Hypertensive Nephropathy: Unveiling the Possible Involvement of Hemichannels and Pannexons
Abstract
1. Introduction
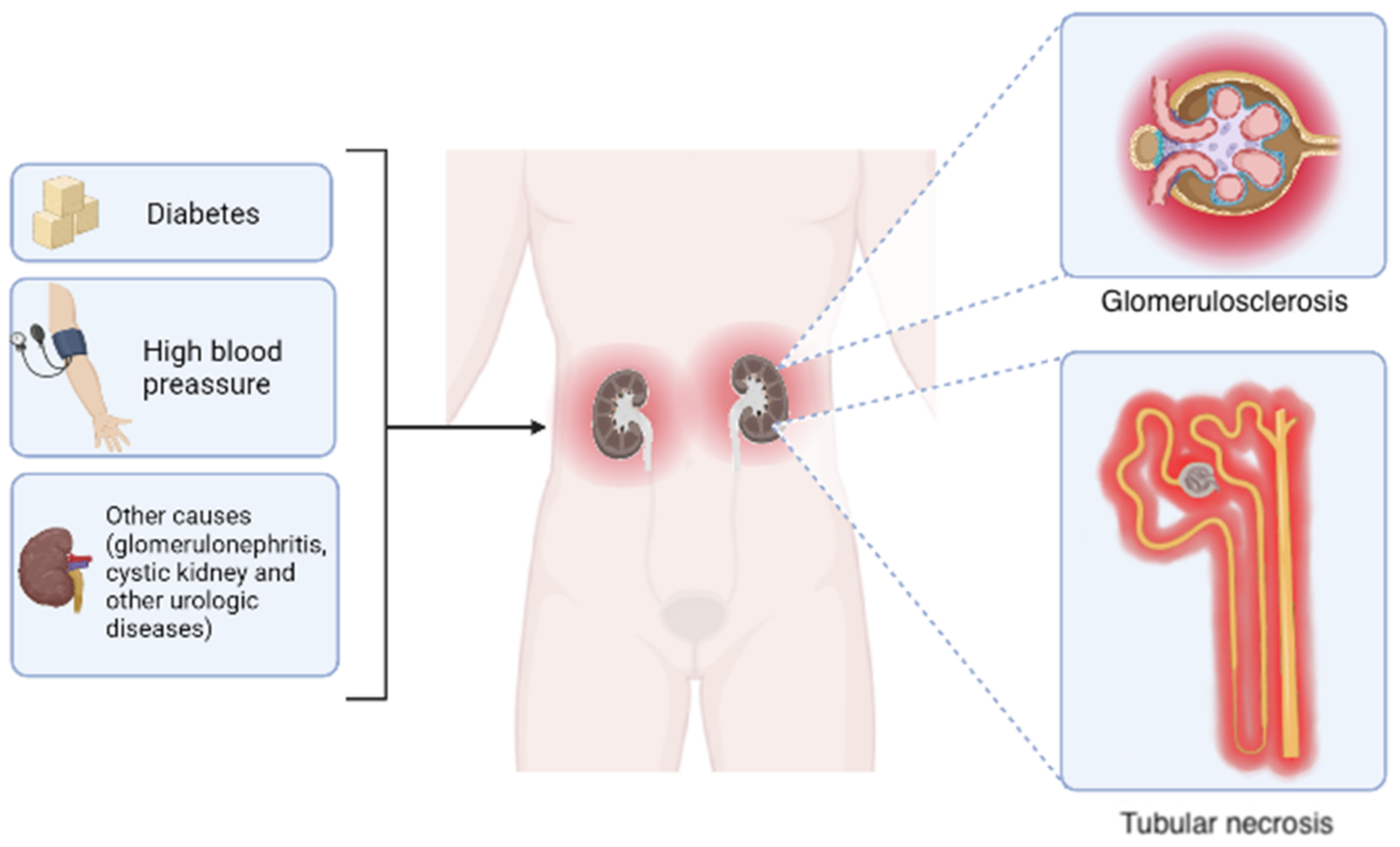
1.1. Chronic Kidney Disease, General Aspects
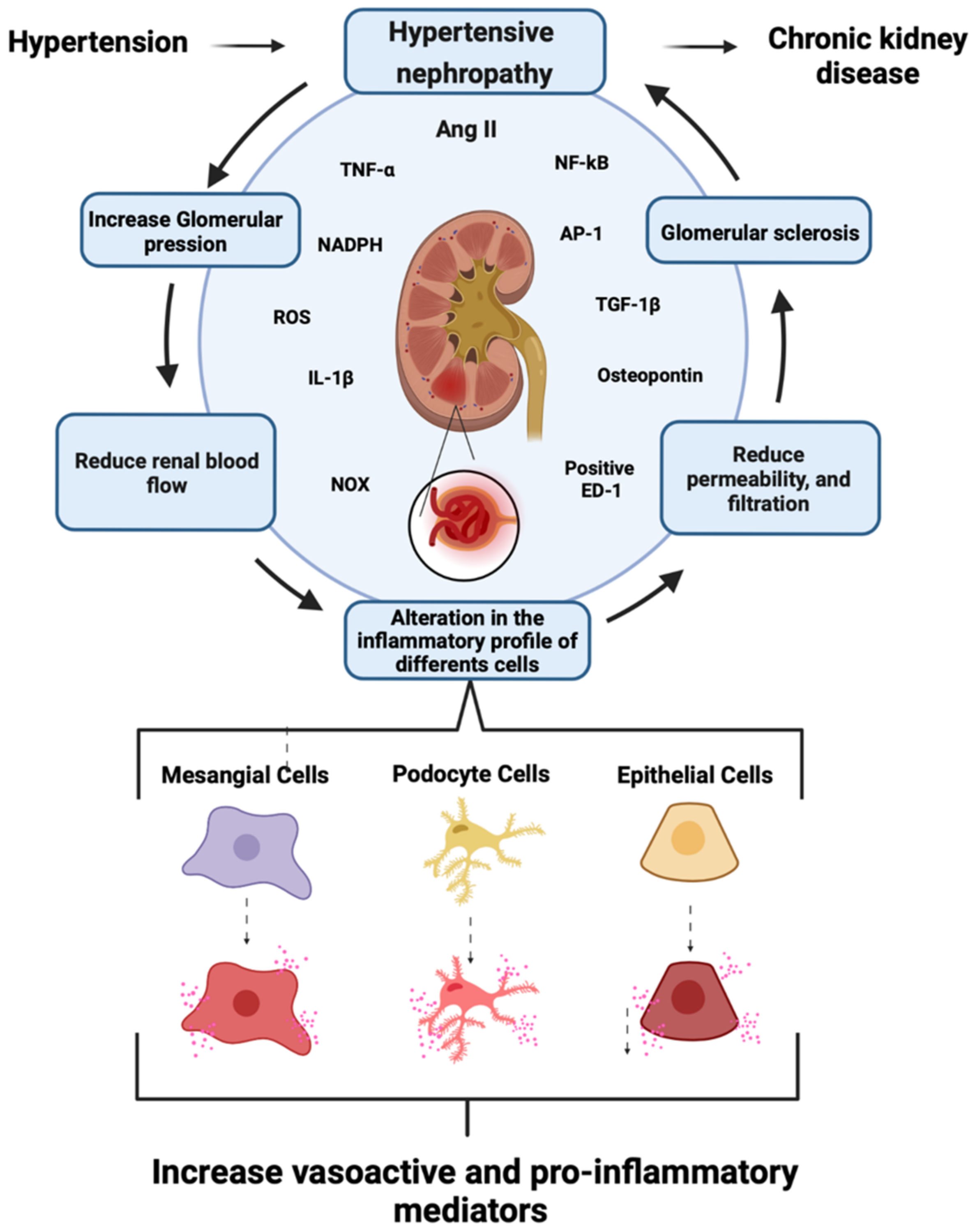
1.2. Role of Renin–Angiotensin System in CKD Development
1.3. Role of Mesangial Cells in CKD
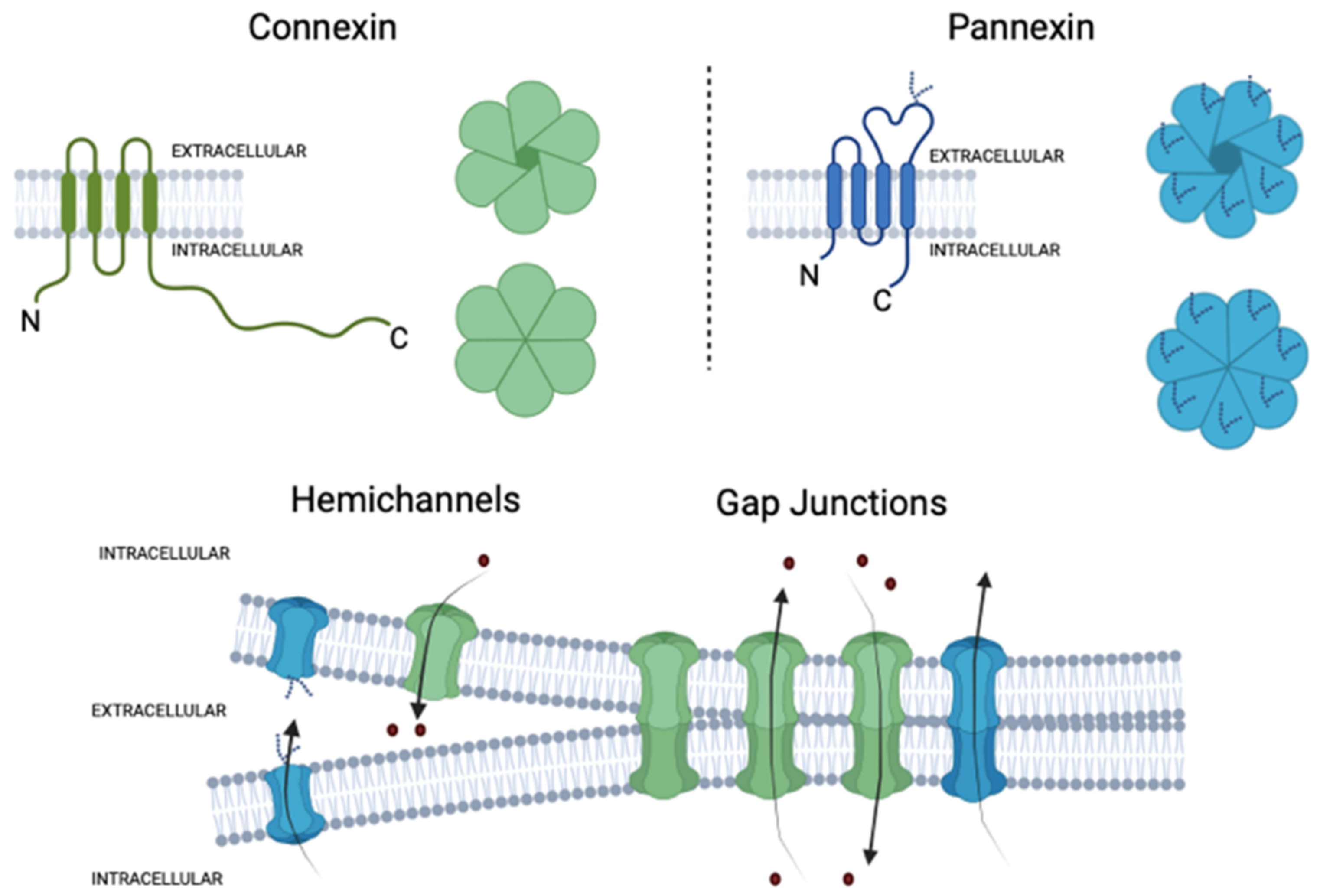
2. Connexins and Pannexins
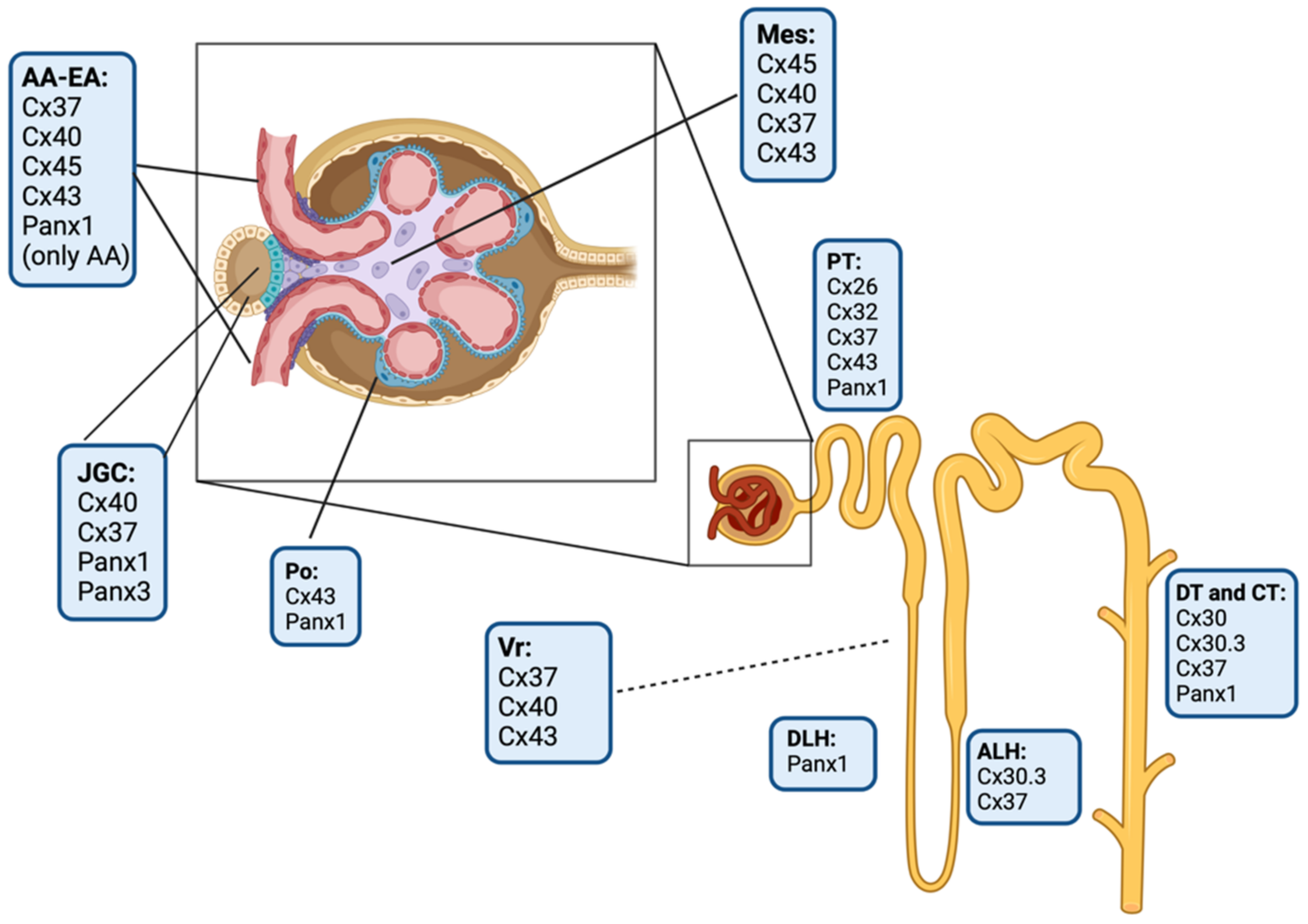
3. Connexins and Pannexin-Based Channels in the Kidney
3.1. Glomerular Connexin and Pannexin-Based Channels
3.2. Tubular Connexin and Pannexin-Based Channels
4. Cx43 and Panx 1 in Hypertensive Nephropathy
| Renal Hypertension Model | Experimental Model | Effect on Cx43 | Renal Site | Technique | Reference |
|---|---|---|---|---|---|
| 2K1C | KI32 mice | Without changes | G, aa, ila | IF, WB | Haefliger et al., 2006 [130] |
| Hypertension-induced CKD | RenTg mice | ↓ | Rc | qPCR, IF | Abed et al., 2014 [131] |
| Uninephrectomized, 1%NaCl and aldosterone infusion | SD rats | ↑ | Po | IF, WB, DCFDA fluorescence | Yang et al., 2014 [133] |
| RenTg, anti-glomerular basement membrane, unilateral ureteral obstruction | Mice | ↑ | G, T | rt-PCR, IF | Toubas et al., 2011 [134] |
| Collagen I and TGFβ1 treatment | HK2 cells | ↑ | PT (cells) | Carboxyfluorescein dye uptake | Potter et al., 2021 [135] |
| The cisplatin-induced kidney injury model | HK2 cells, mice | ↑ | Rc | IF, WB, IHC | Yu et al., 2021 [136] |
| ATP depletion | hPTC | ↑ | PT (cells) | IF, WB, fluorescein dextran dye uptake | Vergara et al., 2003 [118] |
| High glucose | hCDC | ↑ | CD | IF, WB, Lucifer yellow dye uptake | Hills et al., 2006 [138] |
| Angiotensin II infusion | SD rats | ↑ | Rc | WB | Gómez et al., 2019 [139] |
| TGFβ1 treatment | hPTECs, HK2 cells | ↑ | PT (cells) | Carboxyfluorescein dye uptake | Price et al., 2020 [142] |
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2K1C | Two kidneys-one clip model |
| AngII | Angiotensin II |
| AP-1 | Apoliprotein-1 |
| AT1R | Angiotensin membrane G-protein-coupled receptors type I |
| AT2R | Angiotensin membrane G-protein-coupled receptors type II |
| ATP | Adenosine triphosphate |
| BMMC | Bone marrow mononuclear cell |
| cAMP | Cyclic adenosine monophosphate |
| CKD | Chronic kidney disease |
| Cx43 | Connexin 43 |
| eNOS | Endothelial nitric oxide synthase |
| ESRD | End-stage renal disease |
| GFR | Glomerular filtration rate |
| IL-1β | Interleukin-1β |
| IP3 | Inositol trisphosphate |
| MCP-1 | Macrophage chemoattractant protein-1 |
| MCs | Mesangial cells |
| MES-13 | Mesangial cells line |
| NFκB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NO | Nitric oxide |
| NOX | NADPH oxidase |
| OS | Oxidative stress |
| P2X7Rs | P2X7 receptors |
| Panx1 | Pannexin-1 |
| PGE2 | Prostaglandin E2 |
| RAS | Renin–angiotensin system |
| ROS | Reactive oxidative species |
| TGF-β1 | Transforming growth factor β1 |
| TNF-α | Tumor necrosis factor-α |
References
- Atherton, J.C. Renal physiology. Br. J. Anaesth. 1972, 44, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Mohandas, R.; Douma, L.G.; Scindia, Y.; Gumz, M.L. Circadian Rhythms and Renal Pathophysiology. J. Clin. Investig. 2022, 132, e148277. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.; Bouley, R.; Păunescu, T.; Breton, S.; Lu, H. New Insights into the Dynamic Regulation of Water and Acid-Base Balance by Renal Epithelial Cells.o Title. Am. J. Physiol. Cell Physiol. 2012, 302, C1421–C1433. [Google Scholar] [CrossRef]
- Lv, J.-C.; Zhang, L.-X. Prevalence and Disease Burden of Chronic Kidney Disease. In Renal Fibrosis: Mechanisms and Therapies; Springer: Berlin, Germany, 2019; pp. 3–15. [Google Scholar]
- de Zeeuw, D.; Hillege, H.L.; de Jong, P.E. The Kidney, a Cardiovascular Risk Marker, and a New Target for Therapy. Kidney Int. Suppl. 2005, 68, S25–S29. [Google Scholar] [CrossRef] [PubMed]
- Gómez, G.I.; Velarde, V. Boldine Improves Kidney Damage in the Goldblatt 2K1C Model Avoiding the Increase in TGF-β. Int. J. Mol. Sci. 2018, 19, 1864. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.C.; Zhang, L.X. Prevalence and Disease Burden of Chronic Kidney Disease; Springer: Singapore, 2019; Volume 1165, ISBN 9789811388712. [Google Scholar]
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, Regional, and National Burden of Chronic Kidney Disease, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [PubMed]
- Coresh, J.; Byrd-Holt, D.; Astor, B.C.; Briggs, J.P.; Eggers, P.W.; Lacher, D.A.; Hostetter, T.H. Chronic Kidney Disease Awareness, Prevalence, and Trends among U.S. Adults, 1999 to 2000. J. Am. Soc. Nephrol. 2004, 16, 180–188. [Google Scholar] [CrossRef]
- Nangaku, M. Chronic Hypoxia and Tubulointerstitial Injury: A Final Common Pathway to End-Stage Renal Failure. J. Am. Soc. Nephrol. 2005, 17, 17–25. [Google Scholar] [CrossRef]
- Gómez, G.I.; Velarde, V.; Sáez, J.C. Connexin-Based Channels and RhoA/ROCK Pathway in Angiotensin II-Induced Kidney Damage. In Selected Chapters from the Renin-Angiotensin System; IntechOpen: London, UK, 2020. [Google Scholar]
- Fleck, C.; Appenroth, D.; Jonas, P.; Koch, M.; Kundt, G.; Nizze, H.; Stein, G. Suitability of 5/6 Nephrectomy (5/6NX) for the Induction of Interstitial Renal Fibrosis in Rats--Influence of Sex, Strain, and Surgical Procedure. Exp. Toxicol. Pathol. 2006, 57, 195–205. [Google Scholar] [CrossRef]
