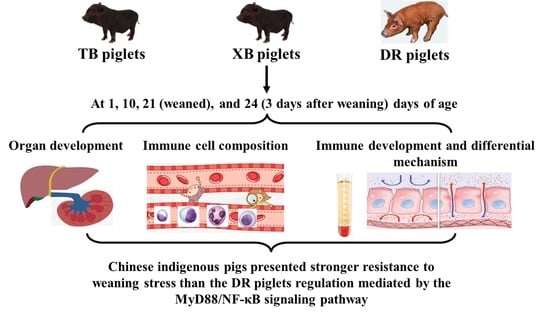
Developmental Changes of Immunity and Different Responses to Weaning Stress of Chinese Indigenous Piglets and Duroc Piglets during Suckling and Weaning Periods
Abstract
1. Introduction
2. Results
2.1. Changes in Liver and Spleen Indexes of Different Pig Breeds during Suckling and Weaning Periods
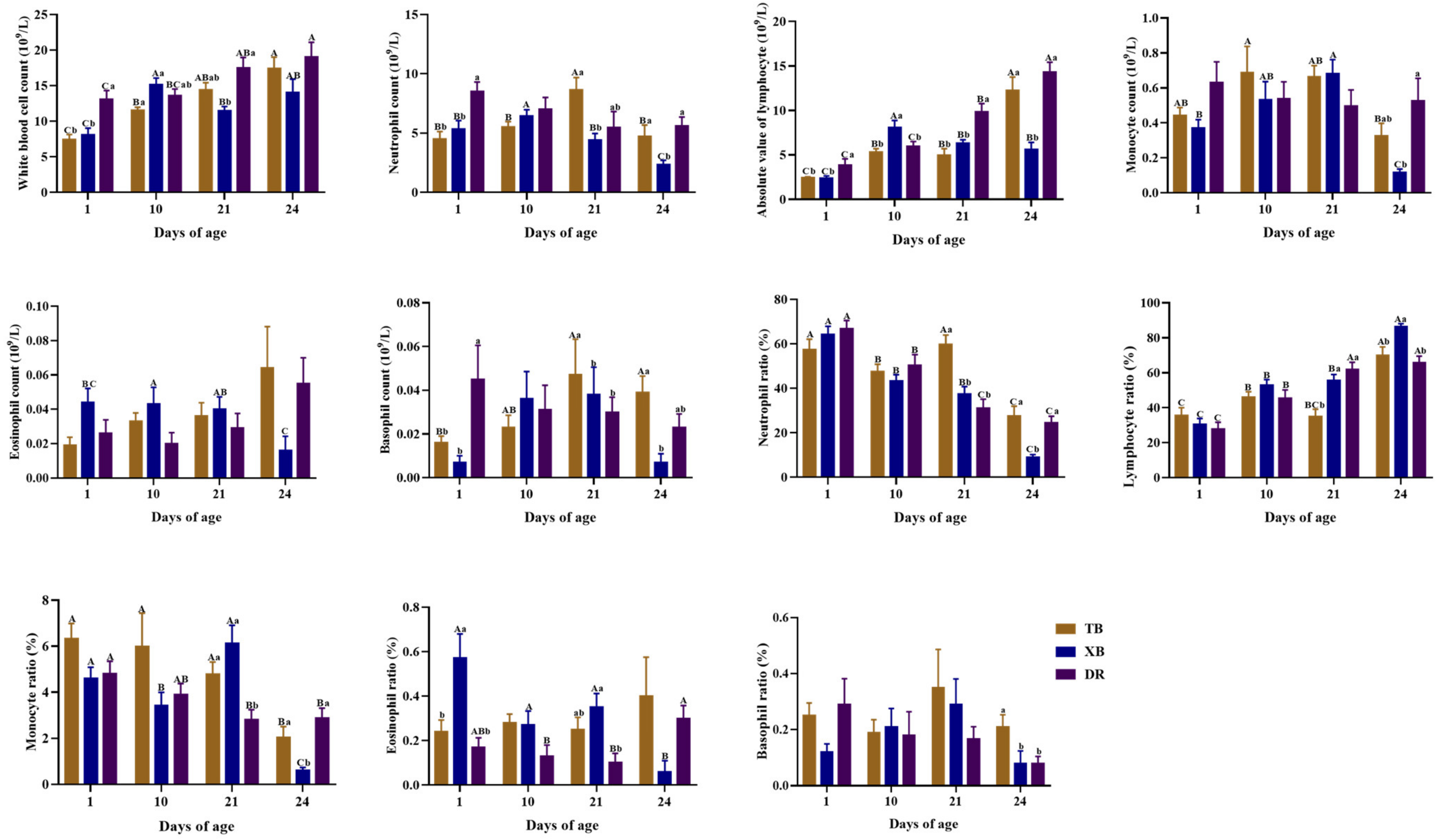
2.2. Changes in Hematological Parameters of Different Pig Breeds during Suckling and Weaning Periods
2.3. Changes in Plasma and Ileal Immunoglobulin Levels of Different Pig Breeds during Suckling and Weaning Periods
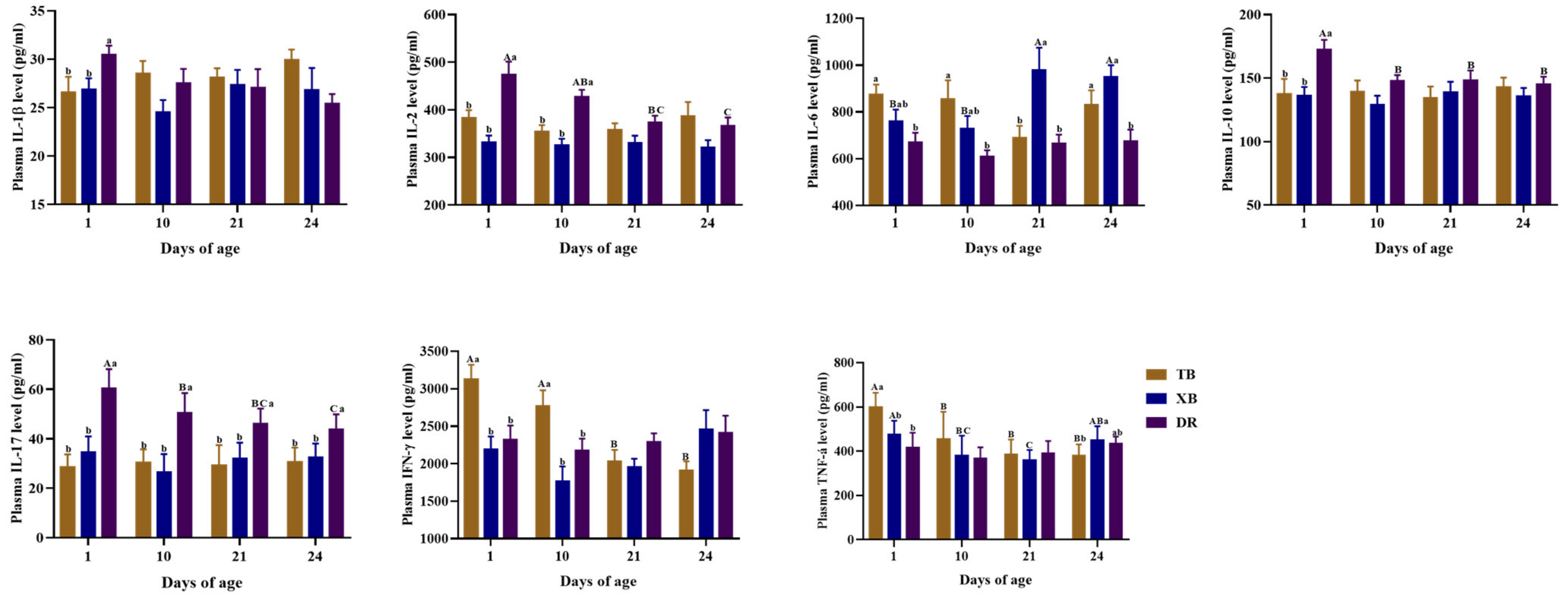
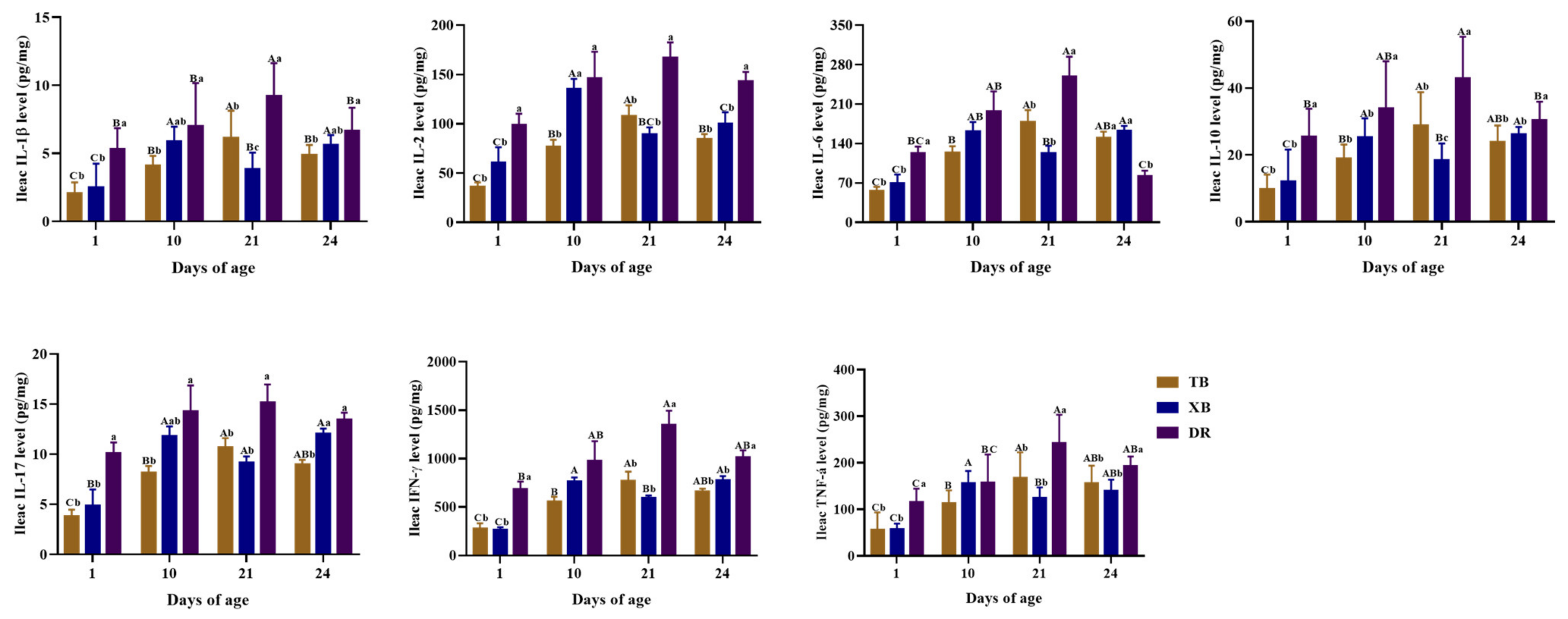
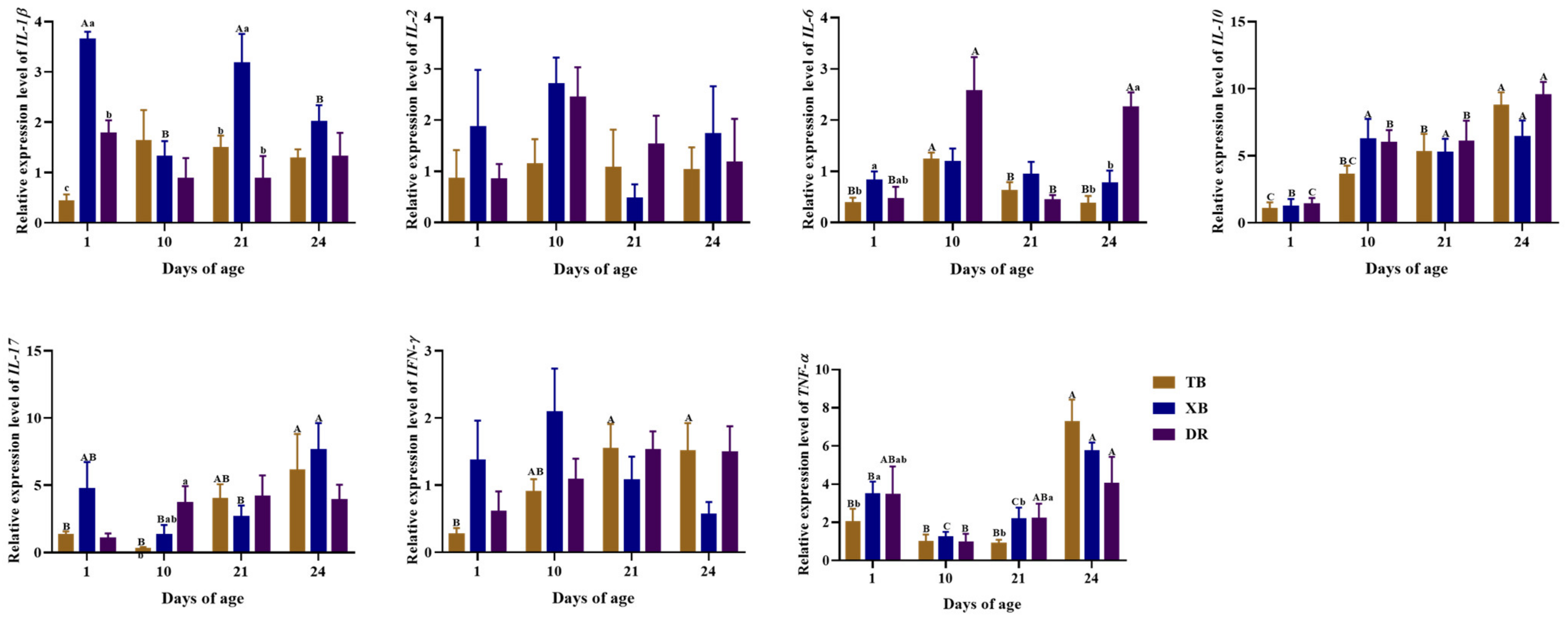
2.4. Changes in Plasma and Ileal Immuno-Cytokine Levels of Different Pig Breeds during Suckling and Weaning Periods
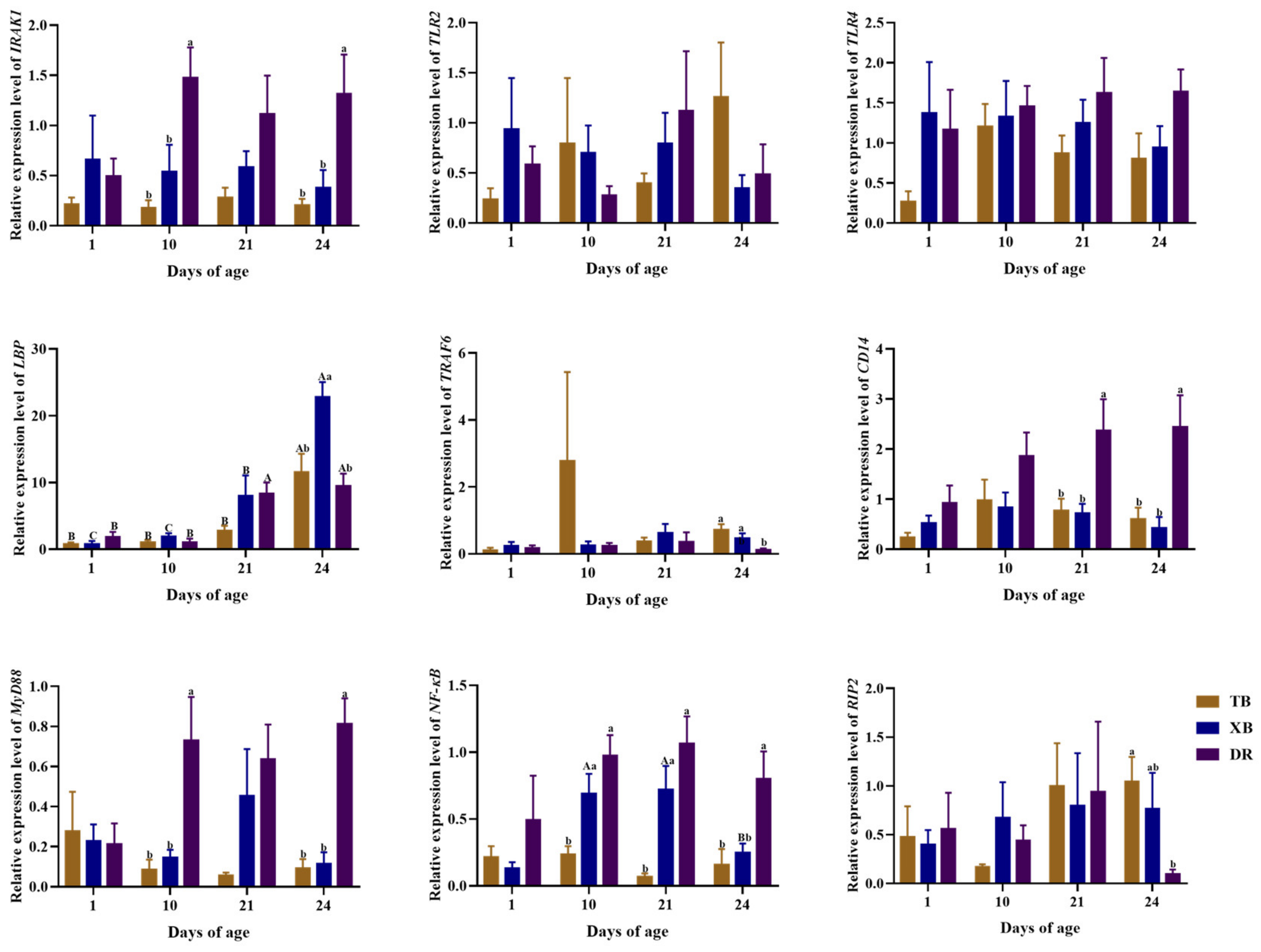
2.5. Changes in Ileal Gene Expressions Related to Immune Function of Different Pig Breeds during Suckling and Weaning Periods
3. Discussion
4. Materials and Methods
4.1. Animals and Study Design
4.2. Sample Collection
4.3. Analysis of Visceral Organ Index
4.4. Analysis of Blood Hematological Parameters
4.5. Analysis of Plasma and Ileal Immunoglobulin and Immuno-Cytokine Contents
4.6. Analysis of Immune-Related Gene Expressions in the Ileum
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morris, N.L.; Choudhry, M.A. Maintenance of gut barrier integrity after injury: Trust your gut microRNAs. J. Leukoc. Biol. 2021, 110, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Levast, B.; Berri, M.; Wilson, H.L.; Meurens, F.; Salmon, H. Development of gut immunoglobulin a production in piglet in response to innate and environmental factors. Dev. Comp. Immunol. 2014, 44, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Bæk, O.; Skadborg, K.; Muk, T.; Amdi, C.; Heegaard, P.M.H.; Thymann, T.; Nguyen, D.N. Infant formula based on milk fat affects immune development in both normal birthweight and fetal growth restricted neonatal piglets. Nutrients 2021, 13, 3310. [Google Scholar] [CrossRef] [PubMed]
