Yeast Protein Asf1 Possesses Modulating Activity towards Protein Kinase CK2
Abstract
1. Introduction
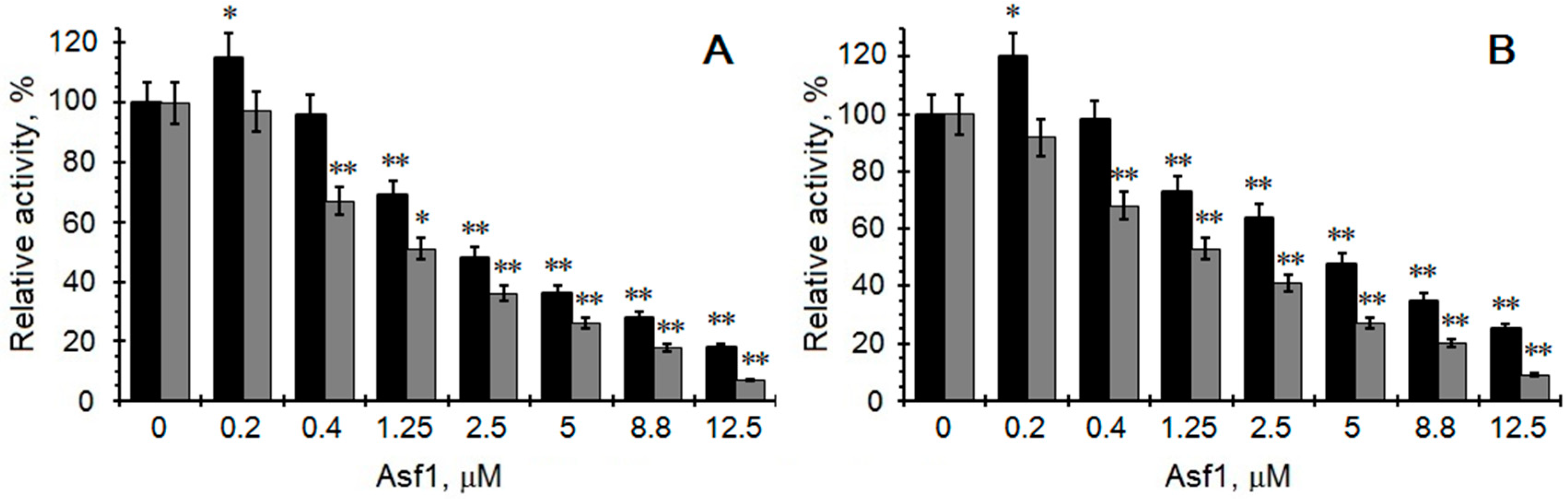
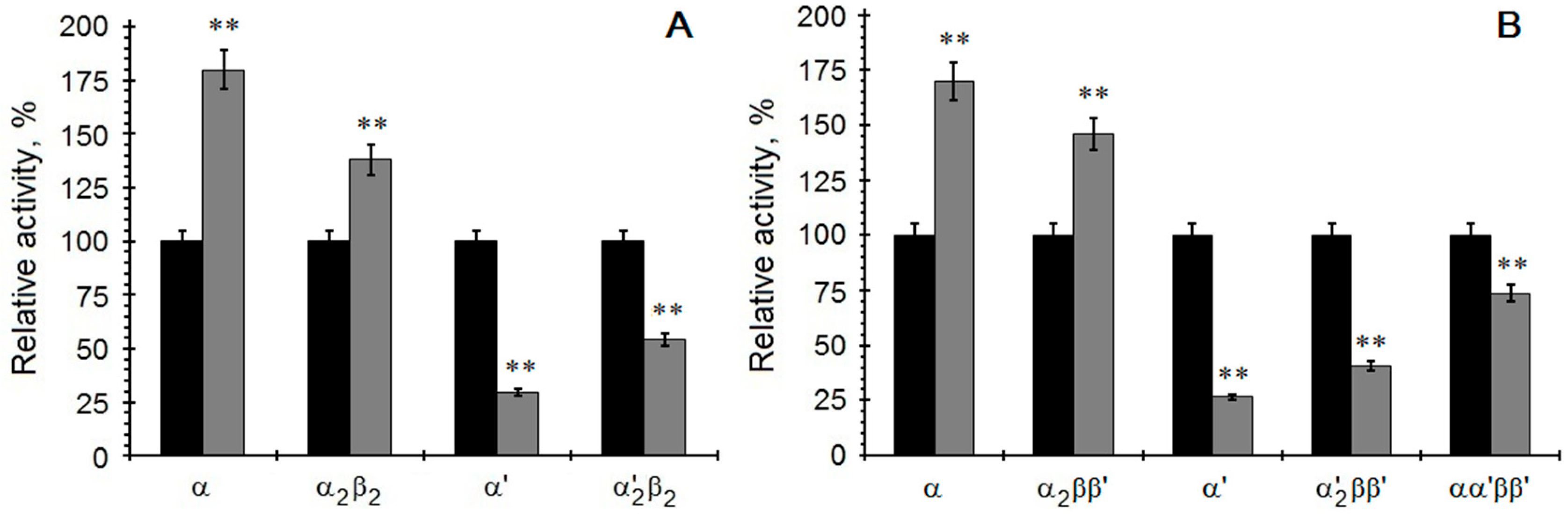
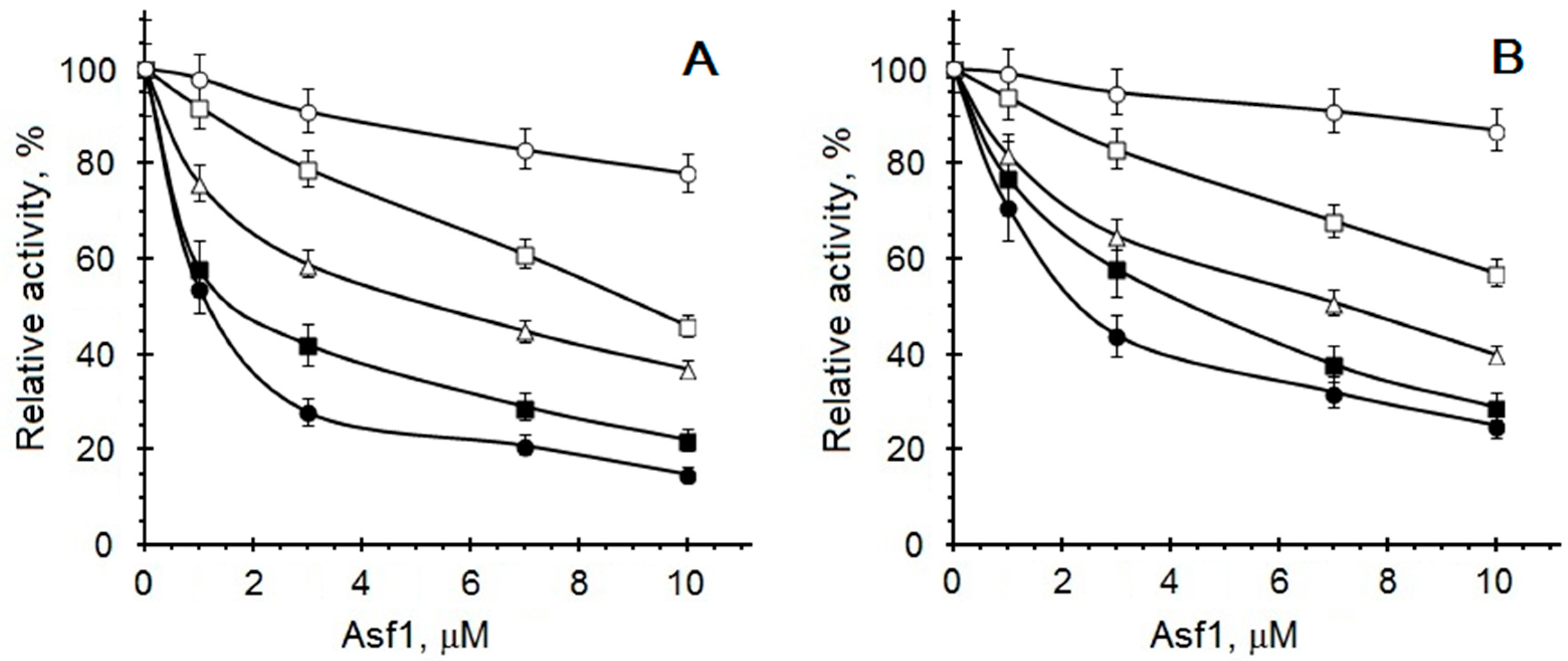
2. Results
3. Materials and Methods
3.1. Materials
3.2. Purification of Yeast CK2
3.3. Overexpression and Purification of Human CK2α and CK2α’ Subunits
3.4. Purification of Human CK2 Holoenzymes
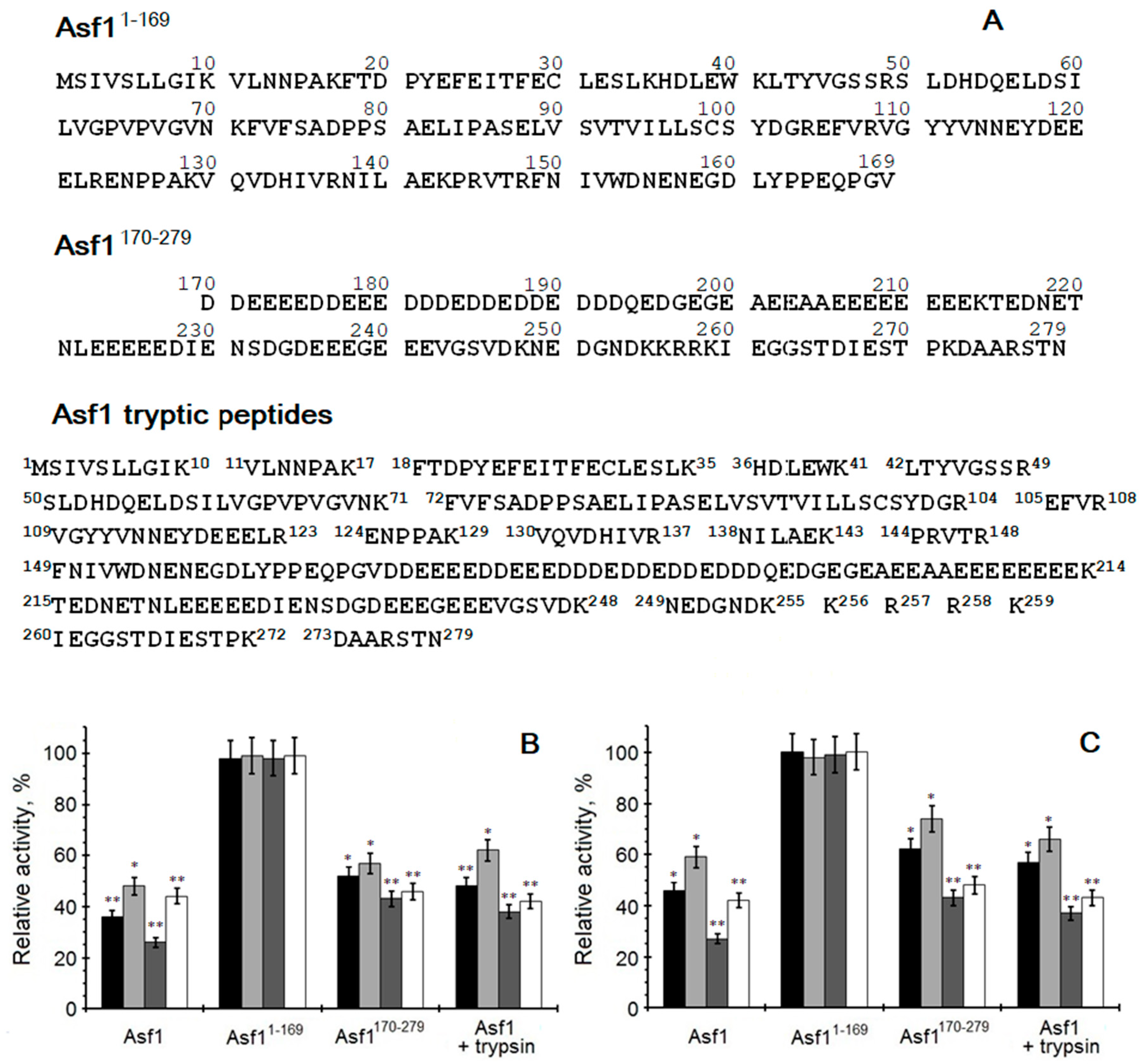
3.5. Cloning of the Asf1, Asf11-169, and Asf1170-279 Proteins into Bacterial Expression Vector pET28a
3.6. Cloning of Asf1 Protein and the C-Terminal Fragment Asf1170-279 into Yeast Expression Vector pYES2/CT
3.7. Overexpression and Purification of Asf1, Asf11-169, and Asf1170-279 Proteins
3.8. Protein Substrates
3.9. Protein Phosphorylation
3.10. Asf1 Digestion by Trypsin
3.11. In Vivo Analysis
3.12. Western Blot Analysis
3.13. Statistical Analysis and Management Data
3.14. CABS-Dock Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Litchfield, D.W. Protein kinase CK2: Structure, regulation and role in cellular decisions of life and death. Biochem. J. 2003, 369, 1–15. [Google Scholar] [CrossRef]
- Faust, M.; Montenarh, M. Subcellular localization of protein kinase CK2. A key to its function? Cell Tissue Res. 2000, 301, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Meggio, F.; Pinna, L.A. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 2003, 17, 349–368. [Google Scholar] [CrossRef]
- Donella-Deana, A.; Cesaro, L.; Sarno, S.; Brunatti, A.M.; Ruzzene, M.; Pinna, L.A. Autocatalytic tyrosine-phosphorylation of protein kinase CK2α and α’ subunits: Implication of Tyr182. Biochem. J. 2001, 357, 563–567. [Google Scholar] [CrossRef]
- Vilk, G.; Weber, J.E.; Turowec, J.P.; Duncan, J.S.; Wu, C.; Derksen, D.R.; Zień, P.; Sarno, S.; Donella-Deana, A.; Lajoie, G.; et al. Protein kinase CK2 catalyzes tyrosine phosphorylation in mammalian cells. Cell Signal. 2008, 20, 1942–1951. [Google Scholar] [CrossRef]
