Le Quesne, J.; Spiteri, I.; Karagavriilidou, K.; Watson, C.J.; Tavaré, S.; Miska, E.A.; et al. Characterisation of microRNA expression in post-natal mouse mammary gland development. BMC Genom. 2009, 10, 548. [Google Scholar] [CrossRef]
- Peng, Y.; Croce, C.M. The role of microRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef]
- Macias, H.; Hinck, L. Mammary gland development. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 533–557. [Google Scholar] [CrossRef]
- Morra, A.; Jung, A.Y.; Behrens, S.; Keeman, R.; Ahearn, T.U.; Anton-Culver, H.; Arndt, V.; Augustinsson, A.; Auvinen, P.K.; Beane Freeman, L.E.; et al. Breast Cancer Risk Factors and Survival by Tumor Subtype: Pooled Analyses from the Breast Cancer Association Consortium. Cancer Epidemiol. Biomark. Prev. 2021, 30, 623–642. [Google Scholar] [CrossRef] [PubMed]
- Bilkovski, R.; Schulte, D.M.; Oberhauser, F.; Gomolka, M.; Udelhoven, M.; Hettich, M.M.; Roth, B.; Heidenreich, A.; Gutschow, C.; Krone, W.; et al. Role of WNT-5a in the determination of human mesenchymal stem cells into preadipocytes. J. Biol. Chem. 2010, 285, 6170–6178. [Google Scholar] [CrossRef]
- Neville, M.C.; Medina, D.; Monks, J.; Hovey, R.C. The Mammary Fat Pad. J. Mammary Gland Biol. Neoplasia 1998, 3, 109–116. [Google Scholar] [CrossRef]
- Slepicka, P.F.; Cyrill, S.L.; dos Santos, C.O. Pregnancy and Breast Cancer: Pathways to Understand Risk and Prevention. Trends Mol. Med. 2019, 25, 866–881. [Google Scholar] [CrossRef]
- Paine, I.S.; Lewis, M.T. The Terminal End Bud: The Little Engine that Could. J. Mammary Gland Biol. Neoplasia 2017, 22, 93–108. [Google Scholar] [CrossRef]
- Watson, C.J. Key stages in mammary gland development—Involution: Apoptosis and tissue remodelling that convert the mammary gland from milk factory to a quiescent organ. Breast Cancer Res. 2006, 8, 203. [Google Scholar] [CrossRef]
- Voduc, K.D.; Cheang, M.C.U.; Tyldesley, S.; Gelmon, K.; Nielsen, T.O.; Kennecke, H. Breast cancer subtypes and the risk of local and regional relapse. J. Clin. Oncol. 2010, 28, 1684–1691. [Google Scholar] [CrossRef]
- Almansour, N.M. Triple-Negative Breast Cancer: A Brief Review About Epidemiology, Risk Factors, Signaling Pathways, Treatment and Role of Artificial Intelligence. Front. Mol. Biosci. 2022, 9, 836417. [Google Scholar] [CrossRef]
- Atashgaran, V.; Wrin, J.; Barry, S.C.; Dasari, P.; Ingman, W.V. Dissecting the Biology of Menstrual Cycle-Associated Breast Cancer Risk. Front. Oncol. 2016, 6, 267. [Google Scholar] [CrossRef]
- Boyd, N.F.; Martin, L.J.; Yaffe, M.J.; Minkin, S. Mammographic density and breast cancer risk: Current understanding and future prospects. Breast Cancer Res. 2011, 13, 223. [Google Scholar] [CrossRef]
- Dave, B.; Mittal, V.; Tan, N.M.; Chang, J.C. Epithelial-mesenchymal transition, cancer stem cells and treatment resistance. Breast Cancer Res. 2012, 14, 202. [Google Scholar] [CrossRef] [PubMed]
- Wellings, S.R.; Jensen, H.M.; Marcum, R.G. An atlas of subgross pathology of the human breast with special reference to possible precancerous lesions. J. Natl. Cancer Inst. 1975, 55, 231–273. [Google Scholar] [PubMed]
- Russo, J. Significance of rat mammary tumors for human risk assessment. Toxicol. Pathol. 2015, 43, 145–170. [Google Scholar] [CrossRef]
- Russo, I.H.; Russo, J. Developmental stage of the rat mammary gland as determinant of its susceptibility to 7,12-dimethylbenz[a]anthracene. J. Natl. Cancer Inst. 1978, 61, 1439–1449. [Google Scholar]
- Russo, J.; Wilgus, G.; Russo, I.H. Susceptibility of the mammary gland to carcinogenesis: I Differentiation of the mammary gland as determinant of tumor incidence and type of lesion. Am. J. Pathol. 1979, 96, 721–736. [Google Scholar]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- DeVeale, B.; Swindlehurst-Chan, J.; Blelloch, R. The roles of microRNAs in mouse development. Nat. Rev. Genet. 2021, 22, 307–323. [Google Scholar] [CrossRef]
- Serpico, D.; Molino, L.; Di Cosimo, S. microRNAs in breast cancer development and treatment. Cancer Treat. Rev. 2014, 40, 595–604. [Google Scholar] [CrossRef]
- Loh, H.-Y.; Norman, B.P.; Lai, K.-S.; Rahman, N.M.A.N.A.; Alitheen, N.B.M.; Osman, M.A. The Regulatory Role of MicroRNAs in Breast Cancer. Int. J. Mol. Sci. 2019, 20, 4940. [Google Scholar] [CrossRef]
- Lakshmi, S.; Hughes, T.A.; Priya, S. Exosomes and exosomal RNAs in breast cancer: A status update. Eur. J. Cancer 2021, 144, 252–268. [Google Scholar] [CrossRef]
- Silveri, L.; Tilly, G.; Vilotte, J.-L.L.; Le Provost, F. MicroRNA involvement in mammary gland development and breast cancer. Reprod. Nutr. Dev. 2006, 46, 549–556. [Google Scholar] [CrossRef][Green Version]
- Jena, M.K. MicroRNAs in the development and neoplasia of the mammary gland. F1000Research 2017, 6, 1018. [Google Scholar] [CrossRef]
- Spina, E.; Cowin, P. Embryonic mammary gland development. Semin. Cell Dev. Biol. 2021, 114, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.R.; Durrans, A.; Lee, S.; Sheng, J.; Li, F.; Wong, S.T.C.; Choi, H.; El Rayes, T.; Ryu, S.; Troeger, J.; et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 2015, 527, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y. Embryonic Programs in Cancer and Metastasis-Insights From the Mammary Gland. Front. Cell Dev. Biol. 2022, 10, 938625. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-M.; Cho, K.-W.; Kim, E.-J.; Tang, Q.; Kim, K.-S.; Tickle, C.; Jung, H.-S. A contrasting function for miR-137 in embryonic mammogenesis and adult breast carcinogenesis. Oncotarget 2015, 6, 22048–22059. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grünert, S.; Jechlinger, M.; Beug, H. Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nat. Rev. Mol. Cell Biol. 2003, 4, 657–665. [Google Scholar] [CrossRef]
- Han, Y.; Bi, Y.; Bi, H.; Diao, C.; Zhang, G.; Cheng, K.; Yang, Z. miR-137 suppresses the invasion and procedure of EMT of human breast cancer cell line MCF-7 through targeting CtBP1. Hum. Cell 2016, 29, 30–36. [Google Scholar] [CrossRef]
- Du, F.; Yu, L.; Wu, Y.; Wang, S.; Yao, J.; Zheng, X.; Xie, S.; Zhang, S.; Lu, X.; Liu, Y.; et al. miR-137 alleviates doxorubicin resistance in breast cancer through inhibition of epithelial-mesenchymal transition by targeting DUSP4. Cell Death Dis. 2019, 10, 922. [Google Scholar] [CrossRef]
- Lee, S.J.; Jeong, J.-H.; Kang, S.H.; Kang, J.; Kim, E.A.; Lee, J.; Jung, J.H.; Park, H.Y.; Chae, Y.S.; SJ, L.; et al. MicroRNA-137 Inhibits Cancer Progression by Targeting Del-1 in Triple-Negative Breast Cancer Cells. Int. J. Mol. Sci. 2019, 20, 6162. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Lou, G.; Zhao, L.; Xu, Z.; Zhang, Y.; He, F. MiR-137 targets estrogen-related receptor alpha and impairs the proliferative and migratory capacity of breast cancer cells. PLoS ONE 2012, 7, e39102. [Google Scholar] [CrossRef]
- Visvader, J.E.; Stingl, J. Mammary stem cells and the differentiation hierarchy: Current status and perspectives. Genes Dev. 2014, 28, 1143–1158. [Google Scholar] [CrossRef]
- Lee, M.J.; Yoon, K.S.; Cho, K.W.; Kim, K.S.; Jung, H.S. Expression of miR-206 during the initiation of mammary gland development. Cell Tissue Res. 2013, 353, 425–433. [Google Scholar] [CrossRef]
- Wang, J.; Aydoğdu, E.; Mukhopadhyay, S.; LA, H.; Williams, C. A miR-206 regulated gene landscape enhances mammary epithelial differentiation. J. Cell. Physiol. 2019, 234, 22220–22233. [Google Scholar] [CrossRef]