- Rothkötter, H.J.; Sowa, E.; Pabst, R. The pig as a model of developmental immunology. Hum. Exp. Toxicol. 2002, 21, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Lallès, J.P.; Bosi, P.; Smidt, H.; Stokes, C.R. Nutritional management of gut health in pigs around weaning. Proc. Nutr. Soc. 2007, 66, 260–268. [Google Scholar] [CrossRef]
- Bailey, M.; Haverson, K.; Inman, C.; Harris, C.; Jones, P.; Corfield, G.; Miller, B.; Stokes, C. The development of the mucosal immune system pre- and post-weaning: Balancing regulatory and effector function. Proc. Nutr. Soc. 2005, 64, 451–457. [Google Scholar] [CrossRef]
- Guevarra, R.B.; Lee, J.H.; Lee, S.H.; Seok, M.J.; Kim, D.W.; Kang, B.N.; Johnson, T.J.; Isaacson, R.E.; Kim, H.B. Piglet gut microbial shifts early in life: Causes and effects. J. Anim. Sci. Biotechnol. 2019, 10, 1. [Google Scholar] [CrossRef]
- Pluske, J.R.; Turpin, D.L.; Kim, J.C. Gastrointestinal tract (gut) health in the young pig. Anim. Nutr. 2018, 4, 187–196. [Google Scholar] [CrossRef]
- Cao, S.; Hou, L.; Sun, L.; Gao, J.; Gao, K.; Yang, X.; Jiang, Z.; Wang, L. Intestinal morphology and immune profiles are altered in piglets by early-weaning. Int. Immunopharmacol. 2022, 105, 1567–1570. [Google Scholar] [CrossRef]
- Wang, L.; Yan, S.; Li, J.; Li, Y.; Ding, X.; Yin, J.; Xiong, X.; Yin, Y.; Yang, H. Rapid Communication: The relationship of enterocyte proliferation with intestinal morphology and nutrient digestibility in weaning piglets. J. Anim. Sci. 2019, 97, 353–358. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Kim, I.H. The impact of weaning stress on gut health and the mechanistic aspects of several feed additives contributing to improved gut health function in weanling piglets—A review. Animals 2021, 11, 2418. [Google Scholar] [CrossRef] [PubMed]