- Salvi, M.; Sarno, S.; Cesaro, L.; Nakamura, H.; Pinna, L.A. Extraordinary pleiotropy of protein kinase CK2 revealed by weblogo phosphoproteome analysis. Biochim. Biophys. Acta 2009, 1793, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Gowda, C.; Song, C.; Kapadia, M.; Payne, J.L.; Hu, T.; Ding, Y.; Dovat, S. Regulation of cellular proliferation in acute lymphoblastic leukemia by Casein Kinase II (CK2) and Ikaros. Adv. Biol. Regul. 2017, 63, 71–80. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Akashi, M.; Matsuda, M.; Goto, K.; Miyata, Y.; Node, K.; Nishida, E. Involvement of the protein kinase CK2 in the regulation of mammalian circadian rhythms. Sci. Signal. 2009, 2, ra26. [Google Scholar] [CrossRef]
- Duncan, J.S.; Turowec, J.P.; Vilk, G.; Li, S.S.; Gloor, G.B.; Litchfield, D.W. Regulation of cell proliferation and survival: Convergence of protein kinases and caspases. Biochim. Biophys. Acta 2010, 1804, 505–510. [Google Scholar] [CrossRef]
- Dominguez, I.; Degano, I.R.; Chea, K.; Cha, J.; Toselli, P.; Seldin, D.C. CK2α is essential for embryonic morphogenesis. Mol. Cell. Biochem. 2011, 356, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Homma, M.K.; Homma, Y. Cell cycle and activation of CK2. Mol. Cell. Biochem. 2008, 316, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.S.; Litchfield, D.W. Too much a good thing: The role of protein kinase CK2 in tumorigenesis and prospects for therapeutic inhibition. Biochim. Biophys. Acta 2008, 1784, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Trembley, J.H.; Chen, Z.; Unger, G.; Slaton, J.; Van Waes, C.; Ahmed, K. Emergence of protein kinase CK2 as a key target in cancer therapy. BioFactors 2010, 36, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Ortega, C.E.; Seidner, Y.; Dominguez, I. Mining CK2 in cancer. PLoS ONE 2014, 9, e115609. [Google Scholar] [CrossRef]
- Firnau, M.B.; Brieger, A. CK2 and the Hallmarks of Cancer. Biomedicines 2022, 10, 1987. [Google Scholar] [CrossRef]