- Singri, N.; Ahya, S.N.; Levin, M.L. Acute Renal Failure. JAMA 2003, 289, 747–751. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J.; Balk, E.; Kausz, A.T.; Levin, A.; Steffes, M.W.; Hogg, R.J.; Perrone, R.D.; Lau, J.; Eknoyan, G.; et al. National Kidney Foundation Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. Ann. Intern. Med. 2003, 139, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Gómez, G.; Fernández, P.; Velarde, V.; Sáez, J. Angiotensin II-Induced Mesangial Cell Damage Is Preceded by Cell Membrane Permeabilization Due to Upregulation of Non-Selective Channels. Int. J. Mol. Sci. 2018, 19, 957. [Google Scholar] [CrossRef] [PubMed]
- López-Novoa, J.M.; Martínez-Salgado, C.; Rodríguez-Peña, A.B.; López-Hernández, F.J. Common Pathophysiological Mechanisms of Chronic Kidney Disease: Therapeutic Perspectives. Pharmacol. Ther. 2010, 128, 61–81. [Google Scholar] [CrossRef] [PubMed]
- Petreski, T.; Piko, N.; Ekart, R.; Hojs, R.; Bevc, S. Review on Inflammation Markers in Chronic Kidney Disease. Biomedicines 2021, 9, 182. [Google Scholar] [CrossRef] [PubMed]
- Rosivall, L. Intrarenal Renin–Angiotensin System. Mol. Cell Endocrinol. 2009, 302, 185–192. [Google Scholar] [CrossRef]
- Seikaly, M.G.; Arant, B.S.; Seney, F.D. Endogenous Angiotensin Concentrations in Specific Intrarenal Fluid Compartments of the Rat. J. Clin. Investig. 1990, 86, 1352–1357. [Google Scholar] [CrossRef]
- Rightsel, W.A.; Okamura, T.; Inagami, T.; Pitcock, J.A.; Takii, Y.; Brooks, B.; Brown, P.; Muirhead, E.E. Juxtaglomerular Cells Grown as Monolayer Cell Culture Contain Renin, Angiotensin I-Converting Enzyme, and Angiotensin I and II/III. Circ. Res. 1982, 50, 822–829. [Google Scholar] [CrossRef]
- Costantino, V.V.; Gil Lorenzo, A.F.; Bocanegra, V.; Vallés, P.G. Molecular Mechanisms of Hypertensive Nephropathy: Renoprotective Effect of Losartan through Hsp70. Cells 2021, 10, 3146. [Google Scholar] [CrossRef]
- Border, W.A.; Noble, N.A. Interactions of Transforming Growth Factor-Beta and Angiotensin II in Renal Fibrosis. Hypertension 1998, 31, 181–188. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Rodriguez-Vita, J.; Cartier, C.; Rupérez, M.; Esteban, V.; Carvajal, G.; Díez, R.R.; Plaza, J.J.; Egido, J.; Ruiz-Ortega, M. Inhibitory effect of interleukin-1β on angiotensin II-induced connective tissue growth factor and type IV collagen production in cultured mesangial cells. Am. J. Physiol. Physiol. 2008, 294, F149–F160. [Google Scholar] [CrossRef]
- Singh, P.; Bahrami, L.; Castillo, A.; Majid, D.S.A. TNF-α Type 2 Receptor Mediates Renal Inflammatory Response to Chronic Angiotensin II Administration with High Salt Intake in Mice. Am. J. Physiol. Ren. Physiol. 2013, 304, F991–F999. [Google Scholar] [CrossRef] [PubMed]
- Mezzano, S.A.; Ruiz-Ortega, M.; Egido, J. Angiotensin II and Renal Fibrosis. Hypertension 2001, 38, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Mezzano, S.A.; Aros, C.A.; Droguett, A.; Burgos, M.E.; Ardiles, L.G.; Flores, C.A.; Carpio, D.; Vío, C.P.; Ruiz-Ortega, M. Jes Renal angiotensin II up-regulation and myofibroblast activation in human membranous nephropathy. Kidney Int. 2003, 64, S39–S45. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, Y.; Kobori, H.; Suzaki, Y.; Navar, L.G. Sustained renal interstitial macrophage infiltration following chronic angiotensin II infusions. Am. J. Physiol. Physiol. 2007, 292, F330–F339. [Google Scholar] [CrossRef] [PubMed]
- Rivera, P.; Ocaranza, M.P.; Lavandero, S.; Jalil, J.E. Rho Kinase Activation and Gene Expression Related to Vascular Remodeling in Normotensive Rats with High Angiotensin I Converting Enzyme Levels. Hypertension 2007, 50, 792–798. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, S.S.; Chen, Y.; Ahokas, R.A.; Sun, Y. Kidney Fibrosis in Hypertensive Rats: Role of Oxidative Stress. Am. J. Nephrol. 2008, 28, 548–554. [Google Scholar] [CrossRef]
- Clermont, G.; Lecour, S.; Lahet, J.-J.; Siohan, P.; Vergely, C.; Chevet, D.; Rifle, G.; Rochette, L. Alteration in plasma antioxidant capacities in chronic renal failure and hemodialysis patients: A possible explanation for the increased cardiovascular risk in these patients. Cardiovasc. Res. 2000, 47, 618–623. [Google Scholar] [CrossRef]
- Johnson, R.J.; Alpers, C.E.; Yoshimura, A.; Lombardi, D.; Pritzl, P.; Floege, J.; Schwartz, S.M. Renal Injury from Angiotensin II-Mediated Hypertension. Hypertension 1992, 19, 464–474. [Google Scholar] [CrossRef]
- Lohmeier, T.E. Angiotensin II Infusion Model of Hypertension. Hypertension 2012, 59, 539–541. [Google Scholar] [CrossRef]
- Hernández-Salinas, R.; Vielma, A.Z.; Arismendi, M.N.; Boric, M.P.; Sáez, J.C.; Velarde, V. Boldine Prevents Renal Alterations in Diabetic Rats. J. Diabetes Res. 2013, 2013, 593672. [Google Scholar] [CrossRef]
- Herrera, G.A. Plasticity of Mesangial Cells: A Basis for Understanding Pathological Alterations. Ultrastruct. Pathol. 2006, 30, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Sequeira-Lopez, M.L.S.; Gomez, R.A. Renin Cells, the Kidney, and Hypertension. Circ. Res. 2021, 128, 887–907. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-Y.; Tanimoto, M.; Gohda, T.; Hagiwara, S.; Yamazaki, T.; Ohara, I.; Murakoshi, M.; Aoki, T.; Ishikawa, Y.; Lee, S.-H.; et al. Attenuating effect of angiotensin-(1–7) on angiotensin II-mediated NAD(P)H oxidase activation in type 2 diabetic nephropathy of KK-Ay/Ta mice. Am. J. Physiol. Physiol. 2011, 300, F1271–F1282. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Wang, W.; Wang, Y.; Du, T.; Shen, W.; Tang, H.; Wang, Y.; Yin, H. Bletilla Striata Polysaccharide Inhibits Angiotensin II-Induced ROS and Inflammation via NOX4 and TLR2 Pathways. Int. J. Biol. Macromol. 2016, 89, 376–388. [Google Scholar] [CrossRef]