- Andek-Potokar, M.; Nieto, R. European Local Pig Breeds—Diversity and Performance a Study of Project Treasure; Intech Open: Rijeka, Croatia, 2019; pp. 1–303. [Google Scholar]
- Xu, D.; Chai, Y.L.; Jiang, J.; He, C.Q.; He, J.; Ma, H.M. The complete mitochondrial genome of the Taoyuan black pig. Mitochondrial DNA 2015, 26, 779–780. [Google Scholar] [CrossRef] [PubMed]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut microbiota dysbiosis in postweaning piglets: Understanding the keys to health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Ingale, S.L.; Kim, J.S.; Kim, K.H.; Lohakare, J.; Park, Y.K.; Park, J.C.; Kwon, I.K.; Chae, B.J. Effects of dietary supplementation with antimicrobial peptide-P5 on growth performance, apparent total tract digestibility, faecal and intestinal microflora and intestinal morphology of weanling pigs. J. Sci. Food Agric. 2013, 93, 587–592. [Google Scholar] [CrossRef]
- Pomorska-Mól, M.; Markowska-Daniel, I. Immunological status of piglets during the first weeks of life. Med. Weter. 2010, 66, 593–596. [Google Scholar]
- Martínez-Boixaderas, N.; Garza-Moreno, L.; Sibila, M.; Segalés, J. Impact of maternally derived immunity on immune responses elicited by piglet early vaccination against the most common pathogens involved in porcine respiratory disease complex. Porc. Health Manag. 2022, 8, 11–24. [Google Scholar] [CrossRef]
- Shuster, A.; Patlas, M.; Pinthus, J.H.; Mourtzakis, M. The clinical importance of visceral adiposity: A critical review of methods for visceral adipose tissue analysis. Br. J. Radiol. 2012, 85, 1–10. [Google Scholar] [CrossRef]
- Lu, T.; Harper, A.F.; Zhao, J.; Estienne, M.J.; Dalloul, R.A. Supplementing antioxidants to pigs fed diets high in oxidants: I. Effects on growth performance, liver function, and oxidative status. J. Anim. Sci. 2014, 92, 5455–5463. [Google Scholar] [CrossRef]
