Vulgarin, a Sesquiterpene Lactone from Artemisia judaica, Improves the Antidiabetic Effectiveness of Glibenclamide in Streptozotocin-Induced Diabetic Rats via Modulation of PEPCK and G6Pase Genes Expression
Abstract
1. Introduction
2. Results
2.1. Effect on Fasting Blood Glucose (FBG) and Insulin Levels
2.2. Effect on Total Hemoglobin (Hb) and Glycosylated Hemoglobin (HbA1c) Levels
2.3. Effect on Blood Lipid Profile
2.4. Effect on Oxidative Stress and Lipid Peroxidation (LPO) Markers in the Pancreatic Tissues
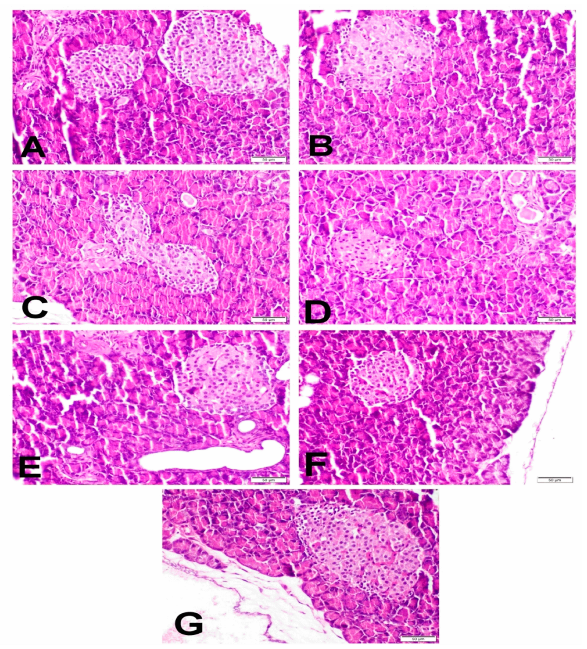
2.5. Histopathological Examination of Pancreas
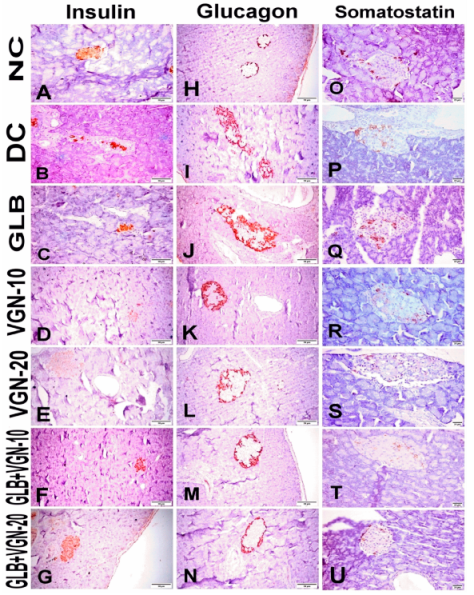
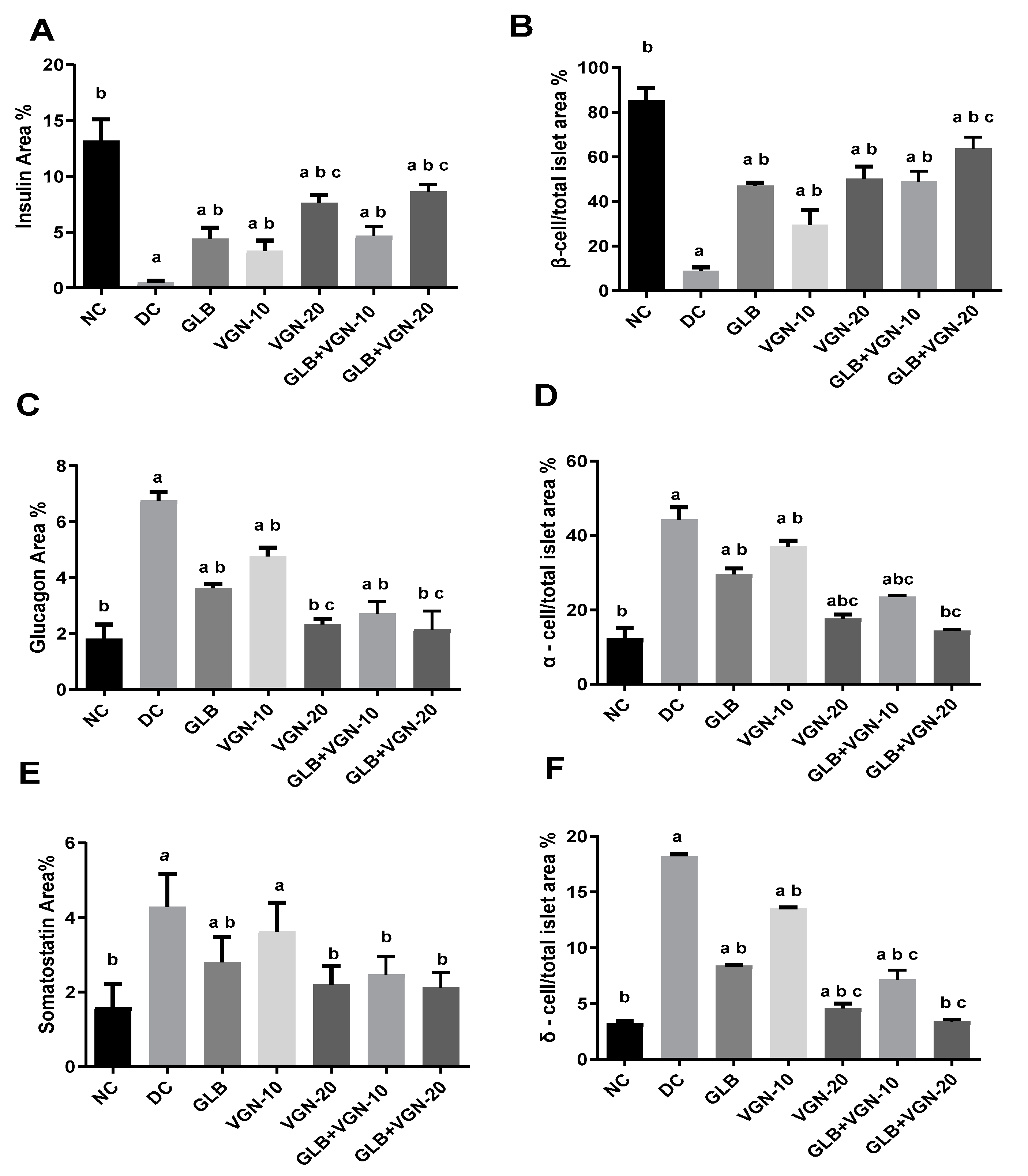
2.6. Immunohistochemistry of Insulin, Glucagon and Somatostatin Contents of Islets of Langerhans
2.7. Quantitative Real-Time PCR (qRT-PCR)
3. Discussion
3.1. Effect on Fasting Blood Glucose (FBG) and Insulin Levels
3.2. Effect on Total Hemoglobin (Hb) and Glycosylated Hemoglobin (HbA1c) Levels
3.3. Effect on Blood Lipid Profile
3.4. Effect on Oxidative Stress and Lipid Peroxidation (LPO) Markers in the Pancreatic Tissues
3.5. Histopathological Examination of Pancreas and Immunohistochemistry of Insulin, Glucagon and Somatostatin Contents of Islets of Langerhans
3.6. Effect on the Gluconeogenic Enzymes PEPCK and G6Pase Gene Expression
4. Materials and Methods
4.1. Plant Material
4.2. Extraction and Isolation
Chloroform Fraction
4.3. Animals
4.4. Induction of Experimental Diabetes
4.5. Experimental Design
4.6. Effect on Fasting Blood Glucose (FBG) and Insulin Levels
4.7. Effect on Total Hemoglobin (Hb) and Glycosylated Hemoglobin (HbA1c) Levels
4.8. Effect on Blood Lipid Profile
4.9. Tissue Collection
4.10. Effect on Oxidative Stress and Lipid Peroxidation (LPO) Markers in the Pancreatic Tissues
4.11. Histopathological Examination of Pancreas
4.12. Immunohistochemistry of Insulin, Glucagon and Somatostatin Contents of Islets of Langerhans
4.13. Quantitative Real-Time PCR (qRT-PCR)