- Hanif, I.M.; Hanif, I.M.; Shazib, I.A.; Pervaiz, S. Casein kinase II: An attractive target for anti-cancer drug design. Int. J. Biochem. Cell Biol. 2010, 42, 1602–1605. [Google Scholar] [CrossRef]
- Battistutta, R. Structural bases of protein kinase CK2 inhibition. Cell. Mol. Life Sci. 2009, 66, 1868–1889. [Google Scholar] [CrossRef]
- Spinello, Z.; Fregnani, A.; Quotti Tubi, L.; Trentin, L.; Piazza, F.; Manni, S. Targeting Protein Kinases in Blood Cancer: Focusing on CK1α and CK2. Int. J. Mol. Sci. 2021, 22, 3716. [Google Scholar] [CrossRef]
- Roffey, S.E.; Litchfield, D.W. CK2 Regulation: Perspectives in 2021. Biomedicines 2021, 9, 1361. [Google Scholar] [CrossRef]
- Baier, A.; Szyszka, R. CK2 and protein kinases of the CK1 superfamily as target for neurodegenerative disorders. Front. Mol. Biosci. 2022, 9, 916063. [Google Scholar] [CrossRef]
- Cochet, C.; Chambaz, E.M. Oligomeric structure and catalytic activity of G-type casein kinase. Isolation of the two subunits and renaturation experiments. J. Biol. Chem. 1983, 258, 1403–1406. [Google Scholar] [CrossRef] [PubMed]
- Filhol, O.; Nueda, A.; Martel, V.; Gerber-Scokaert, D.; Benitez, M.J.; Souchier, C.; Saoudi, Y.; Cochet, C. Live-cell fluorescence imaging reveals the dynamics of protein kinase CK2 subunits. Mol. Cell. Biol. 2003, 23, 975–987. [Google Scholar] [CrossRef] [PubMed]
- St. Denis, N.A.; Litchfield, D.W. Protein kinase CK2 in health and disease: From birth to death: The role of protein kinase CK2 in the regulation of cell proliferation and survival. Cell. Mol. Life Sci. 2009, 66, 1817–1829. [Google Scholar] [CrossRef] [PubMed]
- Poletto, G.; Vilardell, J.; Marin, O.; Pagano, M.A.; Cozza, M.A.; Sarno, S.; Falqués, A.; Itarte, E.; Pinna, L.A.; Meggio, F. The regulatory β subunit of protein kinase CK2 contributes to the recognition of the substrate consensus sequence. A study with an eIF2β-derived peptide. Biochemistry 2008, 47, 8317–8325. [Google Scholar] [CrossRef]
- Bibby, A.C.; Litchfield, D.W. The multiple personalities of the regulatory subunit of protein kinase CK2: CK2 dependent and CK2 independent roles reveal a secret identity for CK2β. Int. J. Biol. Sci. 2005, 1, 67–79. [Google Scholar] [CrossRef]
