PKR-Mediated Phosphorylation of eIF2a and CHK1 Is Associated with Doxorubicin-Mediated Apoptosis in HCC1143 Triple-Negative Breast Cancer Cells
Abstract
1. Introduction
2. Results
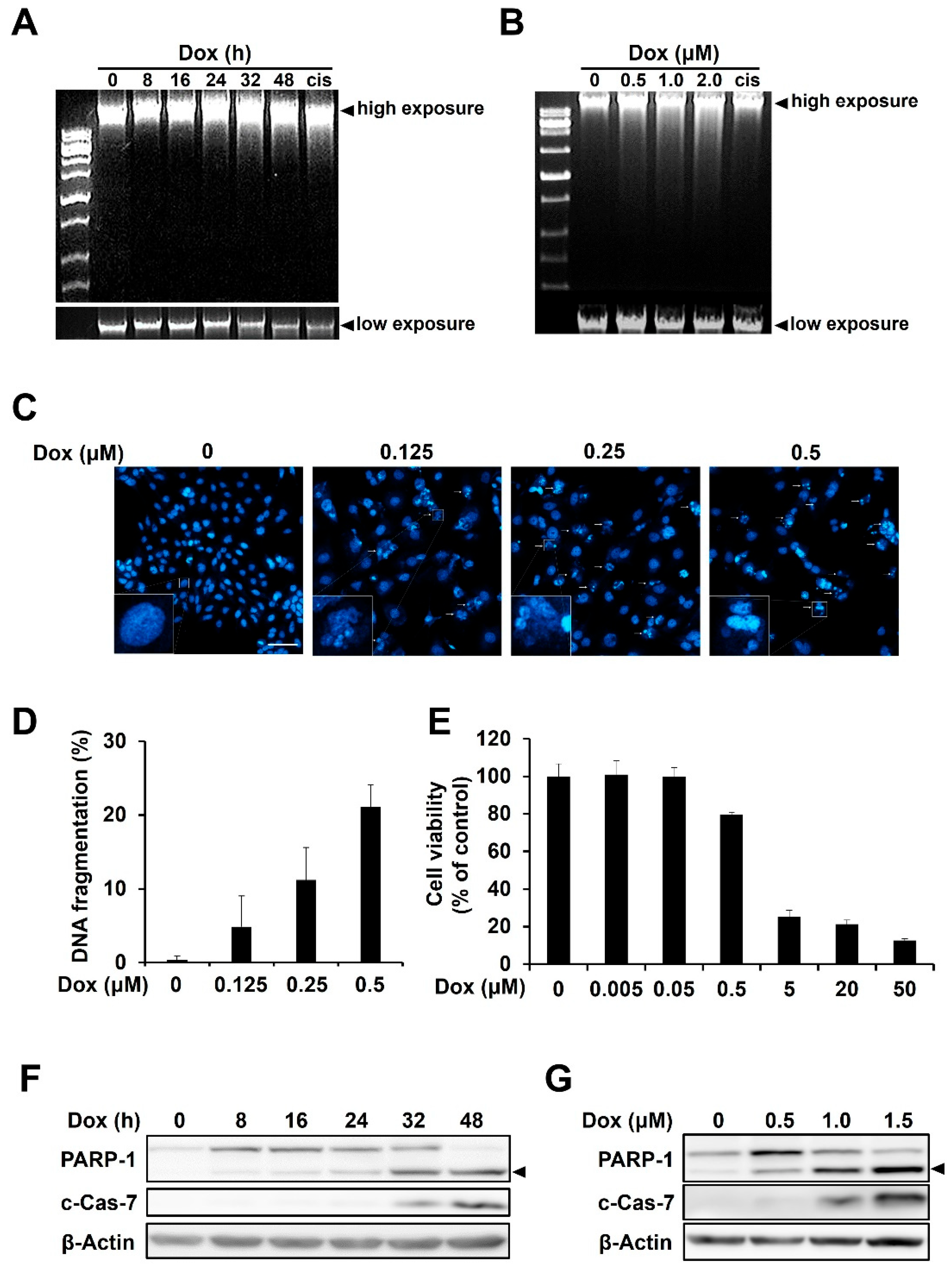
2.1. Doxorubicin Induces DNA Damage and Apoptosis in HCC1143 TNBC Cells
2.2. Doxorubicin-Mediated Activation of PKR, but Not PERK, Is Responsible for eIF2α Phosphorylation in HCC1143 Cells
2.3. Inhibition of PKR Reduces the Doxorubicin-Mediated Apoptosis of HCC1143 Cells
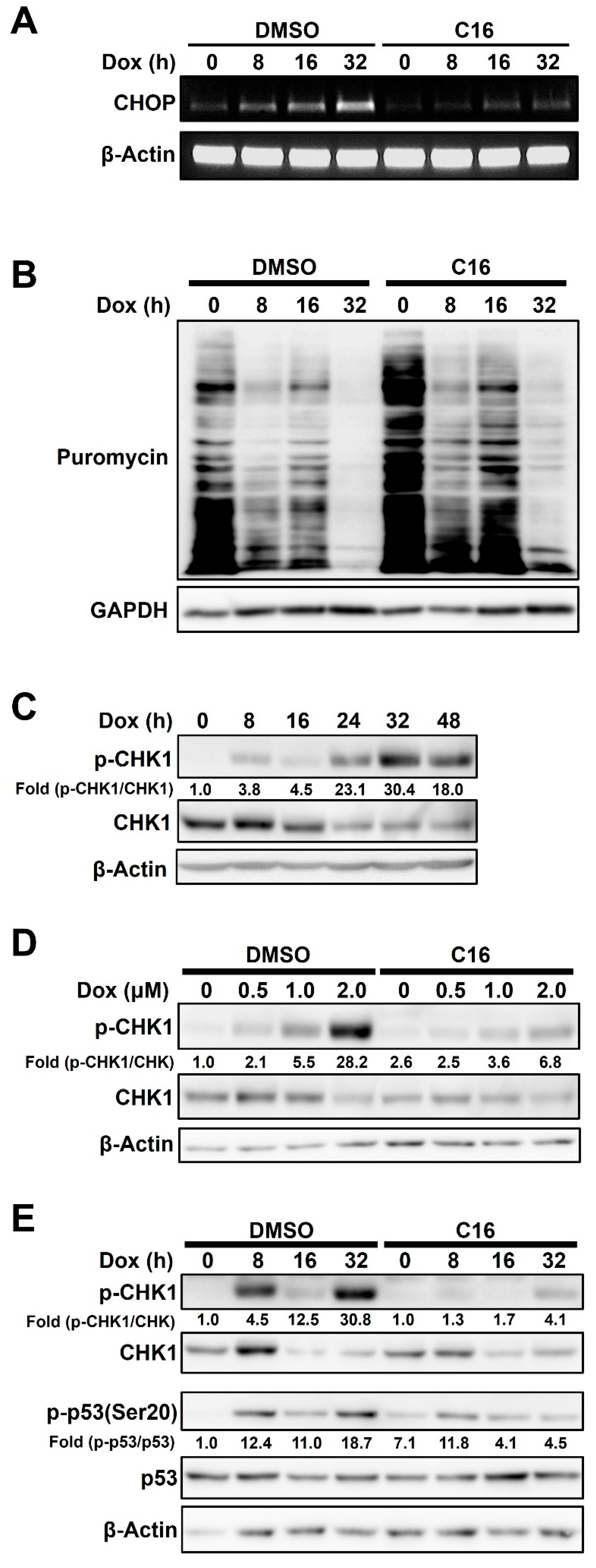
2.4. Doxorubicin-Induced PKR-Mediated Apoptosis Is Caused by eIF2α/CHOP Signaling and CHK1 Phosphorylation in HCC1143 Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Measurement of Apoptosis Using Microscopy
4.4. Measurement of DNA Fragmentation
4.5. Immunoblotting Analysis
4.6. RT-PCR and PCR Primers
4.7. Cell Cycle Analysis
4.8. Statistical Analysis
5. Conclusions
- Doxorubicin-mediated activation of PKR, but not PERK, is responsible for eIF2α phosphorylation in HCC1143 triple-negative breast cancer (TNBC) cells.
- Inhibition of PKR reduces the doxorubicin-mediated apoptosis of HCC1143 TNBC cells.
- Doxorubicin-induced PKR-mediated apoptosis is caused by eIF2α/CHOP signaling in HCC1143 TNBC cells.