- Sun, D.; Li, C.; Zhang, F. MicroRNA-206 suppresses growth and metastasis of breast cancer stem cells via blocking EVI-1-mediated CALR expression. PLoS ONE 2022, 17, e0274919. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, M.; Tong, Y.; Liu, X.; Zhang, L.; Dong, D.; Shao, J. miR-206 Promotes Cancer Progression by Targeting Full-Length Neurokinin-1 Receptor in Breast Cancer. Technol. Cancer Res. Treat. 2019, 18, 1533033819875168. [Google Scholar] [CrossRef]
- Bonetti, P.; Climent, M.; Panebianco, F.; Tordonato, C.; Santoro, A.; MJ, M.; PG, P.; Ventura, A.; Nicassio, F. Dual role for miR-34a in the control of early progenitor proliferation and commitment in the mammary gland and in breast cancer. Oncogene 2019, 38, 360–374. [Google Scholar] [CrossRef]
- Cui, Y.; Sun, X.; Jin, L.; Yu, G.; Li, Q.; Gao, X.; Ao, J.; Wang, C. MiR-139 suppresses β-casein synthesis and proliferation in bovine mammary epithelial cells by targeting the GHR and IGF1R signaling pathways. BMC Vet. Res. 2017, 13, 350. [Google Scholar] [CrossRef]
- Lu, Y.; Cao, J.; Napoli, M.; Xia, Z.; Zhao, N.; Creighton, C.J.; Li, W.; Chen, X.; Flores, E.R.; McManus, M.T.; et al. miR-205 Regulates Basal Cell Identity and Stem Cell Regenerative Potential During Mammary Reconstitution. Stem Cells 2018, 36, 1875–1889. [Google Scholar] [CrossRef]
- Plantamura, I.; Cataldo, A.; Cosentino, G.; Iorio, M.V. miR-205 in Breast Cancer: State of the Art. Int. J. Mol. Sci. 2020, 22, 27. [Google Scholar] [CrossRef]
- Holliday, H.; Baker, L.A.; Junankar, S.R.; Clark, S.J.; Swarbrick, A. Epigenomics of mammary gland development. Breast Cancer Res. 2018, 20, 100. [Google Scholar] [CrossRef]
- El-Osaily, H.H.; Ibrahim, I.H.; Essawi, M.L.; Salem, S.M. Impact of miRNAs expression modulation on the methylation status of breast cancer stem cell-related genes. Clin. Transl. Oncol. 2021, 23, 1440–1451. [Google Scholar] [CrossRef]
- Phua, Y.W.; Nguyen, A.; Roden, D.L.; Elsworth, B.; Deng, N.; Nikolic, I.; Yang, J.; Mcfarland, A.; Russell, R.; Kaplan, W.; et al. MicroRNA profiling of the pubertal mouse mammary gland identifies miR-184 as a candidate breast tumour suppressor gene. Breast Cancer Res. 2015, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, M.; Xu, F.; Jiang, S. Wnt signaling in breast cancer: Biological mechanisms, challenges and opportunities. Mol. Cancer 2020, 19, 165. [Google Scholar] [CrossRef] [PubMed]
- Miricescu, D.; Totan, A.; Badoiu, S.C.; Stefani, C.; Greabu, M. PI3K/AKT/mTOR Signaling Pathway in Breast Cancer: From Molecular Landscape to Clinical Aspects. Int. J. Mol. Sci. 2021, 22, 173. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Dong, L. Inhibitory effect of miR-184 on the potential of proliferation and invasion in human glioma and breast cancer cells in vitro. Int. J. Clin. Exp. Pathol. 2015, 8, 9376–9382. [Google Scholar] [PubMed]
- Feuermann, Y.; Robinson, G.W.; Zhu, B.M.; Kang, K.; Raviv, N.; Yamaji, D.; Hennighausen, L. The miR-17/92 cluster is targeted by STAT5 but dispensable for mammary development. Genesis 2012, 50, 665–671. [Google Scholar] [CrossRef]
- Feuermann, Y.; Kang, K.; Shamay, A.; Robinson, G.W.; Hennighausen, L. MiR-21 is under control of STAT5 but is dispensable for mammary development and lactation. PLoS ONE 2014, 9, e85123. [Google Scholar] [CrossRef]
- Liu, S.; Goldstein, R.H.; Scepansky, E.M.; Rosenblatt, M. Inhibition of Rho-Associated Kinase Signaling Prevents Breast Cancer Metastasis to Human Bone. Cancer Res. 2009, 69, 8742–8751. [Google Scholar] [CrossRef]
- Maryam, M.; Naemi, M.; Hasani, S.S. A comprehensive review on oncogenic miRNAs in breast cancer. J. Genet. 2021, 100, 15. [Google Scholar]
- Cai, Q.; Yang, H.-S.; Li, Y.-C.; Zhu, J. Dissecting the Roles of PDCD4 in Breast Cancer. Front. Oncol. 2022, 12, 855807. [Google Scholar] [CrossRef]
- LeMay-Nedjelski, L.; Mason-Ennis, J.K.; Taibi, A.; Comelli, E.M.; Thompson, L.U. Omega-3 Polyunsaturated Fatty Acids Time-Dependently Reduce Cell Viability and Oncogenic MicroRNA-21 Expression in Estrogen Receptor-Positive Breast Cancer Cells (MCF-7). Int. J. Mol. Sci. 2018, 19, 244. [Google Scholar] [CrossRef]
- Dangat, K.; Khaire, A.; Joshi, S. Cross talk of vascular endothelial growth factor and neurotrophins in mammary gland development. Growth Factors 2020, 38, 16–24. [Google Scholar] [CrossRef]
- Boudreau, N.; Myers, C. Breast cancer-induced angiogenesis: Multiple mechanisms and the role of the microenvironment. Breast Cancer Res. 2003, 5, 140–146. [Google Scholar] [CrossRef]
- Lim, D.; Cho, J.G.; Yun, E.; Lee, A.; Ryu, H.-Y.; Lee, Y.J.; Yoon, S.; Chang, W.; Lee, M.-S.; Kwon, B.S.; et al. MicroRNA 34a-AXL Axis Regulates Vasculogenic Mimicry Formation in Breast Cancer Cells. Genes 2020, 12, 9. [Google Scholar] [CrossRef]
- Yoo, K.H.; Kang, K.; Feuermann, Y.; Jang, S.J.; Robinson, G.W.; Hennighausen, L. The STAT5-regulated miR-193b locus restrains mammary stem and progenitor cell activity and alveolar differentiation. Dev. Biol. 2014, 395, 245–254. [Google Scholar] [CrossRef]
- Yang, X.; Meyer, K.; Friedl, A. STAT5 and Prolactin Participate in a Positive Autocrine Feedback Loop That Promotes Angiogenesis. J. Biol. Chem. 2013, 288, 21184–21196. [Google Scholar] [CrossRef]
- Hulin, J.-A.; Tommasi, S.; Elliot, D.; Hu, D.G.; Lewis, B.C.; Mangoni, A.A. MiR-193b regulates breast cancer cell migration and vasculogenic mimicry by targeting dimethylarginine dimethylaminohydrolase. Sci. Rep. 2017, 7, 13996. [Google Scholar] [CrossRef]
- Parton, M.; Dowsett, M.; Smith, I. Studies of apoptosis in breast cancer. BMJ 2001, 322, 1528–1532. [Google Scholar] [CrossRef]
- Radisky, D.C.; Hartmann, L.C. Mammary involution and breast cancer risk: Transgenic models and clinical studies. J. Mammary Gland Biol. Neoplasia 2009, 14, 181–191. [Google Scholar] [CrossRef]
- Tian, L.; Zhang, L.; Cui, Y.; Li, H.; Xie, X.; Li, Y.; Wang, C. miR-142-3p Regulates Milk Synthesis and Structure of Murine Mammary Glands via PRLR-Mediated Multiple Signaling Pathways. J. Agric. Food Chem. 2019, 67, 9532–9542. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; MF, G.; Shirjang, S.; Asadzadeh, Z.; Khaze, V.; Holmskov, U.; Kazemi, T.; Duijf, P.H.G.; Baradaran, B. miR-142-3p is a tumor suppressor that inhibits estrogen receptor expression in ER-positive breast cancer. J. Cell. Physiol. 2019, 234, 16043–16053. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, B.; Duijf, P.H.; Mohammadi, A.; Safarzadeh, E.; Ditzel, H.J.; Gjerstorff, M.F.; Cho, W.C.; Baradaran, B. MiR-142-3p targets HMGA2 and suppresses breast cancer malignancy. Life Sci. 2021, 276, 119431. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Yadav, P.; Sundaram, S.; Venkatraman, G.; Bera, A.K.; Karunagaran, D. HMGB3 inhibition by miR-142-3p/sh-RNA modulates autophagy and induces apoptosis via ROS accumulation and mitochondrial dysfunction and reduces the tumorigenic potential of human breast cancer cells. Life Sci. 2022, 304, 120727. [Google Scholar] [CrossRef] [PubMed]
- Llobet-Navas, D.; Rodríguez-Barrueco, R.; Castro, V.; Ugalde, A.P.; Sumazin, P.; Jacob-Sendler, D.; Demircan, B.; Castillo-Martín, M.; Putcha, P.; Marshall, N.; et al. The miR-424(322)/503 cluster orchestrates remodeling of the epithelium in the involuting mammary gland. Genes Dev. 2014, 28, 765–782. [Google Scholar] [CrossRef] [PubMed]
- Dastmalchi, N.; Safaralizadeh, R.; Hosseinpourfeizi, M.A.; Baradaran, B.; Khojasteh, S.M.B. MicroRNA-424-5p enhances chemosensitivity of breast cancer cells to Taxol and regulates cell cycle, apoptosis, and proliferation. Mol. Biol. Rep. 2021, 48, 1345–1357. [Google Scholar] [CrossRef] [PubMed]