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bahmanzadeh, M.; Vahidinia, A.; Mehdinejadiani, S.; Shokri, S.; Alizadeh, Z. Dietary supplementation with astaxanthin may ameliorate sperm parameters and DNA integrity in streptozotocin-induced diabetic rats. Clin. Exp. Reprod. Med. 2016, 43, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Eshetu, N. Evaluation of the Acute and Sub-chronic Toxic Effects of Aqueous Leaf Extracts of Artemisia afra on Liver, Kidney and Some Blood Parameters in Wistar Rats. Adv. Biosci. Bioeng. 2016, 4, 1–9. [Google Scholar] [CrossRef][Green Version]
- Efferth, T. Willmar Schwabe Award 2006: Antiplasmodial and antitumor activity of artemisinin--from bench to bedside. Planta Med. 2007, 73, 299–309. [Google Scholar] [CrossRef]
- Bhat, S.; Ullah, M.; Abu-Duhier, F. Bioactive extract of Artemisia judaica causes in vitro inhibition of dipeptidyl peptidase IV and pancreatic/intestinal enzymes of the carbohydrate absorption cascade: Implication for anti-diabetic new molecular entities (NMEs). Orient. Pharm. Exp. Med. 2018, 19, 71–80. [Google Scholar] [CrossRef]
- Nofal, S.M.; Mahmoud, S.S.; Ramadan, A.; Soliman, G.A.; Fawzy, R. Anti-diabetic effect of Artemisia judaica extracts. Res. J. Med. Med. Sci. 2009, 4, 42–48. [Google Scholar]
- Alzweiri, M.; Al Sarhan, A.; Mansi, K.; Hudaib, M.; Aburjai, T. Ethnopharmacological survey of medicinal herbs in Jordan, the Northern Badia region. J. Ethnopharmacol. 2011, 137, 27–35. [Google Scholar] [CrossRef]
- Saeedan, A.S.; Soliman, G.A.; Abdel-Rahman, R.F.; Abd-Elsalam, R.M.; Ogaly, H.A.; Alharthy, K.M.; Abdel-Kader, M.S. Possible synergistic antidiabetic effects of quantified Artemisia judaica extract and glyburide in streptozotocin-induced diabetic rats via restoration of PPAR-α mRNA expression. Biology 2021, 10, 796. [Google Scholar] [CrossRef]
- Rodriguez de Vera, C.; de Diego, A.M.; Villar del Fresno, A. Preliminary study on the effects produced in the lipid fraction of various rat organs after administration of vulgarin (a new oral hypoglycemic agent of natural origin). Boll. Chim. Farm. 1976, 115, 445–456. [Google Scholar]
- Abegaz, B.; Camps, F.; Coll, J.; Feliz, M.; Jacobsson, U.; Miravitlles, C.; Molins, E.; Torramilans, J. The structures of vulgarin and its isomers:—A reinvestigation. Tetrahedron 1986, 42, 6003–6009. [Google Scholar] [CrossRef]
- Mahimainathan, L.; Das, F.; Venkatesan, B.; Choudhury, G.G. Mesangial cell hypertrophy by high glucose is mediated by downregulation of the tumor suppressor PTEN. Diabetes 2006, 55, 2115–2125. [Google Scholar] [CrossRef]