- Guerra, B.; Siemer, S.; Boldyreff, B.; Issinger, O.-G. Protein kinase CK2: Evidence for a protein kinase CK2β subunit fraction devoid from the catalytic CK2α subunit, in mouse brain and testicles. FEBS Lett. 1999, 462, 353–357. [Google Scholar] [CrossRef]
- Seldin, D.C.; Lou, D.Y.; Toselli, P.; Landesman-Bollag, E.; Dominguez, I. Gene targeting of CK2 catalytic subunits. Mol. Cell. Biochem. 2008, 316, 141–147. [Google Scholar] [CrossRef]
- Landesman-Bollag, E.; Belkina, A.; Hovey, B.; Connors, E.; Cox, C.; Seldin, D.C. Developmental and growth defects in mice with combined deficiency of CK2 catalytic genes. Mol. Cell. Biochem. 2011, 356, 227–231. [Google Scholar] [CrossRef][Green Version]
- Buchou, T.; Vernet, M.; Blond, O.; Jensen, H.H.; Pointu, H.; Olsen, B.B.; Cochet, C.; Issinger, O.G.; Boldyreff, B. Disruption of the regulatory beta subunit of protein kinase CK2 in mice leads to a cell-autonomous defect and early embryonic lethality. Mol. Cell. Biol. 2003, 23, 908–915. [Google Scholar] [CrossRef]
- Glover III, C.V. On the physiological role of casein kinase II in Saccharomyces cerevisiae. Prog. Nucleic Acid Res. Mol. Biol. 1999, 59, 95–133. [Google Scholar] [CrossRef]
- Tripodi, F.; Nicastro, R.; Busnelli, S.; Cirulli, C.; Maffioli, E.; Tedeschi, G.; Alberghina, L.; Coccetti, P. Protein Kinase CK2 Holoenzyme Promotes Start-Specific Transcription in Saccharomyces cerevisiae. Eukaryot. Cell 2013, 12, 1271–1280. [Google Scholar] [CrossRef]
- Vilk, G.; Saulnier, R.B.; St. Pierre, R.; Litchfield, D.W. Inducible expression of protein kinase CK2 in mammalian cells. Evidence for functional specialization of CK2 isoforms. J. Biol. Chem. 1999, 274, 14406–14414. [Google Scholar] [CrossRef] [PubMed]