- Dastmalchi, N.; Hosseinpourfeizi, M.A.; Khojasteh, S.M.; Baradaran, B.; Safaralizadeh, R. Tumor suppressive activity of miR-424-5p in breast cancer cells through targeting PD-L1 and modulating PTEN/PI3K/AKT/mTOR signaling pathway. Life Sci. 2020, 259, 118239. [Google Scholar] [CrossRef]
- Ying, X.; Sun, Y.; He, P. MicroRNA-137 inhibits BMP7 to enhance the epithelial-mesenchymal transition of breast cancer cells. Oncotarget 2017, 8, 18348–18358. [Google Scholar] [CrossRef]
- Cheng, S.; Huang, Y.; Lou, C.; He, Y.; Zhang, Y.; Zhang, Q. FSTL1 enhances chemoresistance and maintains stemness in breast cancer cells via integrin β3/Wnt signaling under miR-137 regulation. Cancer Biol. Ther. 2019, 20, 328–337. [Google Scholar] [CrossRef]
- Zeng, J.-S.; Zhang, Z.-D.; Pei, L.; Bai, Z.-Z.; Yang, Y.; Yang, H.; Tian, Q.-H. CBX4 exhibits oncogenic activities in breast cancer via Notch1 signaling. Int. J. Biochem. Cell. Biol. 2018, 95, 1–8. [Google Scholar] [CrossRef]
- Black, J.C.; Zhang, H.; Kim, J.; Getz, G.; Whetstine, J.R. Regulation of Transient Site-specific Copy Gain by MicroRNA. J. Biol. Chem. 2016, 291, 4862–4871. [Google Scholar] [CrossRef]
- Denis, H.; Van Grembergen, O.; Delatte, B.; Dedeurwaerder, S.; Putmans, P.; Calonne, E.; Rothé, F.; Sotiriou, C.; Fuks, F.; Deplus, R. MicroRNAs regulate KDM5 histone demethylases in breast cancer cells. Mol. Biosyst. 2016, 12, 404–413. [Google Scholar] [CrossRef]
- Vimalraj, S.; Miranda, P.J.; Ramyakrishna, B.; Selvamurugan, N. Regulation of breast cancer and bone metastasis by microRNAs. Dis. Markers 2013, 35, 369–387. [Google Scholar] [CrossRef]
- Mobini, K.; Tamaddon, G.; Fardid, R.; Keshavarzi, M.; Mohammadi-Bardbori, A. Aryl hydrocarbon-estrogen alpha receptor-dependent expression of miR-206, miR-27b, and miR-133a suppress cell proliferation and migration in MCF-7 cells. J. Biochem. Mol. Toxicol. 2019, 33, e22304. [Google Scholar] [CrossRef]
- Oltra, S.S.; Peña-Chilet, M.; Vidal-Tomas, V.; Flower, K.; Martinez, M.T.; Alonso, E.; Burgues, O.; Lluch, A.; Flanagan, J.M.; Ribas, G. Methylation deregulation of miRNA promoters identifies miR124-2 as a survival biomarker in Breast Cancer in very young women. Sci. Rep. 2018, 8, 14373. [Google Scholar] [CrossRef]
- Xie, R.; Wang, M.; Zhou, W.; Wang, D.; Yuan, Y.; Shi, H.; Wu, L. Long Non-Coding RNA (LncRNA) UFC1/miR-34a Contributes to Proliferation and Migration in Breast Cancer. Med. Sci. Monit. 2019, 25, 7149–7157. [Google Scholar] [CrossRef]
- Li, Z.-H.; Yu, N.-S.; Deng, Q.; Zhang, Y.; Hu, Y.-Y.; Liu, G.; Huang, K. LncRNA SNHG7 Mediates the Chemoresistance and Stemness of Breast Cancer by Sponging miR-34a. Front. Oncol. 2020, 10, 592757. [Google Scholar] [CrossRef]
- Han, R.; Zhao, J.; Lu, L. MicroRNA-34a expression affects breast cancer invasion in vitro and patient survival via downregulation of E2F1 and E2F3 expression. Oncol. Rep. 2020, 43, 2062–2072. [Google Scholar] [CrossRef]
- Imani, S.; Wu, R.-C.; Fu, J. MicroRNA-34 family in breast cancer: From research to therapeutic potential. J. Cancer 2018, 9, 3765–3775. [Google Scholar] [CrossRef]
- Martini, S.; Zuco, V.; Tortoreto, M.; Percio, S.; Campi, E.; El Bezawy, R.; Doldi, V.; Landesman, Y.; Pennati, M.; Zaffaroni, N. miR-34a-Mediated Survivin Inhibition Improves the Antitumor Activity of Selinexor in Triple-Negative Breast Cancer. Pharmaceuticals 2021, 14, 523. [Google Scholar] [CrossRef]
- Haghi, M.; Taha, M.F.; Javeri, A. Suppressive effect of exogenous miR-16 and miR-34a on tumorigenesis of breast cancer cells. J. Cell. Biochem. 2019, 120, 13342–13353. [Google Scholar] [CrossRef]
- Adams, B.D.; Parsons, C.; Slack, F.J. The tumor-suppressive and potential therapeutic functions of miR-34a in epithelial carcinomas. Expert Opin. Ther. Targets 2016, 20, 737–753. [Google Scholar] [CrossRef]
- Kong, Y.; Geng, C.; Dong, Q. LncRNA PAPAS may promote triple-negative breast cancer by downregulating miR-34a. J. Int. Med. Res. 2019, 47, 3709–3718. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, B.; Silvestris, N.; Mohammadi, A.; Khaze, V.; Baghbani, E.; Mokhtarzadeh, A.; Shanehbandi, D.; Derakhshani, A.; Duijf, P.H.G.; Baradaran, B. miR-34a and miR-200c Have an Additive Tumor-Suppressive Effect on Breast Cancer Cells and Patient Prognosis. Genes 2021, 12, 267. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Huang, T.; Liu, Z.; Sun, M.; Luo, S. LncRNA SNHG7 contributes to tumorigenesis and progression in breast cancer by interacting with miR-34a through EMT initiation and the Notch-1 pathway. Eur. J. Pharmacol. 2019, 856, 172407. [Google Scholar] [CrossRef] [PubMed]
- Irani, S.; Paknejad, M.; Soleimani, M.; Azam, S. Evaluation of miR-34a Effect on CCND1 mRNA Level and Sensitization of Breast Cancer Cell Lines to Paclitaxel. Iran Biomed. J. 2020, 24, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.-G.; Shi, J.-C.; Shang, J.; Hao, J.-G.; Du, X. Effect of miR-34a on resistance to sunitinib in breast cancer by regulating the Wnt/β-catenin signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1151–1157. [Google Scholar]
- Patel, Y.; Soni, M.; Awgulewitsch, A.; Kern, M.J.; Liu, S.; Shah, N.; Sigh, U.P.; Chen, H. Overexpression of miR-489 derails mammary hierarchy structure and inhibits HER2/neu-induced tumorigenesis. Oncogene 2019, 38, 445–453. [Google Scholar] [CrossRef]
- Patel, Y.; Shah, N.; Lee, J.S.; Markoutsa, E.; Jie, C.; Liu, S.; Botbyl, R.; Reisman, D.; Xu, P.; Chen, H. A novel double-negative feedback loop between miR-489 and the HER2-SHP2-MAPK signaling axis regulates breast cancer cell proliferation and tumor growth. Oncotarget 2016, 7, 18295–18308. [Google Scholar] [CrossRef]
- Soni, M.; Saatci, O.; Gupta, G.; Patel, Y.; Keerthi Raja, M.R.; Li, J.; Liu, X.; Xu, P.; Wang, H.; Fan, D.; et al. miR-489 Confines Uncontrolled Estrogen Signaling through a Negative Feedback Mechanism and Regulates Tamoxifen Resistance in Breast Cancer. Int. J. Mol. Sci. 2022, 23, 8086. [Google Scholar] [CrossRef]
- Soni, M.; Patel, Y.; Markoutsa, E.; Jie, C.; Liu, S.; Xu, P.; Chen, H. Autophagy, Cell Viability, and Chemoresistance Are Regulated By miR-489 in Breast Cancer. Mol. Cancer Res. 2018, 16, 1348–1360. [Google Scholar] [CrossRef]
- Jiang, L.; He, D.; Yang, D.; Chen, Z.; Pan, Q.; Mao, A.; Cai, Y.; Li, X.; Xing, H.; Shi, M.; et al. MiR-489 regulates chemoresistance in breast cancer via epithelial mesenchymal transition pathway. FEBS Lett. 2014, 588, 2009–2015. [Google Scholar] [CrossRef]
- Wang, X.; Gu, J.; Zhou, M.; He, Z.; Ferrone, S. Overexpression of miR-489 enhances efficacy of 5-fluorouracil-based treatment in breast cancer stem cells by targeting XIAP. Oncotarget 2017, 8, 113837–113846. [Google Scholar] [CrossRef][Green Version]
- Chen, X.; Wang, Y.W.; Zhu, W.J.; Li, Y.; Liu, L.; Yin, G.; Gao, P. A 4-microRNA signature predicts lymph node metastasis and prognosis in breast cancer. Hum. Pathol. 2018, 76, 122–132. [Google Scholar] [CrossRef]
- Liu, D.; Zhou, Z.; Guo, Y.; Du, Q.; Li, L. CircCDK1 knockdown reduces CDK1 expression by targeting miR-489-3p to suppress the development of breast cancer and strengthen the sensitivity of Tamoxifen. Anticancer Drugs 2022, 33, 286–299. [Google Scholar] [CrossRef]
- Chai, P.; Tian, J.; Zhao, D.; Zhang, H.; Cui, J.; Ding, K.; Liu, B. GSE1 negative regulation by miR-489-5p promotes breast cancer cell proliferation and invasion. Biochem. Biophys. Res. Commun. 2016, 471, 123–128. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.-W.; Xing, A.-Y.; Xiang, S.; Shi, D.-B.; Liu, L.; Li, Y.-X.; Gao, P. Suppression of SPIN1-mediated PI3K-Akt pathway by miR-489 increases chemosensitivity in breast cancer. J. Pathol. 2016, 239, 459–472. [Google Scholar] [CrossRef]