- Doxorubicin-induced PKR-mediated apoptosis is caused by CHK1 phosphorylation in HCC1143 TNBC cells.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Watanabe, T.; Imamura, T.; Hiasa, Y. Roles of protein kinase R in cancer: Potential as a therapeutic target. Cancer Sci. 2018, 109, 919–925. [Google Scholar] [CrossRef]
- Garcia, M.A.; Gil, J.; Ventoso, I.; Guerra, S.; Domingo, E.; Rivas, C.; Esteban, M. Impact of protein kinase PKR in cell biology: From antiviral to antiproliferative action. Microbiol. Mol. Biol. Rev. MMBR 2006, 70, 1032–1060. [Google Scholar] [CrossRef]
- Bennett, R.L.; Carruthers, A.L.; Hui, T.; Kerney, K.R.; Liu, X.; May, W.S., Jr. Increased expression of the dsRNA-activated protein kinase PKR in breast cancer promotes sensitivity to doxorubicin. PLoS ONE 2012, 7, e46040. [Google Scholar] [CrossRef]
- Cheng, X.; Byrne, M.; Brown, K.D.; Konopleva, M.Y.; Kornblau, S.M.; Bennett, R.L.; May, W.S. PKR inhibits the DNA damage response, and is associated with poor survival in AML and accelerated leukemia in NHD13 mice. Blood 2015, 126, 1585–1594. [Google Scholar] [CrossRef]
- Martinez, N.W.; Gomez, F.E.; Matus, S. The Potential Role of Protein Kinase R as a Regulator of Age-Related Neurodegeneration. Front. Aging Neurosci. 2021, 13, 638208. [Google Scholar] [CrossRef]
- Blalock, W.L.; Bavelloni, A.; Piazzi, M.; Faenza, I.; Cocco, L. A role for PKR in hematologic malignancies. J. Cell. Physiol. 2010, 223, 572–591. [Google Scholar] [CrossRef]
- Scheuner, D.; Patel, R.; Wang, F.; Lee, K.; Kumar, K.; Wu, J.; Nilsson, A.; Karin, M.; Kaufman, R.J. Double-stranded RNA-dependent protein kinase phosphorylation of the alpha-subunit of eukaryotic translation initiation factor 2 mediates apoptosis. J. Biol. Chem. 2006, 281, 21458–21468. [Google Scholar] [CrossRef]
- Donnelly, N.; Gorman, A.M.; Gupta, S.; Samali, A. The eIF2alpha kinases: Their structures and functions. Cell. Mol. Life Sci. CMLS 2013, 70, 3493–3511. [Google Scholar] [CrossRef]
- Wek, R.C. Role of eIF2alpha Kinases in Translational Control and Adaptation to Cellular Stress. Cold Spring Harb. Perspect. Biol. 2018, 10, a032870. [Google Scholar] [CrossRef]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–438. [Google Scholar] [CrossRef]
- Oyadomari, S.; Mori, M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004, 11, 381–389. [Google Scholar] [CrossRef]
- Cuddihy, A.R.; Li, S.; Tam, N.W.; Wong, A.H.; Taya, Y.; Abraham, N.; Bell, J.C.; Koromilas, A.E. Double-stranded-RNA-activated protein kinase PKR enhances transcriptional activation by tumor suppressor p53. Mol. Cell. Biol. 1999, 19, 2475–2484. [Google Scholar] [CrossRef]
- Fremont, M.; Vaeyens, F.; Herst, C.V.; De Meirleir, K.L.; Englebienne, P. Double-stranded RNA-dependent protein kinase (PKR) is a stress-responsive kinase that induces NFkappaB-mediated resistance against mercury cytotoxicity. Life Sci. 2006, 78, 1845–1856. [Google Scholar] [CrossRef]
- Meurs, E.F.; Galabru, J.; Barber, G.N.; Katze, M.G.; Hovanessian, A.G. Tumor suppressor function of the interferon-induced double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. USA 1993, 90, 232–236. [Google Scholar] [CrossRef]
- Blalock, W.L.; Bavelloni, A.; Piazzi, M.; Tagliavini, F.; Faenza, I.; Martelli, A.M.; Follo, M.Y.; Cocco, L. Multiple forms of PKR present in the nuclei of acute leukemia cells represent an active kinase that is responsive to stress. Leukemia 2011, 25, 236–245. [Google Scholar] [CrossRef]
- McGowan, J.V.; Chung, R.; Maulik, A.; Piotrowska, I.; Walker, J.M.; Yellon, D.M. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc. Drugs Ther. 2017, 31, 63–75. [Google Scholar] [CrossRef]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharm. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef]
- Gewirtz, D.A. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem. Pharmacol. 1999, 57, 727–741. [Google Scholar] [CrossRef]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef]