- Yusufoglu, H.S.; Soliman, G.A.; Abdel-Rahman, R.F.; Abdel-Kader, M.S.; Ganaie, M.A.; Bedir, E.; Baykan, S.; Ozturk, B. Antihyperglycemic and antihyperlipidemic effects of Ferula duranii in experimental type 2 diabetic rats. Int. J. Pharmacol. 2015, 11, 532–541. [Google Scholar] [CrossRef]
- Serrano-Martín, X.; Payares, G.; Mendoza-León, A. Glibenclamide, a blocker of K+ ATP channels, shows antileishmanial activity in experimental murine cutaneous leishmaniasis. Antimicrob. Agents Chemother. 2006, 50, 4214–4216. [Google Scholar] [CrossRef]
- Sharma, N.; Kar, A. Combined effects Gymnema sylvestre and glibenclamide on alloxan induced diabetic mice. Int. J. Appl. Pharm. 2014, 6, 11–14. [Google Scholar]
- Lorenzo-Medina, M.; De-La-Iglesia, S.; Ropero, P.; Nogueira-Salgueiro, P.; Santana-Benitez, J. Effects of hemoglobin variants on hemoglobin a1c values measured using a high-performance liquid chromatography method. J. Diabetes Sci. Technol. 2014, 8, 1168–1176. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of medical care in diabetes--2014. Diabetes Care 2014, 37 (Suppl. S1), S14–S80. [Google Scholar] [CrossRef]
- Shah, N.A.; Khan, M.R. Antidiabetic effect of Sida cordata in alloxan induced diabetic rats. Biomed. Res. Int. 2014, 2014, 671294. [Google Scholar] [CrossRef]
- Mohammadi, H.; Abdelouahed, E.; Hassar, M.; Bouchrif, B.; Qarbal, B.; Dahbi, F.; Hilal, L.; Ghalim, N. Glycaemic control, HbA1c, and lipid profile in children with type 1 diabetes mellitus. Eur. J. Sci. Res. 2009, 29, 289–294. [Google Scholar]
- Manna, P.; Sinha, M.; Sil, P.C. Protective role of arjunolic acid in response to streptozotocin-induced type-I diabetes via the mitochondrial dependent and independent pathways. Toxicology 2009, 257, 53–63. [Google Scholar] [CrossRef]
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The impact of oxidative stress in human pathology: Focus on gastrointestinal disorders. Antioxidants 2021, 10, 201. [Google Scholar] [CrossRef]
- Rabbani, S.I.; Devi, K.; Khanam, S. Protective role of glibenclamide against nicotinamidestreptozotocin induced nuclear damage in diabetic Wistar rats. J. Pharmacol. Pharmacother. 2010, 1, 18–23. [Google Scholar] [CrossRef]