- O’Bryan, S.; Dong, S.; Mathis, J.M.; Alahari, S.K. The roles of oncogenic miRNAs and their therapeutic importance in breast cancer. Eur. J. Cancer 2017, 72, 1–11. [Google Scholar] [CrossRef]
- Hashemi, Z.S.; Forouzandeh Moghadam, M.; Khalili, S.; Ghavami, M.; Salimi, F.; Sadroddiny, E. Additive effect of metastamiR-193b and breast cancer metastasis suppressor 1 as an anti-metastatic strategy. Breast Cancer 2019, 26, 215–228. [Google Scholar] [CrossRef]
- Sun, L.; He, M.; Xu, N.; Xu, D.-H.; Ben-David, Y.; Yang, Z.-Y.; Li, Y.-J. Regulation of RAB22A by mir-193b inhibits breast cancer growth and metastasis mediated by exosomes. Int. J. Oncol. 2018, 53, 2705–2714. [Google Scholar] [CrossRef]
- Long, J.; Ji, Z.; Jiang, K.; Wang, Z.; Meng, G. miR-193b Modulates Resistance to Doxorubicin in Human Breast Cancer Cells by Downregulating MCL-1. Biomed. Res. Int. 2015, 2015, 373574. [Google Scholar] [CrossRef]
- Yang, Z.; Zhuang, Q.; Hu, G.; Geng, S. MORC4 is a novel breast cancer oncogene regulated by miR-193b-3p. J. Cell. Biochem. 2019, 120, 4634–4643. [Google Scholar] [CrossRef]
- Giacomelli, C.; Jung, J.; Wachter, A.; Ibing, S.; Will, R.; Uhlmann, S.; Mannsperger, H.; Sahin, Ö.; Yarden, Y.; Beißbarth, T.; et al. Coordinated regulation of WNT/β-catenin, c-Met, and integrin signalling pathways by miR-193b controls triple negative breast cancer metastatic traits. BMC Cancer 2021, 21, 1296. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; He, M.; Wang, K.; Sun, G.; Tang, L.; Xu, Z. Tumor suppressive microRNA-193b promotes breast cancer progression via targeting DNAJC13 and RAB22A. Int. J. Clin. Exp. Pathol. 2014, 7, 7563–7570. [Google Scholar] [PubMed]
- Hu, S.; Cao, M.; He, Y.; Zhang, G.; Liu, Y.; Du, Y.; Yang, C.; Gao, F. CD44v6 Targeted by miR-193b-5p in the Coding Region Modulates the Migration and Invasion of Breast Cancer Cells. J. Cancer 2020, 11, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Petrović, N.; Nakashidze, I.; Nedeljković, M. Breast Cancer Response to Therapy: Can microRNAs Lead the Way? J. Mammary Gland Biol. Neoplasia 2021, 26, 157–178. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, A.M.; Salvatore, M.; Incoronato, M. miRNA-Based Therapeutics in Breast Cancer: A Systematic Review. Front. Oncol. 2021, 11, 668464. [Google Scholar] [CrossRef]
- Campos, A.; Sharma, S.; Obermair, A.; Salomon, C. Extracellular Vesicle-Associated miRNAs and Chemoresistance: A Systematic Review. Cancers 2021, 13, 4608. [Google Scholar] [CrossRef]
- Oghabi Bakhshaiesh, T.; Esmaeili, R. Effects of noncoding RNAs in radiotherapy response in breast cancer: A systematic review. Cell Cycle 2022, 21, 883–893. [Google Scholar] [CrossRef]
- Lin, X.; Luo, J.; Zhang, L.; Wang, W.; Shi, H.; Zhu, J. MiR-27a suppresses triglyceride accumulation and affects gene mRNA expression associated with fat metabolism in dairy goat mammary gland epithelial cells. Gene 2013, 521, 15–23. [Google Scholar] [CrossRef]
- Wu, H.; Qiu, J.; Wu, Z.; He, T.; Zhou, C.; Lv, Q. MiR-27a-3p binds to TET1 mediated DNA demethylation of ADCY6 regulates breast cancer progression via epithelial-mesenchymal transition. Front. Oncol. 2022, 12, 957511. [Google Scholar] [CrossRef]
- Zhang, H.-D.; Sun, D.-W.; Mao, L.; Zhang, J.; Jiang, L.-H.; Li, J.; Wu, Y.; Ji, H.; Chen, W.; Wang, J.; et al. MiR-139-5p inhibits the biological function of breast cancer cells by targeting Notch1 and mediates chemosensitivity to docetaxel. Biochem. Biophys. Res. Commun. 2015, 465, 702–713. [Google Scholar] [CrossRef]
- Li, H.-C.; Chen, Y.-F.; Feng, W.; Cai, H.; Mei, Y.; Jiang, Y.-M.; Chen, T.; Xu, K.; Feng, D.-X. Loss of the Opa interacting protein 5 inhibits breast cancer proliferation through miR-139-5p/NOTCH1 pathway. Gene 2017, 603, 1–8. [Google Scholar] [CrossRef]
- Gu, S.Q.; Luo, J.H.; Yao, W.X. The regulation of miR-139-5p on the biological characteristics of breast cancer cells by targeting COL11A1. Math. Biosci. Eng. 2019, 17, 1428–1441. [Google Scholar] [CrossRef]
- Pajic, M.; Froio, D.; Daly, S.; Doculara, L.; Millar, E.; Graham, P.H.; Drury, A.; Steinmann, A.; de Bock, C.E.; Boulghourjian, A.; et al. miR-139-5p Modulates Radiotherapy Resistance in Breast Cancer by Repressing Multiple Gene Networks of DNA Repair and ROS Defense. Cancer Res. 2018, 78, 501–515. [Google Scholar] [CrossRef]
- Fang, J.; Huang, C.; Ke, J.; Li, J.; Zhang, W.; Xue, H.; Chen, J. LncRNA TTN-AS1 facilitates proliferation, invasion, and epithelial-mesenchymal transition of breast cancer cells by regulating miR-139-5p/ZEB1 axis. J. Cell. Biochem. 2020, 121, 4772–4784. [Google Scholar] [CrossRef]
- Zhang, J.; Ke, S.; Zheng, W.; Zhu, Z.; Wu, Y. Hsa_circ_0003645 Promotes Breast Cancer Progression by Regulating miR-139-3p/HMGB1 Axis. OncoTargets Ther. 2020, 13, 10361–10372. [Google Scholar] [CrossRef]
- Dai, H.; Gallagher, D.; Schmitt, S.; Pessetto, Z.Y.; Fan, F.; Godwin, A.K.; Tawfik, O. Role of miR-139 as a surrogate marker for tumor aggression in breast cancer. Hum. Pathol. 2017, 61, 68–77. [Google Scholar] [CrossRef]
- Dong, L.; Zhou, D.; Xin, C.; Liu, B.; Sun, P. MicroRNA-139 Suppresses the Tumorigenicity of Triple Negative Breast Cancer Cells by Targeting SOX8. Cancer Manag. Res. 2020, 12, 9417–9428. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, J.; Wang, K.; Tang, X.; He, J. miR-139-3p suppresses the invasion and migration properties of breast cancer cells by targeting RAB1A. Oncol. Rep. 2019, 42, 1699–1708. [Google Scholar] [CrossRef]
- Hua, W.; Sa, K.D.; Zhang, X.; Jia, L.T.; Zhao, J.; Yang, A.G.; Zhang, R.; Fan, J.; Bian, K. MicroRNA-139 suppresses proliferation in luminal type breast cancer cells by targeting Topoisomerase II alpha. Biochem. Biophys. Res. Commun. 2015, 463, 1077–1083. [Google Scholar] [CrossRef]
- Cheng, C.W.; Liao, W.L.; Chen, P.M.; Yu, J.C.; Shiau, H.P.; Hsieh, Y.H.; Lee, H.J.; Cheng, Y.C.; Wu, P.E.; Shen, C.Y.; et al. MiR-139 Modulates Cancer Stem Cell Function of Human Breast Cancer through Targeting CXCR4. Cancers 2021, 13, 2582. [Google Scholar] [CrossRef]
- Heinz, R.E.; Rudolph, M.C.; Ramanathan, P.; Spoelstra, N.S.; Butterfield, K.T.; Webb, P.G.; Babbs, B.L.; Gao, H.; Chen, S.; Gordon, M.A.; et al. Constitutive expression of microRNA-150 in mammary epithelium suppresses secretory activation and impairs de novo lipogenesis. Development 2016, 143, 4236–4248. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Guo, Z.; Qian, H. Role of microRNA-150-5p/SRCIN1 axis in the progression of breast cancer. Exp. Ther. Med. 2019, 17, 2221–2229. [Google Scholar] [CrossRef] [PubMed]
- Sugita, B.M.; Rodriguez, Y.; Fonseca, A.S.; Nunes Souza, E.; Kallakury, B.; Cavalli, I.J.; Ribeiro, E.M.S.F.; Aneja, R.; Cavalli, L.R.; BM, S.; et al. MiR-150-5p Overexpression in Triple-Negative Breast Cancer Contributes to the In Vitro Aggressiveness of This Breast Cancer Subtype. Cancers 2022, 14, 2156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cao, M.; Kong, L.; Liu, J.; Wang, Y.; Song, C.; Chen, X.; Lai, M.; Fang, X.; Chen, H.; et al. MiR-204-5p promotes lipid synthesis in mammary epithelial cells by targeting SIRT1. Biochem. Biophys. Res. Commun. 2020, 533, 1490–1496. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Shen, H.; Gu, B.; Zhu, N. miR-204-5p Hampers Breast Cancer Malignancy and Affects the Cell Cycle by Targeting PRR11. Comput. Math. Methods Med. 2022, 2022, 4010947. [Google Scholar] [CrossRef]