- Olsen, B.B.; Guerra, B. Ability of CK2β to selectively regulate cellular protein kinases. Mol. Cell. Biochem. 2008, 316, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Montenarh, M. Cellular regulators of protein kinase CK2. Cell Tissue Res. 2010, 342, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Krogan, N.J.; Cagney, G.; Yu, H.; Zhong, G.; Guo, X.; Ignatchenko, A.; Li, J.; Pu, S.; Datta, N.; Tikuisis, A.P.; et al. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature 2006, 440, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Daganzo, S.M.; Erzberger, J.P.; Lam, W.M.; Skordalakes, E.; Zhang, R.; Franco, A.A.; Brill, S.J.; Adams, P.D.; Berger, J.M.; Kaufman, P.D. Structure and function of the conserved core of histone deposition protein ASF1. Curr. Biol. 2003, 13, 2148–2158. [Google Scholar] [CrossRef]
- Sanematsu, F.; Takami, Y.; Barman, H.K.; Fukagawa, T.; Ono, T.; Shibahara, K.; Nakayama, T. Asf1 is Required for viability and chromatin assembly during DNA replication in vertebrate cells. J. Biol. Chem. 2006, 281, 13817–13827. [Google Scholar] [CrossRef]
- Relaix, F.; Wei, X.; Li, W.; Pan, J.; Lin, Y.; Bowtell, D.D.; Sassoon, D.A.; Wu, X. Pw1/Peg3 is a potential cell death mediator and cooperates with Siah1a in p53-mediated apoptosis. Proc. Natl. Acad. Sci. USA 2000, 97, 2105–2110. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, H.; Zhang, X.; He, X.; Xu, X. Comprehensive analysis of pan-cancer reveals potential of ASF1B as a prognostic and immunological biomarker. Cancer Med. 2021, 10, 6897–6916. [Google Scholar] [CrossRef]
- Mousson, F.; Ochsenbein, F.; Mann, C. The histone chaperone Asf1 at the crossroads of chromatin and DNA checkpoint pathways. Chromosoma 2007, 116, 79–93. [Google Scholar] [CrossRef]
- Yamaki, M.; Umehara, T.; Chimura, T.; Horikoshi, M. Cell death with predominant apoptotic features in Saccharomyces cerevisiae mediated by deletion of the histone chaperone ASF1/CIA1. Genes Cells 2001, 6, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Alcasabas, A.A.; Elledge, S.J. Asf1 links Rad53 to control of chromatin assembly. Genes Dev. 2001, 15, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Jach, M.; Kubiński, K.; Zieliński, R.; Sajnaga, E.; Szyszka, R. Asf1–yeast inductor of apoptosis inhibits CK2 activity in vitro. Cell. Mol. Biol. Lett. 2005, 10 (Suppl. 2), 121–122. [Google Scholar]