- Ding, G.-X.; Zhang, A.-H.; Huang, S.-M.; Pan, X.-Q.; Chen, R.-H. SP600125, an inhibitor of c-Jun NH2-terminal kinase, blocks expression of angiotensin II-induced monocyte chemoattractant protein-1 in human mesangial cells. World J. Pediatr. 2010, 6, 169–176. [Google Scholar] [CrossRef]
- Bolick, D.T.; Hatley, M.E.; Srinivasan, S.; Hedrick, C.C.; Nadler, J.L. Lisofylline, a Novel Antiinflammatory Compound, Protects Mesangial Cells from Hyperglycemia- and Angiotensin II-Mediated Extracellular Matrix Deposition. Endocrinology 2003, 144, 5227–5231. [Google Scholar] [CrossRef]
- Söhl, G.; Maxeiner, S.; Willecke, K. Expression and functions of neuronal gap junctions. Nat. Rev. Neurosci. 2005, 6, 191–200. [Google Scholar] [CrossRef]
- Goodenough, D.A.; Paul, D.L. Gap Junctions. Cold Spring Harb. Perspect. Biol. 2009, 1, a002576. [Google Scholar] [CrossRef]
- Nielsen, M.S.; Nygaard Axelsen, L.; Sorgen, P.L.; Verma, V.; Delmar, M.; Holstein-Rathlou, N. Gap Junctions. In Comprehensive Physiology; Wiley: New York, NY, USA, 2012; pp. 1981–2035. [Google Scholar]
- Scemes, E.; Spray, D.C.; Meda, P. Connexins, Pannexins, Innexins: Novel Roles of “Hemi-Channels”. Pflug. Arch. 2009, 457, 1207–1226. [Google Scholar] [CrossRef]
- Leybaert, L.; Lampe, P.D.; Dhein, S.; Kwak, B.R.; Ferdinandy, P.; Beyer, E.C.; Laird, D.W.; Naus, C.C.; Green, C.R.; Schulz, R. Connexins in Cardiovascular and Neurovascular Health and Disease: Pharmacological Implications. Pharm. Rev. 2017, 69, 396–478. [Google Scholar] [CrossRef]
- Nielsen, B.S.; Alstrom, J.S.; Nicholson, B.J.; Nielsen, M.S.; MacAulay, N. Permeant-Specific Gating of Connexin 30 Hemichannels. J. Biol. Chem. 2017, 292, 19999–20009. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.B.; Ye, Z.-C.; Calloe, K.; Braunstein, T.H.; Hofgaard, J.P.; Ransom, B.R.; Nielsen, M.S.; MacAulay, N. Activation, Permeability, and Inhibition of Astrocytic and Neuronal Large Pore (Hemi)Channels. J. Biol. Chem. 2014, 289, 26058–26073. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.B.; Braunstein, T.H.; Nielsen, M.S.; MacAulay, N. Distinct Permeation Profiles of the Connexin 30 and 43 Hemichannels. FEBS Lett. 2014, 588, 1446–1457. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, B.S.; Zonta, F.; Farkas, T.; Litman, T.; Nielsen, M.S.; MacAulay, N. Structural Determinants Underlying Permeant Discrimination of the Cx43 Hemichannel. J. Biol. Chem. 2019, 294, 16789–16803. [Google Scholar] [CrossRef]
- Beblo, D.A.; Veenstra, R.D. Monovalent Cation Permeation through the Connexin40 Gap Junction Channel. J. Gen. Physiol. 1997, 109, 509–522. [Google Scholar] [CrossRef]
- Suchyna, T.M.; Nitsche, J.M.; Chilton, M.; Harris, A.L.; Veenstra, R.D.; Nicholson, B.J. Different Ionic Selectivities for Connexins 26 and 32 Produce Rectifying Gap Junction Channels. Biophys. J. 1999, 77, 2968–2987. [Google Scholar] [CrossRef]
- Wang, H.-Z.; Veenstra, R.D. Monovalent Ion Selectivity Sequences of the Rat Connexin43 Gap Junction Channel. J. Gen. Physiol. 1997, 109, 491–507. [Google Scholar] [CrossRef]
- Goldberg, G.S.; Moreno, A.P.; Lampe, P.D. Gap Junctions between Cells Expressing Connexin 43 or 32 Show Inverse Permselectivity to Adenosine and ATP. J. Biol. Chem. 2002, 277, 36725–36730. [Google Scholar] [CrossRef]
- Belliveau, D.J.; Bani-Yaghoub, M.; McGirr, B.; Naus, C.C.G.; Rushlow, W.J. Enhanced Neurite Outgrowth in PC12 Cells Mediated by Connexin Hemichannels and ATP. J. Biol. Chem. 2006, 281, 20920–20931. [Google Scholar] [CrossRef]
- Sarieddine, M.Z.R.; Scheckenbach, K.E.L.; Foglia, B.; Maass, K.; Garcia, I.; Kwak, B.R.; Chanson, M. Connexin43 Modulates Neutrophil Recruitment to the Lung. J. Cell Mol. Med. 2009, 13, 4560–4570. [Google Scholar] [CrossRef]
- Hanner, F.; Schnichels, M.; Zheng-Fischhöfer, Q.; Yang, L.E.; Toma, I.; Willecke, K.; McDonough, A.A.; Peti-Peterdi, J. Connexin 30.3 Is Expressed in the Kidney But Not Regulated by Dietary Salt or High Blood Pressure. Cell Commun. Adhes. 2008, 15, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Peracchia, C. Chemical gating of gap junction channels: Roles of calcium, pH and calmodulin. Biochim. Biophys. Acta (BBA) Biomembr. 2004, 1662, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Taffet, S.; Stoner, L.; Delmar, M.; Vallano, M.L.; Jalife, J. A Structural Basis for the Unequal Sensitivity of the Major Cardiac and Liver Gap Junctions to Intracellular Acidification: The Carboxyl Tail Length. Biophys. J. 1993, 64, 1422–1433. [Google Scholar] [CrossRef] [PubMed]
- Saez, J.C.; Spray, D.C.; Nairn, A.C.; Hertzberg, E.; Greengard, P.; Bennett, M. v CAMP Increases Junctional Conductance and Stimulates Phosphorylation of the 27-KDa Principal Gap Junction Polypeptide. Proc. Natl. Acad. Sci. USA 1986, 83, 2473–2477. [Google Scholar] [CrossRef] [PubMed]
- Retamal, M.A.; Cortes, C.J.; Reuss, L.; Bennett, M.V.L.; Saez, J.C. S-Nitrosylation and Permeation through Connexin 43 Hemichannels in Astrocytes: Induction by Oxidant Stress and Reversal by Reducing Agents. Proc. Natl. Acad. Sci. USA 2006, 103, 4475–4480. [Google Scholar] [CrossRef]