- Huang, Z.; Li, X.; Xie, T.; Gu, C.; Ni, K.; Yin, Q.; Cao, X.; Zhang, C. Elevated Expression of RIOK1 Is Correlated with Breast Cancer Hormone Receptor Status and Promotes Cancer Progression. Cancer Res. Treat. 2020, 52, 1067–1083. [Google Scholar] [CrossRef]
- Liang, Y.; Ye, F.; Wang, Y.; Li, Y.; Song, X.; Luo, D.; Long, L.; Han, D.; Liu, Y.; Wang, Z.; et al. DGUOK-AS1 acts as a tumorpromoter through regulatingmiR-204-5p/IL-11 axis in breast cancer. Mol. Ther. Nucleic Acids 2021, 26, 1079–1091. [Google Scholar] [CrossRef]
- Bian, Q. Circular RNA PVT1 promotes the invasion and epithelial-mesenchymal transition of breast cancer cells through serving as a competing endogenous RNA for miR-204-5p. Onco Targets Ther. 2019, 12, 11817–11826. [Google Scholar] [CrossRef]
- Li, D.; Xie, X.; Wang, J.; Bian, Y.; Li, Q.; Gao, X.; Wang, C. MiR-486 regulates lactation and targets the PTEN gene in cow mammary glands. PLoS ONE 2015, 10, e0118284. [Google Scholar] [CrossRef]
- Mocavini, I.; Pippa, S.; Licursi, V.; Paci, P.; Trisciuoglio, D.; Mannironi, C.; Presutti, C.; Negri, R. JARID1B expression and its function in DNA damage repair are tightly regulated by miRNAs in breast cancer. Cancer Sci. 2019, 110, 1232–1243. [Google Scholar] [CrossRef]
- Mansoori, B.; Najafi, S.; Mohammadi, A.; AsadollahSeraj, H.; Savadi, P.; Mansoori, B.; Nazari, A.; Mokhtarzadeh, A.; Roshani, E.; Duijf, P.H.; et al. The synergy between miR-486-5p and tamoxifen causes profound cell death of tamoxifen-resistant breast cancer cells. Biomed. Pharmacother. 2021, 141, 111925. [Google Scholar] [CrossRef]
- Li, H.; Mou, Q.; Li, P.; Yang, Z.; Wang, Z.; Niu, J.; Liu, Y.; Sun, Z.; Lv, S.; Zhang, B.; et al. MiR-486-5p inhibits IL-22-induced epithelial-mesenchymal transition of breast cancer cell by repressing Dock1. J. Cancer 2019, 10, 4695–4706. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Z.; Cui, G.; Wang, X.; Yang, Z. MicroRNA-486-5p targeting PIM-1 suppresses cell proliferation in breast cancer cells. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2014, 35, 11137–11145. [Google Scholar] [CrossRef]
- Lin, X.; Luo, J.; Zhang, L.; Wang, W.; Gou, D. MiR-103 controls milk fat accumulation in goat (Capra hircus) mammary gland during lactation. PLoS ONE 2013, 8, e79258. [Google Scholar] [CrossRef]
- Xiong, B.; Lei, X.; Zhang, L.; Fu, J. miR-103 regulates triple negative breast cancer cells migration and invasion through targeting olfactomedin. Biomed. Pharmacother. 2017, 89, 1401–1408. [Google Scholar] [CrossRef]
- Martello, G.; Rosato, A.; Ferrari, F.; Manfrin, A.; Cordenonsi, M.; Dupont, S.; Enzo, E.; Guzzardo, V.; Rondina, M.; Spruce, T.; et al. A MicroRNA targeting dicer for metastasis control. Cell 2010, 141, 1195–1207. [Google Scholar] [CrossRef]
- Jiang, N.; Wu, C.; Li, Y.; Liu, J.; Yuan, Y.; Shi, H. Identification and profiling of microRNAs involved in the regenerative involution of mammary gland. Genomics 2022, 114, 110442. [Google Scholar] [CrossRef]
- Maimaitiming, A.; Wusiman, A.; Aimudula, A.; Kuerban, X.; Su, P. MicroRNA-152 Inhibits Cell Proliferation, Migration, and Invasion in Breast Cancer. Oncol. Res. 2020, 28, 13–19. [Google Scholar] [CrossRef]
- Du, C.; Zhang, J.; Zhang, L.; Zhang, Y.; Wang, Y.; Li, J. Hsa_circRNA_102229 facilitates the progression of triple-negative breast cancer via regulating the miR-152-3p/PFTK1 pathway. J. Gene Med. 2021, 23, e3365. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, M.; Lu, M.M.; Qu, L.Y.; Xu, S.G.; Li, Y.Z.; Wang, M.Y.; Zhu, H.F.; Zhang, Z.Y.; He, G.Y.; et al. EPAS1 targeting by miR-152-3p in Paclitaxel-resistant Breast Cancer. J. Cancer 2020, 11, 5822–5830. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.-W.; Gao, P. SPIN1, negatively regulated by miR-148/152, enhances Adriamycin resistance via upregulating drug metabolizing enzymes and transporter in breast cancer. J. Exp. Clin. Cancer Res. 2018, 37, 100. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, D.; Deb, M.; Rath, S.K.; Kar, S.; Parbin, S.; Pradhan, N.; Patra, S.K. DNA methylation and not H3K4 trimethylation dictates the expression status of miR-152 gene which inhibits migration of breast cancer cells via DNMT1/CDH1 loop. Exp. Cell Res. 2016, 346, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Jiang, Y.; Yin, Y.; Li, Q.; He, J.; Jing, Y.; Qi, Y.-T.; Li, W.; Lu, B.; Peiper, S.S.; et al. A regulatory circuit of miR-148a/152 and DNMT1 in modulating cell transformation and tumor angiogenesis through IGF-IR and IRS1. J. Mol. Cell Biol. 2013, 5, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.-Y.; Liu, W.-T.; Sun, H.-R.; Ge, X.; Shi, Z.-M.; Wang, M.; Li, W.; Zhang, J.-Y.; Liu, L.-Z.; Jiang, B.-H.; et al. IGF-1-mediated PKM2/β-catenin/miR-152 regulatory circuit in breast cancer. Sci. Rep. 2017, 7, 15897. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Wang, D.; Kong, Q.; Gao, W.; Sun, J. Function of miR-152 as a Tumor Suppressor in Human Breast Cancer by Targeting PIK3CA. Oncol. Res. 2017, 25, 1363–1371. [Google Scholar] [CrossRef]
- Chen, M.-J.; Cheng, Y.-M.; Chen, C.-C.; Chen, Y.-C.; Shen, C.-J. MiR-148a and miR-152 reduce tamoxifen resistance in ER+ breast cancer via downregulating ALCAM. Biochem. Biophys. Res. Commun. 2017, 483, 840–846. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, L.; Yin, Y.; He, J.; Li, Q.; Qian, X.; You, Y.; Lu, Z.; Peiper, S.; Shu, Y.; et al. Regulatory circuit of PKM2/NF-κB/miR-148a/152-modulated tumor angiogenesis and cancer progression. Oncogene 2015, 34, 5482–5493. [Google Scholar] [CrossRef]
- Liu, X.; Cao, M.; Palomares, M.; Wu, X.; Li, A.; Yan, W.; Fong, M.Y.; Chan, W.; Wang, S.E. Metastatic breast cancer cells overexpress and secrete miR-218 to regulate type I collagen deposition by osteoblasts. Breast Cancer Res. 2018, 20, 127. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Chen, C.; Zhu, X.; Zhang, C.; Xia, Y.; Zhao, Y.; Andrisani, O.M.; Kong, L. A double-negative feedback loop between DEAD-box protein DDX21 and Snail regulates epithelial-mesenchymal transition and metastasis in breast cancer. Cancer Lett. 2018, 437, 67–78. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, F.; Chen, P. miR-7 and miR-218 epigenetically control tumor suppressor genes RASSF1A and Claudin-6 by targeting HoxB3 in breast cancer. Biochem. Biophys. Res. Commun. 2012, 424, 28–33. [Google Scholar] [CrossRef]
- Hassan, M.Q.; Maeda, Y.; Taipaleenmaki, H.; Zhang, W.; Jafferji, M.; Gordon, J.A.R.; Li, Z.; Croce, C.M.; van Wijnen, A.J.; Stein, J.L.; et al. miR-218 directs a Wnt signaling circuit to promote differentiation of osteoblasts and osteomimicry of metastatic cancer cells. J. Biol. Chem. 2012, 287, 42084–42092. [Google Scholar] [CrossRef]
- Taipaleenmäki, H.; Farina, N.H.; van Wijnen, A.J.; Stein, J.L.; Hesse, E.; Stein, G.S.; Lian, J.B. Antagonizing miR-218-5p attenuates Wnt signaling and reduces metastatic bone disease of triple negative breast cancer cells. Oncotarget 2016, 7, 79032–79046. [Google Scholar] [CrossRef]
- Han, C.; Li, X.; Fan, Q.; Liu, G.; Yin, J. CCAT1 promotes triple-negative breast cancer progression by suppressing miR-218/ZFX signaling. Aging 2019, 11, 4858–4875. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, K.; Yagüe, E. miR-218 targets survivin and regulates resistance to chemotherapeutics in breast cancer. Breast Cancer Res. Treat. 2015, 151, 269–280. [Google Scholar] [CrossRef]
- He, X.; Xiao, X.; Dong, L.; Wan, N.; Zhou, Z.; Deng, H.; Zhang, X. MiR-218 regulates cisplatin chemosensitivity in breast cancer by targeting BRCA1. Tumor Biol. 2015, 36, 2065–2075. [Google Scholar] [CrossRef]