- Nakhaee, A.; Bokaeian, M.; Saravani, M.; Farhangi, A.; Akbarzadeh, A. Attenuation of oxidative stress in streptozotocin-induced diabetic rats by Eucalyptus globulus. Indian J. Clin. Biochem. 2009, 24, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, N.; Halifeoglu, I.; Ozercan, H.I.; Ilhan, N. Tissue malondialdehyde and adenosine triphosphatase level after experimental liver ischaemia–reperfusion damage. Cell Biochem. Funct. 2001, 19, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Saddala, R.R.; Thopireddy, L.; Ganapathi, N.; Kesireddy, S.R. Regulation of cardiac oxidative stress and lipid peroxidation in streptozotocin-induced diabetic rats treated with aqueous extract of Pimpinella tirupatiensis tuberous root. Exp. Toxicol. Pathol. 2013, 65, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Nugent, D.A.; Smith, D.M.; Jones, H.B. A review of islet of Langerhans degeneration in rodent models of type 2 diabetes. Toxicol. Pathol. 2008, 36, 529–551. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. Mechanisms for insulin resistance: Common threads and missing links. Cell 2012, 148, 852–871. [Google Scholar] [CrossRef]
- Abdel-Rahman, R.F.; Ezzat, S.M.; Ogaly, H.A.; Abd-Elsalam, R.M.; Hessin, A.F.; Fekry, M.I.; Mansour, D.F.; Mohamed, S.O. Ficus deltoidea extract down-regulates protein tyrosine phosphatase 1B expression in a rat model of type 2 diabetes mellitus: A new insight into its antidiabetic mechanism. J. Nutr. Sci. 2020, 9. [Google Scholar] [CrossRef]
- Gómez-Valadés, A.G.; Vidal-Alabró, A.; Molas, M.; Boada, J.; Bermúdez, J.; Bartrons, R.; Perales, J.C. Overcoming diabetes-induced hyperglycemia through inhibition of hepatic phosphoenolpyruvate carboxykinase (GTP) with RNAi. Mol. Ther. 2006, 13, 401–410. [Google Scholar] [CrossRef]
- Hutton, J.C.; O’Brien, R.M. Glucose-6-phosphatase catalytic subunit gene family. J. Biol. Chem. 2009, 284, 29241–29245. [Google Scholar] [CrossRef]
- Farsi, E.; Ahmad, M.; Hor, S.Y.; Ahamed, M.B.K.; Yam, M.F.; Asmawi, M.Z. Standardized extract of Ficus deltoidea stimulates insulin secretion and blocks hepatic glucose production by regulating the expression of glucose-metabolic genes in streptozitocin-induced diabetic rats. BMC Complement. Altern. Med. 2014, 14, 1–13. [Google Scholar] [CrossRef]