- Chandrasekhar, A.; Bera, A.K. Hemichannels: Permeants and Their Effect on Development, Physiology and Death. Cell Biochem. Funct. 2012, 30, 89–100. [Google Scholar] [CrossRef]
- Sáez, J.C.; Schalper, K.A.; Retamal, M.A.; Orellana, J.A.; Shoji, K.F.; Bennett, M.V.L. Cell Membrane Permeabilization via Connexin Hemichannels in Living and Dying Cells. Exp. Cell Res. 2010, 316, 2377–2389. [Google Scholar] [CrossRef]
- Retamal, M.A.; Reyes, E.P.; García, I.E.; Pinto, B.; Martínez, A.D.; González, C. Diseases associated with leaky hemichannels. Front. Cell. Neurosci. 2015, 9, 267. [Google Scholar] [CrossRef]
- Prieto-Villalobos, J.; Alvear, T.F.; Liberona, A.; Lucero, C.M.; Martínez-Araya, C.J.; Balmazabal, J.; Inostroza, C.A.; Ramírez, G.; Gómez, G.I.; Orellana, J.A. Astroglial Hemichannels and Pannexons: The Hidden Link between Maternal Inflammation and Neurological Disorders. Int. J. Mol. Sci. 2021, 22, 9503. [Google Scholar] [CrossRef]
- O’Carroll, S.J.; Alkadhi, M.; Nicholson, L.F.B.; Green, C.R. Connexin43 Mimetic Peptides Reduce Swelling, Astrogliosis, and Neuronal Cell Death after Spinal Cord Injury. Cell Commun. Adhes. 2008, 15, 27–42. [Google Scholar] [CrossRef]
- Paul, D.L.; Ebihara, L.; Takemoto, L.J.; Swenson, K.I.; Goodenough, D.A. Connexin46, a Novel Lens Gap Junction Protein, Induces Voltage-Gated Currents in Nonjunctional Plasma Membrane of Xenopus Oocytes. J. Cell Biol. 1991, 115, 1077–1089. [Google Scholar] [CrossRef] [PubMed]
- Fiori, M.C.; Figueroa, V.; Zoghbi, M.E.; Saéz, J.C.; Reuss, L.; Altenberg, G.A. Permeation of Calcium through Purified Connexin 26 Hemichannels. J. Biol. Chem. 2012, 287, 40826–40834. [Google Scholar] [CrossRef] [PubMed]
- Schalper, K.A.; Sánchez, H.A.; Lee, S.C.; Altenberg, G.A.; Nathanson, M.H.; Sáez, J.C. Connexin 43 Hemichannels Mediate the Ca 2+ Influx Induced by Extracellular Alkalinization. Am. J. Physiol. Cell Physiol. 2010, 299, C1504–C1515. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-C.; Pérez-Hernández, M.; Alvarado, F.J.; Maurya, S.R.; Montnach, J.; Yin, Y.; Zhang, M.; Lin, X.; Vasquez, C.; Heguy, A.; et al. Disruption of Ca 2+ i Homeostasis and Connexin 43 Hemichannel Function in the Right Ventricle Precedes Overt Arrhythmogenic Cardiomyopathy in Plakophilin-2–Deficient Mice. Circulation 2019, 140, 1015–1030. [Google Scholar] [CrossRef]
- Yi, C.; Mei, X.; Ezan, P.; Mato, S.; Matias, I.; Giaume, C.; Koulakoff, A. Astroglial connexin43 contributes to neuronal suffering in a mouse model of Alzheimer’s disease. Cell Death Differ. 2016, 23, 1691–1701. [Google Scholar] [CrossRef]
- Orellana, J.A.; Shoji, K.F.; Abudara, V.; Ezan, P.; Amigou, E.; Saez, P.J.; Jiang, J.X.; Naus, C.C.; Saez, J.C.; Giaume, C. Amyloid -Induced Death in Neurons Involves Glial and Neuronal Hemichannels. J. Neurosci. 2011, 31, 4962–4977. [Google Scholar] [CrossRef]
- Barbe, M.T.; Monyer, H.; Bruzzone, R. Cell-Cell Communication Beyond Connexins: The Pannexin Channels. Physiology 2006, 21, 103–114. [Google Scholar] [CrossRef]
- Penuela, S.; Harland, L.; Simek, J.; Laird, D.W. Pannexin Channels and Their Links to Human Disease. Biochem. J. 2014, 461, 371–381. [Google Scholar] [CrossRef]
- Lohman, A.W.; Isakson, B.E. Differentiating Connexin Hemichannels and Pannexin Channels in Cellular ATP Release. FEBS Lett. 2014, 588, 1379–1388. [Google Scholar] [CrossRef]
- Gajardo-Gómez, R.; Labra, V.C.; Orellana, J.A. Connexins and Pannexins: New Insights into Microglial Functions and Dysfunctions. Front. Mol. Neurosci. 2016, 9, 86. [Google Scholar] [CrossRef]
- O’Donnell, B.L.; Penuela, S. Pannexin 3 Channels in Health and Disease. Purinergic Signal. 2021, 17, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.A.; Wright, J.R.; Mahaut-Smith, M.P. Regulation of Pannexin-1 Channel Activity. Biochem. Soc. Trans. 2015, 43, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Michalski, K.; Syrjanen, J.L.; Henze, E.; Kumpf, J.; Furukawa, H.; Kawate, T. The Cryo-EM Structure of Pannexin 1 Reveals Unique Motifs for Ion Selection and Inhibition. Elife 2020, 9, e54670. [Google Scholar] [CrossRef]
- Palacios-Prado, N.; Soto, P.A.; López, X.; Choi, E.J.; Marquez-Miranda, V.; Rojas, M.; Duarte, Y.; Lee, J.; González-Nilo, F.D.; Sáez, J.C. Endogenous Pannexin1 Channels Form Functional Intercellular Cell–Cell Channels with Characteristic Voltage-Dependent Properties. Proc. Natl. Acad. Sci. USA 2022, 119, e2202104119. [Google Scholar] [CrossRef] [PubMed]
- Billaud, M.; Sandilos, J.K.; Isakson, B.E. Pannexin 1 in the Regulation of Vascular Tone. Trends Cardiovasc. Med. 2012, 22, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Krick, S.; Wang, J.; St-Pierre, M.; Gonzalez, C.; Dahl, G.; Salathe, M. Dual Oxidase 2 (Duox2) Regulates Pannexin 1-Mediated ATP Release in Primary Human Airway Epithelial Cells via Changes in Intracellular PH and Not H2O2 Production. J. Biol. Chem. 2016, 291, 6423–6432. [Google Scholar] [CrossRef] [PubMed]
- Chekeni, F.B.; Elliott, M.R.; Sandilos, J.K.; Walk, S.F.; Kinchen, J.M.; Lazarowski, E.R.; Armstrong, A.J.; Penuela, S.; Laird, D.W.; Salvesen, G.S.; et al. Pannexin 1 Channels Mediate “find-Me” Signal Release and Membrane Permeability during Apoptosis. Nature 2010, 467, 863–867. [Google Scholar] [CrossRef]
- Adamson, S.E.; Leitinger, N. The Role of Pannexin1 in the Induction and Resolution of Inflammation. FEBS Lett. 2014, 588, 1416–1422. [Google Scholar] [CrossRef]