- Liu, B.; Tian, Y.; Li, F.; Zhao, Z.; Jiang, X.; Zhai, C.; Han, X.; Zhang, L. Tumor-suppressing roles of miR-214 and miR-218 in breast cancer. Oncol. Rep. 2016, 35, 3178–3184. [Google Scholar] [CrossRef]
- Setijono, S.R.; Park, M.; Kim, G.; Kim, Y.; Cho, K.W.; Song, S.J. miR-218 and miR-129 regulate breast cancer progression by targeting Lamins. Biochem. Biophys. Res. Commun. 2018, 496, 826–833. [Google Scholar] [CrossRef]
- Ren, J.; Chen, Y.; Kong, W.; Li, Y.; Lu, F. Tumor protein D52 promotes breast cancer proliferation and migration via the long non-coding RNA NEAT1/microRNA-218-5p axis. Ann. Transl. Med. 2021, 9, 1008. [Google Scholar] [CrossRef]
- Citron, F.; Segatto, I.; Vinciguerra, G.L.R.; Musco, L.; Russo, F.; Mungo, G.; D’Andrea, S.; Mattevi, M.C.; Perin, T.; Schiappacassi, M.; et al. Downregulation of miR-223 Expression Is an Early Event during Mammary Transformation and Confers Resistance to CDK4/6 Inhibitors in Luminal Breast Cancer. Cancer Res. 2020, 80, 1064–1077. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, S.; Wang, Y.; Zhang, X.; Liu, X.; Li, J.; Li, P.; Du, L.; Wang, C. miR-223-3p targets FBXW7 to promote epithelial-mesenchymal transition and metastasis in breast cancer. Thorac. Cancer 2022, 13, 474–482. [Google Scholar] [CrossRef]
- Favero, A.; Segatto, I.; Perin, T.; Belletti, B. The many facets of miR-223 in cancer: Oncosuppressor, oncogenic driver, therapeutic target, and biomarker of response. Wiley Interdiscip. Rev. RNA 2021, 12, e1659. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Yang, H.; Fang, Q.; Quan, H.; Lu, H.; Wang, X. Circ_ZFR affects FABP7 expression to regulate breast cancer progression by acting as a sponge for miR-223-3p. Thorac. Cancer 2022, 13, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Li, F.; Li, X.; Tian, Y.; Zhang, Y.; Sheng, X.; Song, Y.; Meng, Q.; Yuan, S.; Luan, L.; et al. MiR-31 promotes mammary stem cell expansion and breast tumorigenesis by suppressing Wnt signaling antagonists. Nat. Commun. 2017, 8, 1036. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, H.-Y.; Dai, X.-Y.; Zhang, X.; Huang, Y.-Z.; Shi, L.; Wei, J.-F.; Ding, Q. CircMETTL3, upregulated in a m6A-dependent manner, promotes breast cancer progression. Int. J. Biol. Sci. 2021, 17, 1178–1190. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Lei, J.; Du, L. miR-31-5p may enhance the efficacy of chemotherapy with Taxol and cisplatin in TNBC. Exp. Ther. Med. 2020, 19, 375–383. [Google Scholar] [CrossRef]
- Farokhimanesh, S.; Forouzandeh Moghadam, M.; Ebrahimi, M. Metastasis inhibition by BRMS1 and miR-31 replacement therapy in claudin-low cell lines. Iran. J. Basic Med. Sci. 2020, 23, 264–270. [Google Scholar]
- Chen, Z.; Luo, J.; Sun, S.; Cao, D.; Shi, H.; Loor, J.J. miR-148a and miR-17-5p synergistically regulate milk TAG synthesis via PPARGC1A and PPARA in goat mammary epithelial cells. RNA Biol. 2017, 14, 326–338. [Google Scholar] [CrossRef]
- Okumura, S.; Hirano, Y.; Komatsu, Y. Stable duplex-linked antisense targeting miR-148a inhibits breast cancer cell proliferation. Sci. Rep. 2021, 11, 11467. [Google Scholar] [CrossRef]
- Zhang, K.; Corsa, C.A.; Ponik, S.M.; Prior, J.L.; Piwnica-Worms, D.; Eliceiri, K.W.; Keely, P.J.; Longmore, G.D. The collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to facilitate breast cancer metastasis. Nat. Cell Biol. 2013, 15, 677–687. [Google Scholar] [CrossRef]
- Yu, Z.; Cao, W.; Ren, Y.; Zhang, Q.; Liu, J. ATPase copper transporter A, negatively regulated by miR-148a-3p, contributes to cisplatin resistance in breast cancer cells. Clin. Transl. Med. 2020, 10, 57–73. [Google Scholar] [CrossRef]
- Wu, H.-J.; Hao, M.; Yeo, S.K.; Guan, J.-L. FAK signaling in cancer-associated fibroblasts promotes breast cancer cell migration and metastasis by exosomal miRNAs-mediated intercellular communication. Oncogene 2020, 39, 2539–2549. [Google Scholar] [CrossRef]
- Xie, Y.; Shi, Z.; Qian, Y.; Jiang, C.; Liu, W.; Liu, B.; Jiang, B. HDAC2- and EZH2-Mediated Histone Modifications Induce PDK1 Expression through miR-148a Downregulation in Breast Cancer Progression and Adriamycin Resistance. Cancers 2022, 14, 3600. [Google Scholar] [CrossRef]
- Han, L.; Yan, Y.; Zhao, L.; Liu, Y.; Lv, X.; Zhang, L.; Zhao, Y.; Zhao, H.; He, M.; Wei, M. LncRNA HOTTIP facilitates the stemness of breast cancer via regulation of miR-148a-3p/WNT1 pathway. J. Cell. Mol. Med. 2020, 24, 6242–6252. [Google Scholar] [CrossRef]
- Shupp, A.B.; Neupane, M.; Agostini, L.C.; Ning, G.; Brody, J.R.; Bussard, K.M. Stromal-Derived Extracellular Vesicles Suppress Proliferation of Bone Metastatic Cancer Cells Mediated by ERK2. Mol. Cancer Res. 2021, 19, 1763–1777. [Google Scholar] [CrossRef]
- Hossain, A.; Kuo, M.T.; Saunders, G.F. Mir-17-5p regulates breast cancer cell proliferation by inhibiting translation of AIB1 mRNA. Mol. Cell. Biol. 2006, 26, 8191–8201. [Google Scholar] [CrossRef]
- Liao, X.-H.; Xiang, Y.; Yu, C.-X.; Li, J.-P.; Li, H.; Nie, Q.; Hu, P.; Zhou, J.; Zhang, T.-C. STAT3 is required for MiR-17-5p-mediated sensitization to chemotherapy-induced apoptosis in breast cancer cells. Oncotarget 2017, 8, 15763–15774. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, W.; Xu, X.; Lv, S.; Dong, X. miR-17-5p promotes migration and invasion in breast cancer cells by repressing netrin. Int. J. Clin. Exp. Pathol. 2019, 12, 1649–1657. [Google Scholar]
- Li, X.; Wu, B.; Chen, L.; Ju, Y.; Li, C.; Meng, S. Urokinase-type plasminogen activator receptor inhibits apoptosis in triple-negative breast cancer through miR-17/20a suppression of death receptors 4 and 5. Oncotarget 2017, 8, 88645–88657. [Google Scholar] [CrossRef]
- Chen, Z.; Shi, H.; Sun, S.; Xu, H.; Cao, D.; Luo, J. MicroRNA-181b suppresses TAG via target IRS2 and regulating multiple genes in the Hippo pathway. Exp. Cell Res. 2016, 348, 66–74. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.X.; Chen, L.P.; Ji, M.L. Upregulation of microRNA-181b inhibits CCL18-induced breast cancer cell metastasis and invasion via the NF-κB signaling pathway. Oncol. Lett. 2016, 12, 4411–4418. [Google Scholar] [CrossRef]
- Ma, L.; Qiu, H.; Chen, Z.; Li, L.; Zeng, Y.; Luo, J.; Gou, D. miR-25 modulates triacylglycerol and lipid accumulation in goat mammary epithelial cells by repressing PGC-1beta. J. Anim. Sci. Biotechnol. 2018, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Zan, L.; Chen, Q.; Zhang, L.; Li, X. Epigallocatechin gallate (EGCG) suppresses growth and tumorigenicity in breast cancer cells by downregulation of miR-25. Bioengineered 2019, 10, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hu, Y.; Yang, M.; Jat, P.; Li, K.; Lombardo, Y.; Xiong, D.; Coombes, R.C.; Raguz, S.; Yagüe, E. The miR-106b~25 cluster promotes bypass of doxorubicin-induced senescence and increase in motility and invasion by targeting the E-cadherin transcriptional activator EP300. Cell Death Differ. 2014, 21, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-Z.; Cheng, W.-C.; Chen, S.-F.; Nieh, S.; O’Connor, C.; Liu, C.-L.; Tsai, W.-W.; Wu, C.-J.; Martin, L.; Lin, Y.-S.; et al. miR-25/93 mediates hypoxia-induced immunosuppression by repressing cGAS. Nat. Cell Biol. 2017, 19, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Meng, W.; Chin, Y.; Gao, L.; Yang, X.; Sun, S.; Pan, X.; He, L. Identification of miR-25-3p as a tumor biomarker: Regulation of cellular functions via TOB1 in breast cancer. Mol. Med. Rep. 2021, 23, 406. [Google Scholar] [CrossRef]