- Lewis, S.M.; Williams, A.; Eisenbarth, S.C. Structure and function of the immune system in the spleen. Sci. Immunol. 2019, 4, eaau6085. [Google Scholar] [CrossRef]
- Wang, X.; Huang, X.; Yang, Q.; Yan, Z.; Wang, P.; Gao, X.; Luo, R.; Gun, S. TMT labeled comparative proteomic analysis reveals spleen active immune responses during Clostridium perfringens type C infected piglet diarrhea. PeerJ 2022, 10, e13006. [Google Scholar] [CrossRef]
- Chaussabel, D. Assessment of immune status using blood transcriptomics and potential implications for global health. Semin. Immunol. 2015, 27, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Weller, P.F.; Spencer, L.A. Functions of tissue-resident eosinophils. Nat. Rev. Immunol. 2017, 17, 746–760. [Google Scholar] [CrossRef] [PubMed]
- Yamanishi, Y.; Miyake, K.; Iki, M.; Tsutsui, H.; Karasuyama, H. Recent advances in understanding basophil-mediated Th2 immune responses. Immunol. Rev. 2017, 278, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Shahid, S. Review of hematological indices of cancer patients receiving combined chemotherapy & radiotherapy or receiving radiotherapy alone. Crit. Rev. Oncol. Hematol. 2016, 105, 145–155. [Google Scholar] [PubMed]
- Butler, J.E.; Lager, K.M.; Splichal, I.; Francis, D.; Kacskovics, I.; Sinkora, M.; Wertz, N.; Sun, J.; Zhao, Y.; Brown, W.R.; et al. The piglet as a model for B cell and immune system development. Vet. Immunol. Immunopathol. 2009, 128, 147–170. [Google Scholar] [CrossRef]
- Xu, R.J.; Sangild, P.T.; Zhang, Y.Q.; Zhang, S.H. Bioactive compounds in porcine colostrum and milk and their effects on intestinal development in neonatal pigs. In Biology of Growing Animals; Zabielski, R., Gregory, P.C., Weström, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2002; Volume 1, pp. 169–192. [Google Scholar]