- Baier, A.; Galicka, A.; Nazaruk, J.; Szyszka, R. Selected flavonoid compounds as promising inhibitors of protein kinase CK2α and CK2α’, the catalytic subunits of CK2. Phytochemistry 2017, 136, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Janeczko, M.; Orzeszko, A.; Kazimierczuk, Z.; Szyszka, R.; Baier, A. CK2α and CK2α’ subunits differ in their sensitivity to 4,5,6,7-tetrabromo- and 4,5,6,7-tetraiodo-1H-benzimidazole derivatives. Eur. J. Med. Chem. 2012, 47, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Janeczko, M.; Masłyk, M.; Szyszka, R.; Baier, A. Interactions between subunits of protein kinase CK2 and their protein substrates influences its sensitivity to specific inhibitors. Mol. Cell. Biochem. 2011, 356, 121–126. [Google Scholar] [CrossRef]
- Baier, A.; Nazaruk, J.; Galicka, A.; Szyszka, R. Inhibitory influence of natural flavonoids on human protein kinase CK2 isoforms: Effect of the regulatory subunit. Mol. Cell. Biochem. 2018, 444, 35–42. [Google Scholar] [CrossRef]
- Kubiński, K.; Zieliński, R.; Hellman, U.; Mazur, E.; Szyszka, R. Yeast transcription elongation factor–Elf1 is phosphorylated and interact with protein kinase CK2. J. Biochem. Mol. Biol. 2006, 39, 311–318. [Google Scholar] [CrossRef]
- Masłyk, M.; Kochanowicz, E.; Zieliński, R.; Kubiński, K.; Hellman, U.; Szyszka, R. Yeast surviving factor Svf1 as a new interacting partner, regulator and in vitro substrate of protein kinase CK2. Mol. Cell. Biochem. 2008, 312, 61–69. [Google Scholar] [CrossRef]
- Zieliński, R.; Hellman, U.; Kubiński, K.; Szyszka, R. Fip1—an essential component of the Saccharomyces cerevisiae polyadenylation machinery is phosophorylated by protein kinase CK2. Mol. Cell. Biochem. 2006, 286, 191–197. [Google Scholar] [CrossRef]
- Jeffrey, G.A. An Introduction to Hydrogen Bonding (Topics in Physical Chemistry); Oxford University Press: Oxford, UK, 1997; ISBN 0-19-509549-9. [Google Scholar]