- Silverman, W.R.; de Rivero Vaccari, J.P.; Locovei, S.; Qiu, F.; Carlsson, S.K.; Scemes, E.; Keane, R.W.; Dahl, G. The Pannexin 1 Channel Activates the Inflammasome in Neurons and Astrocytes. J. Biol. Chem. 2009, 284, 18143–18151. [Google Scholar] [CrossRef]
- Su, L.; Jiang, X.; Yang, C.; Zhang, J.; Chen, B.; Li, Y.; Yao, S.; Xie, Q.; Gomez, H.; Murugan, R.; et al. Pannexin 1 Mediates Ferroptosis That Contributes to Renal Ischemia/Reperfusion Injury. J. Biol. Chem. 2019, 294, 19395–19404. [Google Scholar] [CrossRef]
- Malik, S.; Eugenin, E.A. Role of Connexin and Pannexin Containing Channels in HIV Infection and NeuroAIDS. Neurosci. Lett. 2019, 695, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Penuela, S.; Gehi, R.; Laird, D.W. The Biochemistry and Function of Pannexin Channels. Biochim. Biophys. Acta (BBA) Biomembr. 2013, 1828, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Whyte-Fagundes, P.; Zoidl, G. Mechanisms of Pannexin1 Channel Gating and Regulation. Biochim. Biophys. Acta (BBA) Biomembr. 2018, 1860, 65–71. [Google Scholar] [CrossRef]
- Volonté, C.; Apolloni, S.; Skaper, S.D.; Burnstock, G. P2X7 Receptors: Channels, Pores and More. CNS Neurol. Disord. Drug Targets 2012, 11, 705–721. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Locovei, S.; Dahl, G. Pannexin Membrane Channels Are Mechanosensitive Conduits for ATP. FEBS Lett. 2004, 572, 65–68. [Google Scholar] [CrossRef]
- Locovei, S.; Wang, J.; Dahl, G. Activation of Pannexin 1 Channels by ATP through P2Y Receptors and by Cytoplasmic Calcium. FEBS Lett. 2006, 580, 239–244. [Google Scholar] [CrossRef]
- Pinheiro, A.R.; Paramos-de-Carvalho, D.; Certal, M.; Costa, C.; Magalhães-Cardoso, M.T.; Ferreirinha, F.; Costa, M.A.; Correia-de-Sá, P. Bradykinin-Induced Ca2+ Signaling in Human Subcutaneous Fibroblasts Involves ATP Release via Hemichannels Leading to P2Y12 Receptors Activation. Cell Commun. Signal. 2013, 11, 70. [Google Scholar] [CrossRef]
- Billaud, M.; Lohman, A.W.; Straub, A.C.; Looft-Wilson, R.; Johnstone, S.R.; Araj, C.A.; Best, A.K.; Chekeni, F.B.; Ravichandran, K.S.; Penuela, S.; et al. Pannexin1 Regulates A1-Adrenergic Receptor–Mediated Vasoconstriction. Circ. Res. 2011, 109, 80–85. [Google Scholar] [CrossRef]
- Kurtz, A. Renal Connexins and Blood Pressure. Biochim. Biophys. Acta 2012, 1818, 1903–1908. [Google Scholar] [CrossRef][Green Version]
- Hanner, F.; Sorensen, C.M.; Holstein-Rathlou, N.-H.; Peti-Peterdi, J. Connexins and the kidney. Am. J. Physiol. Integr. Comp. Physiol. 2010, 298, R1143–R1155. [Google Scholar] [CrossRef]
- Zhang, Q.; Cao, C.; Mangano, M.; Zhang, Z.; Silldorff, E.P.; Lee-Kwon, W.; Payne, K.; Pallone, T.L. Descending vasa recta endothelium is an electrical syncytium. Am. J. Physiol. Integr. Comp. Physiol. 2006, 291, R1688–R1699. [Google Scholar] [CrossRef] [PubMed]
- Zehra, T.; Cupples, W.A.; Braam, B. Tubuloglomerular Feedback Synchronization in Nephrovascular Networks. J. Am. Soc. Nephrol. 2021, 32, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Hwan Seul, K.; Beyer, E.C. Heterogeneous Localization of Connexin40 in the Renal Vasculature. Microvasc. Res. 2000, 59, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Geis, L.; Boudriot, F.-F.; Wagner, C. Connexin MRNA Distribution in Adult Mouse Kidneys. Pflug. Arch. 2021, 473, 1737–1747. [Google Scholar] [CrossRef]
- de Vriese, A.S.; van de Voorde, J.; Lameire, N.H. Effects of Connexin-Mimetic Peptides on Nitric Oxide Synthase- and Cyclooxygenase-Independent Renal Vasodilation. Kidney Int. 2002, 61, 177–185. [Google Scholar] [CrossRef]
- Takenaka, T.; Inoue, T.; Kanno, Y.; Okada, H.; Meaney, K.R.; Hill, C.E.; Suzuki, H. Expression and Role of Connexins in the Rat Renal Vasculature. Kidney Int. 2008, 73, 415–422. [Google Scholar] [CrossRef]
- Krattinger, N.; Capponi, A.; Mazzolai, L.; Aubert, J.-F.; Caille, D.; Nicod, P.; Waeber, G.; Meda, P.; Haefliger, J.-A. Connexin40 Regulates Renin Production and Blood Pressure. Kidney Int. 2007, 72, 814–822. [Google Scholar] [CrossRef]
- Wagner, C.; Jobs, A.; Schweda, F.; Kurtz, L.; Kurt, B.; Lopez, M.L.S.; Gomez, R.A.; van Veen, T.A.B.; de Wit, C.; Kurtz, A. Selective Deletion of Connexin 40 in Renin-Producing Cells Impairs Renal Baroreceptor Function and Is Associated with Arterial Hypertension. Kidney Int. 2010, 78, 762–768. [Google Scholar] [CrossRef][Green Version]
- Wagner, C.; de Wit, C.; Kurtz, L.; Grünberger, C.; Kurtz, A.; Schweda, F. Connexin40 Is Essential for the Pressure Control of Renin Synthesis and Secretion. Circ. Res. 2007, 100, 556–563. [Google Scholar] [CrossRef]
- Schweda, F.; Kurtz, A. Cellular mechanism of renin release. Acta Physiol. Scand. 2004, 181, 383–390. [Google Scholar] [CrossRef]
- Just, A.; Kurtz, L.; de Wit, C.; Wagner, C.; Kurtz, A.; Arendshorst, W.J. Connexin 40 Mediates the Tubuloglomerular Feedback Contribution to Renal Blood Flow Autoregulation. J. Am. Soc. Nephrol. 2009, 20, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Morioka, T.; Okada, S.; Nameta, M.; Kamal, F.; Yanakieva-Georgieva, N.T.; Yao, J.; Sato, A.; Piao, H.; Oite, T. Glomerular Expression of Connexin 40 and Connexin 43 in Rat Experimental Glomerulonephritis. Clin. Exp. Nephrol. 2013, 17, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Kar, R.; Batra, N.; Riquelme, M.A.; Jiang, J.X. Biological Role of Connexin Intercellular Channels and Hemichannels. Arch. Biochem. Biophys. 2012, 524, 2–15. [Google Scholar] [CrossRef]