- Duizer, E.; Gilde, A.J.; Versantvoort, C.H.; Groten, J.P. Effects of cadmium chloride on the paracellular barrier function of intestinal epithelial cell lines. Toxicol. Appl. Pharmacol. 1999, 155, 117–126. [Google Scholar] [CrossRef]
- Sutherland, M.A.; Rodriguez-Zas, S.L.; Ellis, M.; Salak-Johnson, J.L. Breed and age affect baseline immune traits, cortisol and performance in growing pigs. J. Anim. Sci. 2005, 83, 2087–2095. [Google Scholar] [CrossRef]
- Bourges, D.; Meurens, F.; Berri, M.; Chevaleyre, C.; Zanello, G.; Levast, B.; Melo, S.; Gerdts, V.; Salmon, H. New insights into the dual recruitment of IgA+ B cells in the developing mammary gland. Mol. Immunol. 2008, 45, 3354–3362. [Google Scholar] [CrossRef]
- Mehandru, S.; Colombel, J.F. The intestinal barrier, an arbitrator turned provocateur in IBD. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 83–84. [Google Scholar] [CrossRef]
- Jiang, G.; Liu, Y.; Oso, A.; Li, F.; Kong, X.; Ying, Y. The differences of bacteria and bacteria metabolites in the colon between fatty and lean pigs. J. Anim. Sci. 2016, 94, 349–353. [Google Scholar] [CrossRef]
- Chen, K.; Magri, G.; Grasset, E.K.; Cerutti, A. Rethinking mucosal antibody responses: IgM, IgG and IgD join IgA. Nat. Rev. Immunol. 2020, 20, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.; Wang, H.; Luo, J.; Xie, M.; Zhao, Z.; Chen, X.; Wang, D.; Sun, J.; Xi, Q.; Chen, T.; et al. Porcine milk-derived small extracellular vesicles promote intestinal immunoglobulin production through pIgR. Animals 2021, 11, 1522. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.E.; Weber, P.; Sinkora, M.; Baker, D.; Schoenherr, A.; Mayer, B.; Francis, D. Antibody repertoire development in fetal and neonatal piglets. VIII. Colonization is required for newborn piglets to make serum antibodies to T-dependent and type 2 T-independent antigens. J. Immunol. 2002, 169, 6822–6830. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T. Possible factors influencing immunoglobulin A concentration in swine colostrum. Am. J. Vet. Res. 1981, 42, 533–536. [Google Scholar] [PubMed]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 2014, 1843, 2563–2582. [Google Scholar] [CrossRef]