- Xia, X.; Yan, J.; Shen, Y.; Tang, K.; Yin, J.; Zhang, Y.; Yang, D.; Liang, H.; Ye, J.; Weng, J. Berberine improves glucose metabolism in diabetic rats by inhibition of hepatic gluconeogenesis. PLoS ONE 2011, 6, e16556. [Google Scholar] [CrossRef]
- Khan, W.; Parveen, R.; Chester, K.; Parveen, S.; Ahmad, S. Hypoglycemic potential of aqueous extract of Moringa oleifera leaf and in vivo GC-MS metabolomics. Front. Pharmacol. 2017, 8, 577. [Google Scholar] [CrossRef]
- Satyanarayana, S.; Krishnaiah, Y.S.R.; Eswar, K.K.; Injeti, E.R.; Kanthikiran, V.V.S. Influence of quinidine, selegiline and amphotericin-B on the pharmacokinetics and pharmacodynamics of tolbutamide in rabbits. Indian Drugs 1998, 35, 640. [Google Scholar]
- Drabkin, D.L.; Austin, J.H. Spectrophotometric studies: I. Spectrophotometric constants for common hemoglobin derivatives in human, dog, and rabbit blood. J. Biol. Chem. 1932, 98, 719–733. [Google Scholar] [CrossRef]
- Nayak, S.S.; Pattabiraman, T.N. A new colorimetric method for the estimation of glycosylated hemoglobin. Clin. Chim. Acta 1981, 109, 267–274. [Google Scholar] [CrossRef]
- Foster, L.B.; Dunn, R.T. Stable reagents for determination of serum triglycerides by a colorimetric Hantzsch condensation method. Clin. Chem. 1973, 19, 338–340. [Google Scholar] [CrossRef]
- Zlatkis, A.; Zak, B.; Boyle, A.J. A new method for the direct determination of serum cholesterol. J. Lab. Clin. Med. 1953, 41, 486–492. [Google Scholar]
- Burstein, M.; Scholnick, H.R.; Morfin, R. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J. Lipid Res. 1970, 11, 583–595. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Jain, S.K.; Levine, S.N.; Duett, J.; Hollier, B. Elevated lipid peroxidation levels in red blood cells of streptozotocin-treated diabetic rats. Metabolism 1990, 39, 971–975. [Google Scholar] [CrossRef]
- Alsharif, I.A.; Abd-Elsalam, R.M.; Amer, M.S.; El-Desoky, A.H.; Abdel-Rahman, R.F. Crataegus sinaica defatted methanolic extract ameliorated monosodium iodoacetate-induced oxidative stress and inhibited inflammation in a rat model of osteoarthritis. Res. Pharm. Sci. 2022, 17, 493–507. [Google Scholar]