- Zhang, J.; Hill, C.E. Differential Connexin Expression in Preglomerular and Postglomerular Vasculature: Accentuation during Diabetes. Kidney Int. 2005, 68, 1171–1185. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sawai, K.; Mukoyama, M.; Mori, K.; Yokoi, H.; Koshikawa, M.; Yoshioka, T.; Takeda, R.; Sugawara, A.; Kuwahara, T.; Saleem, M.A.; et al. Redistribution of Connexin43 Expression in Glomerular Podocytes Predicts Poor Renal Prognosis in Patients with Type 2 Diabetes and Overt Nephropathy. Nephrol. Dial. Transplant. 2006, 21, 2472–2477. [Google Scholar] [CrossRef]
- Baranova, A.; Ivanov, D.; Petrash, N.; Pestova, A.; Skoblov, M.; Kelmanson, I.; Shagin, D.; Nazarenko, S.; Geraymovych, E.; Litvin, O.; et al. The Mammalian Pannexin Family Is Homologous to the Invertebrate Innexin Gap Junction Proteins. Genomics 2004, 83, 706–716. [Google Scholar] [CrossRef]
- DeLalio, L.J.; Masati, E.; Mendu, S.; Ruddiman, C.A.; Yang, Y.; Johnstone, S.R.; Milstein, J.A.; Keller, T.C.S.; Weaver, R.B.; Guagliardo, N.A.; et al. Pannexin 1 Channels in Renin-Expressing Cells Influence Renin Secretion and Blood Pressure Homeostasis. Kidney Int. 2020, 98, 630–644. [Google Scholar] [CrossRef]
- Jankowski, J.; Perry, H.M.; Medina, C.B.; Huang, L.; Yao, J.; Bajwa, A.; Lorenz, U.M.; Rosin, D.L.; Ravichandran, K.S.; Isakson, B.E.; et al. Epithelial and Endothelial Pannexin1 Channels Mediate AKI. J. Am. Soc. Nephrol. 2018, 29, 1887–1899. [Google Scholar] [CrossRef]
- Hanner, F.; Lam, L.; Nguyen, M.T.X.; Yu, A.; Peti-Peterdi, J. Intrarenal localization of the plasma membrane ATP channel pannexin1. Am. J. Physiol. Physiol. 2012, 303, F1454–F1459. [Google Scholar] [CrossRef][Green Version]
- Lohman, A.W.; Billaud, M.; Straub, A.C.; Johnstone, S.R.; Best, A.K.; Lee, M.; Barr, K.; Penuela, S.; Laird, D.W.; Isakson, B.E. Expression of Pannexin Isoforms in the Systemic Murine Arterial Network. J. Vasc. Res. 2012, 49, 405–416. [Google Scholar] [CrossRef]
- Guan, Z.; Inscho, E.W. Role of Adenosine 5′-Triphosphate in Regulating Renal Microvascular Function and in Hypertension. Hypertension 2011, 58, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Suwa, M.; Li, B.; Kawamura, K.; Morioka, T.; Oite, T. ATP-Dependent Mechanism for Coordination of Intercellular Ca 2+ Signaling and Renin Secretion in Rat Juxtaglomerular Cells. Circ. Res. 2003, 93, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Capisano, M.C.; Atchison, D.K.; Harding, P.; Lasley, R.D.; Beierwaltes, W.H. Adenosine inhibits renin release from juxtaglomerular cells via an A1 receptor-TRPC-mediated pathway. Am. J. Physiol. Physiol. 2013, 305, F1209–F1219. [Google Scholar] [CrossRef] [PubMed]
- Vergara, L.; Bao, X.; Cooper, M.; Bello-Reuss, E.; Reuss, L. Gap-Junctional Hemichannels Are Activated by ATP Depletion in Human Renal Proximal Tubule Cells. J. Membr. Biol. 2003, 196, 173–184. [Google Scholar] [CrossRef]
- Stoessel, A.; Himmerkus, N.; Bleich, M.; Bachmann, S.; Theilig, F. Connexin 37 is localized in renal epithelia and responds to changes in dietary salt intake. Am. J. Physiol. Physiol. 2010, 298, F216–F223. [Google Scholar] [CrossRef]
- Hills, C.; Price, G.W.; Wall, M.J.; Kaufmann, T.J.; Tang, C.-W.; Yiu, W.H.; Squires, P.E. Transforming Growth Factor Beta 1 Drives a Switch in Connexin Mediated Cell-to-Cell Communication in Tubular Cells of the Diabetic Kidney. Cell. Physiol. Biochem. 2018, 45, 2369–2388. [Google Scholar] [CrossRef]
- McCulloch, F.; Chambrey, R.; Eladari, D.; Peti-Peterdi, J. Localization of connexin 30 in the luminal membrane of cells in the distal nephron. Am. J. Physiol. Physiol. 2005, 289, F1304–F1312. [Google Scholar] [CrossRef]
- Sipos, A.; Vargas, S.L.; Toma, I.; Hanner, F.; Willecke, K.; Peti-Peterdi, J. Connexin 30 Deficiency Impairs Renal Tubular ATP Release and Pressure Natriuresis. J. Am. Soc. Nephrol. 2009, 20, 1724–1732. [Google Scholar] [CrossRef]
- Praetorius, H.A.; Leipziger, J. Intrarenal Purinergic Signaling in the Control of Renal Tubular Transport. Annu. Rev. Physiol. 2010, 72, 377–393. [Google Scholar] [CrossRef]
- Vekaria, R.M.; Unwin, R.J.; Shirley, D.G. Intraluminal ATP Concentrations in Rat Renal Tubules. J. Am. Soc. Nephrol. 2006, 17, 1841–1847. [Google Scholar] [CrossRef]
- Bailey, M.A. Inhibition of Bicarbonate Reabsorption in the Rat Proximal Tubule by Activation of Luminal P2Y1 Receptors. Am. J. Physiol. Ren. Physiol. 2004, 287, F789–F796. [Google Scholar] [CrossRef][Green Version]
- Kordowitzki, P.; Kranc, W.; Bryl, R.; Kempisty, B.; Skowronska, A.; Skowronski, M.T. The Relevance of Aquaporins for the Physiology, Pathology, and Aging of the Female Reproductive System in Mammals. Cells 2020, 9, 2570. [Google Scholar] [CrossRef] [PubMed]
- Almomani, E.; Kaur, S.; Alexander, R.; Cordat, E. Intercalated Cells: More than PH Regulation. Diseases 2014, 2, 71–92. [Google Scholar] [CrossRef]
- Svenningsen, P.; Burford, J.; Peti-Peterdi, J. ATP Releasing Connexin 30 Hemichannels Mediate Flow-Induced Calcium Signaling in the Collecting Duct. Front. Physiol. 2013, 4, 292. [Google Scholar] [CrossRef] [PubMed]
- Leipziger, J. Control of Epithelial Transport via Luminal P2 Receptors. Am. J. Physiol. Ren. Physiol. 2003, 284, F419–F432. [Google Scholar] [CrossRef]