- Sun, S.C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, Z.F.; Xu, Y.M.; Li, Y.S.; Song, J.G. N-Butylphthalide (NBP) ameliorated cerebral ischemia reperfusion-induced brain injury via HGF-regulated TLR4/NF-κB signaling pathway. Biomed. Pharmacother. 2016, 83, 658–666. [Google Scholar] [CrossRef]
- Gan, L.; Qin, W.; Wu, S.; Wu, S.; Bao, W. Spatiotemporal expression of MYD88 gene in pigs from birth to adulthood. Genet. Mol. Biol. 2018, 41, 119–124. [Google Scholar] [CrossRef]
- Ma, T.Y.; Iwamoto, G.K.; Hoa, N.T.; Akotia, V.; Pedram, A.; Boivin, M.A.; Said, H.M. TNF-α-induced increase in intestinal epithelial tight junction permeability requires NF-κB activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G367–G376. [Google Scholar] [CrossRef]
| Items | TB | XB | DR | SEM | p Values |
|---|---|---|---|---|---|
| Liver index (g/kg) | |||||
| 1 day of age | 21.62 C | 25.83 | 32.20 | 3.42 | 0.108 |
| 10 days of age | 31.21 Aa | 27.41 b | 25.90 b | 0.84 | <0.001 |
| 21 days of age | 25.98 Bab | 27.37 a | 23.96 b | 0.89 | 0.039 |
| 24 days of age | 25.64 B | 26.59 | 26.85 | 0.92 | 0.625 |
| SEM | 0.81 | 0.97 | 2.99 | ||
| p values | <0.001 | 0.622 | 0.261 | ||
| Spleen index (g/kg) | |||||
| 1 day of age | 1.24 B | 1.55 B | 2.28 | 0.38 | 0.337 |
| 10 days of age | 2.10 A | 2.17 A | 1.95 | 0.15 | 0.567 |
| 21 days of age | 2.04 A | 2.18 A | 2.08 | 0.20 | 0.872 |
| 24 days of age | 1.87 A | 1.75 AB | 1.93 | 0.13 | 0.610 |
| SEM | 0.12 | 0.18 | 0.33 | ||
| p values | <0.001 | 0.035 | 0.868 |
| Items (μg/mL) | TB | XB | DR | SEM | p Values |
|---|---|---|---|---|---|
| IgA | |||||
| 1 day of age | 25.56 a | 25.88 ABa | 17.59 Bb | 1.43 | <0.001 |