- Kubiński, K.; Domańska, K.; Sajnaga, E.; Mazur, E.; Zieliński, R.; Szyszka, R. Yeast holoenzyme of protein kinase CK2 requires both β and β’ regulatory subunits for its activity. Mol. Cell. Biochem. 2007, 295, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Sajnaga, E.; Kubiński, K.; Szyszka, R. Catalytic activity of mutants of yeast protein kinase CK2α. Acta Biochim. Pol. 2008, 55, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Turowec, J.P.; Duncan, J.S.; French, A.C.; Gyenis, L.; St. Denis, N.A.; Vilk, G.; Litchfield, D.W. Protein kinase CK2 is a constitutively active enzyme that promotes cell survival: Strategies to identify CK2 substrates and manipulate its activity in mammalian cells. Meth. Enzymol. 2010, 484, 471–493. [Google Scholar] [CrossRef]
- Kurcinski, M.; Jamroz, M.; Blaszczyk, M.; Kolinski, A.; Kmiecik, S. CABS-dock web server for the flexible docking of peptides to proteins without prior knowledge of the binding site. Nucleic Acids Res. 2015, 43, W419–W424. [Google Scholar] [CrossRef]
- Blaszczyk, M.; Kurcinski, M.; Kouza, M.; Wieteska, L.; Debinski, A.; Kolinski, A.; Kmiecik, S. Modeling of protein–peptide interactions using the CABS-dock web server for binding site search and flexible docking. Methods 2016, 93, 72–83. [Google Scholar] [CrossRef]
- Ciemny, M.P.; Kurcinski, M.; Kozak, J.K.; Kolinski, A.; Kmiecik, K. Highly flexible protein–peptide docking using CABS-Dock. Methods Mol. Biol. 2017, 1561, 69–94. [Google Scholar] [CrossRef]
- London, N.; Nir, L.; Barak, R.; Schueler-Furman, O. Peptide docking and structure-based characterization of peptide binding: From knowledge to know-how. Curr. Opin. Struct. Biol. 2013, 23, 894–902. [Google Scholar] [CrossRef]
- Baier, A.; Kokel, A.; Horton, W.; Gizińska, E.; Pandey, G.; Szyszka, R.; Török, B.; Török, M. Organofluorine hydrazone derivatives as multifunctional Alzheimer’s agents with CK2 inhibitory and antioxidant features. ChemMedChem 2021, 16, 1927–1932. [Google Scholar] [CrossRef]

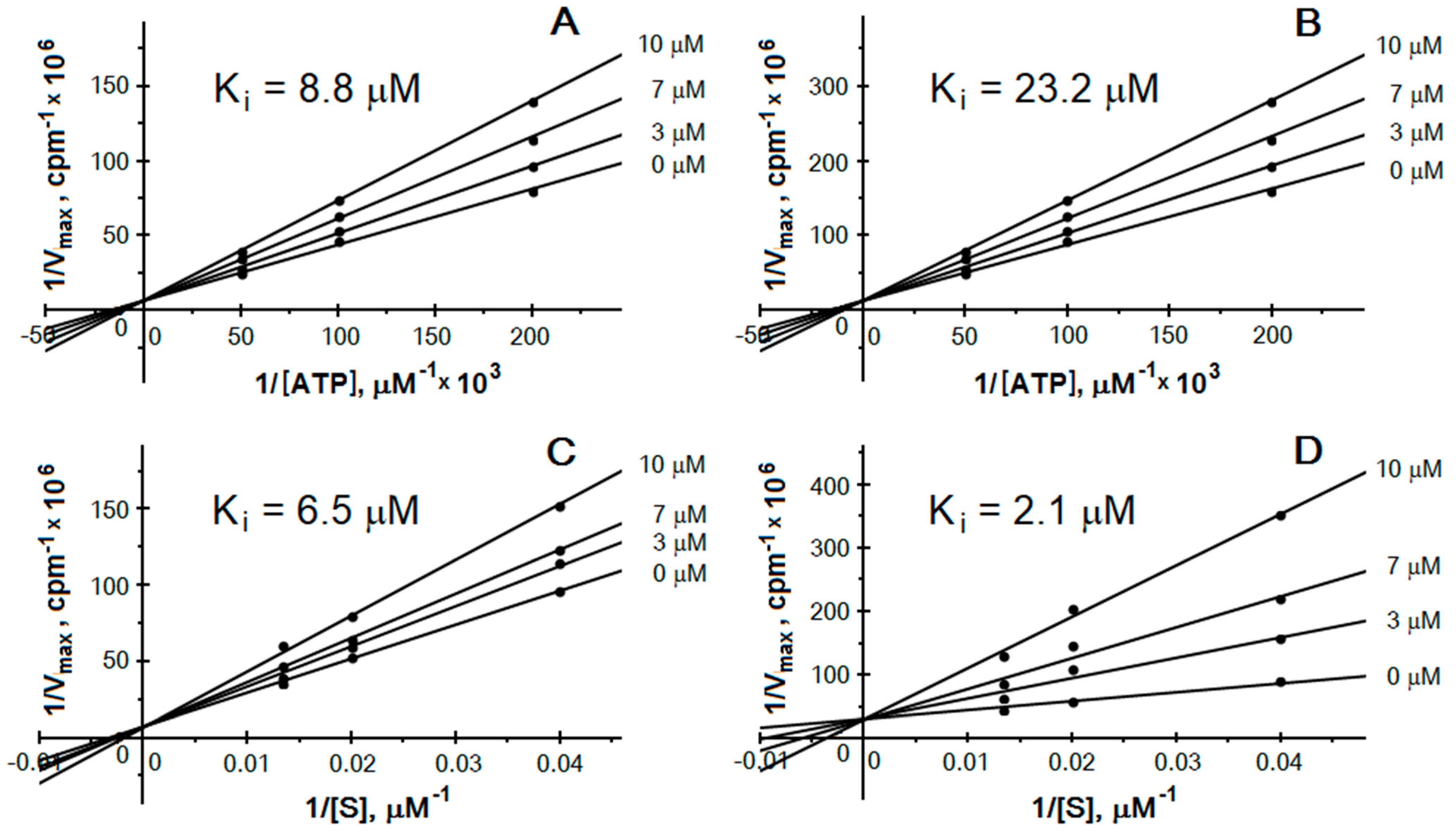