- Haefliger, J.-A.; Krattinger, N.; Martin, D.; Pedrazzini, T.; Capponi, A.; Döring, B.; Plum, A.; Charollais, A.; Willecke, K.; Meda, P. Connexin43-Dependent Mechanism Modulates Renin Secretion and Hypertension. J. Clin. Investig. 2006, 116, 405–413. [Google Scholar] [CrossRef]
- Abed, A.; Toubas, J.; Kavvadas, P.; Authier, F.; Cathelin, D.; Alfieri, C.; Boffa, J.-J.; Dussaule, J.-C.; Chatziantoniou, C.; Chadjichristos, C.E. Targeting Connexin 43 Protects against the Progression of Experimental Chronic Kidney Disease in Mice. Kidney Int. 2014, 86, 768–779. [Google Scholar] [CrossRef]
- Alonso, F.; Krattinger, N.; Mazzolai, L.; Simon, A.; Waeber, G.; Meda, P.; Haefliger, J.-A. An Angiotensin II- and NF-ΚB-Dependent Mechanism Increases Connexin 43 in Murine Arteries Targeted by Renin-Dependent Hypertension. Cardiovasc. Res. 2010, 87, 166–176. [Google Scholar] [CrossRef]
- Yang, M.; Wang, B.; Li, M.; Jiang, B. Connexin 43 Is Involved in Aldosterone-Induced Podocyte Injury. Cell. Physiol. Biochem. 2014, 34, 1652–1662. [Google Scholar] [CrossRef]
- Toubas, J.; Beck, S.; Pageaud, A.-L.; Huby, A.-C.; Mael-Ainin, M.; Dussaule, J.-C.; Chatziantoniou, C.; Chadjichristos, C.E. Alteration of Connexin Expression Is an Early Signal for Chronic Kidney Disease. Am. J. Physiol. Ren. Physiol. 2011, 301, F24–F32. [Google Scholar] [CrossRef]
- Potter, J.A.; Price, G.W.; Cliff, C.L.; Green, C.R.; Squires, P.E.; Hills, C.E. Collagen I Modifies Connexin-43 Hemichannel Activity via Integrin A2β1 Binding in TGFβ1-Evoked Renal Tubular Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 3644. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Lin, Z.; Tian, X.; Chen, S.; Liang, X.; Qin, M.; Zhu, Q.; Wu, Y.; Zhong, S. Downregulation of Cx43 Reduces Cisplatin-Induced Acute Renal Injury by Inhibiting Ferroptosis. Food Chem. Toxicol. 2021, 158, 112672. [Google Scholar] [CrossRef] [PubMed]
- Retamal, M.A.; Froger, N.; Palacios-Prado, N.; Ezan, P.; Sáez, P.J.; Sáez, J.C.; Giaume, C. Cx43 Hemichannels and Gap Junction Channels in Astrocytes Are Regulated Oppositely by Proinflammatory Cytokines Released from Activated Microglia. J. Neurosci. 2007, 27, 13781–13792. [Google Scholar] [CrossRef] [PubMed]
- Hills, C.E.; Bland, R.; Wheelans, D.C.; Bennett, J.; Ronco, P.M.; Squires, P.E. Glucose-Evoked Alterations in Connexin43-Mediated Cell-to-Cell Communication in Human Collecting Duct: A Possible Role in Diabetic Nephropathy. Am. J. Physiol. Ren. Physiol. 2006, 291, F1045–F1051. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gómez, G.I.; Velarde, V.; Sáez, J.C. Role of a RhoA/ROCK-Dependent Pathway on Renal Connexin43 Regulation in the Angiotensin II-Induced Renal Damage. Int. J. Mol. Sci. 2019, 20, 4408. [Google Scholar] [CrossRef] [PubMed]
- Prakoura, N.; Kavvadas, P.; Chadjichristos, C.E. Connexin 43: A New Therapeutic Target Against Chronic Kidney Disease. Cell. Physiol. Biochem. 2018, 49, 985. [Google Scholar] [CrossRef]
- Cliff, C.L.; Williams, B.M.; Chadjichristos, C.E.; Mouritzen, U.; Squires, P.E.; Hills, C.E. Connexin 43: A Target for the Treatment of Inflammation in Secondary Complications of the Kidney and Eye in Diabetes. Int. J. Mol. Sci. 2022, 23, 600. [Google Scholar] [CrossRef]
- Price, G.W.; Chadjichristos, C.E.; Kavvadas, P.; Tang, S.C.W.; Yiu, W.H.; Green, C.R.; Potter, J.A.; Siamantouras, E.; Squires, P.E.; Hills, C.E. Blocking Connexin-43 Mediated Hemichannel Activity Protects against Early Tubular Injury in Experimental Chronic Kidney Disease. Cell Commun. Signal. 2020, 18, 79. [Google Scholar] [CrossRef] [PubMed]
- Kolavennu, V.; Zeng, L.; Peng, H.; Wang, Y.; Danesh, F.R. Targeting of RhoA/ROCK Signaling Ameliorates Progression of Diabetic Nephropathy Independent of Glucose Control. Diabetes 2008, 57, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Rupérez, M.; Sánchez-López, E.; Blanco-Colio, L.M.; Esteban, V.; Rodríguez-Vita, J.; Plaza, J.J.; Egido, J.; Ruiz-Ortega, M. The Rho-Kinase Pathway Regulates Angiotensin II-Induced Renal Damage. Kidney Int. Suppl. 2005, 68, S39–S45. [Google Scholar] [CrossRef]
- Ilatovskaya, D.V.; Blass, G.; Palygin, O.; Levchenko, V.; Pavlov, T.S.; Grzybowski, M.N.; Winsor, K.; Shuyskiy, L.S.; Geurts, A.M.; Cowley, A.W.; et al. A NOX4/TRPC6 Pathway in Podocyte Calcium Regulation and Renal Damage in Diabetic Kidney Disease. J. Am. Soc. Nephrol. 2018, 29, 1917–1927. [Google Scholar] [CrossRef] [PubMed]
- van Kats, J.P.; de Lannoy, L.M.; Jan Danser, A.H.; van Meegen, J.R.; Verdouw, P.D.; Schalekamp, M.A. Angiotensin II Type 1 (AT1) Receptor-Mediated Accumulation of Angiotensin II in Tissues and Its Intracellular Half-Life in Vivo. Hypertension 1997, 30, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Wei, C.; Chen, X.; Wang, J.; Cheng, H.; Zhang, X.; Hong, Q.; Shi, S.; Fu, B.; Wei, R. Essential Role of Ca2+ Release Channels in Angiotensin II-Induced Ca2+ Oscillations and Mesangial Cell Contraction. Kidney Int. 2006, 70, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Ji, Z. AngII-Induced Glomerular Mesangial Cell Proliferation Inhibited by Losartan via Changes in Intracellular Calcium Ion Concentration. Clin. Exp. Med. 2014, 14, 169–176. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, M.; Piskuric, N.A.; Vollmer, C.; Nurse, C.A. P2Y2 Receptor Activation Opens Pannexin-1 Channels in Rat Carotid Body Type II Cells: Potential Role in Amplifying the Neurotransmitter ATP. J. Physiol. 2012, 590, 4335–4350. [Google Scholar] [CrossRef]