| 10 days of age | 24.30 a | 22.93 Ba | 18.84 Bb | 1.03 | 0.002 |
| 21 days of age | 24.71 a | 27.31 Aa | 17.56 Bb | 1.05 | <0.001 |
| 24 days of age | 23.75 b | 28.05 Aa | 23.00 Ab | 1.37 | 0.031 |
| SEM | 1.05 | 1.19 | 1.44 | ||
| p values | 0.667 | 0.022 | 0.035 | ||
| IgG | |||||
| 1 day of age | 323.29 a | 207.00 Bb | 340.81 a | 17.11 | <0.001 |
| 10 days of age | 322.48 a | 221.64 Bb | 322.61 a | 9.95 | <0.001 |
| 21 days of age | 311.81 a | 206.66 Bb | 333.64 a | 10.30 | <0.001 |
| 24 days of age | 324.95 a | 270.43 Ab | 321.69 a | 15.71 | 0.035 |
| SEM | 9.71 | 16.85 | 13.43 | ||
| p values | 0.769 | 0.035 | 0.706 | ||
| IgM | |||||
| 1 day of age | 33.33 Aab | 29.32 b | 34.41 Aa | 1.43 | 0.043 |
| 10 days of age | 32.00 AB | 28.63 | 30.03 B | 1.25 | 0.179 |
| 21 days of age | 28.26 B | 31.61 | 29.26 B | 1.06 | 0.090 |
| 24 days of age | 28.66 B | 30.55 | 29.07 B | 1.13 | 0.473 |
| SEM | 1.37 | 1.30 | 0.97 | ||
| p values | 0.030 | 0.390 | 0.001 |
| Items (μg/mg) | TB | XB | DR | SEM | p Values |
|---|---|---|---|---|---|
| IgA | |||||
| 1 day of age | 1.88 Cb | 2.91 Bab | 3.88 Ba | 0.45 | 0.014 |
| 10 days of age | 4.39 B | 5.73 A | 6.88 A | 0.97 | 0.212 |
| 21 days of age | 6.08 A | 5.19 A | 6.67 A | 0.67 | 0.298 |
| 24 days of age | 4.89 ABb | 6.30 Aab | 8.13 Aa | 0.64 | 0.005 |
| SEM | 0.56 | 0.59 | 0.92 | ||
| p values | <0.001 | 0.001 | 0.018 | ||
| IgG | |||||
| 1 day of age | 19.42 Cb | 25.64 Cb | 65.13 a | 7.81 | 0.001 |
| 10 days of age | 46.65 B | 66.57 A | 69.93 | 9.81 | 0.212 |
| 21 days of age | 61.37 ABb | 46.81 Bb | 98.91 a | 8.36 | 0.001 |
| 24 days of age | 68.42 A | 59.14 AB | 88.10 | 8.10 | 0.051 |
| SEM | 6.94 | 6.01 | 11.63 | ||
| p values | <0.001 | <0.001 | 0.158 | ||
| IgM | |||||
| 1 day of age | 1.74 Cb | 1.91 Cb | 5.81 Ba | 0.51 | <0.001 |
| 10 days of age | 3.94 Bb | 5.54 Ab | 8.27 ABa | 0.89 | 0.007 |