- Kunkeaw, N.; Lee, Y.S.; Im, W.R.; Jang, J.J.; Song, M.J.; Yang, B.; Park, J.L.; Kim, S.Y.; Ku, Y.; Kim, Y.; et al. Mechanism mediated by a noncoding RNA, nc886, in the cytotoxicity of a DNA-reactive compound. Proc. Natl. Acad. Sci. USA 2019, 116, 8289–8294. [Google Scholar] [CrossRef]
- Diana, A.; Carlino, F.; Franzese, E.; Oikonomidou, O.; Criscitiello, C.; De Vita, F.; Ciardiello, F.; Orditura, M. Early Triple Negative Breast Cancer: Conventional Treatment and Emerging Therapeutic Landscapes. Cancers 2020, 12, 819. [Google Scholar] [CrossRef]
- Nofech-Mozes, S.; Trudeau, M.; Kahn, H.K.; Dent, R.; Rawlinson, E.; Sun, P.; Narod, S.A.; Hanna, W.M. Patterns of recurrence in the basal and non-basal subtypes of triple-negative breast cancers. Breast Cancer Res. Treat. 2009, 118, 131–137. [Google Scholar] [CrossRef] [PubMed]
- De Ruijter, T.C.; Veeck, J.; de Hoon, J.P.; van Engeland, M.; Tjan-Heijnen, V.C. Characteristics of triple-negative breast cancer. J. Cancer Res. Clin. Oncol. 2011, 137, 183–192. [Google Scholar] [CrossRef]
- Tan, A.R.; Swain, S.M. Therapeutic strategies for triple-negative breast cancer. Cancer J. 2008, 14, 343–351. [Google Scholar] [CrossRef]
- Carey, L.A.; Dees, E.C.; Sawyer, L.; Gatti, L.; Moore, D.T.; Collichio, F.; Ollila, D.W.; Sartor, C.I.; Graham, M.L.; Perou, C.M. The triple negative paradox: Primary tumor chemosensitivity of breast cancer subtypes. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 2329–2334. [Google Scholar] [CrossRef]
- Peidis, P.; Papadakis, A.I.; Muaddi, H.; Richard, S.; Koromilas, A.E. Doxorubicin bypasses the cytoprotective effects of eIF2alpha phosphorylation and promotes PKR-mediated cell death. Cell Death Differ. 2011, 18, 145–154. [Google Scholar] [CrossRef]
- Gil, J.; Esteban, M. Induction of apoptosis by the dsRNA-dependent protein kinase (PKR): Mechanism of action. Apoptosis Int. J. Program. Cell Death 2000, 5, 107–114. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Y.; Tang, J.; Jiang, J.; Chen, Z. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim. Et Biophys. Sin. 2014, 46, 629–640. [Google Scholar] [CrossRef]
- Koromilas, A.E. Roles of the translation initiation factor eIF2alpha serine 51 phosphorylation in cancer formation and treatment. Biochim. Biophys. Acta 2015, 1849, 871–880. [Google Scholar] [CrossRef]
- Krishnamoorthy, T.; Pavitt, G.D.; Zhang, F.; Dever, T.E.; Hinnebusch, A.G. Tight binding of the phosphorylated alpha subunit of initiation factor 2 (eIF2alpha) to the regulatory subunits of guanine nucleotide exchange factor eIF2B is required for inhibition of translation initiation. Mol. Cell. Biol. 2001, 21, 5018–5030. [Google Scholar] [CrossRef]
- Levine, A.J. p53, the cellular gatekeeper for growth and division. Cell 1997, 88, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wan, X.; Gao, L.; Pan, Y.; Xie, W.; Wang, H.; Guo, J. Activated PKR inhibits pancreatic beta-cell proliferation through sumoylation-dependent stabilization of P53. Mol. Immunol. 2015, 68, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Abramson, V.G.; Lehmann, B.D.; Ballinger, T.J.; Pietenpol, J.A. Subtyping of triple-negative breast cancer: Implications for therapy. Cancer 2015, 121, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N.; Adams, M.J.; Ganatra, S.; Colan, S.D.; Aggarwal, S.; Steiner, R.; Amdani, S.; Lipshultz, E.R.; Lipshultz, S.E. Strategies to prevent anthracycline-induced cardiotoxicity in cancer survivors. Cardio-Oncology 2019, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Diehl, J.A. PERK Integrates Oncogenic Signaling and Cell Survival During Cancer Development. J. Cell. Physiol. 2016, 231, 2088–2096. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lv, Y.; Zhao, N.; Guan, G.; Wang, J. Protein kinase R-like ER kinase and its role in endoplasmic reticulum stress-decided cell fate. Cell Death Dis. 2015, 6, e1822. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Chi, Y.; Xue, J.; Ma, L.; Shao, Z.; Wu, J. Phosphorylated eIF2alpha predicts disease-free survival in triple-negative breast cancer patients. Sci. Rep. 2017, 7, 44674. [Google Scholar] [CrossRef] [PubMed]