- Althurwi, H.N.; Abdel-Rahman, R.F.; Soliman, G.A.; Ogaly, H.A.; Alkholifi, F.K.; Abd-Elsalam, R.M.; Alqasoumi, S.I.; Abdel-Kader, M.S. Protective Effect of Beta-Carotene against Myeloperoxidase-Mediated Oxidative Stress and Inflammation in Rat Ischemic Brain Injury. Antioxidants 2022, 11, 2344. [Google Scholar] [CrossRef]
- Abu-Elala, N.M.; Abd-Elsalam, R.M.; Marzouk, M.S. Molecular and immunohistochemical diagnosis of Photobacterium damselae subspecies piscicida during naturally occurring disease in Egypt. J. World Aquac. Soc. 2015, 46, 583–595. [Google Scholar] [CrossRef]
- Abdel-Rahman, R.F.; Soliman, G.A.; Saeedan, A.S.; Ogaly, H.A.; Abd-Elsalam, R.M.; Alqasoumi, S.I.; Abdel-Kader, M.S. Molecular and biochemical monitoring of the possible herb-drug interaction between Momordica charantia extract and glibenclamide in diabetic rats. Saudi Pharm. J. 2019, 27, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Ogaly, H.A.; Abdel-Rahman, R.F.; Mohamed, M.A.E.; Ahmed-Farid, O.A.; Khattab, M.S.; Abd-Elsalam, R.M. Thymol ameliorated neurotoxicity and cognitive deterioration in a thioacetamide-induced hepatic encephalopathy rat model; involvement of the BDNF/CREB signaling pathway. Food Funct. 2022, 13, 6180–6194. [Google Scholar] [CrossRef]
- Abdel-Rahman, R.F.; Fayed, H.M.; Ogaly, H.A.; Hussein, R.A.; Raslan, M.A. Phytoconstituents of Sansevieria suffruticosa NE Br. Leaves and its Hepatoprotective Effect via Activation of the NRF2/ARE Signaling Pathway in an Experimentally Induced Liver Fibrosis Rat Model. Chem. Biodivers. 2022, 19, e202100960. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]



| Groups | FBG (mg/dL) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | 2 Weeks | 4 Weeks | 8 Weeks | ||||
| M ± SEM | M ± SEM | % | M ± SEM | % | M ± SEM | % | |
| NC | 95.7 ± 4.92 | 98.5 ± 4.39 | −3.14 | 97.80 ± 4.25 | −2.47 | 97.8 ± 4.96 | −2.28 |
| DC | 344.2 ± 9.56 a | 361.5 ± 9.77 a | −5.08 | 362.7 ± 12.57 a | −5.31 | 314.0 ± 4.59 a | 8.40 |
| GLB | 344.3 ± 16.24 a | 189.0 ± 7.65 ab | 44.96 | 179.2 ± 10.77 ab | 48.12 | 138.3 ± 7.30 ab | 59.88 |
| VGN-10 | 340.3 ± 11.30 a | 194.5 ± 11.04 ab | 43.04 | 184.5 ± 4.36 ab | 45.68 | 146.8 ± 3.27 ab | 56.77 |
| VGN-20 | 345.5 ± 11.03 a | 170.8 ± 5.84 ab | 50.51 | 155.3 ± 6.24 ab | 55.10 | 117.5 ± 7.14 ab | 66.03 |