| 21 days of age | 5.67 Ab | 4.14 Bb | 11.48 Aa | 1.04 | <0.001 |
| 24 days of age | 4.94 ABc | 6.14 Ab | 7.80 Ba | 0.38 | <0.001 |
| SEM | 0.49 | 0.37 | 1.15 | ||
| p values | <0.001 | <0.001 | 0.013 | ||
| sIgA | |||||
| 1 day of age | 1.41 Cb | 1.82 Cb | 5.01 a | 0.45 | <0.001 |
| 10 days of age | 2.52 Bb | 5.45 Aa | 5.19 a | 0.57 | 0.002 |
| 21 days of age | 3.41 Ab | 3.73 Bb | 6.98 a | 0.57 | <0.001 |
| 24 days of age | 4.10 Ab | 5.87 Aa | 4.73 ab | 0.41 | 0.016 |
| SEM | 0.30 | 0.43 | 0.69 | ||
| p values | <0.001 | <0.001 | 0.109 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, S.; Cheng, Y.; Azad, M.A.K.; Zhu, Q.; Huang, P.; Kong, X. Developmental Changes of Immunity and Different Responses to Weaning Stress of Chinese Indigenous Piglets and Duroc Piglets during Suckling and Weaning Periods. Int. J. Mol. Sci. 2022, 23, 15781. https://doi.org/10.3390/ijms232415781
Ding S, Cheng Y, Azad MAK, Zhu Q, Huang P, Kong X. Developmental Changes of Immunity and Different Responses to Weaning Stress of Chinese Indigenous Piglets and Duroc Piglets during Suckling and Weaning Periods. International Journal of Molecular Sciences. 2022; 23(24):15781. https://doi.org/10.3390/ijms232415781
Chicago/Turabian StyleDing, Sujuan, Yating Cheng, Md. Abul Kalam Azad, Qian Zhu, Pan Huang, and Xiangfeng Kong. 2022. "Developmental Changes of Immunity and Different Responses to Weaning Stress of Chinese Indigenous Piglets and Duroc Piglets during Suckling and Weaning Periods" International Journal of Molecular Sciences 23, no. 24: 15781. https://doi.org/10.3390/ijms232415781
APA StyleDing, S., Cheng, Y., Azad, M. A. K., Zhu, Q., Huang, P., & Kong, X. (2022). Developmental Changes of Immunity and Different Responses to Weaning Stress of Chinese Indigenous Piglets and Duroc Piglets during Suckling and Weaning Periods. International Journal of Molecular Sciences, 23(24), 15781. https://doi.org/10.3390/ijms232415781