- Iurlaro, R.; Munoz-Pinedo, C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016, 283, 2640–2652. [Google Scholar] [CrossRef]
- McCullough, K.D.; Martindale, J.L.; Klotz, L.O.; Aw, T.Y.; Holbrook, N.J. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol. Cell. Biol. 2001, 21, 1249–1259. [Google Scholar] [CrossRef]
- Yang, F.; Teves, S.S.; Kemp, C.J.; Henikoff, S. Doxorubicin, DNA torsion, and chromatin dynamics. Biochim. Biophys. Acta 2014, 1845, 84–89. [Google Scholar] [CrossRef]
- Roos, W.P.; Thomas, A.D.; Kaina, B. DNA damage and the balance between survival and death in cancer biology. Nat. Rev. Cancer 2016, 16, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Piwnica-Worms, H. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol. Cell. Biol. 2001, 21, 4129–4139. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.C.; Siu, W.Y.; Chow, J.P.; Lau, A.; Arooz, T.; Tong, H.Y.; Ng, I.O.; Poon, R.Y. The relative contribution of CHK1 and CHK2 to Adriamycin-induced checkpoint. Exp. Cell Res. 2005, 304, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Shieh, S.Y.; Ahn, J.; Tamai, K.; Taya, Y.; Prives, C. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 2000, 14, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.W.; Ho, C.J.; Chen, H.W.; Pao, Y.H.; Chen, L.E.; Yang, M.C.; Huang, S.B.; Wang, S.; Chen, C.H.; Wang, C. P53 enhances apoptosis induced by doxorubicin only under conditions of severe DNA damage. Cell Cycle 2018, 17, 2175–2186. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.H.; Lee, E.S.; Lim, D.S.; Bae, Y.S. PKR, a p53 target gene, plays a crucial role in the tumor-suppressor function of p53. Proc. Natl. Acad. Sci. USA 2009, 106, 7852–7857. [Google Scholar] [CrossRef] [PubMed]
- Vera, J.; Raatz, Y.; Wolkenhauer, O.; Kottek, T.; Bhattacharya, A.; Simon, J.C.; Kunz, M. Chk1 and Wee1 control genotoxic-stress induced G2-M arrest in melanoma cells. Cell. Signal. 2015, 27, 951–960. [Google Scholar] [CrossRef]
- Kung, C.P.; Cottrell, K.A.; Ryu, S.; Bramel, E.R.; Kladney, R.D.; Bao, E.A.; Freeman, E.C.; Sabloak, T.; Maggi, L., Jr.; Weber, J.D. Evaluating the therapeutic potential of ADAR1 inhibition for triple-negative breast cancer. Oncogene 2021, 40, 189–202. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Jee, H.-Y.; Lee, Y.-G.; Shin, J.-I.; Jeon, Y.-J.; Kim, J.-B.; Seo, H.-e.; Lee, J.-Y.; Lee, K. PKR-Mediated Phosphorylation of eIF2a and CHK1 Is Associated with Doxorubicin-Mediated Apoptosis in HCC1143 Triple-Negative Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 15872. https://doi.org/10.3390/ijms232415872
Lee S, Jee H-Y, Lee Y-G, Shin J-I, Jeon Y-J, Kim J-B, Seo H-e, Lee J-Y, Lee K. PKR-Mediated Phosphorylation of eIF2a and CHK1 Is Associated with Doxorubicin-Mediated Apoptosis in HCC1143 Triple-Negative Breast Cancer Cells. International Journal of Molecular Sciences. 2022; 23(24):15872. https://doi.org/10.3390/ijms232415872
Chicago/Turabian StyleLee, Sol, Ha-Yeon Jee, Yoon-Gyeong Lee, Jong-Il Shin, Yong-Joon Jeon, Ji-Beom Kim, Hye-eun Seo, Ji-Yeon Lee, and Kyungho Lee. 2022. "PKR-Mediated Phosphorylation of eIF2a and CHK1 Is Associated with Doxorubicin-Mediated Apoptosis in HCC1143 Triple-Negative Breast Cancer Cells" International Journal of Molecular Sciences 23, no. 24: 15872. https://doi.org/10.3390/ijms232415872
APA StyleLee, S., Jee, H.-Y., Lee, Y.-G., Shin, J.-I., Jeon, Y.-J., Kim, J.-B., Seo, H.-e., Lee, J.-Y., & Lee, K. (2022). PKR-Mediated Phosphorylation of eIF2a and CHK1 Is Associated with Doxorubicin-Mediated Apoptosis in HCC1143 Triple-Negative Breast Cancer Cells. International Journal of Molecular Sciences, 23(24), 15872. https://doi.org/10.3390/ijms232415872