| GLB+VGN-10 | 361.7 ± 15.99 a | 189.2 ± 9.99 ab | 47.70 | 167.5 ± 6.01 ab | 53.60 | 128.7 ± 4.42 ab | 64.33 |
| GLB+VGN-20 | 349.8 ± 15.68 a | 160.7 ± 7.94 abc | 54.00 | 118.5 ± 4.96 abc | 66.08 | 94.7 ± 5.56 abc | 73.00 |
| Groups | Insulin (mIU/L) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | 2 Weeks | 4 Weeks | 8 Weeks | ||||
| M ± SEM | M ± SEM | % | M ± SEM | % | M ± SEM | % | |
| NC | 8.5 ± 0.47 | 8.6 ± 0.31 | −1.56 | 8.5 ± 0.35 | −0.22 | 8.6 ± 0.65 | −1.68 |
| DC | 3.4 ± 0.22 a | 3.2 ± 0.15 a | 4.85 | 3.3 ± 0.16 a | 4.61 | 3.1 ± 0.14 a | 4.63 |
| GLB | 3.5 ± 0.13 a | 3.9 ± 0.27 ab | −11.13 | 4.6 ± 0.26 ab | −33.38 | 5.1 ± 0.28 ab | −46.64 |
| VGN-10 | 3.3 ± 0.15 a | 3.6 ± 0.09 ab | −10.94 | 4.2 ± 0.19 ab | −27.95 | 4.6 ± 0.22 ab | −41.64 |
| VGN-20 | 3.4 ± 0.16 a | 4.3 ± 0.27 ab | −28.32 | 5.3 ± 0.25 ab | −56.78 | 5.9 ± 0.24 ab | −74.98 |
| GLB+VGN-10 | 3.4 ± 0.16 a | 4.1 ± 0.27 ab | −20.78 | 5.0 ± 0.24 ab | −49.11 | 5.7 ± 0.22 ab | −70.08 |
| GLB+VGN-20 | 3.6 ± 0.13 a | 4.7 ± 0.15 abc | −31.29 | 5.8 ± 0.17 abc | −62.28 | 7.1 ± 0.61 bc | −97.80 |
| Groups | Total Hemoglobin (g/dL) | HbA1c (%) |
|---|---|---|
| NC | 14.6 ± 0.71 | 4.9 ± 0.34 |
| DC | 9.9 ± 0.25 a | 9.0 ± 0.32 a |
| GLB | 11.8 ± 0.37 ab | 6.7 ± 0.30 ab |
| VGN-10 | 11.3 ± 0.32 ab | 7.1 ± 0.10 ab |
| VGN-20 | 12.6 ± 0.24 ab | 5.9 ± 0.21 ab |
| GLB+VGN-10 | 12.1 ± 0.44 ab | 6.3 ± 0.20 ab |
| GLB+VGN-20 | 13.5 ± 0.39 bc | 5.2 ± 0.27 bc |
| Groups | TG (mg/dL) | TC (mg/dL) | HDL-C (mg/dL) | LDL-C (mg/dL) |
|---|---|---|---|---|
| NC | 30.3 ± 1.14 | 43.3 ± 1.01 | 27.8 ± 1.05 | 14.6 ± 0.94 |
| DC | 56.3 ± 2.75 a | 72.0 ± 1.18 a | 16.6 ± 0.24 a | 36.9 ± 1.17 a |
| GLB | 41.4 ± 2.75 ab | 61.1 ± 1.71 ab | 19.5 ± 1.08 ab | 27.8 ± 1.21 ab |
| VGN-10 | 45.3 ± 1.18 ab | 64.5 ± 2.61 ab | 18.3 ± 0.71 ab | 29.9 ± 0.66 ab |
| VGN-20 | 34.8 ± 1.21 ab | 53.8 ± 2.99 ab | 22.6 ± 1.27 ab | 23.9 ± 1.51 ab |
| GLB+VGN-10 | 37.9 ± 0.94 ab | 57.6 ± 2.82 ab | 20.4 ± 1.45 ab | 26.8 ± 1.28 ab |
| GLB+VGN-20 | 27.9 ± 1.63 bc | 48.3 ± 2.32 bc | 25.6 ± 1.91 bc | 18.4 ± 1.65 bc |
| Groups | SOD (U/mg Protein) | GPx (U/mg Protein) | CAT (U/mg Protein) | MDA (nmol/g Tissue) |
|---|---|---|---|---|
| NC | 51.7 ± 2.43 | 8.7 ± 0.42 | 14.3 ± 0.98 | 30.2 ± 1.86 |
| DC | 26.7 ± 0.79 a | 4.8 ± 0.22 a | 5.8 ± 0.12 a | 54.9 ± 1.14 a |