- Qattan, A.; Al-Tweigeri, T.; Alkhayal, W.; Suleman, K.; Tulbah, A.; Amer, S. Clinical Identification of Dysregulated Circulating microRNAs and Their Implication in Drug Response in Triple Negative Breast Cancer (TNBC) by Target Gene Network and Meta-Analysis. Genes 2021, 12, 549. [Google Scholar] [CrossRef]
- Chen, H.; Pan, H.; Qian, Y.; Zhou, W.; Liu, X. MiR-25-3p promotes the proliferation of triple negative breast cancer by targeting BTG2. Mol. Cancer 2018, 17, 4. [Google Scholar] [CrossRef]
- Zhou, Y.; Yamamoto, Y.; Takeshita, F.; Yamamoto, T.; Xiao, Z.; Ochiya, T. Delivery of miR-424-5p via Extracellular Vesicles Promotes the Apoptosis of MDA-MB-231 TNBC Cells in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 844. [Google Scholar] [CrossRef]
- Vilquin, P.; Donini, C.F.; Villedieu, M.; Grisard, E.; Corbo, L.; Bachelot, T.; Vendrell, J.A.; Cohen, P.A. MicroRNA-125b upregulation confers aromatase inhibitor resistance and is a novel marker of poor prognosis in breast cancer. Breast Cancer Res. 2015, 17, 13. [Google Scholar] [CrossRef]
- Tao, Q.; Lu, Y.; Qi, Y.; Yu, D.; Gu, J.; Zhu, Y.; Shi, C.; Liang, X. Hypoxia promotes the expression of Von Willebrand factor in breast cancer cells by up-regulating the transcription factor YY1 and down-regulating the hsa-miR-424. Eur. J. Pharmacol. 2022, 934, 175308. [Google Scholar] [CrossRef]
- Rodriguez-Barrueco, R.; Nekritz, E.A.; Bertucci, F.; Yu, J.; Sanchez-Garcia, F.; Zeleke, T.Z.; Gorbatenko, A.; Birnbaum, D.; Ezhkova, E.; Cordon-Cardo, C.; et al. miR-424(322)/503 is a breast cancer tumor suppressor whose loss promotes resistance to chemotherapy. Genes Dev. 2017, 31, 553–566. [Google Scholar] [CrossRef]
- Drasin, D.; Guarnieri, A.; Neelakantan, D.; Kim, J.; Cabrera, J.; Wang, C.-A.; Zaberezhnyy, V.; Gasparini, P.; Cascione, L.; Huebner, K.; et al. TWIST1-Induced miR-424 Reversibly Drives Mesenchymal Programming while Inhibiting Tumor Initiation. Cancer Res. 2015, 75, 1908–1921. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S.; Zhou, J.; Qian, Q. miR-424-5p regulates cell proliferation, migration and invasion by targeting doublecortin-like kinase 1 in basal-like breast cancer. Biomed. Pharmacother. 2018, 102, 147–152. [Google Scholar] [CrossRef]
- Xie, D.; Song, H.; Wu, T.; Li, D.; Hua, K.; Xu, H.; Zhao, B.; Wu, C.; Hu, J.; Ji, C.; et al. MicroRNA-424 serves an anti-oncogenic role by targeting cyclin-dependent kinase 1 in breast cancer cells. Oncol. Rep. 2018, 40, 3416–3426. [Google Scholar] [CrossRef]
- Bose Nandy, S.; Orozco, A.; Lopez-Valdez, R.; Roberts, R.; Subramani, R.; Arumugam, A.; Dwivedi, A.; Stewart, V.; Prabhakar, G.; Jones, S.; et al. Glucose insult elicits hyperactivation of cancer stem cells through miR-424-cdc42-prdm14 signalling axis. Br. J. Cancer 2017, 117, 1665–1675. [Google Scholar] [CrossRef]
- Chu, M.; Zhao, Y.; Feng, Y.; Zhang, H.; Liu, J.; Cheng, M.; Li, L.; Shen, W.; Cao, H.; Li, Q.; et al. MicroRNA-126 participates in lipid metabolism in mammary epithelial cells. Mol. Cell. Endocrinol. 2017, 454, 77–86. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, P.; Sun, T.; Li, D.; Xu, X.; Rui, Y.; Li, C.; Chong, M.; Ibrahim, T.; Mercatali, L.; et al. miR-126 and miR-126* repress recruitment of mesenchymal stem cells and inflammatory monocytes to inhibit breast cancer metastasis. Nat. Cell Biol. 2013, 15, 284–294. [Google Scholar] [CrossRef]
- Zhang, J.; Du, Y.; Lin, Y.; Chen, Y.; Yang, L.; Wang, H.; Ma, D. The cell growth suppressor, mir-126, targets IRS-1. Biochem. Biophys. Res. Commun. 2008, 377, 136–140. [Google Scholar] [CrossRef]
- Alhasan, L. MiR-126 Modulates Angiogenesis in Breast Cancer by Targeting VEGF-A-mRNA. Asian Pac. J. Cancer Prev. 2019, 20, 193–197. [Google Scholar] [CrossRef]
- Sibilano, M.; Tullio, V.; Adorno, G.; Savini, I.; Gasperi, V.; Catani, M.V. Platelet-Derived miR-126-3p Directly Targets AKT2 and Exerts Anti-Tumor Effects in Breast Cancer Cells: Further Insights in Platelet-Cancer Interplay. Int. J. Mol. Sci. 2022, 23, 5484. [Google Scholar] [CrossRef]
- Baldassari, F.; Zerbinati, C.; Galasso, M.; Corrà, F.; Minotti, L.; Agnoletto, C.; Previati, M.; Croce, C.M.; Volinia, S. Screen for MicroRNA and Drug Interactions in Breast Cancer Cell Lines Points to miR-126 as a Modulator of CDK4/6 and PIK3CA Inhibitors. Front. Genet. 2018, 9, 174. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Z.; Yuan, P.; Li, Y. MiR-126 regulated breast cancer cell invasion by targeting ADAM9. Int. J. Clin. Exp. Pathol. 2015, 8, 6547–6553. [Google Scholar] [PubMed]
- Fu, R.; Tong, J.-S. miR-126 reduces trastuzumab resistance by targeting PIK3R2 and regulating AKT/mTOR pathway in breast cancer cells. J. Cell. Mol. Med. 2020, 24, 7600–7608. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Lu, J.; Fan, B.; Sun, L. MicroRNA-126-5p Inhibits the Migration of Breast Cancer Cells by Directly Targeting CNOT7. Technol. Cancer Res. Treat. 2020, 19, 1533033820977545. [Google Scholar] [CrossRef] [PubMed]
- Turgut Cosan, D.; Oner, C.; Mutlu Sahin, F. Micro RNA-126 coordinates cell behavior and signaling cascades according to characteristics of breast cancer cells. Bratisl. Lek. Listy 2016, 117, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Tahiri, A.; Leivonen, S.-K.; Lüders, T.; Steinfeld, I.; Aure, M.; Geisler, J.; Mäkelä, R.; Nord, S.; Riis, M.; Yakhini, Z.; et al. Deregulation of cancer-related miRNAs is a common event in both benign and malignant human breast tumors. Carcinogenesis 2014, 35, 76–85. [Google Scholar] [CrossRef]
- Tavazoie, S.; Alarcón, C.; Oskarsson, T.; Padua, D.; Wang, Q.; Bos, P.; Gerald, W.; Massagué, J. Endogenous human microRNAs that suppress breast cancer metastasis. Nature 2008, 451, 147–152. [Google Scholar] [CrossRef]
- Msheik, Z.; Nassar, F.; Chamandi, G.; Itani, A.; Gadaleta, E.; Chalala, C.; Alwan, N.; Nasr, R. miR-126 Decreases Proliferation and Mammosphere Formation of MCF-7 and Predicts Prognosis of ER+ Breast Cancer. Diagnostics 2022, 12, 745. [Google Scholar] [CrossRef]
- Png, K.; Halberg, N.; Yoshida, M.; Tavazoie, S. A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature 2011, 481, 190–194. [Google Scholar] [CrossRef]
- Cui, W.; Li, Q.; Feng, L.; Ding, W. MiR-126-3p regulates progesterone receptors and involves development and lactation of mouse mammary gland. Mol. Cell. Biochem. 2011, 355, 17–25. [Google Scholar] [CrossRef]
- Koh, M.; Ho, W.Y.; Yeap, S.K.; Ali, N.; Yong, C.; Boo, L.; Alitheen, N. Exosomal-microRNA transcriptome profiling of Parental and CSC-like MDA-MB-231 cells in response to cisplatin treatment. Pathol. Res. Pract. 2022, 233, 153854. [Google Scholar] [CrossRef]
- Xu, T.; He, B.; Pan, B.; Pan, Y.-Q.; Sun, H.; Liu, X.; Xu, X.; Chen, X.; Zeng, K.; Xu, M.; et al. MiR-142-3p functions as a tumor suppressor by targeting RAC1/PAK1 pathway in breast cancer. J. Cell. Physiol. 2019, 235, 4928–4940. [Google Scholar] [CrossRef]
- Troschel, F.; Böhly, N.; Borrmann, K.; Braun, T.; Paping, A.; Kiesel, L.; Eich, H.; Götte, M.; Greve, B.; FM, T.; et al. miR-142-3p attenuates breast cancer stem cell characteristics and decreases radioresistance in vitro. Tumour Biol. 2018, 40, 1010428318791887. [Google Scholar] [CrossRef]
- Liang, L.; Fu, J.; Wang, S.; Cen, H.; Zhang, L.; Mandukhail, S.R.; Du, L.; Wu, Q.; Zhang, P.; Yu, X. MiR-142-3p enhances chemosensitivity of breast cancer cells and inhibits autophagy by targeting HMGB1. Acta Pharm. Sin. B 2020, 10, 1036–1046. [Google Scholar] [CrossRef]