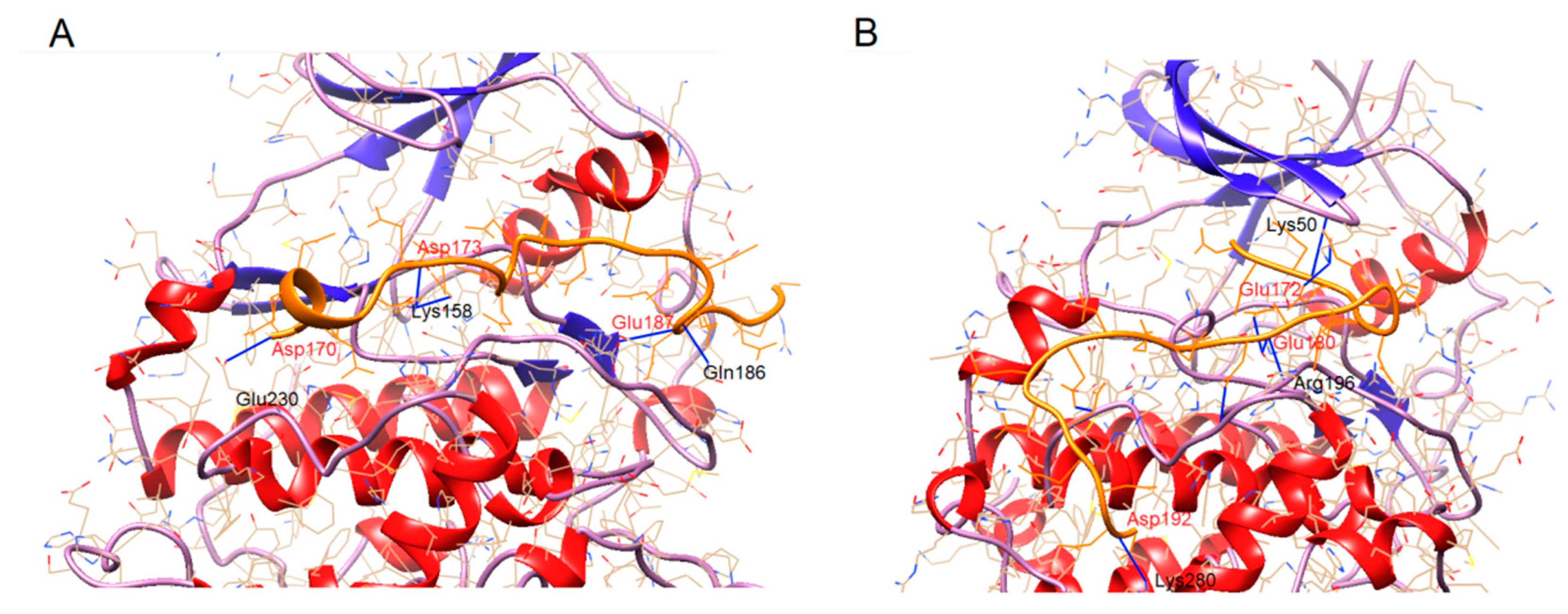
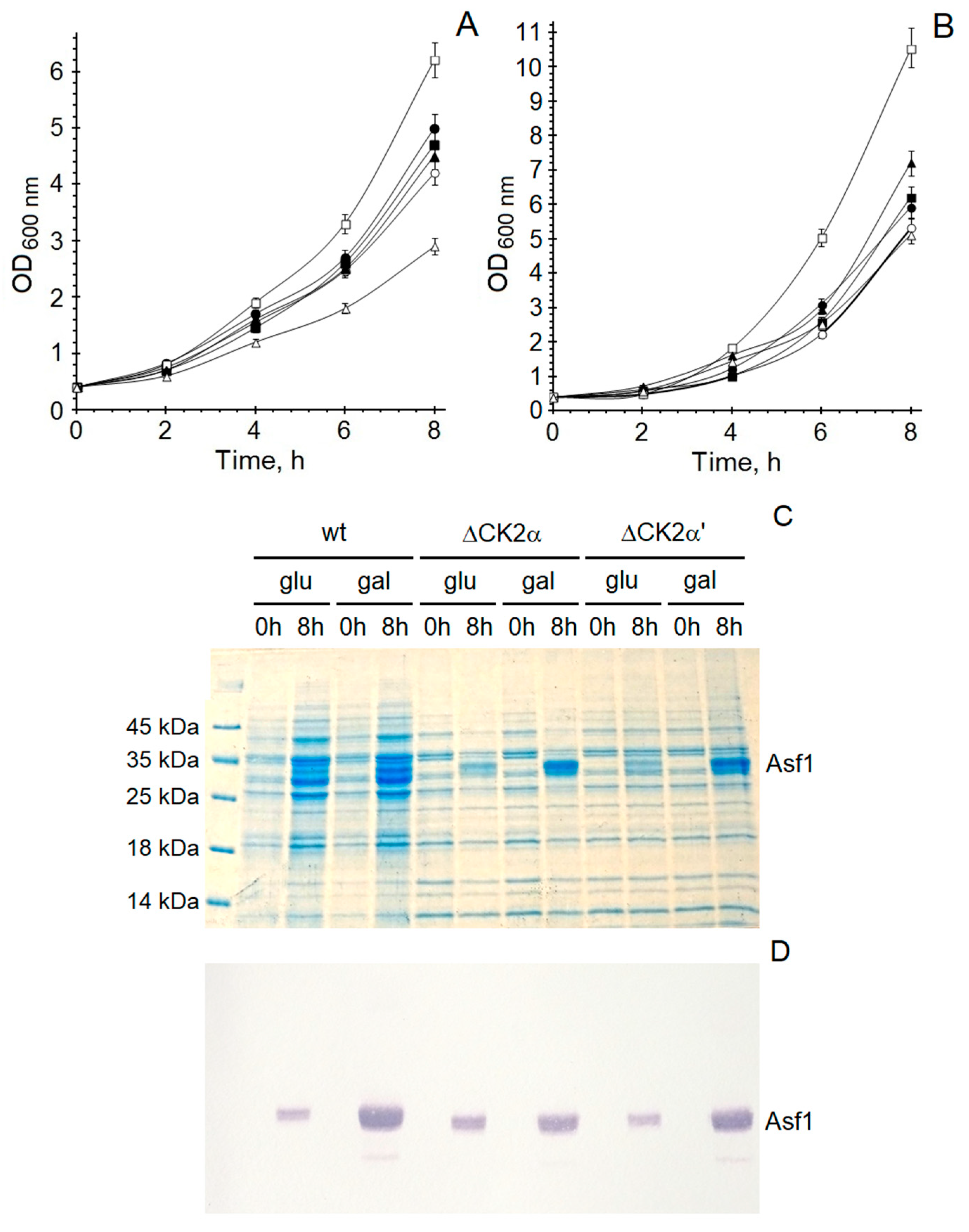
| CK2α | Asf1 170-193 | Distance (Å) | CK2α’ | Asf1 170-193 | Distance (Å) |
|---|---|---|---|---|---|
| K158 | D177 | 3.553 | K50 | E174 | 3.291 |
| K158 | E180 | 3.295 | K50 | E175 | 3.061 |
| E180 | D189 | 2.938 | K50 | E178 | 3.141 |
| Q186 | E187 | 3.302 | S195 | E180 | 2.826 |
| E230 | D170 | 2.747 | S195 | E180 | 2.845 |
| R196 | E180 | 3.362 | |||
| E231 | D186 | 3.084 | |||
| G236 | D181 | 2.983 | |||
| K280 | D192 | 3.249 |
| Protein | Expression Vector | Primer Sequences * |
|---|---|---|
| Asf1 | pET28a | 5′-CCGGATCCTCAATTGTTTCACTGTTAGGCA-3′ 5′-ACGTCGACTTAATTCGTTGAACGTGCCGCA-3′ |
| Asf11-169 | pET28a | 5′-CCGGATCCTCAATTGTTTCACTGTTAGGCA-3′ 5′-CGGTCGACTTATAC GCCGGGCTGTTCAG-3′ |
| Asf1170-279 | pET28a | 5′-ACGGATCCGATGATGAAGAGGAGGAGGACG-3′ 5′-ACGTCGACTTAATTCGTTGAACGTGCCGCA-3′ |
| Asf1 | pYES2/CT | 5′-CCAAGCTTGGAAAAATGTCAATTGTTTCACTG-3′ 5′-CCGAATTCTTAATTCGTTGAACGTGCCGC-3′ |
| Asf1170-279 | pYES2/CT | 5′-CCAAGCTTGGAAAAATGGATGATGAAGAGGAG-3′ 5′-CCGAATTCTTAATTCGTTGAACGTGCCGC-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baier, A.; Szyszka, R.; Jach, M.E. Yeast Protein Asf1 Possesses Modulating Activity towards Protein Kinase CK2. Int. J. Mol. Sci. 2022, 23, 15764. https://doi.org/10.3390/ijms232415764
Baier A, Szyszka R, Jach ME. Yeast Protein Asf1 Possesses Modulating Activity towards Protein Kinase CK2. International Journal of Molecular Sciences. 2022; 23(24):15764. https://doi.org/10.3390/ijms232415764
Chicago/Turabian StyleBaier, Andrea, Ryszard Szyszka, and Monika Elżbieta Jach. 2022. "Yeast Protein Asf1 Possesses Modulating Activity towards Protein Kinase CK2" International Journal of Molecular Sciences 23, no. 24: 15764. https://doi.org/10.3390/ijms232415764
APA StyleBaier, A., Szyszka, R., & Jach, M. E. (2022). Yeast Protein Asf1 Possesses Modulating Activity towards Protein Kinase CK2. International Journal of Molecular Sciences, 23(24), 15764. https://doi.org/10.3390/ijms232415764