| GLB | 31.4 ± 1.93 ab | 5.7 ± 0.33 ab | 7.2 ± 0.58 ab | 48.2 ± 2.76 ab |
| VGN-10 | 34.1 ± 2.14 ab | 6.3 ± 0.39 ab | 7.8 ± 0.39 ab | 45.4 ± 1.41 ab |
| VGN-20 | 39.7 ± 3.68 ab | 7.1 ± 0.55 ab | 9.1 ± 0.73 ab | 40.8 ± 2.89 ab |
| GLB+VGN-10 | 37.8 ± 2.26 ab | 6.5 ± 0.20 ab | 8.9 ± 0.68 ab | 43.6 ± 2.53 ab |
| GLB+VGN-20 | 46.7 ± 2.85 bc | 7.6 ± 0.30 bc | 12.1 ± 0.31 bc | 36.4 ± 2.62 bc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Althurwi, H.N.; Soliman, G.A.; Abdel-Rahman, R.F.; Abd-Elsalam, R.M.; Ogaly, H.A.; Alqarni, M.H.; Albaqami, F.F.; Abdel-Kader, M.S. Vulgarin, a Sesquiterpene Lactone from Artemisia judaica, Improves the Antidiabetic Effectiveness of Glibenclamide in Streptozotocin-Induced Diabetic Rats via Modulation of PEPCK and G6Pase Genes Expression. Int. J. Mol. Sci. 2022, 23, 15856. https://doi.org/10.3390/ijms232415856
Althurwi HN, Soliman GA, Abdel-Rahman RF, Abd-Elsalam RM, Ogaly HA, Alqarni MH, Albaqami FF, Abdel-Kader MS. Vulgarin, a Sesquiterpene Lactone from Artemisia judaica, Improves the Antidiabetic Effectiveness of Glibenclamide in Streptozotocin-Induced Diabetic Rats via Modulation of PEPCK and G6Pase Genes Expression. International Journal of Molecular Sciences. 2022; 23(24):15856. https://doi.org/10.3390/ijms232415856
Chicago/Turabian StyleAlthurwi, Hassan N., Gamal A. Soliman, Rehab F. Abdel-Rahman, Reham M. Abd-Elsalam, Hanan A. Ogaly, Mohammed H. Alqarni, Faisal F. Albaqami, and Maged S. Abdel-Kader. 2022. "Vulgarin, a Sesquiterpene Lactone from Artemisia judaica, Improves the Antidiabetic Effectiveness of Glibenclamide in Streptozotocin-Induced Diabetic Rats via Modulation of PEPCK and G6Pase Genes Expression" International Journal of Molecular Sciences 23, no. 24: 15856. https://doi.org/10.3390/ijms232415856
APA StyleAlthurwi, H. N., Soliman, G. A., Abdel-Rahman, R. F., Abd-Elsalam, R. M., Ogaly, H. A., Alqarni, M. H., Albaqami, F. F., & Abdel-Kader, M. S. (2022). Vulgarin, a Sesquiterpene Lactone from Artemisia judaica, Improves the Antidiabetic Effectiveness of Glibenclamide in Streptozotocin-Induced Diabetic Rats via Modulation of PEPCK and G6Pase Genes Expression. International Journal of Molecular Sciences, 23(24), 15856. https://doi.org/10.3390/ijms232415856