- Dahl, G.; Qiu, F.; Wang, J. The Bizarre Pharmacology of the ATP Release Channel Pannexin1. Neuropharmacology 2013, 75, 583–593. [Google Scholar] [CrossRef]
- Orellana, J.A.; von Bernhardi, R.; Giaume, C.; Sáez, J.C. Glial Hemichannels and Their Involvement in Aging and Neurodegenerative Diseases. Rev. Neurosci. 2012, 23, 163–177. [Google Scholar] [CrossRef]
- Abudara, V.; Retamal, M.A.; Del Rio, R.; Orellana, J. Synaptic Functions of Hemichannels and Pannexons: A Double-Edged Sword. Front. Mol. Neurosci. 2018, 11, 435. [Google Scholar] [CrossRef]
- Sáez, P.J.; Orellana, J.A.; Vega-Riveros, N.; Figueroa, V.A.; Hernández, D.E.; Castro, J.F.; Klein, A.D.; Jiang, J.X.; Zanlungo, S.; Sáez, J.C. Disruption in Connexin-Based Communication Is Associated with Intracellular Ca2+ Signal Alterations in Astrocytes from Niemann-Pick Type C Mice. PLoS ONE 2013, 8, e71361. [Google Scholar] [CrossRef]
- Garre, J.M.; Yang, G.; Bukauskas, F.F.; Bennett, M.V.L. FGF-1 Triggers Pannexin-1 Hemichannel Opening in Spinal Astrocytes of Rodents and Promotes Inflammatory Responses in Acute Spinal Cord Slices. J. Neurosci. 2016, 36, 4785–4801. [Google Scholar] [CrossRef]
- Sun, H.; Sun, Z.; Varghese, Z.; Guo, Y.; Moorhead, J.F.; Unwin, R.J.; Ruan, X.Z. Nonesterified Free Fatty Acids Enhance the Inflammatory Response in Renal Tubules by Inducing Extracellular ATP Release. Am. J. Physiol. Ren. Physiol. 2020, 319, F292–F303. [Google Scholar] [CrossRef] [PubMed]
- Menzies, R.I.; Howarth, A.R.; Unwin, R.J.; Tam, F.W.K.; Mullins, J.J.; Bailey, M.A. Inhibition of the Purinergic P2X7 Receptor Improves Renal Perfusion in Angiotensin-II-Infused Rats. Kidney Int. 2015, 88, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Zheng, P.; Zhao, L.; Wang, Y.; Miao, N.; Zhou, Z.; Cheng, Q.; Chen, P.; Xie, H.; Li, J.; et al. Caspase-11 Promotes NLRP3 Inflammasome Activation via the Cleavage of Pannexin1 in Acute Kidney Disease. Acta Pharm. Sin. 2022, 43, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shen, Y.; Huang, L.; Wang, J. TLR2/Caspase-5/Panx1 Pathway Mediates Necrosis-Induced NLRP3 Inflammasome Activation in Macrophages during Acute Kidney Injury. Cell Death Discov. 2022, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Graciano, M.L.; Nishiyama, A.; Jackson, K.; Seth, D.M.; Ortiz, R.M.; Prieto-Carrasquero, M.C.; Kobori, H.; Navar, L.G. Purinergic Receptors Contribute to Early Mesangial Cell Transformation and Renal Vessel Hypertrophy during Angiotensin II-Induced Hypertension. Am. J. Physiol. Ren. Physiol. 2008, 294, F161–F169. [Google Scholar] [CrossRef] [PubMed]
- Vonend, O.; Turner, C.M.; Chan, C.M.; Loesch, A.; Dell’Anna, G.C.; Srai, K.S.; Burnstock, G.; Unwin, R.J. Glomerular Expression of the ATP-Sensitive P2X Receptor in Diabetic and Hypertensive Rat Models. Kidney Int. 2004, 66, 157–166. [Google Scholar] [CrossRef][Green Version]
- Díaz, E.F.; Labra, V.C.; Alvear, T.F.; Mellado, L.A.; Inostroza, C.A.; Oyarzún, J.E.; Salgado, N.; Quintanilla, R.A.; Orellana, J.A. Connexin 43 hemichannels and pannexin-1 channels contribute to the α-synuclein-induced dysfunction and death of astrocytes. Glia 2019, 67, 1598–1619. [Google Scholar] [CrossRef]
- Pelegrin, P.; Surprenant, A. Pannexin-1 Couples to Maitotoxin- and Nigericin-Induced Interleukin-1β Release through a Dye Uptake-Independent Pathway. J. Biol. Chem. 2007, 282, 2386–2394. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Q.; Hong, J.; Ritter, J.K.; Li, P.-L. Inhibition of Pannexin-1 Channel Activity by Adiponectin in Podocytes: Role of Acid Ceramidase Activation. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2018, 1863, 1246–1256. [Google Scholar] [CrossRef]
- Kang, J.; Kang, N.; Lovatt, D.; Torres, A.; Zhao, Z.; Lin, J.; Nedergaard, M. Connexin 43 Hemichannels Are Permeable to ATP. J. Neurosci. 2008, 28, 4702–4711. [Google Scholar] [CrossRef]
- Abed, A.B.; Kavvadas, P.; Chadjichristos, C.E. Functional Roles of Connexins and Pannexins in the Kidney. Cell Mol. Life Sci. 2015, 72, 2869–2877. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucero, C.M.; Prieto-Villalobos, J.; Marambio-Ruiz, L.; Balmazabal, J.; Alvear, T.F.; Vega, M.; Barra, P.; Retamal, M.A.; Orellana, J.A.; Gómez, G.I. Hypertensive Nephropathy: Unveiling the Possible Involvement of Hemichannels and Pannexons. Int. J. Mol. Sci. 2022, 23, 15936. https://doi.org/10.3390/ijms232415936
Lucero CM, Prieto-Villalobos J, Marambio-Ruiz L, Balmazabal J, Alvear TF, Vega M, Barra P, Retamal MA, Orellana JA, Gómez GI. Hypertensive Nephropathy: Unveiling the Possible Involvement of Hemichannels and Pannexons. International Journal of Molecular Sciences. 2022; 23(24):15936. https://doi.org/10.3390/ijms232415936
Chicago/Turabian StyleLucero, Claudia M., Juan Prieto-Villalobos, Lucas Marambio-Ruiz, Javiera Balmazabal, Tanhia F. Alvear, Matías Vega, Paola Barra, Mauricio A. Retamal, Juan A. Orellana, and Gonzalo I. Gómez. 2022. "Hypertensive Nephropathy: Unveiling the Possible Involvement of Hemichannels and Pannexons" International Journal of Molecular Sciences 23, no. 24: 15936. https://doi.org/10.3390/ijms232415936
APA StyleLucero, C. M., Prieto-Villalobos, J., Marambio-Ruiz, L., Balmazabal, J., Alvear, T. F., Vega, M., Barra, P., Retamal, M. A., Orellana, J. A., & Gómez, G. I. (2022). Hypertensive Nephropathy: Unveiling the Possible Involvement of Hemichannels and Pannexons. International Journal of Molecular Sciences, 23(24), 15936. https://doi.org/10.3390/ijms232415936