- Dastmalchi, N.; Safaralizadeh, R.; Khojasteh, S.M.B.; Shadbad, M.A.; Hosseinpourfeizi, M.A.; Azarbarzin, S.; Rajabi, A.; Baradaran, B. The combined restoration of miR-424-5p and miR-142-3p effectively inhibits MCF-7 breast cancer cell line via modulating apoptosis, proliferation, colony formation, cell cycle and autophagy. Mol. Biol. Rep. 2022, 49, 8325–8335. [Google Scholar] [CrossRef]
- Saatci, O.; Kaymak, A.; Raza, U.; Ersan, P.G.; Akbulut, O.; Banister, C.E.; Sikirzhytski, V.; Tokat, U.M.; Aykut, G.; Ansari, S.A.; et al. Targeting lysyl oxidase (LOX) overcomes chemotherapy resistance in triple negative breast cancer. Nat. Commun. 2020, 11, 2416. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Ghasabi, M.; Shirjang, S.; Dehghan, R.; Montazeri, V.; Holmskov, U.; Kazemi, T.; Duijf, P.; Gjerstorff, M.; et al. miR-142-3p as tumor suppressor miRNA in the regulation of tumorigenicity, invasion and migration of human breast cancer by targeting Bach-1 expression. J. Cell. Physiol. 2019, 234, 9816–9825. [Google Scholar] [CrossRef]
- Cao, X.-C.; Yu, Y.; Hou, L.-K.; Sun, X.-H.; Ge, J.; Zhang, B.; Wang, X. miR-142-3p inhibits cancer cell proliferation by targeting CDC25C. Cell Prolif. 2016, 49, 58–68. [Google Scholar] [CrossRef]
- Schwickert, A.; Weghake, E.; Brüggemann, K.; Engbers, A.; Brinkmann, B.F.; Kemper, B.; Seggewiß, J.; Stock, C.; Ebnet, K.; Kiesel, L.; et al. microRNA miR-142-3p Inhibits Breast Cancer Cell Invasiveness by Synchronous Targeting of WASL, Integrin Alpha V, and Additional Cytoskeletal Elements. PLoS ONE 2015, 10, e0143993. [Google Scholar] [CrossRef]
- Mathsyaraja, H.; Thies, K.; Taffany, D.A.; Deighan, C.; Liu, T.; Yu, L.; Fernandez, S.A.; Shapiro, C.; Otero, J.; Timmers, C.; et al. CSF1-ETS2-induced microRNA in myeloid cells promote metastatic tumor growth. Oncogene 2015, 34, 3651–3661. [Google Scholar] [CrossRef]
- Chu, M.; Zhao, Y.; Yu, S.; Hao, Y.; Zhang, P.; Feng, Y.; Zhang, H.; Ma, D.; Liu, J.; Cheng, M.; et al. miR-15b negatively correlates with lipid metabolism in mammary epithelial cells. Am. J. Physiol. Cell Physiol. 2018, 314, C43–C52. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Liu, G.; Jin, Y.; Yang, T.; Zhang, D.; Ding, L.; Zhou, F.; Pan, Y.; Wei, Y. miR-15b-5p Promotes Growth and Metastasis in Breast Cancer by Targeting HPSE2. Front. Oncol. 2020, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.-Q.; Sun, B.; Yang, B.-B.; Lu, S. MiR-15b facilitates breast cancer progression via repressing tumor suppressor PAQR3. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 740–748. [Google Scholar] [PubMed]
- Kedmi, M.; Ben-Chetrit, N.; Körner, C.; Mancini, M.; Ben-Moshe, N.B.; Lauriola, M.; Lavi, S.; Biagioni, F.; Carvalho, S.; Cohen-Dvashi, H.; et al. EGF induces microRNAs that target suppressors of cell migration: miR-15b targets MTSS1 in breast cancer. Sci. Signal. 2015, 8, ra29. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Yang, X.; Wang, G.; Li, H.; Li, S.; Xu, T.; Wu, Y.; Zhang, Z.; Li, X.; Du, Y.; et al. Regulation of antitumor miR-205 targets oncogenes: Direct regulation of lymphoid specific helicase and its clinical significance. Life Sci. 2022, 309, 120993. [Google Scholar] [CrossRef]
- Lin, L.-F.; Li, Y.-T.; Han, H.; Lin, S.-G. MicroRNA-205-5p targets the HOXD9-Snail1 axis to inhibit triple negative breast cancer cell proliferation and chemoresistance. Aging 2021, 13, 3945–3956. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, Y.; Huang, L.; Chi, Y.; Meng, L. MiR-205 suppressed the malignant behaviors of breast cancer cells by targeting CLDN11 via modulation of the epithelial-to-mesenchymal transition. Aging 2021, 13, 13073–13086. [Google Scholar] [CrossRef]
- Tao, Q.; Qi, Y.; Gu, J.; Yu, D.; Lu, Y.; Liu, J.; Liang, X. Breast cancer cells-derived Von Willebrand Factor promotes VEGF-A-related angiogenesis through PI3K/Akt-miR-205-5p signaling pathway. Toxicol. Appl. Pharmacol. 2022, 440, 115927. [Google Scholar] [CrossRef]
- Xu, Y.; Qian, C.; Liu, C.; Fu, Y.; Zhu, K.; Niu, Z.; Liu, J. Investigation of the Mechanism of hsa_circ_000 1429 Adsorbed miR-205 to Regulate KDM4A and Promote Breast Cancer Metastasis. Contrast Media Mol. Imaging 2022, 2022, 4657952. [Google Scholar] [CrossRef]
- Wronski, A.; Sandhu, G.; Milevskiy, M.; Brewster, B.; Bridge, J.; Shewan, A.; Edwards, S.; French, J.; Brown, M. MicroRNA-206 is differentially expressed in Brca1-deficient mice and regulates epithelial and stromal cell compartments of the mouse mammary gland. Oncogenesis 2016, 5, e218. [Google Scholar] [CrossRef]
- Chu, M.; Zhao, Y.; Yu, S.; Hao, Y.; Zhang, P.; Feng, Y.; Zhang, H.; Ma, D.; Liu, J.; Cheng, M.; et al. MicroRNA-221 may be involved in lipid metabolism in mammary epithelial cells. Int. J. Biochem. Cell Biol. 2018, 97, 118–127. [Google Scholar] [CrossRef]
- Di Martino, M.T.; Arbitrio, M.; Caracciolo, D.; Cordua, A.; Cuomo, O.; Grillone, K.; Riillo, C.; Caridà, G.; Scionti, F.; Labanca, C.; et al. miR-221/222 as biomarkers and targets for therapeutic intervention on cancer and other diseases: A systematic review. Mol. Ther. Nucleic Acids 2022, 27, 1191–1224. [Google Scholar] [CrossRef]
- Le Guillou, S.; Sdassi, N.; Laubier, J.; Passet, B.; Vilotte, M.; Castille, J.; Laloë, D.; Polyte, J.; Bouet, S.; Jaffrézic, F.; et al. Overexpression of miR-30b in the developing mouse mammary gland causes a lactation defect and delays involution. PLoS ONE 2012, 7, e45727. [Google Scholar] [CrossRef]
- Wu, T.; Song, H.; Xie, D.; Hua, K.; Hu, J.; Deng, Y.; Ji, C.; Fang, L. Mir-30b-5p Promotes Proliferation, Migration, and Invasion of Breast Cancer Cells via Targeting ASPP2. Biomed. Res. Int. 2020, 2020, 7907269. [Google Scholar] [CrossRef]
- Tacconelli, E. Systematic reviews: CRD’s guidance for undertaking reviews in health care. Lancet Infect. Dis. 2010, 10, 226. [Google Scholar] [CrossRef]
| PICOS Component | Search Strategy/Terms |
|---|---|
| Population | ((mammary development) OR (mammary gland development) OR (breast development)) |
| Intervention | (miRNA OR microRNA) |
| Comparisons | Mammary gland stages |
| Outcomes | Changes in miRNA expression, miRNA functional analysis in mammary gland |
| Study design | Primary research in animal models, human studies |
| PICOS Component | Search Strategy/Terms |
|---|---|
| Population | Breast cancer cell lines, xenografts, mouse models, biopsies |
| Intervention | miR-x |
| Comparisons | Normal/healthy/non-cancerous mammary cells/tissue vs. cancerous OR Non-metastatic cells/tissue vs. metastatic OR Treatment resistant cells/tissue vs. non-treatment resistant |
| Outcomes | Breast cancer occurrence, severity, metastatic potential, treatment resistance |
| Study design | Primary research and reviews on miRNAs in breast cancer cell lines, murine models, human studies |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Thompson, L.U.; Comelli, E.M. MicroRNAs: A Link between Mammary Gland Development and Breast Cancer. Int. J. Mol. Sci. 2022, 23, 15978. https://doi.org/10.3390/ijms232415978
Wu D, Thompson LU, Comelli EM. MicroRNAs: A Link between Mammary Gland Development and Breast Cancer. International Journal of Molecular Sciences. 2022; 23(24):15978. https://doi.org/10.3390/ijms232415978
Chicago/Turabian StyleWu, Diana, Lilian U. Thompson, and Elena M. Comelli. 2022. "MicroRNAs: A Link between Mammary Gland Development and Breast Cancer" International Journal of Molecular Sciences 23, no. 24: 15978. https://doi.org/10.3390/ijms232415978
APA StyleWu, D., Thompson, L. U., & Comelli, E. M. (2022). MicroRNAs: A Link between Mammary Gland Development and Breast Cancer. International Journal of Molecular Sciences, 23(24), 15978. https://doi.org/10.3390/ijms232415978






